Optimized Synthesis of Dinitrochalcones via Ultrasonic Bath in a Cyclohexane–Methanol Solvent System
Abstract
1. Introduction
2. Materials and Methods
2.1. General Approach
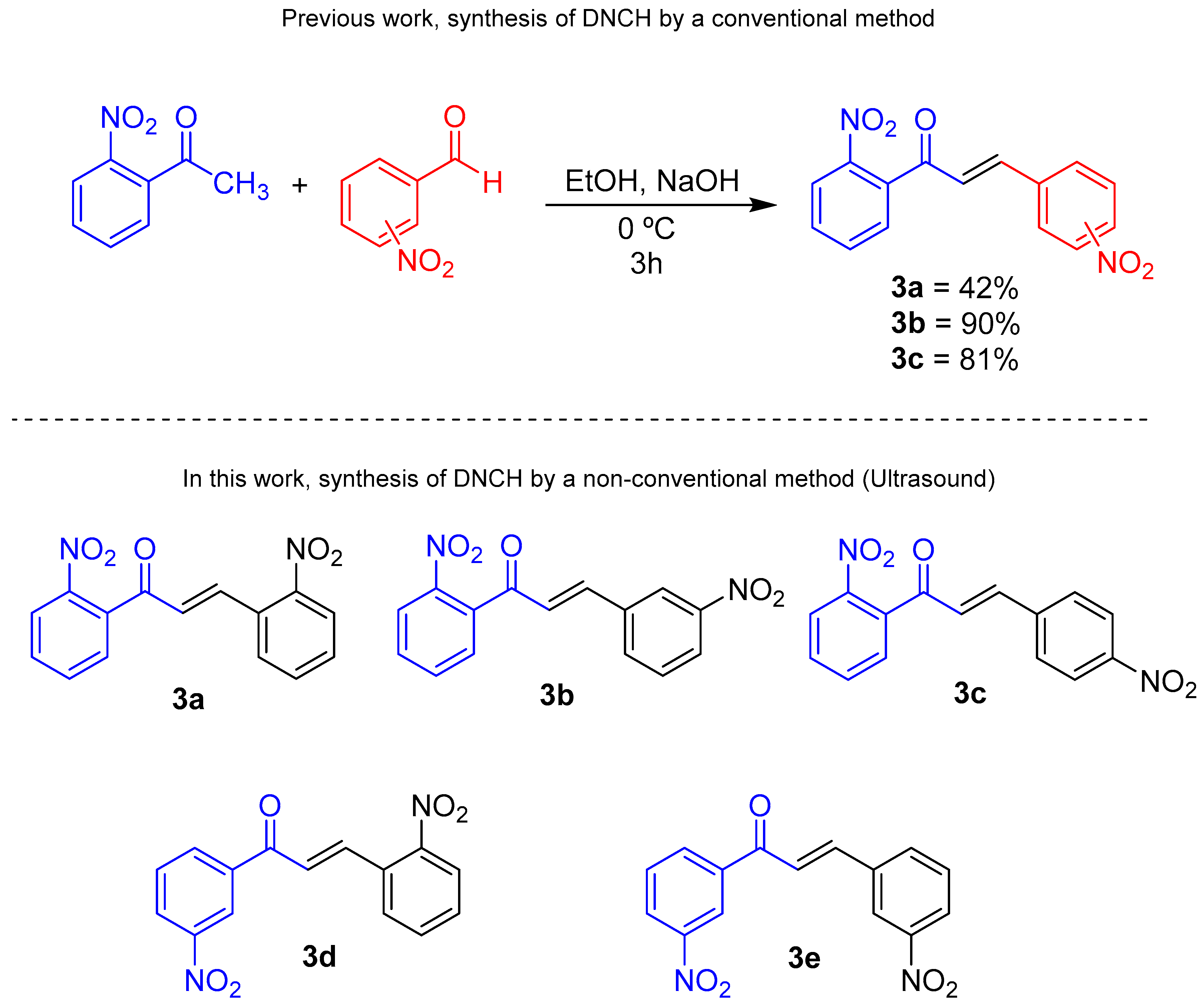
2.2. Procedure for the Synthesis of Dinitrochalcones (DNCHs)
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campos, K.R.; Coleman, P.J.; Alvarez, J.C.; Dreher, S.D.; Garbaccio, R.M.; Terrett, N.K.; Tillyer, R.D.; Truppo, M.D.; Parmee, E.R. The Importance of Synthetic Chemistry in the Pharmaceutical Industry. Science 2019, 363, eaat0805. [Google Scholar] [PubMed]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone Synthesis, Properties and Medicinal Applications: A Review. Environ. Chem. Lett. 2020, 18, 433–458. [Google Scholar]
- Rajendran, G.; Bhanu, D.; Aruchamy, B.; Ramani, P.; Pandurangan, N.; Bobba, K.N.; Oh, E.J.; Chung, H.Y.; Gangadaran, P.; Ahn, B.-C. Chalcone: A Promising Bioactive Scaffold in Medicinal Chemistry. Pharmaceuticals 2022, 15, 1250. [Google Scholar] [CrossRef] [PubMed]
- Narwal, S.; Devi, B.; Dhanda, T.; Kumar, S.; Tahlan, S. Exploring Chalcone Derivatives: Synthesis and Their Therapeutic Potential. J. Mol. Struct. 2024, 1303, 137554. [Google Scholar]
- Salem, M.S.; Hussein, R.A.; El-Sayed, W.M. Substitution at Phenyl Rings of Chalcone and Schiff Base Moieties Accounts for Their Antiproliferative Activity. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Agents) 2019, 19, 620–626. [Google Scholar]
- Noriega, S.; Cardoso-Ortiz, J.; López-Luna, A.; Cuevas-Flores, M.D.R.; Flores De La Torre, J.A. The Diverse Biological Activity of Recently Synthesized Nitro Compounds. Pharmaceuticals 2022, 15, 717. [Google Scholar] [CrossRef]
- Boguszewska-Czubara, A.; Kula, K.; Wnorowski, A.; Biernasiuk, A.; Popiołek, Ł.; Miodowski, D.; Demchuk, O.M.; Jasiński, R. Novel Functionalized β-Nitrostyrenes: Promising Candidates for New Antibacterial Drugs. Saudi Pharm. J. 2019, 27, 593–601. [Google Scholar]
- Boguszewska-Czubara, A.; Lapczuk-Krygier, A.; Rykala, K.; Biernasiuk, A.; Wnorowski, A.; Popiolek, L.; Maziarka, A.; Hordyjewska, A.; Jasiński, R. Novel Synthesis Scheme and in Vitro Antimicrobial Evaluation of a Panel of (E)-2-Aryl-1-Cyano-1-Nitroethenes. J. Enzym. Inhib. Med. Chem. 2016, 31, 900–907. [Google Scholar]
- Gómez-Rivera, A.; Aguilar-Mariscal, H.; Romero-Ceronio, N.; Roa-de la Fuente, L.F.; Lobato-García, C.E. Synthesis and Anti-Inflammatory Activity of Three Nitro Chalcones. Bioorg. Med. Chem. Lett. 2013, 23, 5519–5522. [Google Scholar]
- Hidalgo, A.Y.; Romero-Ceronio, N.; Lobato-García, C.E.; Herrera-Ruiz, M.; Vázquez-Cancino, R.; Peña-Morán, O.A.; Vilchis-Reyes, M.Á.; Gallegos-García, A.J.; Medrano-Sánchez, E.J.; Hernández-Abreu, O. Position Matters: Effect of Nitro Group in Chalcones on Biological Activities and Correlation via Molecular Docking. Sci. Pharm. 2024, 92, 54. [Google Scholar] [CrossRef]
- Arévalo, J.M.C.; Feuser; Rossi, G.R.; Trindade, E.S.; da Silva Córneo, E.; Machado-de-Ávila, R.A.; Sayer, C.; Cadena, S.M.S.C.; Noleto, G.R.; Martinez, G.R. Preparation and Characterization of 4-Nitrochalcone-Folic Acid-Poly (Methyl Methacrylate) Nanocapsules and Cytotoxic Activity on HeLa and NIH3T3 Cells. J. Drug Deliv. Sci. Technol. 2019, 54, 101300. [Google Scholar]
- Damazio, R.G.; Zanatta, A.P.; Cazarolli, L.H.; Mascarello, A.; Chiaradia, L.D.; Nunes, R.J.; Yunes, R.A.; Silva, F.R.M.B. Nitrochalcones: Potential in Vivo Insulin Secretagogues. Biochimie 2009, 91, 1493–1498. [Google Scholar] [PubMed]
- Batista, A.S.; Oliveira, S.D.S.; Pomel, S.; Commere, P.-H.; Mazan, V.; Lee, M.; Loiseau, P.M.; Rossi-Bergmann, B.; Prina, E.; Duval, R. Targeting Chalcone Binding Sites in Living Leishmania Using a Reversible Fluorogenic Benzochalcone Probe. Biomed. Pharmacother. 2022, 149, 112784. [Google Scholar]
- Emeri, F.T.D.S.D.; Rosalen, P.L.; Paganini, E.R.; Garcia, M.A.R.; Nazare, A.C.; Lazarini, J.G.; de Alencar, S.M.; Regasini, L.O.; Sardi, J.D.C.O. Antimicrobial Activity of Nitrochalcone and Pentyl Caffeate against Hospital Pathogens Results in Decreased Microbial Adhesion and Biofilm Formation. Biofouling 2019, 35, 129–142. [Google Scholar]
- Leitão, E. Chalcones: Retrospective Synthetic Approaches and Mechanistic Aspects of a Privileged Scaffold. Curr. Pharm. Des. 2020, 26, 2843–2858. [Google Scholar]
- Marotta, L.; Rossi, S.; Ibba, R.; Brogi, S.; Calderone, V.; Butini, S.; Campiani, G.; Gemma, S. The Green Chemistry of Chalcones: Valuable Sources of Privileged Core Structures for Drug Discovery. Front. Chem. 2022, 10, 988376. [Google Scholar]
- Rosa, G.P.; Seca, A.M.L.; Barreto, M.D.C.; Pinto, D.C.G.A. Chalcone: A Valuable Scaffold Upgrading by Green Methods. ACS Sustain. Chem. Eng. 2017, 5, 7467–7480. [Google Scholar]
- Mason, T.J. Ultrasound in Synthetic Organic Chemistry. Chem. Soc. Rev. 1997, 26, 443–451. [Google Scholar]
- Suslick, K.S.; Price, G.J. Applications of Ultrasound to Materials Chemistry. Annu. Rev. Mater. Sci. 1999, 29, 295–326. [Google Scholar]
- Yao, Y.; Pan, Y.; Liu, S. Power Ultrasound and Its Applications: A State-of-the-Art Review. Ultrason. Sonochem. 2020, 62, 104722. [Google Scholar]
- Lepoint, T.; Mullie, F. What Exactly Is Cavitation Chemistry? Ultrason. Sonochem. 1994, 1, S13–S22. [Google Scholar]
- Martínez, R.F.; Cravotto, G.; Cintas, P. Organic Sonochemistry: A Chemist’s Timely Perspective on Mechanisms and Reactivity. J. Org. Chem. 2021, 86, 13833–13856. [Google Scholar] [PubMed]
- Cravotto, G.; Cintas, P. Introduction to Sonochemistry: A Historical and Conceptual Overview. In Handbook on Applications of Ultrasound: Sonochemistry for Sustainability; Chen, D., Sharma, S.K., Mudhoo, A., Eds.; CRC Press, Inc.: Boca Raton, FL, USA, 2011; pp. 23–40. [Google Scholar]
- Cravotto, G.; Cintas, P. Power Ultrasound in Organic Synthesis: Moving Cavitational Chemistry from Academia to Innovative and Large-Scale Applications. Chem. Soc. Rev. 2006, 35, 180–196. [Google Scholar] [PubMed]
- Brahmachari, G.; Nayek, N.; Mandal, M.; Bhowmick, A.; Karmakar, I. Ultrasound-Promoted Organic Synthesis-A Recent Update. Curr. Org. Chem. 2021, 25, 1539–1565. [Google Scholar]
- Gharat, N.N.; Rathod, V.K. Ultrasound-Assisted Organic Synthesis. In Green Sustainable Process for Chemical And Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–41. [Google Scholar]
- Draye, M.; Chatel, G.; Duwald, R. Ultrasound for Drug Synthesis: A Green Approach. Pharmaceuticals 2020, 13, 23. [Google Scholar] [CrossRef]
- Hidalgo, A.Y.; Velasco, M.; Sánchez-Lara, E.; Gómez-Rivera, A.; Vilchis-Reyes, M.A.; Alvarado, C.; Herrera-Ruiz, M.; López-Rodríguez, R.; Romero-Ceronio, N.; Lobato-García, C.E. Synthesis, Crystal Structures, and Molecular Properties of Three Nitro-Substituted Chalcones. Crystals 2021, 11, 1589. [Google Scholar] [CrossRef]
- Kinkle, P.; Gibian, H. Uber Chalkone. Chem. Ber. 1961, 94, 26–38. [Google Scholar]
- Wei, W.; Qunrong, W.; Liqin, D.; Aiqing, Z.; Duoyuan, W. Synthesis of Dinitrochalcones by Using Ultrasonic Irradiation in the Presence of Potassium Carbonate. Ultrason. Sonochem. 2005, 12, 411–414. [Google Scholar]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar]
- Merouani, S.; Hamdaoui, O.; Rezgui, Y.; Guemini, M. Modeling of Ultrasonic Cavitation as an Advanced Technique for Water Treatment. Desalin. Water Treat. 2015, 56, 1465–1475. [Google Scholar]
- Andrade, S.M.; Costa, S.M.B.; Pansu, R. Structural Changes in W/O Triton X-100/Cyclohexane-Hexanol/Water Microemulsions Probed by a Fluorescent Drug Piroxicam. J. Colloid. Interface Sci. 2000, 226, 260–268. [Google Scholar] [CrossRef]
- Cheng, S.; Han, F.; Wang, Y.; Yan, J. Effect of Cosurfactant on Ionic Liquid Solubilization Capacity in Cyclohexane/TX-100/1-Butyl-3-Methylimidazolium Tetrafluoroborate Microemulsions. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 317, 457–461. [Google Scholar] [CrossRef]
- Khanal, H.D.; Perumal, M.; Lee, Y.R. Annulation Strategies for Diverse Heterocycles via the Reductive Transformation of 2-Nitrostyrenes. Org. Biomol. Chem. 2022, 20, 7675–7693. [Google Scholar] [CrossRef] [PubMed]


| Entry | Activation Method | Reaction Conditions | Solvent | Time | Yield |
|---|---|---|---|---|---|
| 1 * | Magnetic stirring | NaOH (0.6 Equiv.), rt | Methanol | 180 | 33 |
| 2 * | Reflux | NaOH (2 Equiv.) | Methanol | 240 | 25 |
| 3 * | Reflux | K2CO3 (0.3 Equiv.) | Methanol | 240 | 29 |
| 4 | Ultrasound | K2CO3 (0.3 Equiv.) | Methanol | 60 | 22 |
| 5 | Ultrasound | K2CO3 (0.4 Equiv.) | Methanol | 60 | 25 |
| 6 | Ultrasound | K2CO3 (0.4 Equiv.) | Methanol/ cyclohexane | 60 | 52 |
| 7 | Ultrasound | K2CO3 (0.6 Equiv.) | Methanol/ cyclohexane | 60 | 49 |
| 8 | Ultrasound | K2CO3 (0.9 Equiv.) | Methanol/ cyclohexane | 60 | 44 |
| 9 | Ultrasound | Na2CO3 (0.4 Equiv.) | Methanol/ cyclohexane | 30 | 88 |
| 10 | Ultrasound | Li2CO3 (0.4 Equiv.) | Methanol/ cyclohexane | 30 | 48 |
| 11 | Ultrasound | Cs2CO3 (0.4 Equiv.) | Methanol/ cyclohexane | 15 | 80 |
| 12 | Ultrasound | CaCO3 (0.4 Equiv.) | Methanol/ cyclohexane | 60 | ---- |
| DNCH | Time (min) | Temperature (°C) | Yield (%) | m.p. (°C) |
|---|---|---|---|---|
| 3a | 60 | 0 | 56 | 140–142 |
| 3b | 60 | 0 | 92 | 145–147 |
| 3c | 60 | 0 | 86 | 175–177 |
| 3d | 60 | 0 | 65 | 160–162 |
| 3e | 30 | 60 | 88 | 214–216 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo, A.Y.; Torres-Sauret, Q.; Lobato-García, C.E.; Ramos-Rivera, E.M.; Roa de la Fuente, L.F.; Gómez-Rivera, A.; Vilchis-Reyes, M.Á.; Alarcón-Matus, E.; Hernández-Abreu, O.; Romero-Ceronio, N. Optimized Synthesis of Dinitrochalcones via Ultrasonic Bath in a Cyclohexane–Methanol Solvent System. Organics 2025, 6, 14. https://doi.org/10.3390/org6020014
Hidalgo AY, Torres-Sauret Q, Lobato-García CE, Ramos-Rivera EM, Roa de la Fuente LF, Gómez-Rivera A, Vilchis-Reyes MÁ, Alarcón-Matus E, Hernández-Abreu O, Romero-Ceronio N. Optimized Synthesis of Dinitrochalcones via Ultrasonic Bath in a Cyclohexane–Methanol Solvent System. Organics. 2025; 6(2):14. https://doi.org/10.3390/org6020014
Chicago/Turabian StyleHidalgo, Alam Yair, Quirino Torres-Sauret, Carlos Ernesto Lobato-García, Erika Madeleyne Ramos-Rivera, Luis Fernando Roa de la Fuente, Abraham Gómez-Rivera, Miguel Ángel Vilchis-Reyes, Erika Alarcón-Matus, Oswaldo Hernández-Abreu, and Nancy Romero-Ceronio. 2025. "Optimized Synthesis of Dinitrochalcones via Ultrasonic Bath in a Cyclohexane–Methanol Solvent System" Organics 6, no. 2: 14. https://doi.org/10.3390/org6020014
APA StyleHidalgo, A. Y., Torres-Sauret, Q., Lobato-García, C. E., Ramos-Rivera, E. M., Roa de la Fuente, L. F., Gómez-Rivera, A., Vilchis-Reyes, M. Á., Alarcón-Matus, E., Hernández-Abreu, O., & Romero-Ceronio, N. (2025). Optimized Synthesis of Dinitrochalcones via Ultrasonic Bath in a Cyclohexane–Methanol Solvent System. Organics, 6(2), 14. https://doi.org/10.3390/org6020014







