Abstract
A graft reaction of polyvinyl chloride (PVC) with furfural was conducted in a tetrahydrofuran solution. The resulting graft structure (FF-g-PVC) was characterized using UV spectroscopy, photoluminescence (PL), Fourier Transform Infrared (FTIR) spectroscopy, Raman spectroscopy, and proton nuclear magnetic resonance (1H NMR) spectroscopy. The grafting efficiency was determined through ultraviolet spectrophotometry. Thermal stability analysis via thermogravimetric (TG) testing revealed that furfural was successfully grafted onto the PVC chain. In a nitrogen atmosphere, the temperature of the maximum weight loss rate during the first stage of pyrolysis increased from 296.3 °C to 301.7 °C, while the activation energy for the second stage increased from 199.4 kJ/mol to 294.4 kJ/mol, indicating enhanced stability and delayed degradation of the PVC. Additionally, microwave irradiation markedly improved the graft reaction, achieving a grafting rate of 57.76‰ compared to only 1.808‰ with water bath heating. The optimal conditions were found to be a PVC/FF/Zn ratio of 1:1:0.9, with microwave irradiation for 20 min at 40 °C.
1. Introduction
Polyvinyl chloride (PVC) is one of the five major plastics and serves as an irreplaceable product in the chlor-alkali industry and chlorine balance. However, during its production, PVC is prone to side reactions such as chain transfer and chain termination, leading to the formation of unstable and abnormal structures [1], including branched-chain tertiary chlorides, allyl chlorides, and conjugated double bonds. These abnormal structures—branched tertiary chlorides, allyl chlorides, double bonds, and other unstable configurations—are recognized as significant contributors to the thermal degradation of PVC.As a result, the presence of these defective structures adversely affects thermal stability and reduces impact strength, which represents a major bottleneck for the PVC industry. Addressing these challenges is essential for achieving higher performance, functionalization, and recyclability of PVC, which are vital for the sustainable development of the industry [2,3,4].
To mitigate the degradation of PVC and enhance its thermal stability, heat stabilizers are typically employed to stabilize these abnormal structures. These stabilizers function by absorbing the hydrochloric acid (HCl) produced during degradation, disrupting the microenvironment conducive to HCl homolysis, which forms free radicals, and thereby eliminating the catalytic effects of HCl on degradation. Jiaying [5] prepared a new ethylenediamine bismaleimide--metal complex (M-DMA) heat stabilizer. Not only does the cross-linking between PVC and the heat stabilizer change from the original linear molecule into a three-dimensional network structure, but M-DMA also has the potential to cause a thermoreversible Diels-Alder (D-A) reaction with conjugated diolefins such as furan. While this covalent cross-linking facilitates the repairable recycling of PVC, it also complicates the recovery and recycling process [6,7]. If we can achieve cross-linking enhancement with the help of reversible reactions, combine molecular structure modification with heat stabilization, and achieve covalent cross-linking and repairable recycling of PVC through reversible reactions [8], we can solve the problem of PVC reuse, recovery, and recycling.
Shaban A. Darwish et al. [9] attached benzimidazole groups directly to PVC using short spacer arms through a nucleophilic substitution reaction, so that the PVC had an antibacterial function. Swastika Choudhury et al. [10] significantly improved the organophilic properties of polyvinyl chloride (PVC) films by grafting 4-vinyl pyridine with different mass fractions and 4-vinyl pyridine with different molar ratios to vinyl acetate (VA). During the polymerization process, the grafted copolymer was further filled with organic clay in situ. The PVC film showed good mechanical stability, and showed high adsorption selectivity, flux selectivity, and pervaporation selectivity for tetrahydrofuran (THF) without using clay and with clay filling film, which successfully achieved the selective permeability of the PVC film.
Since its industrialization in 1922, furfural (FF) has been widely used because its raw materials are mainly natural products such as wheat bran and wood chips [11]. Inspired by the Reformatsky reaction, we propose enhancing the stability of PVC at the molecular level by substituting furan groups for unstable chlorine atoms, such as those in allyl chloride, with furan groups, thus enhancing the stability of PVC at the molecular level. Additionally, the furan ring on PVC reacts with M-DMA through a thermally reversible Diels--Alder reaction, enabling the self-healing functionality of the modified PVC.
2. Experiments
2.1. Materials
We used the following materials in this study:
PVC (SG-5) originated from Bei yuan Chemical Group Co., Ltd., Yulin City, China.
Furfural is sourced from McLean Biochemical Technology in Shanghai, China.
Zinc powder is sourced from Feng chuan Chemical Reagent Technology Co., Ltd., Tianjin, China.
Tetrahydrofuran is sourced from Fufu Fine Chemical Co., Ltd., in Tianjin, China.
The ethanol is sourced from Fufu Fine Chemical Co., Ltd., in Tianjin, China.
2.2. Experimental Procedure
The reaction was divided into two stages: initiation and grafting. In the first step, an appropriate amount of PVC was dissolved in THF, and zinc of varying qualities was added as a catalyst, followed by stirring for 15 min. The organic zinc reagent coordinated with the chlorine atoms on the PVC, forming a carbocation. In the second stage, the activated PVC solution was mixed with a measured amount of furfural at the appropriate ratio and subjected to a grafting reaction in a microwave synthesizer. Under the action of the catalyst, the aldehyde group on the furfural reacted with the carbocation ions on the PVC molecular chain to form a graft. Upon completion of the reaction, the mixture is poured into a beaker, and a large volume of anhydrous ethanol is added while stirring continuously. The mixture was filtered, washed with distilled water after flocculation, and dried at 60 °C until a constant weight was obtained, resulting in furan FF-g-PVC.
2.3. Characterization
UV-Vis: A TU-1900 double-beam ultraviolet spectrophotometer, produced by Beijing Spectrometer General Instrument Company, based in Beijing, China, was used for the tests.
PL: A 7890A-5975C fluorescence spectrometer, manufactured by Agilent Technologies in the United States (Santa Clara, CA), was employed for the emission spectrum tests. The excitation light wavelength was set to 280 nm, while the emission light wavelength ranged from 300 nm to 540 nm, with an excitation light bandwidth of 3 nm.
FTIR: The KBr tablet method was utilized for testing on a NICOLET iZ10 infrared spectrometer produced by Thermo Fisher Scientific in the United States.
Raoman: The KBr tablet method was utilized for testing on the NICOLET iZ10 infrared spectrometer produced by Thermo Fisher Scientific incorporated of Waltham, MA, USA.
Congo red test: The static heat stability time of the PVC was determined according to the static test method outlined in the Congo red method (GB/T 2917-2002) [12] (the experimental device is shown in Figure 1), and an appropriate amount of FF-g-PVC was weighed and added to the device as shown in Figure 1. The oil bath temperature was kept at 180 ± 1 °C, the test tube was immersed in the constant temperature oil bath at the same height as the sample surface, and the observation and timing began. When the red Congo test paper in the test tube turned obviously blue, the time was recorded.
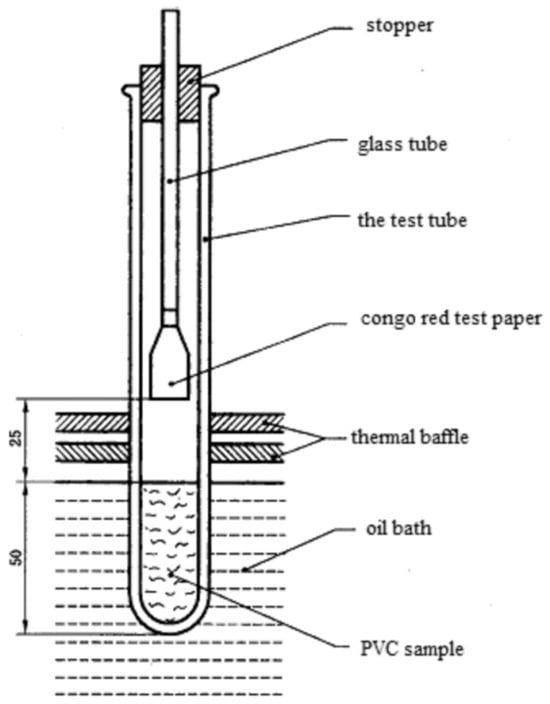
Figure 1.
Congo red test by illustration.
1H NMR: An appropriate amount of samples was weighed and dissolved in deuterated tetrahydrofuran, and the WNMR-1 400 MHz nuclear magnetic resonance instrument from Zhongke Oxford Company, based in Wuhan, China, was used for testing.
TG: The sample was analyzed using a STA 449 F5 thermogravimetric analyzer from Netzsch at heating rates of 5 K/min, 10 K/min, and 15 K/min, in both nitrogen and air atmospheres. The temperature range was from room temperature to 700 °C.
Grafting rate determination: FF-g-PVC was prepared as a 1 g/100 mL standard solution, and its absorbance at 248 nm was measured using a UV-Vis spectrophotometer to obtain a standard curve. The grafting rate was calculated using the following formula, which represents the content of chlorine atoms ClL replaced by furanaldehyde per 1000 vinyl chloride units:
where CIL/1000VC—is the grafting rate (‰); C—is the PVC solution concentration (1 g/100 mL); F—is the amount of furfural substance in the measured solution; and A is the-absorbance of the solution.
CIL/1000VC= (F × 62.5) /C × 1000
F = 0.2391 × A × 10−5
3. Results and Discussion
3.1. Structural Characterization
3.1.1. UV–Visible Absorbance
During the actual processing of plastic samples, the strength and position of the absorption band will be changed due to the addition of additives such as plasticizer and heat stabilizer and the structural state of the polymer [13]. According to El-Hachemi B [14], PVC typically exhibits two electron transition bands around 286 nm and 197 nm [15]. It typically exhibits the π → π* electron transition of an unsaturated C=O bond at the carbonyl allyl site and the C-Cl bond of PVC. Due to the different structural states of PVC, we can see from Figure 2 that at the emission wavelength of 340 nm, the band at 197 nm moves to 228 nm in the scanning range of 200–500 nm. Conjugated dienes show a new absorption peak at 240 nm.
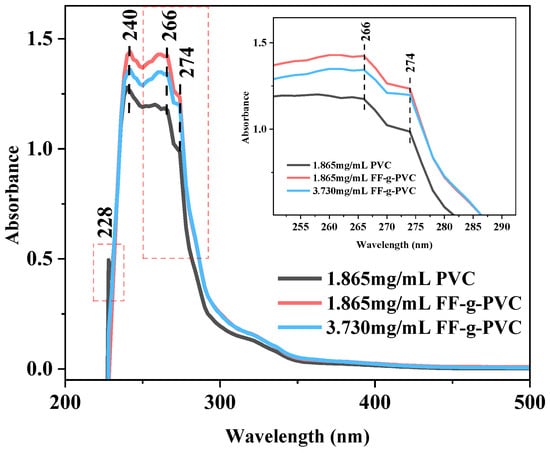
Figure 2.
UV-Vis spectra of PVC and FF-g-PVC.
Compared with PVC, grafted FF-g-PVC showed significantly enhanced absorption at 240 nm and 266 nm, with additional absorption at 274 nm. This enhancement is related to the incorporation of furan rings in FF-g-PVC and the n → π conjugation of chromophore -OH with conjugated diene, which improves the chromophore color ability of PVC.
3.1.2. Photoluminescence Study
The emission spectra of PVC and FF-g-PVC are shown in Figure 3. The emission spectrum of PVC primarily exhibits excimer fluorescence [16]. In their study, El-Hachemi et al. specified that an emission band corresponding to excimer fluorescence, associated with the π* → π transition, was observed at 467 nm for their original PVC under excitation at 467 nm. When excited at 340 nm, a broad peak-like emission band is observed near 402 nm (purple) in pure PVC. For this PVC sample under λ_exc = 280 nm excitation, the photoluminescence (PL) emission spectrum shows a peak at 310 nm, which can be attributed to the excimer fluorescence of PVC corresponding to the π* → π transition [17]. Additionally, the shoulder at 323 nm [18] may be caused by impurities present in PVC. After grafting, the fluorescence intensity of PVC decreases significantly, and the emission peak shifts to 308 nm.
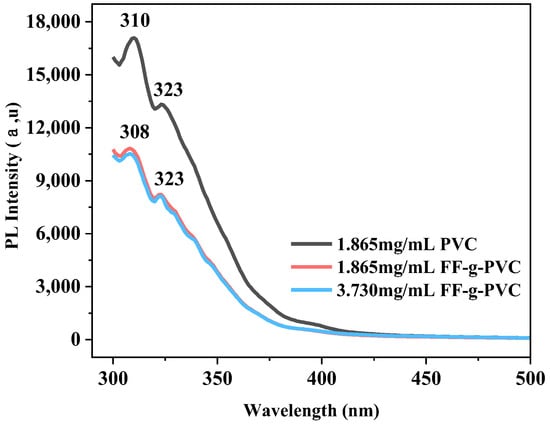
Figure 3.
Photoluminescence spectrum of PVC and FF-g-PVC powder.
3.1.3. FT-IR Analysis
Figure 4 shows the FTIR spectra of PVC and FF-g-PVC. The peak at 2911 cm−1 corresponds to the symmetric stretching vibration of -CH2, while the peak at 2973 cm−1 corresponds to the asymmetric stretching vibration of -CH2. The peak at 640 cm−1 is the characteristic peak of the C-Cl bond. Compared to PVC, FF-g-PVC shows a new absorption peak, the most prominent of which is due to -OH at 3444 cm−1. The characteristic vibration peak of the aromatic skeleton of the furan ring appears at 1605 cm−1, and the stretching vibration of the C-H bond of the furan ring is observed at 3022 cm−1. Bending vibration outside the C-H plane of the furan ring and bending vibration inside the ring appear at 883 cm−1 and 744 cm−1, respectively. The presence of these four characteristic peaks of the furan rings further confirms that the furan rings were successfully grafted onto the PVC molecular chains.
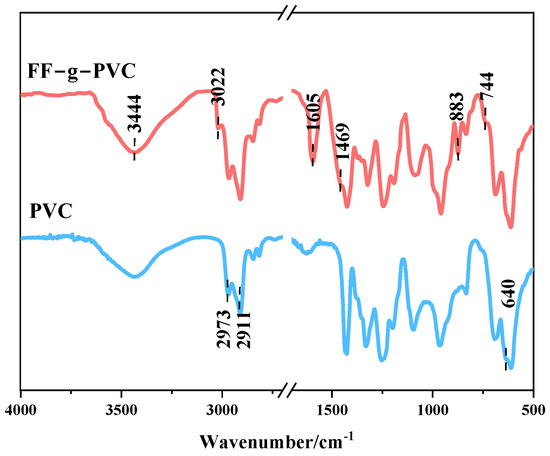
Figure 4.
FTIR spectra of PVC and FF-g-PVC.
3.1.4. Raman Spectroscopy
Figure 5 shows the Raman spectra of PVC and FF-g-PVC, where the peaks at 2975 cm−1 and 2916 cm−1 correspond to the stretching vibrations of CH2 in PVC, while the peaks at 1425 cm−1 and 1325 cm−1 correspond to the bending vibrations of CH2. The peaks at 1167 cm−1, 1100 cm−1, and 904 cm−1 correspond to the stretching vibrations of the C-C bond, and the peaks at 683 cm−1 and 626 cm−1 correspond to the stretching vibrations of C-Cl. The peak at 350 cm−1 corresponds to the bending vibration of C-C, which is consistent with the Raman characteristic peaks of PVC reported in the literature [19].
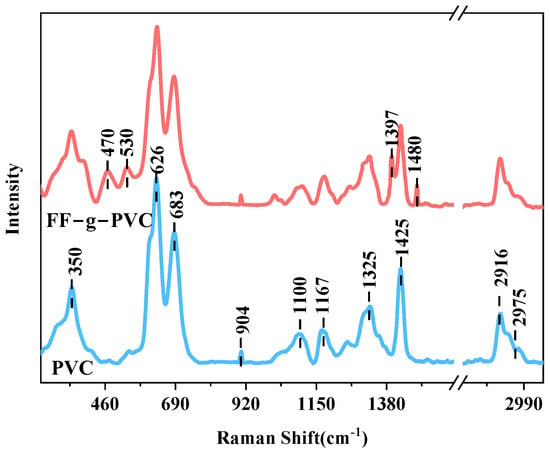
Figure 5.
Raman spectra of PVC and FF-g-PVC.
In contrast, FF-g-PVC exhibits characteristic peaks of the furan ring. The peak at 1480 cm−1 is associated with the in-plane bending vibration of the heterocyclic ring, accompanied by the stretching vibrations of C2=C3 and C4=C5, as well as C-O-C in-plane bending vibration. The peak at 1397 cm−1 corresponds to the in-plane bending vibration of the heterocyclic ring, which is accompanied by the stretching vibrations of C3 and C4, as well as the bending vibration of the C=C-C plane. Additionally, the peaks at 530 cm−1 and 470 cm−1 are attributed to the stretching vibrations of the C2-C6 plane, indicating that the furan ring was successfully grafted onto the PVC chain.
3.1.5. Nuclear Magnetic Hydrogen Spectrum
Figure 6 shows a comparison of the nuclear magnetic resonance (NMR) hydrogen spectra of PVC and FF-g-PVC, and the results are summarized in Table 1. The peak at δ 3.58 corresponds to the characteristic peak of deuterated tetrahydrofuran, the range δ 3.84~1.73 corresponds to the characteristic peak of -CH2 for PVC, and the range δ 4.72~4.08 corresponds to the characteristic peak of -CHCl.
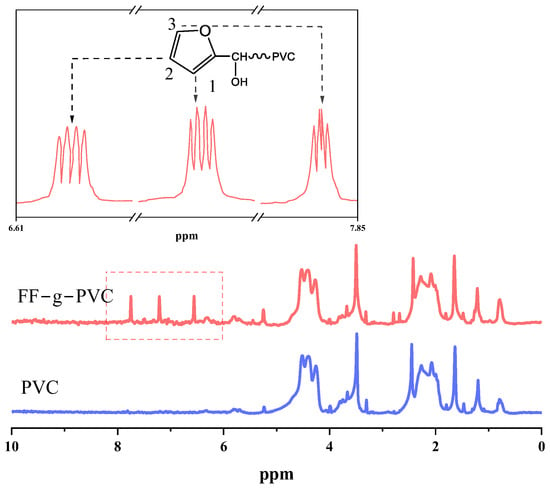
Figure 6.
1H NMR spectra of FF-g-PVC.

Table 1.
1H NMR (400 MHz) data for PVC and FF-g-PVC in THF-d8.
In comparison to PVC, FF-g-PVC exhibits a new characteristic peak at δ 6.64, which corresponds to the hydrogen at position 2. This peak is split into a doublet of doublets (dd) due to the influence of the hydrogens at positions 1 and 3, indicating the presence of the =CH- characteristic peak on the furan ring. The hydrogens at positions 1 and 3 are in the same environment, with chemical shifts at δ 7.29 and δ 7.87.
According to the absorption peak intensity (ordinate) of the nuclear magnetic hydrogen spectrum, the peak area is integrated to quantitatively calculate the number of protons, and the number of hydrogen atoms is shown in Table 1. It is calculated that there are 4 furan rings replacing chlorine atoms on every 68 chain links (-CHCl-), that is, the grafting rate is about 58.82‰. When furfural reacts with PVC, the first reaction involves the highest activity of allyl chloride atoms and normal chlorine atoms, and the reaction of defective chlorine atoms in other positions does not easily occur; for example, the branch chain of tertiary chlorine is not easily replaced due to large steric resistance. The normal chlorine atom position reaction equation is shown in Scheme 1, and the allyl chloride atom reaction equation is shown in Scheme 2.
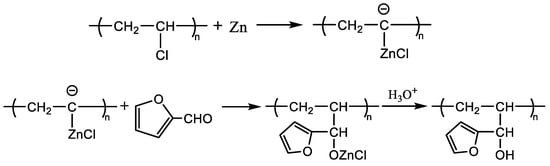
Scheme 1.
PVC and furfural reaction equation (normal position chlorine atom).
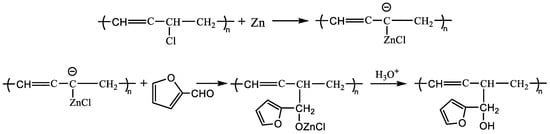
Scheme 2.
PVC and furfural reaction equation (allyl chloride atom).
3.2. Thermal Stability
3.2.1. Congo Red Static Experiment
As one of the most widely used general purpose plastics at present, PVC is limited in terms of application due to its own structure and processing technology. Therefore, the thermal stability of PVC is a key indicator of its processing performance.
The causes and mechanisms of the thermal degradation of PVC are very complex, and the most widely recognized degradation theories include the free radical mechanism, ionic mechanism, and single–molecular mechanism, of which the free radical mechanism is the most popular. According to the principle of the PVC thermal stability test, its thermal stability can be divided into static thermal stability and dynamic thermal stability. There are two main principles of the PVC thermal stability test: a. The observation principle, that is, observing the color change in PVC when it is heated and decomposed; the test method based on this principle is relatively limited, and can only observe materials such as white ones for which it is easy to observe color changes. b. The Cl release measurement principle, that is, measuring the release of HCl to study the thermal decomposition reaction process of PVC; the test method based on this principle (Congo red test paper method and general pH test paper method) has fewer limitations, and has become the current most commonly used PVC thermal stability test method.
The thermal stability of PVC was tested according to the static test method for the thermal stability of rigid PVC (GB/T 2917.1-2002) [12], as shown in Table 2. The relative deviation between the individual measurement results and the average value was within 10%, which satisfies the requirements of the test standard.

Table 2.
Congo red static test duration.
3.2.2. Thermogravimetric Analysis
To further investigate whether the thermal properties of PVC improved after grafting, a thermogravimetric (TG) analysis was conducted on the PVC grafts. Based on the TG and DTG curves shown in Figure 7 and Figure 8, as well as the data presented in Table 3, it is evident that the rapid pyrolysis of PVC under a nitrogen atmosphere can be divided into two degradation stages: the HCl removal stage of PVC and the cracking and aromatization reactions of PVC olefins following dechlorination [20,21].
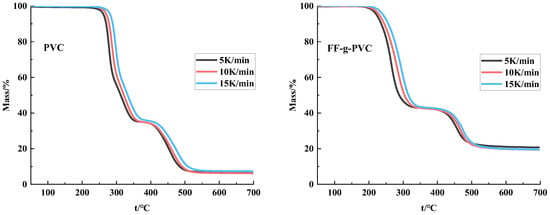
Figure 7.
TG curve of PVC and FF-g-PVC (in nitrogen).
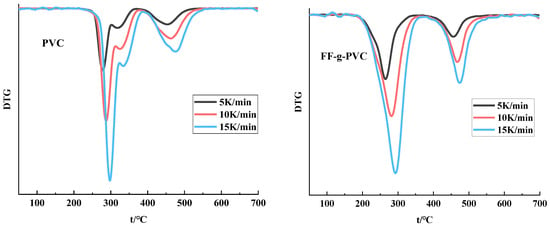
Figure 8.
DTG curve of PVC and FF-g-PVC (in nitrogen).

Table 3.
Characteristics of pyrolysis in nitrogen atmosphere at different rates.
At a heating rate of 15 K/min, the significant weight loss in the first stage occurs between 279.07 °C and 326.54 °C, with the maximum weight loss temperature at 296.31 °C and a weight loss rate of 64.28%. This weight loss is roughly equivalent to the mass of chlorine atoms in the PVC sample [22]. During this stage, most chlorine atoms are removed, forming HCl molecules, while the remaining polymers convert into π-conjugated polyenes.
The significant weight loss in the second stage occurs between 423.09 °C and 509.63 °C, with the maximum weight loss temperature at 474.38 °C and a weight loss rate of 28.69%. Compared to the rapid weight loss in the first stage, the weight loss at this stage is relatively stable, as the main chain of the dechlorinated PVC begins to crack and decompose [23]. Isomerization, crosslinking, and aromatization reactions occur during this stage [24]. As the temperature continues to rise, the sample further decomposes and carbonizes, resulting in residues that account for 7.03%.
To further analyze the effect of the grafting reaction on the thermal release of HCl from PVC, a kinetic analysis of thermal degradation was conducted. The Arrhenius equation, a common tool for this analysis, is expressed as follows:
where is the reaction rate constant;
refers to the pre-factor or the total frequency factor;
is the activation energy;
is the gas constant;
is the temperature.
The Arrhenius equation is suitable for almost all elementary reactions and most complex reactions in homogeneous reactions. The Arrhenius equation is suitable for gas phase and liquid phase reactions and most multinomial catalytic reactions. The most commonly used dynamic thermal analysis methods include the Ozawa method [25], Friedman method [26], and Kissinger method [27]. The thermal degradation kinetics of FF-g-PVC was analyzed using the Friedman method.
The following is a common kinetic equation for heterogeneous systems under non-constant temperature conditions:
Taking the natural logarithm of both sides of Equation (2), we obtain:
We plot from , fit the data using the least squares method, and find E based on the slope; the results are listed in Table 4.

Table 4.
Calculation results of activation energy of degradation reaction.
The activation energy reflects the difficulty of thermal degradation of PVC. According to the fitting curve of degradation kinetic parameters shown in Table 4, the activation energies for PVC and FF-g-PVC in the first stage of thermal degradation are 149.0 kJ/mol and 100.6 kJ/mol, respectively, indicating a decrease of 48.40 kJ/mol for FF-g-PVC compared to pure PVC. The main reason for this reduction is that furfural replaced some of the unstable chlorine atoms in PVC, resulting in the formation of alcohols. During the process of R–OH bond breaking, tertiary alcohols undergo cleavage the fastest, followed by primary alcohols. However, tertiary chlorine in the branched-chain position exhibits significant steric hindrance, making it less susceptible to replacement.
In the second stage, the activation energies for PVC and FF-g-PVC are 199.3 kJ/mol and 294.4 kJ/mol, respectively, showing an increase of 95.1 kJ/mol. This second stage primarily involves C–C bond cleavage, with the PVC main chain continuing to crack at elevated temperatures. The degree of cleavage of the hydroxyl group at the allyl group and the adjacent carbon–carbon double bond increases with temperature, leading to higher degrees of cyclization, aromatization, and condensation. Consequently, the removal processes require higher activation energy, which reflects the increase in activation energy for pyrolysis during the second stage.
3.3. Optimization of Grafted Reaction Conditions for FF-g-PVC
The reaction conditions greatly affect the grafting rate of furfural. The microwave irradiation method [28] can provide more high–frequency reciprocating motion to the reaction system through microwaves so that internal and external heating can be carried out at the same time; this method is simple to operate and has fewer by-products. Meanwhile, polar polymer PVC can quickly absorb a large amount of microwaves. This method is simple to operate and produces fewer by-products. In our experiments, PVC, FF, and Zn were reacted using water bath heating and microwave irradiation at a ratio of 1:1:0.9. It was observed that the grafting rate using water bath heating was only 1.808‰, while the rate achieved with microwave irradiation was significantly higher at 57.76‰. Therefore, microwave irradiation was selected as the heating method for the reaction in subsequent experiments.
In addition, the appropriate reaction time can ensure the maximum reaction grafting rate. Too long a reaction time for irradiation will cause degradation and disproportionation of the PVC molecular chain and affect the grafting rate. The appropriate reaction temperature can improve the reaction activity, and the reaction temperature can not easily to exceed the boiling point of tetrahydrofuran. Zinc powder, as a solid catalyst, will have a synergistic catalyst carrier surface covering, and a synergistic effect cannot be exerted; in addition, the number of reaction materials and molecules is certain, and there is a limited number of active numbers involved in the reaction. If the catalyst content is too high, it will cause active site inactivation, resulting in PVC degradation. The effects of reaction time, heating temperature and catalyst dosage on the grafting rate are shown in Figure 9.
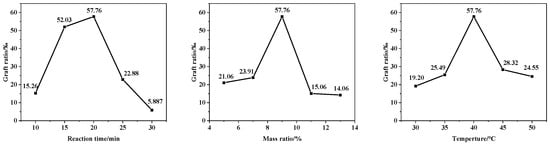
Figure 9.
The effect of reaction time, heating temperature and catalyst dosage on the grafting rate.
In summary, the reaction mode, reaction temperature, reaction time, and mass ratio of the catalyst all significantly affect the grafting reaction of furfural with PVC. The optimal reaction conditions were determined to be a PVC/Fur/Zn ratio of 1:1:0.9, with a microwave irradiation grafting rate of 57.76‰ at 40 °C for 20 min.
4. Conclusions
- (1)
- Microwave irradiation can promote the grafting reaction between furfural and PVC. The grafting rate under water bath heating is only 1.808‰, while the grafting rate achieved through microwave irradiation is 57.76‰. The optimal reaction conditions are as follows: a PVC/FF/Zn ratio of 1:1:0.9, with microwave irradiation at 40 °C for 20 min.
- (2)
- In the first stage of pyrolysis, the bond energy of the hydroxyl group is low, making it easy to break off; the fracture rate of primary alcohols is high, and the activation energy is slightly reduced. In the second stage of pyrolysis, the degree of cleavage of the hydroxyl group and the adjacent carbon–carbon double bond increases with temperature. This leads to a greater degree of cyclization, aromatization, and condensation, resulting in a 47.64% increase in activation energy for the second stage of pyrolysis.
- (3)
- In this experiment, a furan ring was grafted onto PVC through a chemical reaction. The introduction of a furan ring brought many new functions to the PVC molecular chain, such as giving PVC fluorescent luminescence characteristics; in addition, furan rings can be subjected to a REDOX reaction by through photocatalysis. The aromatic properties of furan rings can lead to electrophilic addition reactions such as halogenation, nitration, and sulfonation, and a D-A addition reaction occurs with dienophile (e.g., maleimide).
Author Contributions
Conceptualization, K.L. and M.K.; formal analysis, M.K.; investigation, K.L.; resources, K.L.; data curation, M.K.; writing—original draft preparation, M.K.; writing—review and editing, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ait-Touchente, Z.; Khellaf, M.; Raffin, G.; Lebaz, N.; Elaissari, A. Recent advances in polyvinyl chloride (PVC) recycling. Adv. Technol. 2024, 35, e6228. [Google Scholar] [CrossRef]
- Moulay, S. Chemical modification of poly(vinyl chloride)—Still on the run. Prog. Polym. Sci. 2010, 35, 303–331. [Google Scholar] [CrossRef]
- Kameda, T.; Ono, M.; Grause, G.; Mizoguchi, T.; Yoshioka, T. Chemical modification of poly(vinyl chloride) by nucleophilic substitution. Polym. Degrad. Stab. 2009, 94, 107–112. [Google Scholar] [CrossRef]
- Taurino, R.; Sciancalepore, C.; Collini, L.; Bondi, M.; Bondioli, F. Functionalization of PVC by chitosan addition: Compound stability and tensile properties. Compos. Part B Eng. 2018, 149, 240–247. [Google Scholar] [CrossRef]
- Sun, J.; Bai, M.; Wang, R.; He, L.; Li, K. Effect of Ethylene Bismaleimide Acid Derivatives on Thermal Stability and Repair Performance of Polyvinyl Chloride. Polym. Mater. Sci. Eng. 2019, 35, 76–87. [Google Scholar] [CrossRef]
- Leadbitter, J. PVC and sustainability. Prog. Polym. Sci. 2002, 27, 2197–2226. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, M.-Q.; Jiao, Y.; Li, Y.; Sun, B.; Xiao, D.; Wang, M.; Ma, D. Co-upcycling of polyvinyl chloride and polyesters. Nat. Sustain. 2023, 6, 1685–1692. [Google Scholar] [CrossRef]
- Li, B.; Cao, P.-F.; Saito, T.; Sokolov, A.P. Intrinsically Self-Healing Polymers: From Mechanistic Insight to Current Challenges. Chem. Rev. 2023, 123, 701–735. [Google Scholar] [CrossRef]
- Darwish, S.A.; Abdel-Monem, R.A.; Rabie, S.T.; El-Liethy, M.A.; Hemdan, B.A.; Gaballah, S.T. Facile strategy for the synthesis of antimicrobial poly(vinyl chloride) functionalized with benzimidazole. Vinyl Addit. Technol. 2024, 31, 94–108. [Google Scholar] [CrossRef]
- Meng, X.; Li, W.; Tian, Y.; Sun, C.; Huang, J.; Liu, X. Synthesis of trioctyl polyvinyl chloride ammonium, membrane extraction properties, and electrodriven mass transfer behavior of Chromium (VI). Sep. Purif. Technol. 2023, 320, 1383–5866. [Google Scholar] [CrossRef]
- Lv, X.; Luo, X.; Cheng, X.; Liu, J.; Li, C.; Shuai, L. Production of Hydroxymethylfurfural Derivatives From Furfural Derivatives via Hydroxymethylation. Front. Bioeng. Biotechnol. 2022, 10, 851668. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Zahoor, A.F.; Ahmad, S.; Akhtar, R.; Sikandar, S. Reformatsky reaction as a key step in the synthesis of natural products: A review. Synth. Commun. 2022, 52, 317–345. [Google Scholar] [CrossRef]
- Li, D.; Liu, S.; Gu, L.; Li, G.; Ding, Y.; Dong, L. Preparation of rare earth complexes with curcumin and their stabilization for PVC—ScienceDirect. Polym. Degrad. Stab. 2020, 184, 109480. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; Heiba, Z.K.; Kamal, A.M.; Abd-Elkader, O.H.; Mohamed, M.B. Impact of ZnS/Mn on the Structure, Optical, and Electric Properties of PVC Polymer. Polymers 2023, 15, 2091. [Google Scholar] [CrossRef] [PubMed]
- El-Hachemi, B.; Miloud, S.; Sabah, M.; Souad, T.; Zineddine, O.; Boubekeur, B.; Toufik, S.M.; Ouahiba, H. Structural, electrical and optical properties of PVC/ZnTe nanocomposite thin films. Inorg. Organomet. Polym. Mater. 2021, 15, 3637–3648. [Google Scholar] [CrossRef]
- Taha, T.A. Optical and thermogravimetric analysis of-Pb3O4/PVC nanocomposites. Mater. Sci. 2017, 28, 12108–12114. [Google Scholar] [CrossRef]
- Meng, X.; Song, Y.; Lv, Y.; Xin, X.; Ren, T.; Wang, X. Study on stable mass transfer and enrichment of phenol by 1-octanol/kerosene/polyvinyl chloride polymer inclusion membrane. Environ. Pollut. 2019, 253, 1100–1106. [Google Scholar] [CrossRef]
- Aziz, S.B.; Ahmed, H.M.; Hussein, A.M.; Fathulla, A.B.; Wsw, R.M.; Hussein, R.T. Tuning the absorption of ultraviolet spectra and optical parameters of aluminum doped PVA based solid polymer composites. Mater. Sci. Mater. Electron. 2015, 26, 8022–8028. [Google Scholar] [CrossRef]
- Xie, L.; Luo, S.; Liu, Y. Automatic Identification of Individual Nanoplastics by Raman Spectroscopy Based on Machine Learning. Environ. Sci. Technol. EST 2023, 57, 18203–18214. [Google Scholar] [CrossRef]
- Lee, T.; Oh, J.-I.; Baek, K.; Tsang, Y.F.; Kim, K.-H.; Kwon, E.E. Compositional modification of pyrogenic products using CaCO3 and CO2 from the thermolysis of polyvinyl chloride (PVC). Green Chem. 2018, 20, 1583–1593. [Google Scholar] [CrossRef]
- Wu, J.; Papanikolaou, K.G.; Cheng, F.; Addison, B.; Cuthbertson, A.A.; Mavrikakis, M.; Huber, G.W. Kinetic Study of Polyvinyl Chloride Pyrolysis with Characterization of Dehydrochlorinated PVC. ACS Sustain. Chem. Eng. 2024, 19, 12. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, B.; Qiao, Y.; Zhang, J.; Wang, W.; Yao, H.; Yu, Y.; Xu, M. Understanding the pyrolysis mechanism of polyvinylchloride (PVC) by characterizing the chars produced in a wire-mesh reactor. Fuel 2016, 166, 526–532. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Hu, E.; Qu, R.; Li, S.; Xiong, Q. Interaction and characteristics of furfural residues and polyvinyl chloride in fast co-pyrolysis. Front. Chem. Sci. Eng. 2024, 18, 142. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, G.; Wang, S.; Zhang, H.; Xu, F. TG-FTIR and Py-GC/MS study of the pyrolysis mechanism and composition of volatiles from flash pyrolysis of PVC. Energy Inst. 2020, 774, 2362–2370. [Google Scholar] [CrossRef]
- Azam, M.; Ashraf, A.; Jahromy, S.S.; Miran, S.; Raza, N.; Wesenauer, F.; Jordan, C.; Harasek, M.; Winter, F. Co-Combustion Studies of Low-Rank Coal and Refuse-Derived Fuel: Performance and Reaction Kinetics. Energies 2021, 14, 3796. [Google Scholar] [CrossRef]
- Franco-Urquiza, E.A.; May-Crespo, J.F.; Velázquez, C.A.E.; Mora, R.P.; García, P.G. Thermal Degradation Kinetics of ZnO/polyester Nanocomposites. Polymers 2020, 12, 1753. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, D.; Eloussifi, H.; Farjas, J.; Roura, P.; Dammak, M. Thermal gradients in thermal analysis experiments: Criterions to prevent inaccuracies when determining sample temperature and kinetic parameters. Thermochim. Acta 2014, 589, 37–46. [Google Scholar] [CrossRef]
- Goyal, H.; Chen, T.Y.; Chen, W. A review of microwave-assisted process intensified multiphase reactors. Chem. Eng. J. 2022, 430, 133183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).