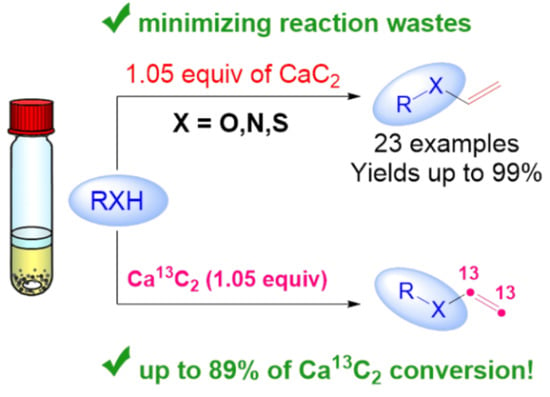
Vinylation of Alcohols, Thiols, and Nitrogen Compounds Using a Stoichiometric Amount of In Situ Generated Acetylene
Abstract
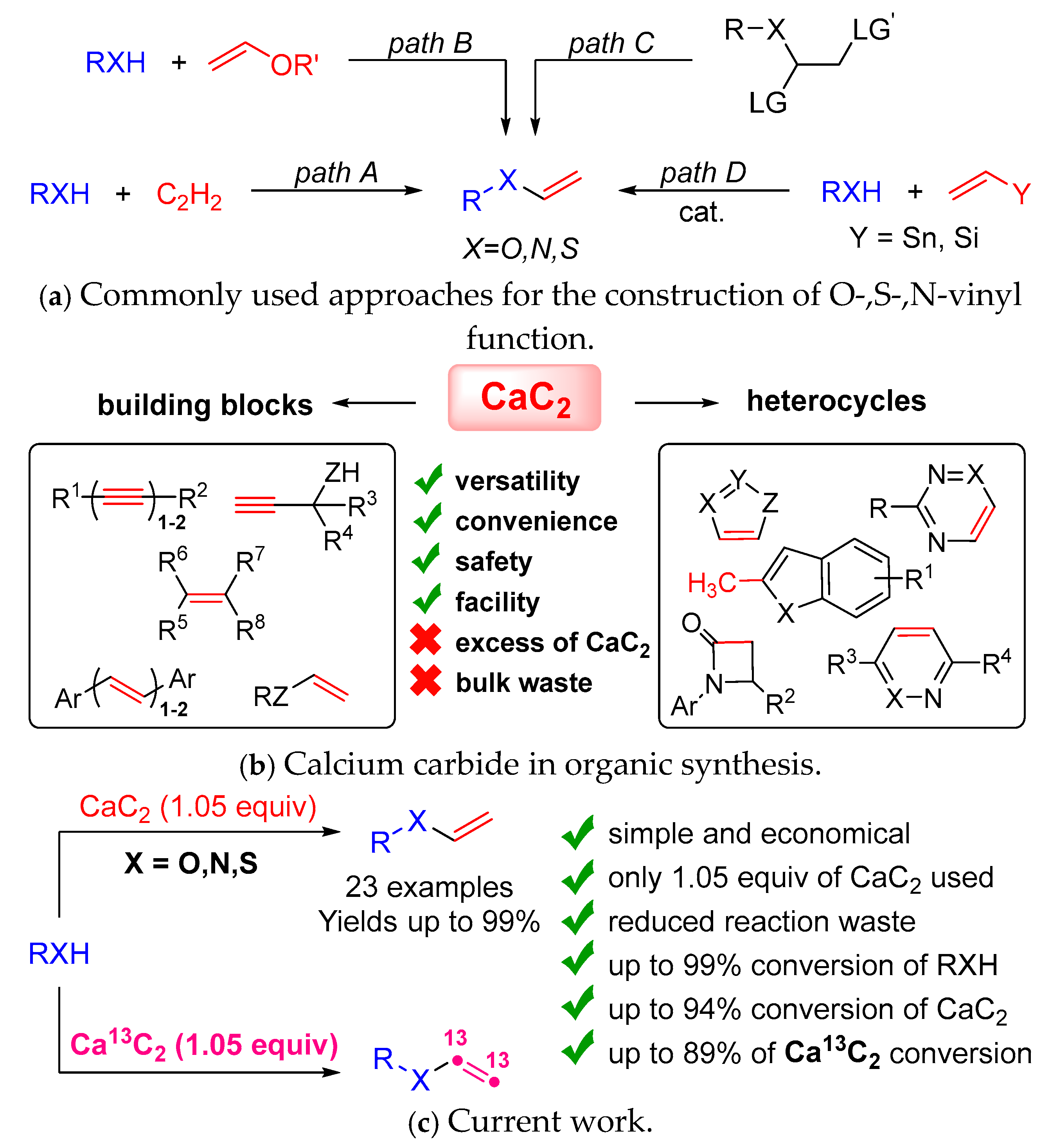
1. Introduction
2. Materials and Methods
2.1. General
2.2. General Procedure for the Synthesis of 2a–w
2.3. Gram-Scale Synthesis of Benzyl Vinyl Sulfide 2n
2.4. General Procedure for the Synthesis of 4a,i
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oesch, F.; Honarvar, N.; Fabian, E.; Berger, F.I.; Landsiedel, R. N-vinyl compounds: Studies on metabolism, genotoxicity, carcinogenicity. Arch. Toxicol. 2021, 95, 3143–3159. [Google Scholar] [CrossRef] [PubMed]
- Ledovskaya, M.S.; Voronin, V.V.; Rodygin, K.S. Methods for the synthesis of O-, S- and N-vinyl derivatives. Russ. Chem. Rev. 2018, 87, 167–191. [Google Scholar] [CrossRef]
- Silva, V.L.M.; Silva, A.M.S. Revisiting the Chemistry of Vinylpyrazoles: Properties, Synthesis, and Reactivity. Molecules 2022, 27, 3493. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Jia, T.; Chen, S.; Pan, M.; Li, X. Ni-catalyzed enantioselective three-component reductive alkylacylation of alkenes: Modular access to structurally complex α-amino ketones. Chem. Sci. 2024, 15, 15489–15495. [Google Scholar] [CrossRef] [PubMed]
- Er, T.K.G.; Lim, X.Y.H.; Oh, X.Y.; Goto, A. Synthesis of Degradable Homopolymer, Gradient and Block Copolymers, and Self-Assembly via RAFT Polymerization of 4,4-Dimethyl-2-methylene-1,3-dioxolan-5-one. Macromolecules 2024, 57, 8983–8997. [Google Scholar] [CrossRef]
- Jiang, B.; Shi, M. Rhodium(ii)-catalyzed intermolecular [3 + 2] annulation of N-vinyl indoles with N-tosyl-1,2,3-triazoles via an aza-vinyl Rh carbene. Org. Chem. Front. 2017, 4, 2459–2464. [Google Scholar] [CrossRef]
- Teator, A.J.; Leibfarth, F.A. Catalyst-controlled stereoselective cationic polymerization of vinyl ethers. Science 2019, 363, 1439–1443. [Google Scholar] [CrossRef]
- Sugihara, S. From controlled radical polymerization of vinyl ether to polymerization-induced self-assembly. Polym. J. 2022, 54, 1407–1418. [Google Scholar] [CrossRef]
- Ledovskaya, M.S.; Polynski, M.V.; Ananikov, V.P. One-Pot and Two-Chamber Methodologies for Using Acetylene Surrogates in the Synthesis of Pyridazines and Their D-Labeled Derivatives. Chem. Asian J. 2021, 16, 2286–2297. [Google Scholar] [CrossRef]
- Ledovskaya, M.S.; Voronin, V.V.; Polynski, M.V.; Lebedev, A.N.; Ananikov, V.P. Primary Vinyl Ethers as Acetylene Surrogate: A Flexible Tool for Deuterium-Labeled Pyrazole Synthesis. Eur. J. Org. Chem. 2020, 2020, 4571–4580. [Google Scholar] [CrossRef]
- Fragis, M.; Deobald, J.L.; Dharavath, S.; Scott, J.; Magolan, J. Aldehyde to Ketone Homologation Enabled by Improved Access to Thioalkyl Phosphonium Salts. Org. Lett. 2021, 23, 4548–4552. [Google Scholar] [CrossRef]
- Lou, J.; Wang, Q.; Wu, P.; Wang, H.; Zhou, Y.-G.; Yu, Z. Transition-metal mediated carbon–sulfur bond activation and transformations: An update. Chem. Soc. Rev. 2020, 49, 4307–4359. [Google Scholar] [CrossRef] [PubMed]
- Schobert, H. Production of Acetylene and Acetylene-based Chemicals from Coal. Chem. Rev. 2014, 114, 1743–1760. [Google Scholar] [CrossRef] [PubMed]
- Voronin, V.V.; Ledovskaya, M.S.; Bogachenkov, A.S.; Rodygin, K.S.; Ananikov, V.P. Acetylene in Organic Synthesis: Recent Progress and New Uses. Molecules 2018, 23, 2442. [Google Scholar] [CrossRef]
- Mu, Y.; Fan, J.; Chu, B.; Zhong, S.; Cheng, Y. Synthesis of N-vinylcarbazole from acetylene by a continuous high-pressure liquid-phase process with inherent safety. Chem. Eng. J. 2024, 493, 152642. [Google Scholar] [CrossRef]
- Mondal, S.; Yashmin, S.; Khan, A.T. Synthesis of vinyl sulfides and thioethers via a hydrothiolation reaction of 4-hydroxydithiocoumarins and arylacetylenes/styrenes. Org. Biomol. Chem. 2021, 19, 9223–9230. [Google Scholar] [CrossRef] [PubMed]
- Trofimov, B.A.; Oparina, L.A.; Kolyvanov, N.A.; Vysotskaya, O.V.; Gusarova, N.K. Nucleophilic addition to acetylenes in superbasic catalytic systems: XVIII. Vinylation of phenols and naphthols with acetylene. Russ. J. Org. Chem. 2015, 51, 188–194. [Google Scholar] [CrossRef]
- Gusarova, N.K.; Chernysheva, N.A.; Yas’ko, S.V.; Trofimov, B.A. Highly efficient atom economical “green chemistry” synthesis of vinyl sulfides from thiols and acetylene in water. Russ. Chem. Bull. 2013, 62, 438–440. [Google Scholar] [CrossRef]
- Shmidt, E.Y.; Protsuk, N.I.; Vasil’tsov, A.M.; Ivanov, A.V.; Mikhaleva, A.I.; Trofimov, B.A. Improved method for the synthesis of 1-vinylindole. Chem. Heterocycl. Compd. 2013, 49, 404–407. [Google Scholar] [CrossRef]
- Rusakov, Y.; Krivdin, L.; Sinegovskaya, L.; Istomina, N.; Ludmila, O.; Stepanov, A.; Trofimov, B. Synthesis and conformational analysis of furfuryl vinyl ethers. Russ. Chem. Bull. 2008, 57, 2132–2138. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Gusarova, N.K. Acetylene: New prospects of classical reactions. Russ. Chem. Rev. 2007, 76, 507. [Google Scholar] [CrossRef]
- Trofimov, B.A. Acetylene and its Derivatives in Reactions with Nucleophiles: Recent Advances and Current Trends. Curr. Org. Chem. 2002, 6, 1121–1162. [Google Scholar] [CrossRef]
- Zyk, N.V.; Beloglazkina, E.K.; Belova, M.A.; Dubinina, N.y.S. Methods for the synthesis of vinyl sulfides. Russ. Chem. Rev. 2003, 72, 769–786. [Google Scholar] [CrossRef]
- Kimura, J.; Nakamichi, S.; Ogawa, S.; Obora, Y. Iridium-Catalyzed Vinylation of Carbazole Derivatives with Vinyl Acetate. Synlett 2017, 28, 719–723. [Google Scholar]
- Queffelec, C.; Ribière, P.; Montchamp, J.-L. Synthesis of P,N-Heterocycles from ω-Amino-H-Phosphinates: Conformationally Restricted α-Amino Acid Analogs. J. Org. Chem. 2008, 73, 8987–8991. [Google Scholar] [CrossRef] [PubMed]
- Guillerm, G.; Muzard, M.; Glapski, C. Inactivation of S-adenosylhomocysteine hydrolase with haloethyl and dihalocyclopropyl esters derived from homoadenosine-6′-carboxylic acid. Bioorganic Med. Chem. Lett. 2004, 14, 5799–5802. [Google Scholar] [CrossRef]
- McKeon, J.E.; Fitton, P. The palladium (II) catalyzed vinyl interchange reaction—II. Tetrahedron 1972, 28, 233–238. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, Q.; Chen, F. Base-Promoted Synthesis of Vinyl Sulfides from Sulfonium Triflates. Org. Lett. 2022, 24, 7769–7773. [Google Scholar] [CrossRef] [PubMed]
- Politanskaya, L.; Khasanov, B.; Potapov, A. Synthetic approaches to fluorinated derivatives of 4-(vinylthio)pyridine. J. Fluor. Chem. 2022, 264, 110063. [Google Scholar] [CrossRef]
- Sitte, N.A.; Menche, M.; Tužina, P.; Bienewald, F.; Schäfer, A.; Comba, P.; Rominger, F.; Hashmi, A.S.K.; Schaub, T. Phosphine-Catalyzed Vinylation at Low Acetylene Pressure. J. Org. Chem. 2021, 86, 13041–13055. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, Q.; Cheng, S.; Zhao, Z.; Li, X. PhI(OAc)2-Mediated Regioselective Hydrothiolation of Allenamides with Thiophenol via a Radical Process: Synthesis of Vinyl Sulfides. J. Org. Chem. 2023, 88, 15626–15638. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.P.; Ananikov, V.P. Transition-Metal-Catalyzed C–S, C–Se, and C–Te Bond Formations via Cross-Coupling and Atom-Economic Addition Reactions. Achievements and Challenges. Chem. Rev. 2022, 122, 16110–16293. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Lv, H.; Yang, T.; Su, M.; Luo, W.; Liu, Q.; Guo, C. Synthesis of Non-Terminal Alkenyl Ethers, Alkenyl Sulfides, and N-Vinylazoles from Arylaldehydes or Diarylketones, DMSO and O, S, N-Nucleophiles. Adv. Synth. Catal. 2022, 364, 1473–1480. [Google Scholar] [CrossRef]
- Bolshan, Y.; Batey, R.A. Enamide Synthesis by Copper-Catalyzed Cross-Coupling of Amides and Potassium Alkenyltrifluoroborate Salts. Angew. Chem. Int. Ed. 2008, 47, 2109–2112. [Google Scholar] [CrossRef]
- Blouin, M.; Frenette, R. A New Method for the Preparation of Aryl Vinyl Ethers. J. Org. Chem. 2001, 66, 9043–9045. [Google Scholar] [CrossRef] [PubMed]
- Matake, R.; Adachi, Y.; Matsubara, H. Synthesis of vinyl ethers of alcohols using calcium carbide under superbasic catalytic conditions (KOH/DMSO). Green Chem. 2016, 18, 2614–2618. [Google Scholar] [CrossRef]
- Rattanangkool, E.; Vilaivan, T.; Sukwattanasinitt, M.; Wacharasindhu, S. An Atom-Economic Approach for Vinylation of Indoles and Phenols Using Calcium Carbide as Acetylene Surrogate. Eur. J. Org. Chem. 2016, 2016, 4347–4353. [Google Scholar] [CrossRef]
- Voronin, V.V.; Ledovskaya, M.S.; Rodygin, K.S.; Ananikov, V.P. Examining the vinyl moiety as a protecting group for hydroxyl (–OH) functionality under basic conditions. Org. Chem. Front. 2020, 7, 1334–1342. [Google Scholar] [CrossRef]
- Parshina, L.N.; Oparina, L.A.; Gusarova, N.K.; Trofimov, B.A. Towards C1 chemistry: Methanol vinylation by CaC2 in water in the presence of potassium or sodium carbonates. J. Chem. Technol. Biotechnol. 2019, 94, 1945–1950. [Google Scholar] [CrossRef]
- Ledovskaya, M.S.; Voronin, V.V.; Rodygin, K.S.; Posvyatenko, A.V.; Egorova, K.S.; Ananikov, V.P. Direct Synthesis of Deuterium-Labeled O-, S-, N-Vinyl Derivatives from Calcium Carbide. Synthesis 2019, 51, 3001. [Google Scholar] [CrossRef]
- Teong, S.P.; Lim, J.; Zhang, Y. Vinylation of Aryl Ether (Lignin β-O-4 Linkage) and Epoxides with Calcium Carbide through C−O Bond Cleavage. ChemSusChem 2017, 10, 3198–3201. [Google Scholar] [CrossRef]
- Teong, S.P.; Chua, A.Y.H.; Deng, S.; Li, X.; Zhang, Y. Direct vinylation of natural alcohols and derivatives with calcium carbide. Green Chem. 2017, 19, 1659–1662. [Google Scholar] [CrossRef]
- Ledovskaya, M.S.; Voronin, V.V. Calcium carbide: Highly potent solid reagent for the construction of heterocycles. Tetrahedron 2023, 149, 133720. [Google Scholar] [CrossRef]
- Rodygin, K.S.; Ledovskaya, M.S.; Voronin, V.V.; Lotsman, K.A.; Ananikov, V.P. Calcium Carbide: Versatile Synthetic Applications, Green Methodology and Sustainability. Eur. J. Org. Chem. 2021, 2021, 43–52. [Google Scholar] [CrossRef]
- Ma, X.; Li, Z. Synthesis of Diarylethynes from Aryldiazonium Salts by Using Calcium Carbide as an Alkyne Source in a Deep Eutectic Solvent. Synlett 2021, 32, 631–635. [Google Scholar]
- Liu, Z.; Li, Z. Synthesis of 1,3-Diynes Using Calcium Carbide as an Alkyne Source. Eur. J. Org. Chem. 2021, 2021, 302–308. [Google Scholar] [CrossRef]
- Liu, S.; Yin, S.; Zhang, Z.; Liu, H.; Liu, M.; Han, B. Synthesis of Bis(trimethylsilyl)acetylene (BTMSA) by Direct Reaction of CaC2 with N-(trimethylsilyl)imidazole. ChemistrySelect 2020, 5, 3644–3646. [Google Scholar] [CrossRef]
- Gao, L.; Li, Z. Synthesis of aromatic terminal allenes and aliphatic terminal alkynes from hydrazones using calcium carbide as an acetylene source. Org. Chem. Front. 2020, 7, 702–708. [Google Scholar] [CrossRef]
- Gao, L.; Li, Z. Direct Synthesis of 1-Arylprop-1-ynes with Calcium Carbide as an Acetylene Source. Synlett 2019, 30, 1580–1584. [Google Scholar] [CrossRef]
- Teong, S.P.; Yu, D.; Sum, Y.N.; Zhang, Y. Copper catalysed alkynylation of tertiary amines with CaC2via sp3 C–H activation. Green Chem. 2016, 18, 3499–3502. [Google Scholar] [CrossRef]
- Wang, B.; You, X.; Wang, J.; Li, Z. Highly Stereoselective Synthesis of 2-Acyl-3-sulfonamidobut-2-enoates Using Solid Calcium Carbide as a Substitute for Gaseous Acetylene. Org. Lett. 2024, 26, 6269–6273. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Sum, Y.N.; Ean, A.C.C.; Chin, M.P.; Zhang, Y. Acetylide Ion (C22−) as a Synthon To Link Electrophiles and Nucleophiles: A Simple Method for Enaminone Synthesis. Angew. Chem. Int. Ed. 2013, 52, 5125–5128. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, Z.; Ma, X.; Li, Z. Direct Synthesis of Propen-2-yl Sulfones through Cascade Reactions Using Calcium Carbide as an Alkyne Source. Org. Lett. 2020, 22, 5246–5250. [Google Scholar] [CrossRef] [PubMed]
- Ledovskaya, M.S.; Voronin, V.V.; Valov, N.R. New Reactions of Acetylene Generated in Two-Chamber Reactor. Russ. J. Gen. Chem. 2023, 93, 235–239. [Google Scholar] [CrossRef]
- Scharnagel, D.; Escofet, I.; Armengol-Relats, H.; de Orbe, M.E.; Korber, J.N.; Echavarren, A.M. Acetylene as a Dicarbene Equivalent for Gold(I) Catalysis: Total Synthesis of Waitziacuminone in One Step. Angew. Chem. Int. Ed. 2020, 59, 4888–4891. [Google Scholar] [CrossRef] [PubMed]
- Shabalin, D.A.; Dubovtsev, A.Y.; Schmidt, E.Y.; Trofimov, B.A. Calcium Carbide as Acetylene Source in Cascade Assemblies of Hydroxypyrrolines and 3H-Pyrroles from Ketoximes. ChemistrySelect 2020, 5, 3434–3437. [Google Scholar] [CrossRef]
- Kaewchangwat, N.; Sukato, R.; Vchirawongkwin, V.; Vilaivan, T.; Sukwattanasinitt, M.; Wacharasindhu, S. Direct synthesis of aryl substituted pyrroles from calcium carbide: An underestimated chemical feedstock. Green Chem. 2015, 17, 460–465. [Google Scholar] [CrossRef]
- Voronin, V.V.; Ledovskaya, M.S.; Rodygin, K.S.; Ananikov, V.P. Cycloaddition Reactions of in situ Generated C2D2 in Dioxane: Efficient Synthetic Approach to D2-Labeled Nitrogen Heterocycles. Eur. J. Org. Chem. 2021, 2021, 5640–5648. [Google Scholar] [CrossRef]
- Voronin, V.V.; Ledovskaya, M.S.; Gordeev, E.G.; Rodygin, K.S.; Ananikov, V.P. [3 + 2]-Cycloaddition of in Situ Generated Nitrile Imines and Acetylene for Assembling of 1,3-Disubstituted Pyrazoles with Quantitative Deuterium Labeling. J. Org. Chem. 2018, 83, 3819–3828. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, Y.; Huang, W.; Wu, W.; Jiang, H. One-Pot Synthesis of Spirocyclic or Fused Pyrazoles from Cyclic Ketones: Calcium Carbide as the Carbon Source in Ring Expansion. J. Org. Chem. 2017, 82, 9479–9486. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, W.; Chen, Y.; Gao, B.; Wu, W.; Jiang, H. Calcium carbide as the acetylide source: Transition-metal-free synthesis of substituted pyrazoles via [1,5]-sigmatropic rearrangements. Green Chem. 2016, 18, 6445–6449. [Google Scholar] [CrossRef]
- Liu, L.; Sun, G.; Zhang, J. Constructing 5-Methyl-2,4-diaryl-1H-imidazoles Using Calcium Carbide as Alkyne Source via A3-Coupling Cyclization. Adv. Synth. Catal. 2023, 365, 1801–1805. [Google Scholar] [CrossRef]
- Gonda, Z.; Lőrincz, K.; Novák, Z. Efficient synthesis of deuterated 1,2,3-triazoles. Tetrahedron Lett. 2010, 51, 6275–6277. [Google Scholar] [CrossRef]
- Ledovskaya, M.S.; Rodygin, K.S.; Ananikov, V.P. Calcium-mediated one-pot preparation of isoxazoles with deuterium incorporation. Org. Chem. Front. 2018, 5, 226–231. [Google Scholar] [CrossRef]
- Hosseini, A.; Schreiner, P.R. Synthesis of Exclusively 4-Substituted β-Lactams through the Kinugasa Reaction Utilizing Calcium Carbide. Org. Lett. 2019, 21, 3746–3749. [Google Scholar] [CrossRef]
- Voronin, V.V.; Polynski, M.V.; Ledovskaya, M.S. 1,2,4-Triazines and Calcium Carbide in the Catalyst-Free Synthesis of 2,3,6-Trisubstituted Pyridines and Their D-, 13C-, and Doubly D2-13C2-Labeled Analogues. Chem. Asian J. 2023, 18, e202300781. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Li, Z. One-Pot Three-Component Synthesis of 4-Arylpyrimidin-2-amines Using Solid Calcium Carbide as a Surrogate of Gaseous Acetylene. ChemistrySelect 2023, 8, e202302154. [Google Scholar] [CrossRef]
- Fu, R.; Li, Z. Direct Synthesis of 2-Methylbenzofurans from Calcium Carbide and Salicylaldehyde p-Tosylhydrazones. Org. Lett. 2018, 20, 2342–2345. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Shao, T.; Li, Z. Construction of 3-Methyl-2-Substituted Benzo[b]furans and 3-Methyl-2-Substituted Benzo[b]thiophenes Using Solid Calcium Carbide as a Substitute for Gaseous Acetylene. J. Org. Chem. 2024, 89, 7182–7186. [Google Scholar] [CrossRef]
- You, X.; Wang, B.; Wen, F.; Li, Z. One-step construction of indolo[2,1-a]isoquinolines using solid calcium carbide as an alternative to gaseous acetylene. Synth. Commun. 2024, 54, 1209–1219. [Google Scholar] [CrossRef]
- Wen, F.; Zhang, Y.; Zhang, Z.; Mu, H.; Cheng, L.; Wang, J.; He, B.; Li, Z.; Fu, R. One-Pot Three-Component Construction of (Z)-3-Benzylidene-2-(quinolin-8-yl)isoindolin-1-ones Through C(sp2)−H Bond Activation Using Calcium Carbide as a Solid Alkyne Source. ChemistrySelect 2024, 9, e202402448. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Wang, B.; Li, Z. Construction of 2-Methylindoles Using Solid Calcium Carbide as a Substitute for Gaseous Acetylene. Eur. J. Org. Chem. 2024, 27, e202301262. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Z.; Li, Z. One-Step Construction of 9,10-Diarylphenanthrenes Using Solid Calcium Carbide as an Alternative of Gaseous Acetylene. Asian J. Org. Chem. 2024, 13, e202400235. [Google Scholar] [CrossRef]
- Ledovskaya, M.S.; Voronin, V.V. The Use of Calcium Carbide for Cyclopentenone Ring Construction. Russ. J. Gen. Chem. 2024, 94, 45–48. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Li, Z. Three-Component One-Pot Construction of 2-Aryl-4H-benzo[4,5]thiazolo[3,2-a]pyrimidines Using Solid Calcium Carbide as a Surrogate of Gaseous Acetylene. Org. Lett. 2022, 24, 5491–5496. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Z. Copper-Catalyzed Construction of Benzo[4,5]imidazo[2,1-a]isoquinolines Using Calcium Carbide as a Solid Alkyne Source. Org. Lett. 2021, 23, 8407–8412. [Google Scholar] [CrossRef]
- Zimmerman, J.B.; Anastas, P.T.; Erythropel, H.C.; Leitner, W. Designing for a green chemistry future. Science 2020, 367, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Wink, K.; Hartmann, I. Recent Progress in Turning Waste into Catalysts for Green Syntheses. Sustain. Chem. 2024, 5, 27–39. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Bode, M.L.; Akakios, S.G. Metrics of green chemistry: Waste minimization. Curr. Opin. Green Sustain. 2022, 33, 100569. [Google Scholar] [CrossRef]
- Pacheco-López, A.; Somoza-Tornos, A.; Graells, M.; Espuña, A. Synthesis and assessment of waste-to-resource routes for circular economy. Comput. Chem. Eng. 2021, 153, 107439. [Google Scholar] [CrossRef]
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A review on circular economy: The expected transition to a balanced interplay of environmental and economic systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Ledovskaya, M.; Voronin, V.; Rodygin, K.; Ananikov, V. Efficient labeling of organic molecules using 13C elemental carbon: Universal access to 13С2-labeled synthetic building blocks, polymers and pharmaceuticals. Org. Chem. Front. 2020, 7, 638–647. [Google Scholar] [CrossRef]
- Werner, G.; Rodygin, K.S.; Kostin, A.A.; Gordeev, E.G.; Kashin, A.S.; Ananikov, V.P. A solid acetylene reagent with enhanced reactivity: Fluoride-mediated functionalization of alcohols and phenols. Green Chem. 2017, 19, 3032–3041. [Google Scholar] [CrossRef]
- Ledovskaya, M.S.; Voronin, V.V.; Valov, N.R.; Samoylenko, D.E. Calcium Carbide: From Elemental Carbon to Isotope-Economic Synthesis of 13C2-Labeled Heterocycles. Chin. J. Chem. 2023, 41, 2810–2818. [Google Scholar] [CrossRef]
- Kutskaya, A.M.; Serkov, S.A.; Voronin, V.V.; Ledovskaya, M.S.; Polynski, M.V. Negligible Substituent Effect as Key to Synthetic Versatility: A Computational-Experimental Study of Vinyl Ethers Addition to Nitrile Oxides. ChemistrySelect 2022, 7, e202200174. [Google Scholar] [CrossRef]

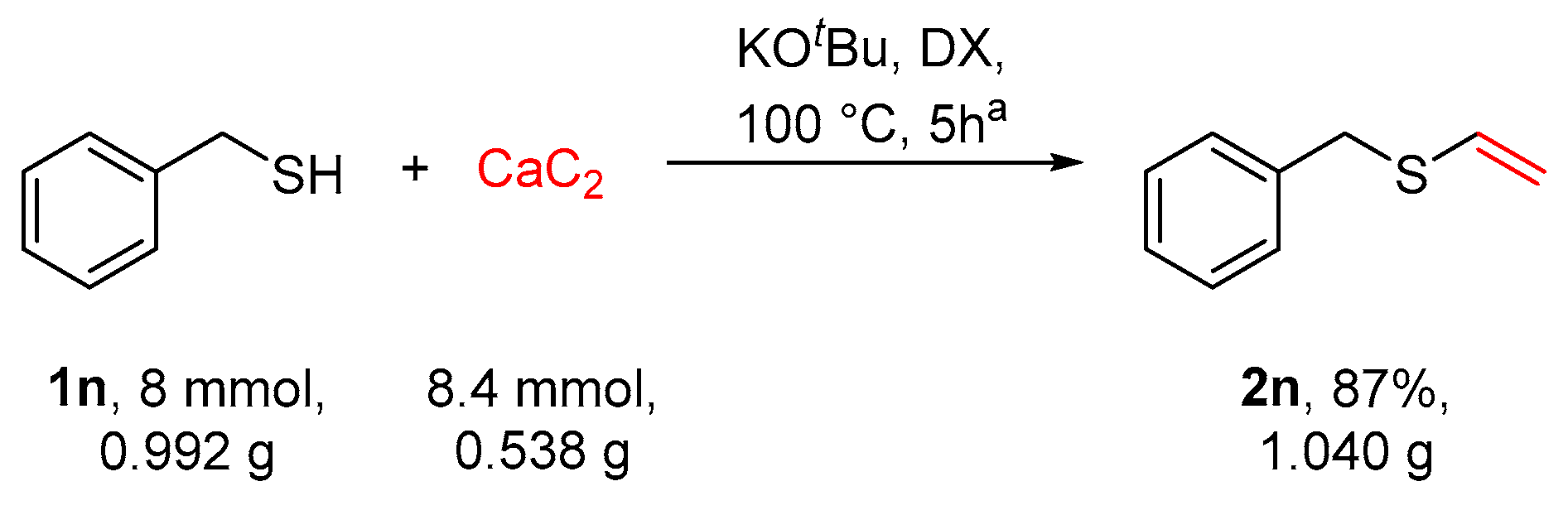

 | |||||||
| Entry | Substrate | KOtBu, mmol | Solvent | T, °C | Time, h | Product | Yield of 2, % b |
| 1 | BnOH (1a) | 1.0 | DMSO | 130 | 5 | 2a | 81 |
| 2 | 1a | 0.75 | DMSO | 130 | 5 | 2a | 82 |
| 3 | 1a | 0.5 | DMSO | 130 | 5 | 2a | 82 |
| 4 | 1a c | 0.5 | DMSO | 130 | 5 | 2a | 86 |
| 5 | 1a c | 0.5 | DMSO | 130 | 7 | 2a | 95 |
| 6 | carbazole (1i) c | 2.0 | DMSO | 130 | 5 | 2i | 64 |
| 7 | 1i c | 1.0 | DMSO | 130 | 5 | 2i | 78 |
| 8 | 1i c | 0.75 | DMSO | 130 | 5 | 2i | 83 |
| 9 | 1i c | 0.5 | DMSO | 130 | 5 | 2i | 85 |
| 10 | 1i c | 0.25 | DMSO | 130 | 5 | 2i | 84 |
| 11 | PhSH (1o) | 1.0 | DX d | 100 | 4 | 2o | 83 |
| 12 | 1o | 1.0 | DX | 100 | 5 | 2o | 88 |
| 13 | 1o c | 1.0 | DX | 100 | 5 | 2o | 94 |
| 14 | n-C12H25SH (1w) | 1.0 | DX | 100 | 4 | 2w | 90 |
| 15 | 1w | 1.0 | DX | 100 | 5 | 2w | 95 |
| 16 | 1w c | 1.0 | DX | 100 | 5 | 2w | 97 |
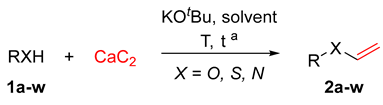 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledovskaya, M.S.; Voronin, V.V.; Reznichenko, A.A.; Reznichenko, E.A. Vinylation of Alcohols, Thiols, and Nitrogen Compounds Using a Stoichiometric Amount of In Situ Generated Acetylene. Organics 2025, 6, 5. https://doi.org/10.3390/org6010005
Ledovskaya MS, Voronin VV, Reznichenko AA, Reznichenko EA. Vinylation of Alcohols, Thiols, and Nitrogen Compounds Using a Stoichiometric Amount of In Situ Generated Acetylene. Organics. 2025; 6(1):5. https://doi.org/10.3390/org6010005
Chicago/Turabian StyleLedovskaya, Maria S., Vladimir V. Voronin, Anna A. Reznichenko, and Ekaterina A. Reznichenko. 2025. "Vinylation of Alcohols, Thiols, and Nitrogen Compounds Using a Stoichiometric Amount of In Situ Generated Acetylene" Organics 6, no. 1: 5. https://doi.org/10.3390/org6010005
APA StyleLedovskaya, M. S., Voronin, V. V., Reznichenko, A. A., & Reznichenko, E. A. (2025). Vinylation of Alcohols, Thiols, and Nitrogen Compounds Using a Stoichiometric Amount of In Situ Generated Acetylene. Organics, 6(1), 5. https://doi.org/10.3390/org6010005