Facile Synthesis of a Cholesterol–Doxorubicin Conjugate Using Cholesteryl-4-nitrophenolate as an Activated Ester and Biological Property Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Theoretical Molecular Parameter and Molecular Electrostatic Potential Analysis
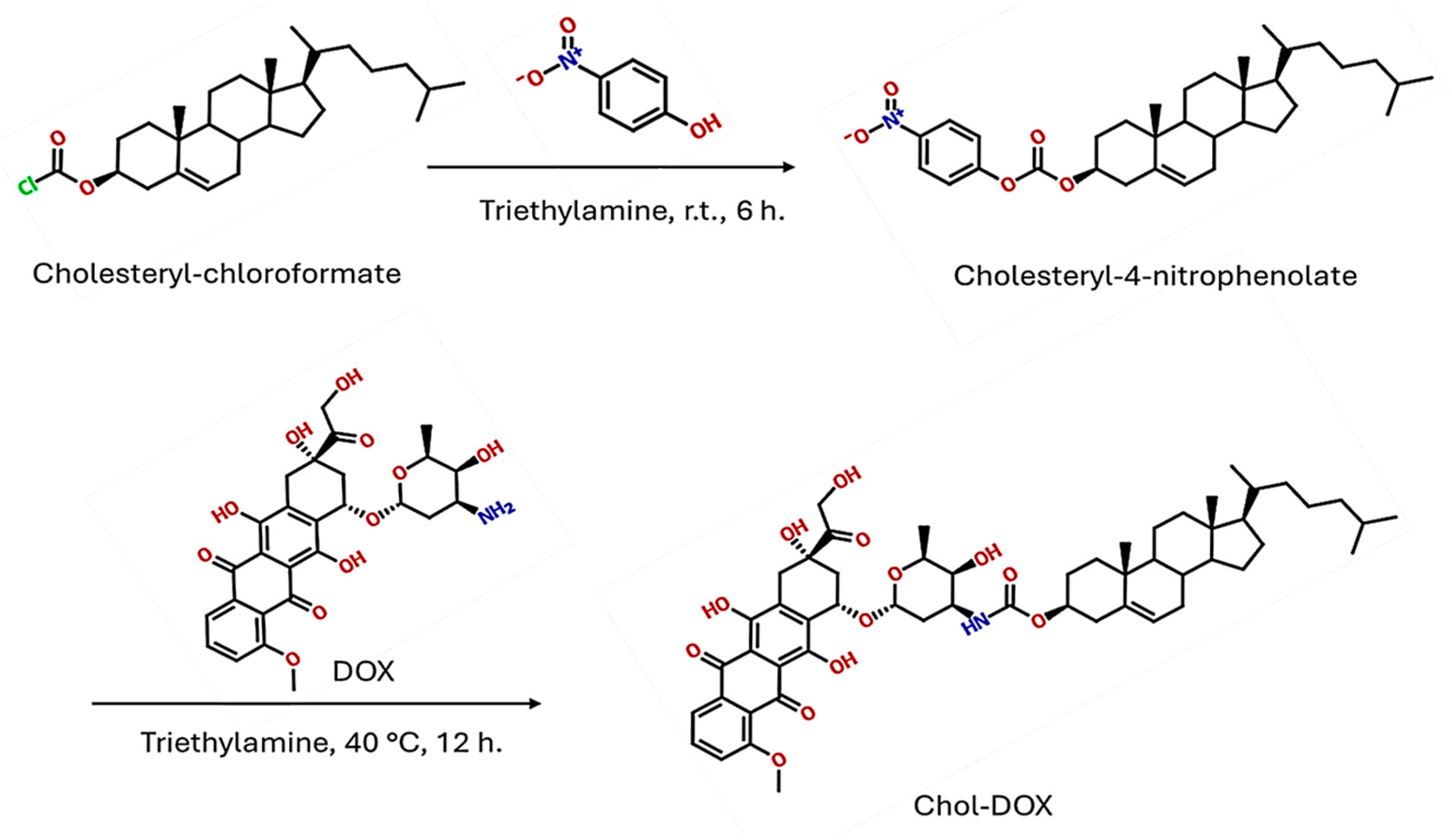
2.2. Chemical Synthesis Experimental Method
2.2.1. Materials and Methods for the Synthesis
2.2.2. Synthesis of Cholesteryl-4-nitrophenolate (IUPAC Name: [(3S,10R,13R,14S,17R)-10,13-Dimethyl-17-(6-methylheptan-2-yl)-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]-phenanthren-3-yl] (4-Nitrophenyl) Carbonate)
- 1H NMR (CDCl3, 400 MHz, δ in ppm) δ 8.26 (2H, Ar-H, 2Jortho = 12.0 Hz, nitrophenolate), 7.40 (2H, Ar-H, 2Jortho = 12.0 Hz, nitrophenolate), 5.42 (1H, -CH=, Chol), 4.60 (1H, COCH), 2.50 (2H, -CH2-C=, Chol) 2.30–0.79 (38 H, cholesterol skeleton), 0.68 (3H, -CH3, Chol).
- 13C NMR (CDCl3, 101 MHz, δ in ppm) 155.79, 151.91, 145.42, 138.98, 125.41, 123.67, 121.93, 79.90, 56.81, 56.27, 50.11, 42.46, 39.84, 39.65, 38.00, 36.93, 36.69, 36.32, 35.93, 32.05, 31.97, 28.36, 28.16, 27.73, 24.42, 23.97, 22.96, 22.71, 21.19, 19.41, 18.86, 12.01.
- FT-IR (ν cm−1) 2936.32 (stretching of aromatic C-H), 1756.13 (stretching of ester C=O), 1519.33 (stretching of aromatic C=C), 1491.74 (bending of aromatic C-H), 1350.03 (bending of C-H), 1257.45, 1230.17, 1165.52, 1110.07 (C-O-C stretching), 972.35, 849.06, 745.34, 681.91 (C-H out-of-plane bending).
2.2.3. Synthesis of Chol-DOX Conjugate (IUPAC Name: [(3S,10R,13R,17R)-10,13-Dimethyl-17-(6-methylheptan-2-yl)-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl] N-[(2S,3S,4S,6R)-3-Hydroxy-2-methyl-6-[[(1S,3S)-3,5,12-trihydroxy-3-(2-hydroxyacetyl)-10-methoxy-6,11-dioxo-2,4-dihydro-1H-tetracen-1-yl]oxy]oxan-4-yl]carbamate)
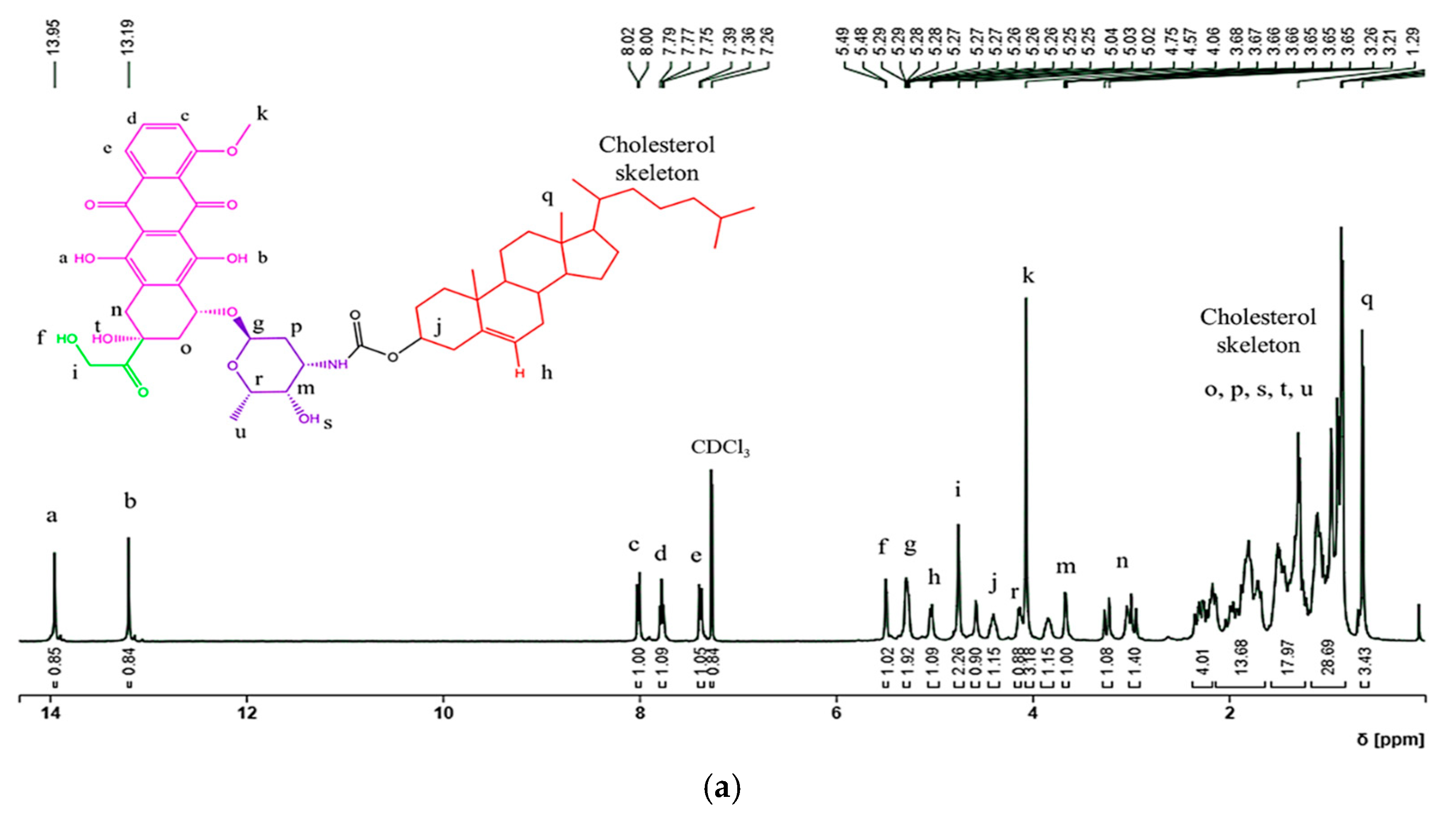
- 1H NMR (CDCl3, 400 MHz, δ in ppm): δ 13.97 (1H, Ar-OH, DOX), 13.24 (1H, Ar-OH, DOX), 8.02 (1H, Ar-H, DOX), 7.78 (1H, Ar-H, DOX), 7.28 (1H, Ar-H, DOX), 5.50 (1H, CH2-O(H), DOX), 5.29 (1H, -CHO(O)-, DOX) and 1H, -CH=, DOX), 5.11 (1H, Ar-C(O)HCH), 4.78 (2H, -CH2CO, DOX), 4.58 (1H, OCOO-CH, Chol), 4.10 (3H, -OCH3, DOX), 3.81 (1H, -CH-NH, DOX), 3.68 (1H, -CH-O-, DOX), 3.18 and 3.06 (2H, Ar-CH2 (Ha and Hb), Dox), 2.23–0.74 (51 H, Chol and Dox skeletons), 0.63 (3H, -CH3, Chol).
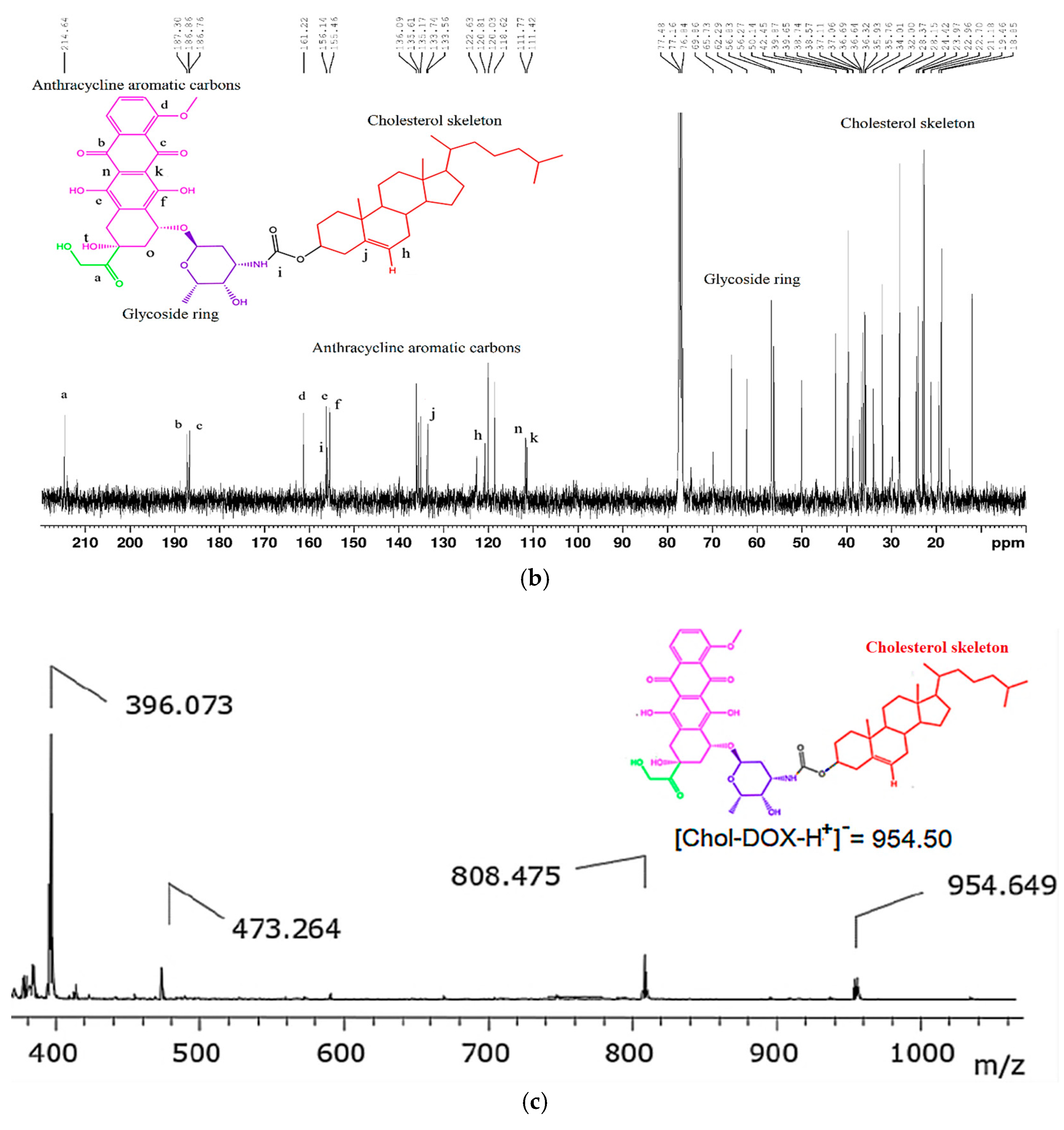
- 13C NMR (CDCl3, 101 MHz, δ in ppm): δ 214.6, 187.3, 186.76, 161.22, 156.14, 155.72, 155.46, 136.09, 135.61, 135.17, 133.74, 133.56, 122.63, 120.81, 120.03, 118.62, 111.78, 111.42, 69.86, 65.73, 62.29, 56.83, 56.27, 50.14, 42.45, 39.87, 39.65, 38.74, 38.57, 37.11, 37.06, 36.69, 36.64, 36.32, 35.93, 35.76, 34.01, 28.37, 28.15, 24.42, 23.97, 22.96, 22.70, 21.18, 19.46, 18.85, 17.03, 12.00.
- MALDI-TOF-MS (negative ion mode): [C55H73NO13-H+]− calculated: 954.50, found: 954.649.
- FT-IR (ν cm−1) 3620~3230 (broad peak, stretching of -OH and -NH), 2935.36 (stretching of aromatic C-H), 1759.12 (stretching of ester C=O), 1614.52, 1578.20, 1518.82 (stretching of aromatic C=C), 1410.78 (bending of aromatic C-H), 1350. 29 (bending of C-H), 1258.31, 1207.10, 1111.54, 1069.62 (C-O-C stretching), 1009.80, 983.15, 765.01 (C-H out-of-plane bending).
3. Results and Discussion
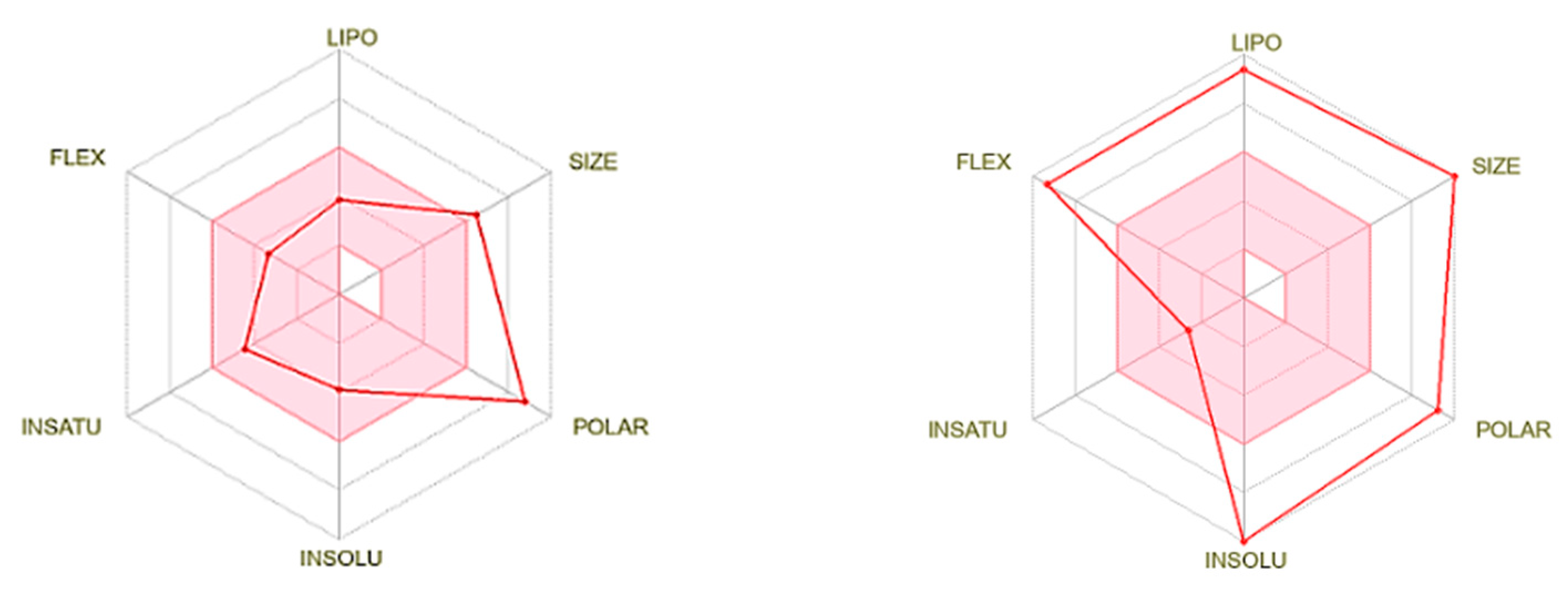
3.1. Theoretical Analysis of Molecular Parameters
3.2. Synthesis and Characterization of the Chol-DOX Conjugate
3.3. Theoretical and Experimental Biological Properties of the Chol-DOX Conjugate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sritharan, S.; Sivalingam, N. A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.; Mignani, S.; Rodrigues, J.; Tomás, H. A glance over doxorubicin based-nanotherapeutics: From proof-of-concept studies to solutions in the market. J. Control. Release 2020, 317, 347–374. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, T.; Cao, A.; Sun, J.; Jia, L.; Sheng, R. Morphology-Variable Aggregates Prepared from Cholesterol-Containing Amphiphilic Glycopolymers: Their Protein Recognition/Adsorption and Drug Delivery Applications. Nanomaterials 2018, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Sheng, R.; Wang, Z.; Luo, T.; Cao, A.; Sun, J.; Kinsella, J.M. Skeleton-Controlled pDNA Delivery of Renewable Steroid-Based Cationic Lipids, the Endocytosis Pathway Analysis and Intracellular Localization. Int. J. Mol. Sci. 2018, 19, 369. [Google Scholar] [CrossRef] [PubMed]
- Sheng, R.; Luo, T.; Li, H.; Sun, J.; Wang, Z.; Cao, A. Cholesterol-based cationic lipids for gene delivery: Contribution of molecular structure factors to physico-chemical and biological properties. Colloids Surf. B Biointerfaces 2014, 116, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, J.; Li, M.; Luo, T.; Shen, Y.; Cao, A.; Sheng, R. Natural steroid-based cationic copolymers cholesterol/diosgenin-r-PDMAEMAs and their pDNA nanoplexes: Impact of steroid structures and hydrophobic/hydrophilic ratios on pDNA delivery. RSC Adv. 2021, 11, 19450–19460. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, L.; Sheng, R.; Chen, J. Microfluidic-Based Cationic Cholesterol Lipid siRNA Delivery Nanosystem: Highly Efficient In Vitro Gene Silencing and the Intracellular Behavior. Int. J. Mol. Sci. 2022, 23, 3999. [Google Scholar] [CrossRef]
- Wang, Z.; Song, W.; Sheng, R.; Guo, X.; Hao, L.; Zhang, X. Controlled preparation of cholesterol-bearing polycations with pendent l-lysine for efficient gene delivery. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 750–758. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, X.; Hao, L.; Zhang, X.; Lin, Q.; Sheng, R. Charge-Convertible and Reduction-Sensitive Cholesterol-Containing Amphiphilic Copolymers for Improved Doxorubicin Delivery. Materials 2022, 15, 6476. [Google Scholar] [CrossRef]
- Olim, F.; Neves, A.R.; Vieira, M.; Tomás, H.; Sheng, R. Self-Assembly of Cholesterol-Doxorubicin and TPGS into Prodrug-Based Nanoparticles with Enhanced Cellular Uptake and Lysosome-Dependent Pathway in Breast Cancer Cells. Eur. J. Lipid Sci. Technol. 2021, 123, 2000337. [Google Scholar] [CrossRef]
- Polik, W.F.; Schmidt, J.R. WebMO: Web-based computational chemistry calculations in education and research. WIREs Comput. Mol. Sci. 2022, 12, e1554. [Google Scholar] [CrossRef]
- Amić, A.; Gotal, E. Selected Thermodynamic Parameters of Antioxidant Activity of Coumarin Based Heterocyclic Compounds. Chem. Proc. 2021, 3, 109. [Google Scholar] [CrossRef]
- Lu, J.; Paci, I.; Leitch, D.C. A broadly applicable quantitative relative reactivity model for nucleophilic aromatic substitution (SNAr) using simple descriptors. Chem. Sci. 2022, 13, 12681–12695. [Google Scholar] [CrossRef] [PubMed]
- Darvishmanesh, S.; Vanneste, J.; Tocci, E.; Jansen, J.C.; Tasselli, F.; Degrève, J.; Drioli, E.; Van der Bruggen, B. Physicochemical Characterization of Solute Retention in Solvent Resistant Nanofiltration: The Effect of Solute Size, Polarity, Dipole Moment, and Solubility Parameter. J. Phys. Chem. B 2011, 115, 14507–14517. [Google Scholar] [CrossRef]
- Brahmachari, G.; Nayek, N.; Nurjamal, K.; Karmakar, I.; Begam, S. Triethylamine—A Versatile Organocatalyst in Organic Transformations: A Decade Update. Synthesis 2018, 50, 4145–4164. [Google Scholar] [CrossRef]
- Cai, L.; Wang, S. Elucidating Colorization in the Functionalization of Hydroxyl-Containing Polymers Using Unsaturated Anhydrides/Acyl Chlorides in the Presence of Triethylamine. Biomacromolecules 2010, 11, 304–307. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, L.; Hang, S.; Wang, S.; Li, N.; Sun, X.; Wang, Z.; Sheng, R.; Wang, F.; Wu, W.; et al. Synthesis of Coumarin Derivatives: A New Class of Coumarin-Based G Protein-Coupled Receptor Activators and Inhibitors. Polymers 2022, 14, 2021. [Google Scholar] [CrossRef]
- Grolier, J.P.E.; Roux-Desgranges, G.; Berkane, M.; Jiménez, E.; Wilhelm, E. Heat capacities and densities of mixtures of very polar substances 2. Mixtures containing N, N-dimethylformamide. J. Chem. Thermodyn. 1993, 25, 41–50. [Google Scholar] [CrossRef]
- Mokbel, I.; Razzouk, A.; Sawaya, T.; Jose, J. Experimental Vapor Pressures of 2-Phenylethylamine, Benzylamine, Triethylamine, and cis-2,6-Dimethylpiperidine in the Range between 0.2 Pa and 75 kPa. J. Chem. Eng. Data 2009, 54, 819–822. [Google Scholar] [CrossRef]
- Bakchi, B.; Krishna, A.D.; Sreecharan, E.; Ganesh, V.B.J.; Niharika, M.; Maharshi, S.; Puttagunta, S.B.; Sigalapalli, D.K.; Bhandare, R.R.; Shaik, A.B. An overview on applications of SwissADME web tool in the design and development of anticancer, antitubercular and antimicrobial agents: A medicinal chemist’s perspective. J. Mol. Struct. 2022, 1259, 132712. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Mei, L.; Quach, T.; Porter, C.; Trevaskis, N. Lipophilic Conjugates of Drugs: A Tool to Improve Drug Pharmacokinetic and Therapeutic Profiles. Pharm. Res. 2021, 38, 1497–1518. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Carballo, K.; Lamoureux, G.V.; Perez, A.L.; Bella Cruz, A.; Cechinel Filho, V. Novel one-pot synthesis of a library of 2-aryloxy-1,4-naphthoquinone derivatives. Determination of antifungal and antibacterial activity. RSC Adv. 2022, 12, 18507–18523. [Google Scholar] [CrossRef] [PubMed]
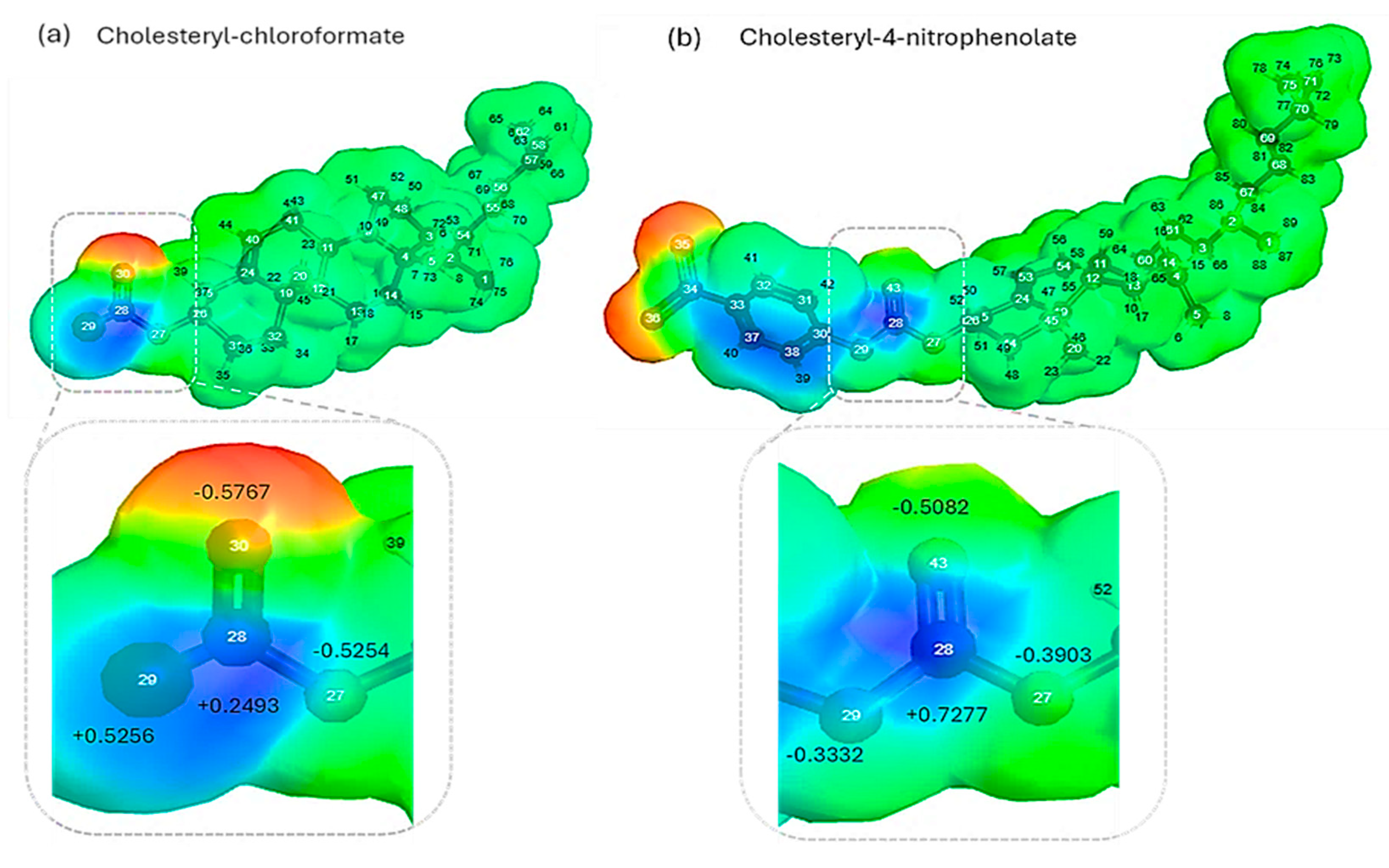
| Molecular Parameters | Cholesteryl Chloroformate | Cholesteryl-4-nitrophenolate |
|---|---|---|
| Molecular formula | C28H45O2Cl | C34H49NO5 |
| Molecular weight (MW) | 449.115 | 551.765 |
| Heat of formation (Kcal/mol) | 98.164 | 469.093 |
| Molecular area (Å2) * | 465.440 | 576.180 |
| Molecular volume (Å2) * | 595.540 | 713.880 |
| LUMO energy (ELUMO, eV) | +0.088 | −12.812 |
| HOMO energy (EHOMO, eV) | −9.000 | −13.946 |
| HOMO-LUMO energy gap (ΔE= ELUMO-EHOMO, eV) | 9.088 | 0.134 |
| Dipole moment (Debye) | 2.855 | 10.721 |
| Biological Properties | Free DOX | Chol-DOX |
|---|---|---|
| GI absorption a | Low | Low |
| BBB permeant a | No | No |
| P-gp substrate a | Yes | Yes |
| CYP1A2 inhibitor a | No | No |
| CYP2C19 inhibitor a | No | No |
| CYP2C9 inhibitor a | No | No |
| CYP2D6 inhibitor a | No | No |
| CYP3A4 inhibitor a | Yes | Yes |
| Log Kp (skin permeation, cm/s) a | −8.71 | −4.37 |
| Log Po/w (iLOGP) a | 2.58 | 5.80 |
| Lipinski druglikeness a | No (3 violations): MW > 500; number of N or O atoms >10; NH or OH groups >5) | No (3 violations): MW > 500; number of N or O atoms >10; NH or OH groups >5 |
| Bioavailability score a | 0.17 | 0.17 |
| MDA-MB-231 cell viability (%) with 2, 6, and 10ug/mL DOX b | 87.1, 74.3, 70.2 | 98.9, 94.3, 82.1 |
| MCF-7 cell viability (%) with 2, 6, and 10ug/mL DOX b | 67.3, 64.1, 60.7 | 95.9, 79.4, 62.4 |
| Intracellular localization b | Cell nuclei | Lysosomes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, P.; Maciel, D.; Jaśkowska, J.; Zeńczak-Tomera, K.; Zhou, Y.; Yin, G.; Sheng, R. Facile Synthesis of a Cholesterol–Doxorubicin Conjugate Using Cholesteryl-4-nitrophenolate as an Activated Ester and Biological Property Analysis. Organics 2025, 6, 6. https://doi.org/10.3390/org6010006
Freitas P, Maciel D, Jaśkowska J, Zeńczak-Tomera K, Zhou Y, Yin G, Sheng R. Facile Synthesis of a Cholesterol–Doxorubicin Conjugate Using Cholesteryl-4-nitrophenolate as an Activated Ester and Biological Property Analysis. Organics. 2025; 6(1):6. https://doi.org/10.3390/org6010006
Chicago/Turabian StyleFreitas, Pedro, Dina Maciel, Jolanta Jaśkowska, Kamila Zeńczak-Tomera, Yanbiao Zhou, Guoyin Yin, and Ruilong Sheng. 2025. "Facile Synthesis of a Cholesterol–Doxorubicin Conjugate Using Cholesteryl-4-nitrophenolate as an Activated Ester and Biological Property Analysis" Organics 6, no. 1: 6. https://doi.org/10.3390/org6010006
APA StyleFreitas, P., Maciel, D., Jaśkowska, J., Zeńczak-Tomera, K., Zhou, Y., Yin, G., & Sheng, R. (2025). Facile Synthesis of a Cholesterol–Doxorubicin Conjugate Using Cholesteryl-4-nitrophenolate as an Activated Ester and Biological Property Analysis. Organics, 6(1), 6. https://doi.org/10.3390/org6010006







