Abstract
Density functional theory calculations were performed to elucidate the mechanistic details and origins of the selectivity of the nickel-catalyzed hydroboration of vinylarenes using B2pin2/MeOH. The catalytic cycles involved four sequential elementary steps: hydronickelation, anion exchange, transmetalation, and reductive elimination. Kinetic analyses identified hydronickelation as the rate-determining step with an activation barrier of 19.8 kcal/mol, while transmetalation proceeded through a stepwise mechanism characterized by two distinct transition states. Comprehensive analyses of the relevant transition structures and energetics demonstrated that the observed R-enantioselectivity (94% ee) originated from favorable nonbonding interactions. Lastly, our calculations suggested that the Markovnikov regioselectivity was predominantly governed by steric factors rather than electronic effects.
1. Introduction
Organoboron compounds have emerged as pivotal synthetic intermediates in constructing various carbon–carbon and carbon–heteroatom bonds, prompting significant interest among organic chemists in developing efficient synthetic routes using readily available precursors [1,2,3,4]. The hydroboration of unsaturated hydrocarbons (alkenes and alkynes) represents one of the most straightforward approaches to accessing structurally diverse organoboron derivatives [5,6]. Nevertheless, the required harsh reaction conditions substantially restrict their wide application.
Transition-metal-catalyzed reactions, particularly those employing palladium (Pd) [7,8,9], copper (Cu) [10,11], rhodium (Rh) [12,13,14,15], and ruthenium (Ru) [16,17], have revolutionized modern organic synthesis, enabling remarkable advances over recent decades. Notably, the Pd- and Rh-catalyzed hydroboration reactions of unsaturated hydrocarbons have exhibited high efficiency and stereoselectivity under mild conditions [18]. However, the exorbitant cost and limited natural abundance of these noble metals have driven the search for sustainable alternatives [19]. In this context, nickel (Ni)—a group 10 transition metal and cost-effective congener of palladium—has shown considerable promise in catalyzing hydroboration reactions with comparable efficacy [20,21,22,23].
In 2021, Stanley and co-workers [24] developed a nickel-catalyzed approach for the enantioselective hydroboration of vinylarenes (1) employing bis(pinacolato)diborane (B2pin2) and methanol (MeOH) as the boron and hydrogen sources, which afforded chiral benzylic boronate ester (3) yields of up to 92%. A catalytic system consisting of bis(1,5-cyclooctadiene)nickel(0) [Ni(cod)2] and a chiral bisoxazoline ligand (L) enabled the transformation to proceed with excellent enantioselectivity, generating an R-configured stereocenter in 3 with up to 94% ee. Notably, the reaction exhibited remarkable Markovnikov selectivity, with the anti-Markovnikov byproduct 3′ merely being formed in trace amounts.
Although a number of computational studies have elucidated the mechanisms of transition-metal-catalyzed hydroboration reactions [25,26,27], the theoretical understanding of nickel-catalyzed variants remains notably underdeveloped. For example, Dang [28] and Meng [29] have independently explored the detailed mechanism of the nickel-catalyzed hydroarylation of styrene with arylboronic acid and MeOH using a density functional theory (DFT) approach; likewise, Ritleng’s group [30] conducted a combined experimental and theoretical study on the nickel-catalyzed hydroboration of styrene with catecholborane. In contrast, mechanistic investigations employing B2pin2 and MeOH as reagents in a nickel-catalyzed hydroboration reaction remain scarce. In this study, we employed systematic DFT calculations to investigate the mechanistic details of the Ni-catalyzed hydroboration of styrene (Figure 1), with a particular emphasis on the origins of both the observed enantioselectivity and Markovnikov regioselectivity.
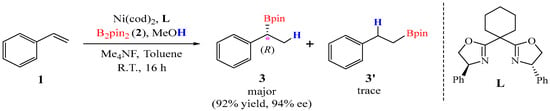
Figure 1.
Ni-catalyzed enantioselective hydroboration of styrene using B2pin2/MeOH.
2. Materials and Methods
All the theoretical calculations were completed using the Gaussian 16 computational program [31]. Geometrical optimizations in the gas phase were carried out using the B3LYP density functional method [32,33,34] with Grimme’s D3(BJ) correction [35,36] and def2SVP basis sets [37,38] (denoted as B3LYP-D3(BJ)/def2SVP in this paper). Frequency analyses were performed at the same level of theory under the conditions of 298.15 K and 1 atm. Intrinsic reaction coordinate calculations employing a Hessian-based predictor–corrector integrator were used to confirm the connectivity of some suspected transition states [39,40]. To further refine the electronic energies obtained, single-point calculations were carried out using the B3LYP-D3(BJ) method and def2TZVPP basis sets [37,38], in which the Solvation Model Density (SMD) [41] method was used to determine the solvation effect of toluene. Since the default translational entropies obtained from the Gaussian 16 output files represented ideal gas-phase values, their direct application would have artificially amplified the entropic decrease and consequently would have led to an overestimation of the free-energy barrier for bimolecular reactions in solution. To address this, we applied a scaling factor of 0.5 to the default entropies in Gibbs free-energy determinations [42,43,44]. Wavefunction analyses based on the Molecular Electron Density Theory [45,46] were performed at the B3LYP-D3(BJ)/def2TZVPP level using the Multiwfn 3.8 software package [47,48]. Molecular visualizations and three-dimensional structure representations were generated using both the CYLview (Version 1.0b) [49] and Visual Molecular Dynamics (Version 1.9.4a55) [50] programs.
3. Results and Discussion
The proposed catalytic cycle for the Ni-catalyzed hydroboration of styrene is depicted in Figure 2. The catalytic process initiates with a ligand exchange, where styrene 1 and the chiral ligand L displace two cod ligands from Ni(cod)2, generating the active precursor complex A. The subsequent hydronickelation of the coordinated styrene yields the nickel–benzyl intermediate B, with methanol serving as the source of the hydrogen transferred to the C2 position of the alkene. The reaction then proceeds through anion exchange, converting the Ni–OMe moiety in B to a Ni–F bond in intermediate C. This is followed by transmetalation with B2pin2 to form intermediate D. The catalytic cycle concludes with reductive elimination from D, which both delivers the desired product, 3, and regenerates the active species, A. The observed kinetic isotope effect (KIE = 2.7) supports the notion that hydronickelation is the rate-determining step [24]. Subsequent sections will provide a detailed mechanistic analysis of both the enantioselectivity and regioselectivity observed in this transformation.
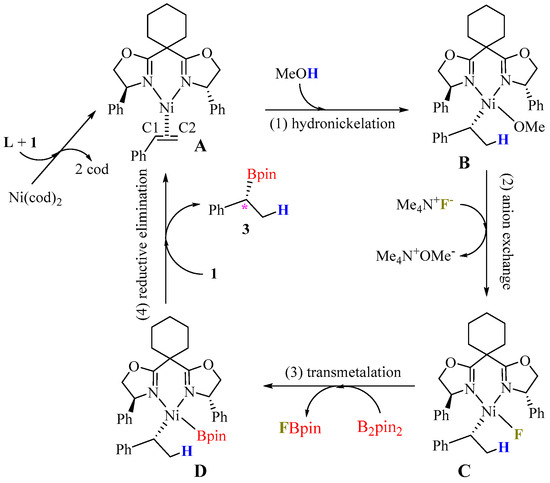
Figure 2.
Proposed catalytic cycles of Ni-catalyzed hydroboration of styrene.
3.1. Reaction Mechanism and Free-Energy Profiles
Figure 3 illustrates the detailed mechanism and free-energy profiles of the nickel-catalyzed hydroboration of styrene 1 with B2pin2/MeOH, calculated at the B3LYP-D3(BJ)/def2TZVPP//B3LYP-D3(BJ)/def2SVP level of theory. The NiL(cod) catalyst is formed through ligand substitution, in which the L ligand coordinates to the nickel center in Ni(cod)2 to displace one cod ligand. Subsequently, the remaining cod ligand in the catalyst is replaced by styrene 1, generating the active precursor complex pre-com. The hydronickelation of styrene 1 proceeds via the transition state TS1Ra, during which a hydrogen-bonded methanol dimer [(HOMe)2] acts as a proton donor to deliver a hydrogen atom to the C2 position of 1. Meanwhile, the metal center of the catalyst assumes the C1 position, providing the chiral nickel–benzyl intermediate INT1Ra. An anion exchange between INT1Ra and [Me4N]F facilitates the conversion of the Ni–OMe coordination bond in INT1Ra to the Ni–F one in INT1-1Ra, with the release of [Me4N]OMe as a co-product. Then, the B–F bond formation between the boron atom of B2pin2 and the catalyst-tethered fluorine atom enables the assembly of the transmetalation precursor INT1-2Ra.
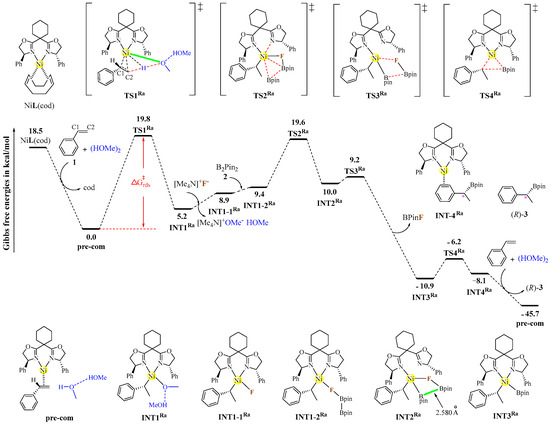
Figure 3.
Identified reaction mechanism and free-energy profiles (in kcal/mol) of the nickel-catalyzed hydroboration of styrene 1 with B2pin2/MeOH, calculated at the B3LYP-D3(BJ)/def2TZVPP//B3LYP-D3(BJ)/def2SVP level of theory. Each transition state is labeled with a “‡” superscript in its molecular structure.
The transmetalation process follows a two-step mechanism involving two transition states. In the first transition state (TS2Ra), concomitant with the movement of two boron atoms toward the nickel center, the dissociation of one N-arm from the L ligand occurs, alongside the partial cleavage of the B–B bond (i.e., the B···B distance is 2.580 Å in the resultant species INT2Ra). The subsequent step via TS3Ra is responsible for complete B–B bond cleavage, with the concomitant liberation of FBpin as a co-product and the formation of intermediate INT3Ra. The final C–B-bond-forming reductive elimination proceeds via a three-center transition state, TS4Ra, to yield INT4Ra, in which the desired hydroboration product, 3, is attached to the nickel center through a Ni···π interaction. Catalyst regeneration is achieved through a ligand exchange between product 3 and styrene 1, releasing the desired product and closing the catalytic cycles.
The conversion of the NiL(cod) catalyst to pre-com is exergonic in free energy by 18.5 kcal/mol, establishing the latter species as the reference point for subsequent free-energy values. The hydronickelation step with an activation barrier of 19.8 kcal/mol was identified as the rate-determining step, which is consistent with experimental observations, including under mild reaction conditions and in the presence of kinetic isotope effects [24]. It should be pointed out that Dang’s group [28] has located and characterized a similar transition state in the initial hydronickelation step of the nickel-catalyzed hydroarylation of styrene with arylboronic acid and MeOH, with an activation barrier of 23.0 kcal/mol. The transmetalation process exhibits two low-lying transition states (TS2Ra: 10.2 kcal/mol; TS3Ra: −0.8 kcal/mol), and the reductive elimination step occurs favorably via TS4Ra with a low barrier of 4.7 kcal/mol. At the B3LYP-D3(BJ)/def2SVP level of theory, TS3Ra is slightly higher in energy than INT2Ra, but this energetic ordering is reversed after single-point calculations at the B3LYP-D3(BJ)/def2TZVPP level of theory. The overall reaction is strongly exergonic in free energy (ΔG = −45.7 kcal/mol), demonstrating that the proposed mechanism is favorable both thermodynamically and kinetically. The Cartesian coordinates of the optimized stationary points and the complete energetic terms are provided in the Supplementary Materials (Tables S1–S4).
Figure 4 displays the optimized three-dimensional geometries of the transition states TS2Ra and TS3Ra involved in the transmetalation process. The B···B distance in TS2Ra (2.139 Å) shows significant elongation compared to that in INT1-2Ra (1.719 Å), indicating the substantial weakening of the B–B bond. A further progression to TS3Ra reveals complete B–B bond cleavage, evidenced by the extended B···B distance of 2.683 Å, which is 0.1 Å longer than that in INT2Ra. After overcoming TS3Ra, a molecule of FBpin is liberated from the complex.
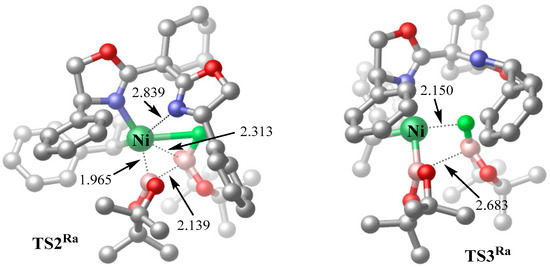
Figure 4.
Optimized three-dimensional geometries of TS2Ra and TS3Ra (interatomic distances in Å; color code: F, light green; O, red; C, gray; N, blue; B, pink; hydrogen atoms are omitted for simplicity).
The experimental results indicated that substituting methanol (MeOH) with n-butanol (nBuOH) or isopropanol (iPrOH) as the hydrogen source decreased the reaction yield to 16% or trace amounts, respectively [24]. To elucidate the alcohol-dependent reactivity trend, we evaluated the activation barriers for the hydronickelation step with nBuOH and iPrOH. The calculated barriers (20.6 and 21.7 kcal/mol for nBuOH and iPrOH, respectively) were qualitatively consistent with the experimentally observed reactivity order (MeOH > nBuOH > iPrOH), supporting the hypothesis that bulkier alcohols hinder the reaction kinetics.
3.2. Selectivity Issues
3.2.1. Enantioselectivity
The hydronickelation of the C=C bond in styrene 1 is the enantioselectivity-determining step, dictating the stereochemical outcome at the chiral center of product 3. Figure 5 illustrates how two distinct hydronickelation pathways bifurcate to form (R)-3 and (S)-3, respectively. (1) Re-face pathway: The coordination of the styrene’s Re-face to the Ni center results in the trans disposition of the two adjacent phenyl groups, and the subsequent transfer of hydrogen from methanol to the C2 position of styrene in this intermediate yields the R-configured benzylic carbon. (2) Si-face pathway: The binding of the Si-face to the Ni center induces a cis arrangement of the two phenyl groups, with the transfer of hydrogen to C2 ultimately generating the S-configured product.
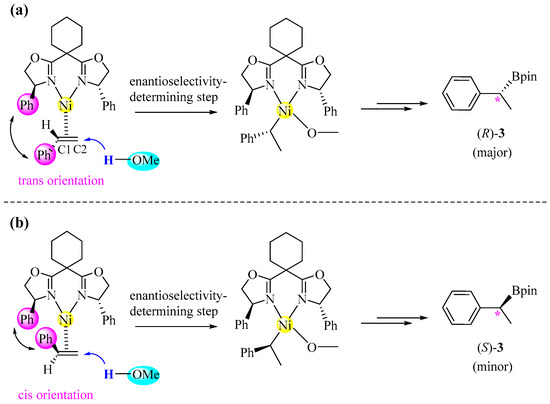
Figure 5.
Proposed stereochemical model for hydronickelation step, showing formation of R- or S-configured product. (a) Re-face pathway producing (R)-3; (b) Si-face pathway producing (S)-3.
The DFT calculations identified two distinct transition states for both the Re-face and Si-face hydronickelation pathways, as visualized in the three-dimensional structural representations in Figure 6. For the Re-face pathway yielding (R)-3, the transition states TS1Ra and TS1Rb differ primarily in the spatial orientation of the methanol dimer (above/below the coordination plane). Analogously, the Si-face pathway yielding (S)-3 exhibits the transition states TS1Sa and TS1Sb with similar geometric distinctions. The calculated activation barriers indicate TS1Ra (ΔG‡ = 19.8 kcal/mol) and TS1Sb (ΔG‡ = 22.6 kcal/mol) to be the dominant transition states for the formation of (R)-3 and (S)-3, respectively. The difference (2.8 kcal/mol) in the activation barriers can be translated to an R/S ratio of 113:1, generally aligning with experimental observations (94% ee). [24] Structural comparisons revealed a shorter Ni···OMe distance in TS1Ra (2.555 Å vs. 2.925 Å in TS1Sb), while reduced density gradient (RDG) analyses [51] supported the idea that the enantioselectivity stems from nonbonding interactions in these transition states.
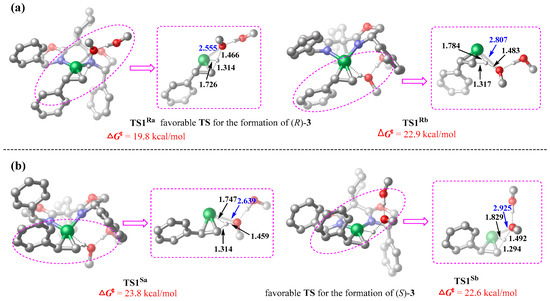
Figure 6.
Optimized three-dimensional geometries of the relevant hydronickelation transition states (interatomic distances in Å; the hydrogen atoms on the carbon atoms are omitted for simplicity) and the corresponding free-energy barriers (in kcal/mol), calculated at the B3LYP-D3(BJ)/def2TZVPP//B3LYP-D3(BJ)/def2SVP level of theory. (a) Transition states on the Re-face pathway leading to (R)-3. (b) Transition states on the Si-face pathway leading to (S)-3.
To elucidate the origin of the enantioselectivity, RDG analyses were carried on the key transition states TS1Ra and TS1Sb (see Figure 7). It was shown that a π-π stacking interaction between two adjacent phenyl groups persists in both transition states. A pronounced C–H···π interaction can be clearly observed in TS1Ra, while this interaction is negligible in TS1Sb. The mixed blue (attractive) and red (repulsive) RDG isosurfaces around Ni···OMe indicate that this interaction contributes minimally to the stereochemical control. Therefore, the enhanced stabilization of TS1Ra originating from favorable noncovalent interactions can explain the observed enantioselectivity.
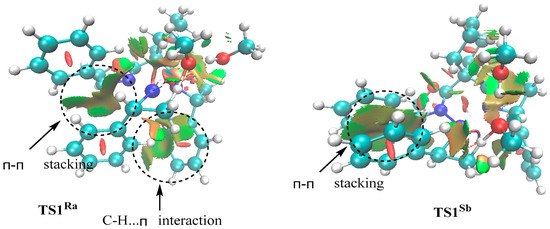
Figure 7.
RDG isosurfaces (0.5 a.u.) of TS1Ra and TS1Sb, colored based on sign(λ2)ρ (blue: strong attraction; green: van der Waals; red: steric repulsion).
To validate the enantioselectivity toward the R-configured product, we comparatively analyzed the free-energy profiles for the formation of (R)-3 and (S)-3 (Figure 8). Notably, the key transition states (TS1 and TS2) on the R-pathway exhibited lower activation barriers compared to their counterparts on the S-pathway. This energetic difference quantitatively accounts for the observed kinetic preference for (R)-3 over (S)-3.
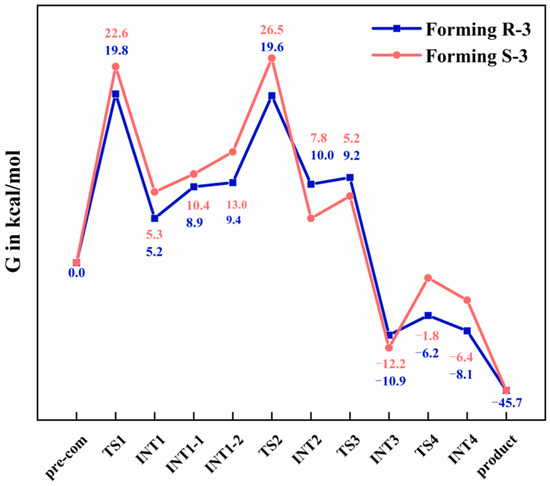
Figure 8.
Free-energy profiles (in kcal/mol) for the formation of (R)-3 (in blue) and (S)-3 (in red), calculated at the B3LYP-D3(BJ)/def2TZVPP//B3LYP-D3(BJ)/def2SVP level of theory.
Stanley and co-workers [24] observed that applying the current hydroboration protocol to 1-methyl-2-vinylbenzene (1a) or buta-1,3-dien-1-ylbenzene (1b) resulted in reduced yields and diminished enantioselectivity, with the ee values decreasing to 89% and 67%, respectively (Figure 9). The activation barriers for the formation of (R)-3a and (S)-3a computed from 1a are 20.5 and 21.9 kcal/mol, respectively, while those for (R)-3b/(S)-3b formation computed from 1b are 20.5 and 21.7 kcal/mol, respectively. The resulting ΔΔG‡values of 2.8 kcal/mol (for 1), 1.4 kcal/mol (for 1a), and 1.2 kcal/mol (for 1b) quantitatively align with the observed enantioselectivity trend: 1 > 1a > 1b.
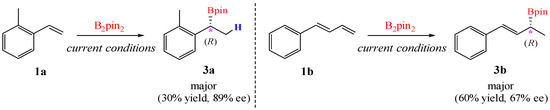
Figure 9.
Test of the substrate scope in this Ni-catalyzed enantioselective hydroboration reaction.
3.2.2. Regioselectivity
The hydronickelation of the C=C bond in styrene 1 governs the regioselectivity, preferentially yielding the Markovnikov product 3 rather than the anti-Markovnikov product 3′. DFT calculations were performed to elucidate the observed regioselectivity, identifying and characterizing the transition state (TS1′) leading to 3′, as illustrated in Figure 10. The computed free-energy barriers demonstrate that TS1 is more kinetically favored by 6.2 kcal/mol than TS1′, which aligns well with the experimental observations.
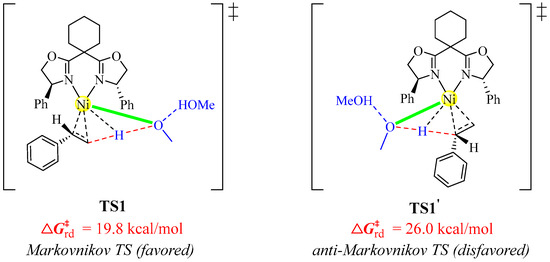
Figure 10.
Identified hydronickelation transition states yielding 3 and 3′. Each transition state is labeled with a “‡” superscript in its molecular structure.
To elucidate the origin of the observed Markovnikov selectivity, we analyzed the Fukui function isosurfaces of pre-com (see Figure 11). The nucleophilic Fukui function (f+(r)) is predominantly localized on the L ligand moiety, with a minimal distribution on the styrene moiety. In contrast, the electrophilic Fukui function (f−(r)) exhibits significant density on the styrene moiety, suggesting that the transfer of hydrogen from methanol to the olefinic carbon proceeds via a proton transfer mechanism rather than a hydride transfer pathway. The subsequent calculation of the electrophilic Fukui indices at the olefinic carbon centers (C1 and C2) of the styrene moiety revealed higher reactivity at the C1 position, which contrasts with the experimentally observed Markovnikov selectivity. This discrepancy suggests that the regioselectivity in the hydronickelation step is governed primarily by steric effects rather than electronic factors. Specifically, the transfer of protons from methanol to the C1 position is sterically hindered by repulsive interactions with adjacent phenyl groups, leading to the destabilization of the corresponding transition state (TS1′).
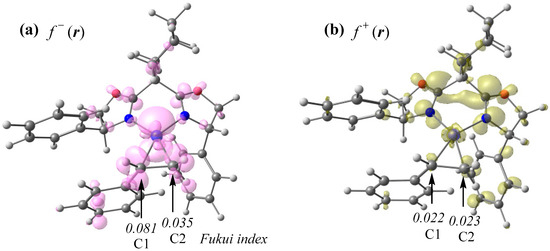
Figure 11.
(a) Electrophilic Fukui function (f−(r)) and (b) nucleophilic Fukui function (f+(r)) isosurfaces (0.003 a.u.) of pre-com, with the corresponding electrophilic Fukui indices calculated at the olefinic carbon centers of the styrene moiety.
4. Conclusions
The mechanistic details of the nickel-catalyzed enantioselective hydroboration of vinylarenes using B2pin2/MeOH were elucidated through comprehensive DFT calculations, which revealed the following key findings:
- (1)
- The catalytic cycle comprises several sequential elementary steps, namely hydronickelation, anion exchange, transmetalation, and reductive elimination. The kinetic analysis identified hydronickelation as the rate-determining step, with an activation barrier of 19.8 kcal/mol. Notably, the transmetalation process follows a stepwise mechanism characterized by two distinct transition states corresponding to B–B bond cleavage.
- (2)
- The enantioselectivity originates during the hydronickelation of styrene. The free-energy difference of 2.8 kcal/mol between the transition states yielding (R)-3 and (S)-3 accounts for the observed 94% ee. The RDG analyses attributed this selectivity to nonbonding interactions in the transition state ensemble.
- (3)
- The observed Markovnikov selectivity appears to be governed by a steric factor, by which the proton transfer process on the anti-Markovnikov pathway is hindered by repulsive interactions with adjacent phenyl groups. This study provides a theoretical basis for the future design of novel chiral ligands and the enhancement of enantioselectivity for nickel-catalyzed hydroboration.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/org6030030/s1: Table S1. Cartesian coordinates of the optimized stationary points in the main reaction pathway, calculated at the B3LYP-D3(BJ)/def2SVP level; Table S2. Cartesian coordinates of the optimized stationary points involved in the discussion of selectivity, calculated at the B3LYP-D3(BJ)/def2SVP level; Table S3. Imaginary frequencies of all the transition states in this work, calculated at the B3LYP-D3(BJ)/def2SVP level; Table S4. Electronic energies (E in au), Gibbs free energies (G in au), and entropies (S in cal·mol−1·K−1) calculated at the B3LYP-D3(BJ)/def2SVP level and single-point energies (E’ in au) calculated at the B3LYP-D3(BJ)/def2TZVPP level.
Author Contributions
Conceptualization, J.W.; methodology, J.Z. and P.S.; investigation, J.W.; data curation, X.W. and C.W.; writing—original draft preparation, J.W. and Y.Z.; writing—review and editing, L.Z. and J.Z.; supervision, Y.Z. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 22003045).
Data Availability Statement
The data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Simonetti, S.O.; Pellegrinet, S.C. Asymmetric organocatalytic C-C bond forming reactions with organoboron compounds: A mechanistic survey. Eur. J. Org. Chem. 2019, 2019, 2956–2970. [Google Scholar] [CrossRef]
- Tian, J.; Li, R.; Tian, G.; Wang, X. Enantioselective C3-allylation of pyridines via tandem borane and palladium catalysis. Angew. Chem. Int. Ed. 2023, 62, e202307697. [Google Scholar] [CrossRef]
- Jiang, N.; Chen, D.; Liu, C. Recent advances in the chemistry of α-oxylboronate reagents. Org. Chem. Front. 2023, 10, 3684–3700. [Google Scholar] [CrossRef]
- Hirva, P.; Turhanen, P.; Timonen, J.M. Synthesis and theoretical studies of aromatic azaborines. Organics 2022, 3, 196–209. [Google Scholar] [CrossRef]
- Xu, G.; Han, H.; Cao, L.; Hong, S.; Hai, L.; Cui, X. Research progress of transition metal-catalyzed synthesis of 1,3-conjugated dienyl boron compounds. Chin. J. Org. Chem. 2024, 44, 1480–1493. [Google Scholar] [CrossRef]
- Feng, Y.L.; Zhang, B.; Xu, Y.; Jin, S.; Mazzarella, D.; Cao, Z. The reactivity of alkenyl boron reagents in catalytic reactions: Recent advances and perspectives. Org. Chem. Front. 2024, 11, 7249–7277. [Google Scholar] [CrossRef]
- D’Andrea, L.; Steinmann, C. Pd EnCat™ 30 recycling in suzuki cross-coupling reactions. Organics 2024, 5, 443–449. [Google Scholar] [CrossRef]
- Kanno, S.; Kakiuchi, F.; Kochi, T. Palladium-catalyzed hydroboration/cyclization of 1,n-dienes. J. Org. Chem. 2023, 88, 2621–2630. [Google Scholar] [CrossRef]
- Yang, M.; Yu, Y.; Ma, W.; Feng, Y.; Zhang, G.; Wu, Y.; Zhou, F.; Yang, Y.; Liu, D. Palladium-catalyzed hydroboration reaction of unactivated alkynes with bis(pinacolato) diboron in water. RSC Adv. 2022, 12, 9815–9820. [Google Scholar] [CrossRef]
- Buchbinder, N.W.; Nguyen, L.H.; Beck, O.N.; Bage, A.D.; Slebodnick, C.; Santos, W.L. Chemo-, regio-, and stereoselective cis-hydroboration of 1,3-enynes: Copper-catalyzed access to (Z,Z)- and (Z,E)-2-boryl-1,3-dienes. Org. Lett. 2024, 26, 6136–6141. [Google Scholar] [CrossRef]
- Zheng, W.; Tan, B.B.; Ge, S.; Lu, Y. Enantioselective copper-catalyzed ring-opening diboration of arylidenecyclopropanes to access chiral skipped 1,4- and 1,3-diboronates. J. Am. Chem. Soc. 2024, 146, 5366–5374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Chen, K.Z.; Li, A.Z.; Li, B.J. Remote stereocenter through amide-directed, rhodium-catalyzed enantioselective hydroboration of unactivated internal alkenes. J. Am. Chem. Soc. 2022, 144, 13071–13078. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Park, S. Rhodium-catalyzed double hydroboration of quinolines. ACS Catal. 2023, 13, 7067–7078. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Z.; Zhao, W. Rhodium-catalyzed remote borylation of alkynes and vinylboronates. Angew. Chem. Int. Ed. 2023, 62, e202215455. [Google Scholar]
- Zhang, B.; Xu, X.; Tao, L.; Lin, Z.; Zhao, W. Rhodium-catalyzed regiodivergent synthesis of alkylboronates via deoxygenative hydroboration of aryl ketones: Mechanism and origin of selectivities. ACS Catal. 2021, 11, 9495–9505. [Google Scholar] [CrossRef]
- Tan, Y.; Li, S.; Song, L.; Zhang, X.; Wu, Y.; Sun, J. Ruthenium-catalyzed geminal hydroborative cyclization of enynes. Angew. Chem. Int. Ed. 2022, 61, e202204319. [Google Scholar] [CrossRef]
- Pradhan, S.; Thiyagarajan, S.; Gunanathan, C. Ruthenium(ii)-catalysed 1,2-selective hydroboration of aldazines. Org. Biomol. Chem. 2021, 19, 7147–7151. [Google Scholar] [CrossRef]
- Choy, P.Y.; Tse, M.H.; Kwong, F.Y. Recent expedition in PD- and RH-catalyzed C(AR)-B bond formations and their applications in modern organic syntheses. Chem. Asian J. 2023, 18, e202300649. [Google Scholar] [CrossRef]
- Ananikov, V.P. Nickel: The “Spirited horse” of transition metal catalysis. ACS Catal. 2015, 5, 1964–1971. [Google Scholar] [CrossRef]
- De, S.K. Applications of nickel(II) compounds in organic synthesis. Curr. Org. Synth. 2021, 18, 517–534. [Google Scholar] [CrossRef]
- Magallón, C.; Griego, L.; Hu, C.H.; Company, A.; Ribas, X.; Mirica, L.M. Organometallic Ni(ii), Ni(iii), and Ni(iv) complexes relevant to carbon-carbon and carbon-oxygen bond formation reactions. Inorg. Chem. Front. 2022, 9, 1016–1022. [Google Scholar] [CrossRef]
- Saunders, T.M.; Shepard, S.B.; Hale, D.J.; Robertson, K.N.; Turculet, L. Highly selective Nickel-catalyzed isomerization-hydroboration of alkenes affords terminal functionalization at remote C-H position. Chem. Eur. J. 2023, 29, e202301946. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Long, J.; Li, Y.; Wang, W.; Pang, H.; Yin, G. Nickel-catalyzed regioselective arylboration of conjugated dienes. Eur. J. Org. Chem. 2021, 2021, 1424–1428. [Google Scholar] [CrossRef]
- Tran, H.N.; Stanley, L.M. Nickel-catalyzed enantioselective hydroboration of vinylarenes. Org. Lett. 2021, 24, 395–399. [Google Scholar] [CrossRef]
- Mi, J.; Huo, S.; Meng, L.; Li, X. Mechanism and regioselectivity of [Cu-Fe] heterobimetallic-catalyzed hydroboration of pyridines: DFT investigation. Mol. Catal. 2021, 511, 111722. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Wen, Y.; Ma, X.; Zhang, J.; Zhou, J.; Meng, X. DFT study on copper-catalyzed hydroboration of 1,3-diynes: Mechanism, selectivity, and comparison with ruthenium. Asian J. Org. Chem. 2025, 14, e202400430. [Google Scholar] [CrossRef]
- Wen, Y.; Gu, Y.; Fei, X.; Kang, J.; Li, G.; Zhang, L. Computational study on the reaction mechanism of phosphine-catalyzed hydroboration of propiolonitriles: With cyano group or not? Tetrahedron 2024, 155, 133933. [Google Scholar] [CrossRef]
- Cheng, Q.; Dang, Y. Mechanistic studies of nickel-catalyzed hydroarylation of styrenes. Org. Lett. 2020, 22, 8998–9003. [Google Scholar] [CrossRef]
- Wang, F.; Meng, Q. Theoretical insight into Ni(0)-catalyzed hydroarylation of alkenes and arylboronic acids. J. Org. Chem. 2020, 85, 13264–13271. [Google Scholar] [CrossRef]
- Ulm, F.; Cornaton, Y.; Djukic, J.-P.; Chetcuti, M.J.; Ritleng, V. Hydroboration of alkenes catalysed by a nickel N-heterocyclic carbene complex: Reaction and mechanistic aspects. Chem. Eur. J. 2020, 26, 8916–8925. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density functional theochemistry. III The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.N.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. A 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange approximation. Phys. Rev. B 1988, 37, 785–789. [Google Scholar]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Fukui, K. The path of chemical reactions-the IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Hratchian, H.P.; Schlegel, H.B. Using Hessian updating to increase the efficiency of a Hessian based predictor-corrector reaction path following method. J. Chem. Theory Comput. 2005, 1, 61–69. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Huang, F.; Lu, G.; Zhao, L.; Li, H.; Wang, Z.X. The catalytic role of N-heterocyclic carbene in a metal-free conversion of carbon dioxide into methanol: A computational mechanism study. J. Am. Chem. Soc. 2010, 132, 12388–12396. [Google Scholar] [CrossRef] [PubMed]
- Plata, R.E.; Singleton, D.A. A case study of the mechanism of alcohol-mediated Morita Baylis-Hillman reactions. The importance of experimental observations. J. Am. Chem. Soc. 2015, 137, 3811–3826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, B.; Chen, Y.; Lv, J.F.; Feng, W.C. A computational study on the reaction mechanisms of nickelcatalyzed diarylation of alkenes. Eur. J. Org. Chem. 2019, 2019, 6217–6224. [Google Scholar] [CrossRef]
- Domingo, L.R. Molecular Electron Density Theory: A modern view of reactivity in organic chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef]
- Sadowski, M.; Dresler, E.; Wróblewska, A.; Jasi’nski, R. A new insight into the molecular mechanism of the reaction between 2-methoxyfuran and ethyl (Z)-3-phenyl-2-nitroprop-2-enoate: An Molecular Electron Density Theory (MEDT) computational study. Molecules 2024, 29, 4876. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
- Legault, C.Y. CYLView, 1.0b; Universite de Sherbrooke: Quebec City, QC, Canada, 2009. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics, Version 1.9.4a55; University of Illinois Urbana-Champaign: Champaign, IL, USA, 2024. [Google Scholar]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).