Synthesis of Indole-Based Derivatives Containing Ammonium Salts, Diamines and Aminoureas for Organocatalysis
Abstract
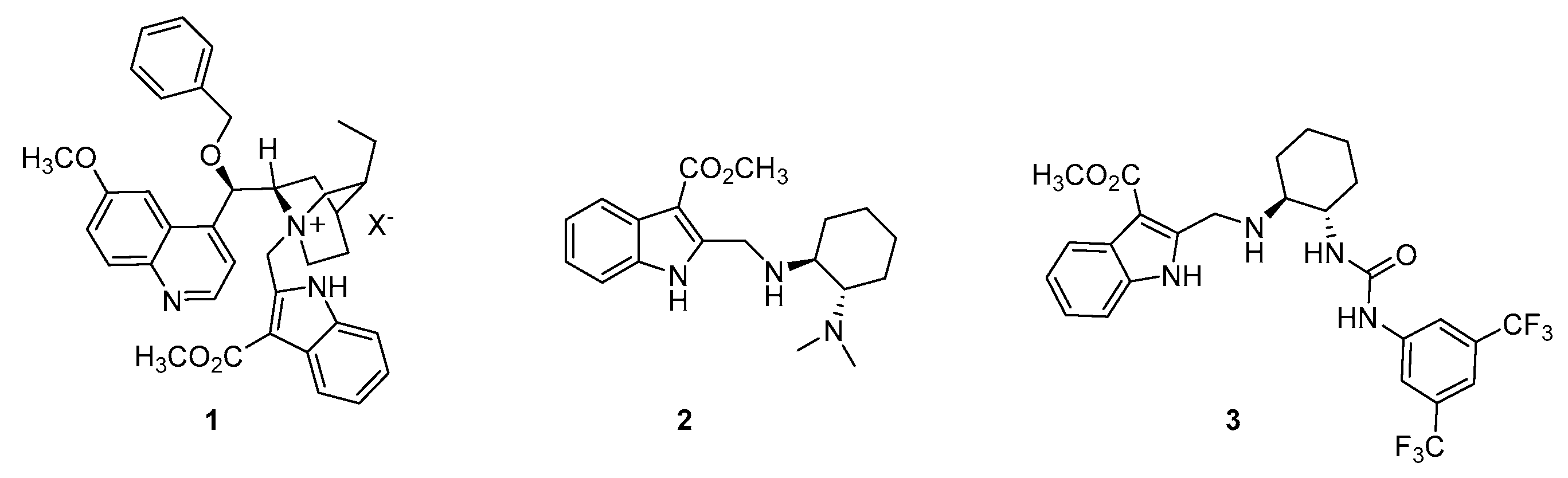
1. Introduction
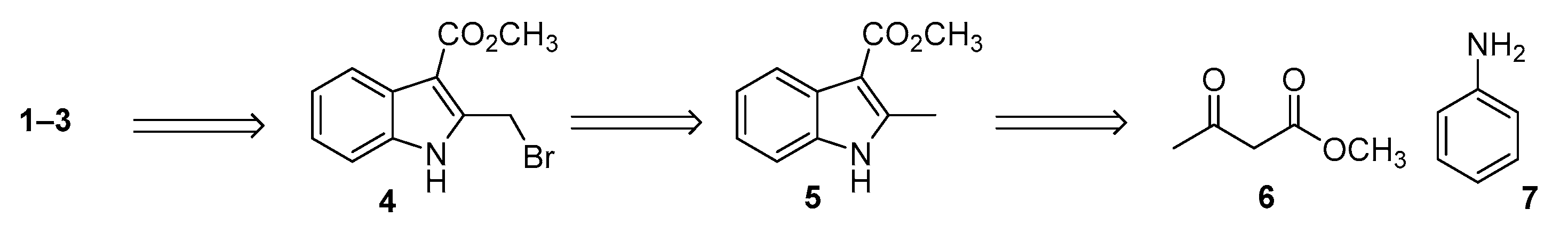
2. Results and Discussion
3. Materials and Methods
3.1. General Experiment
3.2. Synthetic Protocols for the Preparation of Key Synthon 4
3.2.1. Synthesis of Methyl (E)-3-(Phenylimino)butanoate 8
3.2.2. Synthesis of Methyl 2-Methilindole-3-carboxylate 5
3.2.3. Synthesis of 1-(Tert-butyl) 3-Methyl 2-Methyl-1H-indole-1,3-dicarboxylate 9
3.2.4. Synthesis of 1-(Tert-butyl) 3-Methyl 2-(Bromomethyl)-1H-indole-1,3-dicarboxylate 10
3.2.5. Synthesis of Methyl 2-(Bromomethyl)-1H-indole-3-carboxylate 4
3.3. Synthetic Protocols for the Preparation of Quaternary Ammonium Salt 1
3.3.1. Synthesis of O-Benzyl Hydroquinine 12
3.3.2. Synthesis of (1S,2S)-2-((R)-(Benzyloxy)(6-methoxyquinolin-4-yl)methyl)-5-ethyl-1-((3-(methoxycarbonyl)-1H-indol-2-yl)methyl)quinuclidin-1-ium Bromide 1
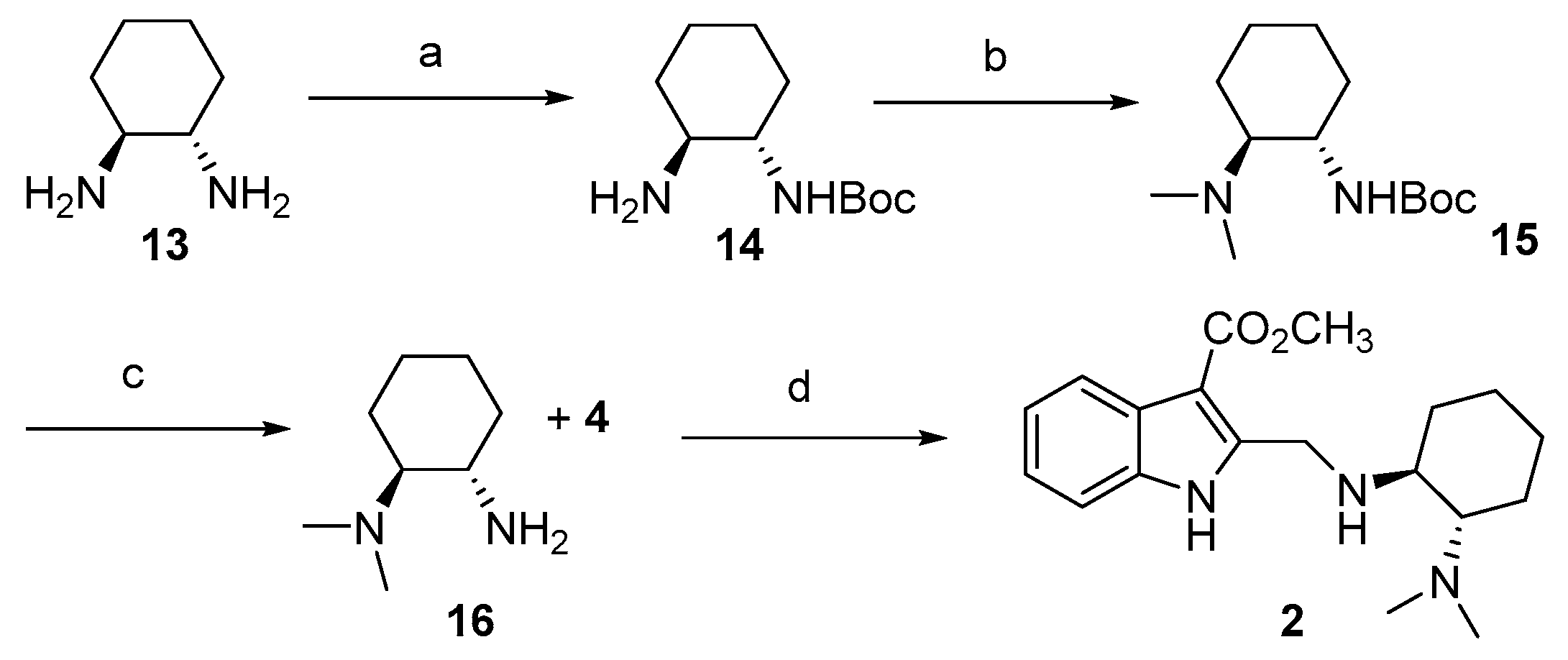
3.4. Synthetic Protocols for the Preparation of Indolediamine 2
3.4.1. Synthesis of (1S,2S)-Trans-N-Boc-1,2-cyclohexanediamine 14
3.4.2. Synthesis of Tert-butyl ((1S,2S)-2-(Dimethylamino)cyclohexyl)carbamate 15
3.4.3. Synthesis of (1S,2S)-N1,N1-Dimethylcyclohexane-1,2-diamine 16
3.4.4. Synthesis of Methyl 2-((((1S,2S)-2-(Dimethylamino)cyclohexyl)amino)methyl)-1H-indole-3-carboxylate 2
3.5. Synthetic Protocols for the Preparation of Indoleaminourea 3
3.5.1. Synthesis of 1-((1S,2S)-2-Aminocyclohexyl)-3-(3,5-bis(trifluoromethyl)phenyl)urea 19
3.5.2. Synthesis of Methyl 2-((((1S,2S)-2-(3-(3,5-Bis(trifluoromethyl)phenyl)ureido)cyclohexyl)amino)methyl)-1H-indole-3-carboxylate 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nagendra, K.K.; Neha, K.; Pankaj, A.; Naresh, K.; Chung, H.K.; Akhilesh, K.V.; Eun, H.C. Biomedical Importance of Indoles. Molecules 2013, 6, 6620–6662. [Google Scholar]
- Archana, K.; Rajesh, K.S. Medicinal chemistry of indole derivatives: Current to future therapeutic perspectives. Bioorg. Chem. 2019, 89, 103021–103036. [Google Scholar]
- James, P.N.; Snyder, H.R. Indole-3-aldehyde. Org. Synth. 1959, 39, 30–32. [Google Scholar]
- Heaney, H.; Ley, S.V. 1-Benzylindole. Org. Synth. 1974, 54, 54–58. [Google Scholar]
- Bergman, J.; Venemalm, L. Efficient synthesis of 2-chloro-,2-bromo-, and 2-iodoindole. J. Org. Chem. 1992, 57, 2495–2497. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Li, J.; Stevens, C. Facile synthesis of 2-substituted indoles and indolo[3,2-b] carbazoles from 2-(benzotriazol-1-ylmethyl) indole. J. Org. Chem. 1995, 60, 3401–3404. [Google Scholar] [CrossRef]
- Ximenes, V.F.; Campa, A.; Catalani, L.H. The oxidation of indole derivatives catalysed by horseradish peroxidase is highly chemiluminescent. Arch. Biochem. Biophys. 2001, 387, 173–179. [Google Scholar]
- Lynch, S.M.; Bur, S.K.; Padwa, A. Intramolecular amidofuran cycloadditions across an indole pi-bond: An efficient approach to the aspidosperma and strychnos ABCE core. Org. Lett. 2002, 4, 4643–4645. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Haldar, S.; Sun, S.; Koeppe II, R.E.; Chattopadhyay, A. Importance of indole NH hydrogen bonding in the organization and dynamics of gramicidin channels. Biochim. Biophys. Acta 2013, 1838, 419–428. [Google Scholar] [CrossRef]
- Chen, T.; Foo, T.J.Y.; Yeung, Y.Y. Indole-Catalyzed Bromolactonization in Lipophilic Solvent: A Solid− Liquid Phase Transfer Approach. ACS Catal. 2015, 5, 4751–4755. [Google Scholar] [CrossRef]
- Shi, Y.; Ke, Z.; Yeung, Y.Y. Environmentally benign indole-catalyzed position- selective halogenation of thioarenes and other aromatic. Green Chem. 2018, 20, 4448–4452. [Google Scholar] [CrossRef]
- Wong, J.; Ke, Z.; Yeung, Y.Y. Lipophilic indole mediated chemoselective a-monobromination of 1,3-dicarbonyl compounds. Tetrahedron Lett. 2020, 61, 151772–151775. [Google Scholar] [CrossRef]
- Shi, Y.; Wong, J.; Ke, Z.; Yeung, Y.Y. Lipophilic Indole-Catalyzed Intermolecular Bromoesterification of Olefins in Nonpolar Media. J. Org. Chem. 2019, 84, 4017–4024. [Google Scholar] [CrossRef] [PubMed]
- Bencivenni, G.; Saraiva Rosa, N.; Grieco, P.; Gillick-Healy, M.W.; Kelly, B.G.; Twamley, B.; Adamo, M.F.A. Quaternary Ammonium Salts Interact with Enolates and Sulfonates via Formation of Multiple +N-C-H Hydrogen Bonding Interactions. Catalysts 2022, 12, 803. [Google Scholar] [CrossRef]
- Bencivenni, G.; Salazar Illera, D.; Moccia, M.; Houk, K.N.; Izzo, J.A.; Novacek, J.; Grieco, P.; Vetticatt, M.J.; Waser, M.; Adamo, M.F.A. Study of Ground State Interactions of Enantiopure Chiral Quaternary Ammonium Salts and Amides, Nitroalkanes, Nitroalkenes, Esters, Heterocycles, Ketones and Fluoroamides. Chem. Eur. J. 2021, 27, 11354–11366. [Google Scholar] [CrossRef]
- Salazar Illera, D.; Pacifico, R.; Adamo, M.F.A. Asymmetric Phase Transfer Catalysed Michael Addition of γ-Butenolide and N-Boc-Pyrrolidone to 4-Nitro-5-styrylisoxazoles. Catalysts 2022, 12, 634. [Google Scholar] [CrossRef]
- Destro, D.; Bottinelli, C.; Ferrari, L.; Albanese, D.C.A.; Bencivenni, G.; Gillick Healy, M.; Kelly, B.; Adamo, M.F.A. Enantioselective Synthesis of 3, 4-Dihydropyran-2-ones via Phase-Transfer-Catalyzed Addition–Cyclization of Acetylacetone to Cinnamic Thioesters. J. Org. Chem. 2020, 85, 5183–5192. [Google Scholar] [CrossRef]
- Del Fiandra, C.; Piras, L.; Fini, F.; Disetti, P.; Moccia, M.; Adamo, M.F.A. Phase transfer catalyzed enantioselective cyclopropanation of 4-nitro-5-styrylisoxazoles. Chem. Commun. 2012, 48, 3863–3865. [Google Scholar]
- Baschieri, A.; Bernardi, L.; Ricci, A.; Suresh, S.; Adamo, M.F.A. Catalytic Asymmetric Conjugate Addition of Nitroalkanes to 4-Nitro-5-styrylisoxazoles. Angew. Chem. Int. Ed. 2009, 48, 9342–9345. [Google Scholar] [CrossRef]
- Pineda, A.; Carr, J.; Rodriguez-Padron, D.; Lazaro Ronco, N.; Fox, K.; Gonzalez-Arellano, C.; Gillick-Healy, M.W.; Kelly, B.G.; Adamo, M.F.A.; Luque, R. A continuous flow approach for the desulfurative bromination of sulfides. Sustain. Chem. Pharm. 2024, 38, 101490–101495. [Google Scholar] [CrossRef]
- Canestrani, D.; Cioffi, C.; Biancofiore, I.; Lancianesi, S.; Ghisu, L.; Reuther, M.; O´Brien, J.; Adamo, M.F.A.; Ibrahim, H. Sulphide as a leaving group: Highly stereoselective bromination of alkyl phenyl sulphides. Chem. Sci. 2019, 10, 9042–9050. [Google Scholar] [CrossRef] [PubMed]
- Alletto, F.; Adamo, M.F.A. Enantiospecific on-water bromination: A mild and efficient protocol for the preparation of alkyl bromides. Green Chem. 2020, 22, 8692–8698. [Google Scholar] [CrossRef]
- Canestrari, D.; Lancianesi, S.; Badiola, E.; Strinna, C.; Ibrahim, H.; Adamo, M.F.A. Desulfurative chlorination of alkyl phenyl sulfides. Org. Lett. 2017, 19, 918–921. [Google Scholar] [PubMed]
- Würtz, S.; Rakshit, S.; Neumann, J.J.; Dröge, T.; Glorius, F. Palladium-Catalyzed Oxidative Cyclization of N-Aryl Enamines: From Anilines to Indoles. Angew. Chem. Int. Ed. 2008, 38, 7230–7233. [Google Scholar]
- Patil, S.A.; Patil, R.; Miller, D.D. Microwave-assisted synthesis of medicinally relevant indoles. Curr. Med. Chem. 2011, 4, 615–637. [Google Scholar] [CrossRef] [PubMed]
- Nigam, V.; Singh, S.; Kasana, S.; Kumar, S.; Das Kurmi, B.; Das Gupta, G.; Patel, P. Revolutionizing Indole Synthesis: A Microwave-Powered Approach. Chem. Select 2024, 9, e202402171. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Y.; Song, H.; Liu, Y.; Wang, Q. Synthesis of Structurally Diverse 2,3-Fused Indoles via Microwave-Assisted AgSbF6-Catalysed Intramolecular Difunctionalization of o-Alkynylanilines. Sci. Rep. 2015, 5, 13516. [Google Scholar] [CrossRef]
- Bellavita, R.; Casertano, M.; Grasso, N.; Gillick-Healy, M.W.; Kelly, B.G.; Adamo, M.F.A.; Menna, M.; Merlino, F.; Grieco, P. Microwave-Assisted synthesis of 2-methyl-1H-indole-3-carboxylate derivatives via Pd-catalysed heterocyclisation. Symmetry 2022, 3, 435–445. [Google Scholar]
- Kohler, M.C.; Yost, M.J.; Garnsey, M.R.; Coltart, D.M. Direct Carbon−Carbon Bond Formation via Soft Enolization: A Biomimetic Asymmetric Mannich Reaction of Phenylacetate Thioesters. Org. Lett. 2010, 15, 3376–3379. [Google Scholar]
- Bennani, Y.L.; Hanessian, S. trans-1,2-Diaminocyclohexane Derivatives as Chiral Reagents, Scaffolds, and Ligands for Catalysis: Applications in Asymmetric Synthesis and Molecular Recognition. Chem. Rev. 1997, 97, 3161–3195. [Google Scholar]
- Kopyt, M.; Glowacki, M.P. Trans-1,2-Diaminocyclohexane and Its Derivatives in Asymmetric Organocatalysis. In Chiral Building Blocks in Asymmetric Synthesis: Synthesis and Applications; Wiley VCH, GmbH: Weinheim, Germany, 2022; ISBN 9783527349463/9783527834204. [Google Scholar]
- Lee, D.W.; Ha, H.J.; Lee, W.K. Selective Mono-BOC Protection of Diamines. Synth. Commun. 2007, 37, 737–742. [Google Scholar]
- Imperatore, C.; Valadan, M.; Tartaglione, L.; Persico, M.; Ramunno, A.; Menna, M.; Casertano, M.; Dell’Aversano, C.; Singh, M.; d’Aulisio Garigliota, M.L.; et al. Exploring the Photodynamic Properties of Two Antiproliferative Benzodiazopyrrole Derivatives. Int. J. Mol. Sci. 2020, 21, 1246. [Google Scholar] [CrossRef] [PubMed]
- Casertano, M.; Genovese, M.; Piazza, L.; Balestri, F.; Del Corso, A.; Vito, A.; Paoli, P.; Santi, A.; Imperatore, C.; Menna, M. Identifying Human PTP1B Enzyme Inhibitors from Marine Natural Products: Perspectives for Developing of Novel Insulin-Mimetic Drugs. Pharmaceuticals 2022, 15, 325. [Google Scholar] [CrossRef]
- Amarasinghe, N.R.; Turner, P.; Todd, M.H. The First Catalytic, Enantioselective Aza-Henry Reaction of an Unactivated Cyclic Imine. Adv. Synth. Catal. 2012, 354, 2954–2958. [Google Scholar]
- Bulman Page, P.; Farah, M.; Buckley, B.; Chan, Y.; Blacker, A. Preparation of C2-Symmetric Biaryl Bisiminium Salts and Their Use as Organocatalysts for Asymmetric Epoxidation. Synlett 2015, 27, 126–130. [Google Scholar] [CrossRef]
- Berkessel, A.; Seeling, B. A simplified synthesis of Takemoto’s catalyst. Synthesis 2009, 12, 2113–2115. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casertano, M.; Kelly, B.G.; Gillick-Healy, M.W.; Grieco, P.; Adamo, M.F.A. Synthesis of Indole-Based Derivatives Containing Ammonium Salts, Diamines and Aminoureas for Organocatalysis. Organics 2025, 6, 15. https://doi.org/10.3390/org6020015
Casertano M, Kelly BG, Gillick-Healy MW, Grieco P, Adamo MFA. Synthesis of Indole-Based Derivatives Containing Ammonium Salts, Diamines and Aminoureas for Organocatalysis. Organics. 2025; 6(2):15. https://doi.org/10.3390/org6020015
Chicago/Turabian StyleCasertano, Marcello, Brian G. Kelly, Malachi W. Gillick-Healy, Paolo Grieco, and Mauro F. A. Adamo. 2025. "Synthesis of Indole-Based Derivatives Containing Ammonium Salts, Diamines and Aminoureas for Organocatalysis" Organics 6, no. 2: 15. https://doi.org/10.3390/org6020015
APA StyleCasertano, M., Kelly, B. G., Gillick-Healy, M. W., Grieco, P., & Adamo, M. F. A. (2025). Synthesis of Indole-Based Derivatives Containing Ammonium Salts, Diamines and Aminoureas for Organocatalysis. Organics, 6(2), 15. https://doi.org/10.3390/org6020015







