Abstract
Six secondary amine derivatives derived from salicylaldehyde (SA) were successfully synthesized in good to excellent yields and evaluated for their biological activities. The synthesized compounds exhibited remarkable antioxidant properties, as determined by ABTS and phenanthroline assays. Notably, compound 2 demonstrated an IC50 value of 5.14 ± 0.11 µM in the ABTS assay, approximately six to nine times lower than the standards BHT and BHA. In the phenanthroline assay, all compounds showed inhibition capacities five to ten times greater than BHT and comparable to BHA, with A0.5 values ranging from 9.42 to 31.73 µM. Among these, compound 5 displayed the lowest A0.5 value of 9.42 ± 1.02 µM. The anti-inflammatory activity, assessed through BSA denaturation, revealed that compounds 2 and 5 were the most promising, although their activity was moderate compared to the standard diclofenac. The insecticidal potential of the compounds was evaluated against the storage insect pest Tribolium castaneum. Among the tested derivatives, compounds 1 and 6 exhibited the highest efficacy, achieving maximum mortality rates of 73.31% and 76.67%, respectively, over a seven-day treatment period. Furthermore, the molecular geometry, electronic properties, and intramolecular interactions of all compounds were investigated using DFT calculations. Thermodynamic analyses of the antioxidant mechanisms suggested that the NH bond is the most likely site for free radical attacks. These findings underscore the significant biological potential of the synthesized salicylaldehyde-derived secondary amines.
1. Introduction
Salicylaldehyde (SA), or 2-hydroxybenzaldehyde, and its derivatives continue to demonstrate exceptional versatility and therapeutic potential. Numerous studies have highlighted their potent activities as antimicrobial [1,2], antioxidant [3], and anticancer [4]. SA has shown significant insecticidal effects against Frankliniella occidentalis, one of the most destructive pests in agriculture and horticulture, by reducing oviposition in females and minimizing crop damage caused by feeding [5]. Additionally, SA exhibits significant antifungal and anti-mycotoxigenic activities against Aspergillus flavus and A. parasiticus, effectively inhibiting fungal growth and reducing aflatoxin production. Notably, its ability to enhance the efficacy of commercial antifungal agents underscores its potential as a practical fumigant for controlling fungal pathogens and mycotoxin contamination [6,7]. Substituted SA and related compounds have shown broad-spectrum antimicrobial efficacy, particularly against microbes such as Candida albicans, Staphylococcus epidermidis, Aspergillus niger, and Saccharomyces cerevisiae [8]. SA serves as a privileged reactive in the synthesis of various biologically active compounds. For example, α-alkenyl-γ and δ-lactam derivatives, prepared using different aromatic aldehydes including SA, exhibited potent antifungal activity against Colletotrichum orbiculare, an agricultural pathogen [9]. Additionally, xanthone and azaxanthone derivatives synthesized from SA have demonstrated diverse biological activities [10]. Benzofuran derivatives, using SA as a starting material, displayed strong antimicrobial properties against Staphylococcus aureus and Escherichia coli [11]. In sustainable materials research, SA-derived compounds have been applied to the development of polyvinyl acetals, which hold promising potential in industrial polymer production [12]. Moreover, these compounds have been incorporated into polymer and nanocomposite technologies. Functionalized block copolymer nano-objects containing SA have shown potential for fluorescence and crosslinking applications [13]. Similarly, polymethyl methacrylate (PMMA) derivatives functionalized with phenyl and adamantyl groups have been explored for their advanced optical, thermal, and mechanical properties [14,15].
Compounds containing secondary amine fragments have attracted significant attention due to their diverse and remarkable biological activities, including antioxidant, anti-inflammatory [16], and insecticidal properties [17]. These compounds, which can be either synthesized or derived from natural sources such as medicinal and aromatic plants, are characterized by two alkyl or aryl substituents attached to an NH group. This structural feature enables effective interactions with biological macromolecules, underpinning their potent bioactivity [18]. Notably, when one substituent is an aromatic fragment, the NH group can donate its hydrogen atom, enhancing their antiradical activity and contributing to their therapeutic potential [19,20].
This study presents the efficient synthesis of a series of secondary amines incorporating the SA fragment. The molecular structures of these compounds were characterized using infrared spectroscopy (IR) and 1H and 13C nuclear magnetic resonance (NMR) spectroscopy. Their biological potential was then evaluated through in vitro experiments. The antioxidant activity of the compounds was assessed using two complementary assays: one measuring free radical scavenging capacity (ABTS assay) and the other evaluating electron donation potential (phenanthroline assay). Anti-inflammatory activity was determined by examining the ability of the compounds to denature bovine serum albumin (BSA). Additionally, insecticidal activity was tested against T. castaneum, a pest responsible for considerable economic losses in stored grain products [21,22]. To further explore the molecular and electronic properties of the synthesized compounds, density functional theory (DFT) calculations were performed, considering the effects of the ethanol environment. Thermodynamic analyses were also conducted to elucidate the compounds’ antioxidant mechanisms, with a particular focus on the contributions of OH and NH bonds to their activity.
2. Materials and Methods
2.1. General Information
Thin-layer chromatography (TLC) was performed using plastic plates coated with a 0.2 mm layer of silica gel 60F254. The melting points of the various compounds were determined using the Köfler apparatus available at the laboratory of the National School of Biotechnology in Constantine. Carbon and proton nuclear magnetic resonance (13C and 1H NMR) spectroscopy were carried out at the NMR facility of the LCP-A2MC laboratory at the University of Lorraine. The multiplicity of the peaks is described as follows: singlet (s), doublet (d), doublet of doublets (dd), triplet (t), multiplet (m). The measurements of the infrared (IR) spectra were conducted using an infrared spectrometer available at the laboratory of the National School of Biotechnology in Constantine.
2.2. Synthesis
2.2.1. Synthesis of Imine Intermediates a–f
In a 100 mL round-bottom flask, 1 mmol of the corresponding aromatic amine was dissolved in 5 mL of absolute ethanol. Subsequently, 1 mmol of salicylaldehyde was added dropwise. Once the addition was complete, the solution was left to react at room temperature for 10 min. After cooling the solution in an ice bath, the resulting imine was filtered using a Büchner funnel, washed with a small amount of cold ethanol, and dried under vacuum.
2.2.2. Synthesis of Compounds 1–6
The entire quantity of the previously synthesized imine 150 mg (0.7 mmol) was transferred into a 100 mL round-bottom flask containing 5 mL of absolute ethanol. The solution was cooled to 5 °C. While maintaining stirring, sodium borohydride 15 mg (0.4 mmol) was added in small portions, corresponding to 1/10 of the amount of imine used, ensuring that the temperature did not exceed 10 °C. The solution was then allowed to rest for 5 min. The resulting secondary amine was filtered using a Büchner funnel, washed with a small amount of cold ethanol, and the final product was dried under vacuum.
2-((p-Tolylamino)methyl)phenol (1)
Compound 1 was obtained as a white solid (70%). M.p: 136 °C; FTIR (νmax, cm−1): 3358 (NH); 1H NMR (400 MHz, CDCl3) δ (ppm): 8.78 (s, 1H, NH), 7.26–7.21 (m, 1H), 7.15 (d, J = 8Hz, 1H), 7.07 (d, J = 8Hz, 2H), 6.91–6.86 (m, 2H), 6.79–6.76 (m, 2H), 4.40 (s, 2H, CH2), 3.85 (s, 1H, OH), 2.29 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ (ppm): 157.0, 144.7, 130.4, 130.0, 129.9, 129.2, 128.6, 122.9, 119.9, 116.7, 116.2, 49.4, 20.5.
2-(((4-Chlorophenyl)amino)methyl)phenol (2)
Compound 2 was obtained as a white solid (53%). M.p: 136 °C; FTIR (νmax, cm−1): 3254 (NH); 1H NMR (400 MHz, CDCl3) δ (ppm): 7.97 (s, 1H, NH), 7.26–7.15 (m, 4H), 6.91–6.87 (m, 2H), 6.77–6.74 (m, 2H), 4.38 (s, 2H, CH2), 3.98 (s, 1H, OH); 13C NMR (100 MHz, CDCl3): δ (ppm): 156.4, 145.8, 129.4, 129.3, 128.8, 125.5, 122.6, 120.3, 116.9, 116.6, 48.4.
2-(((3-Chlorophenyl)amino)methyl)phenol (3)
Compound 3 was obtained as a white solid (52%). M.p: 120 °C; FTIR (νmax, cm−1): 3255 (NH); 1H NMR (400 MHz, CDCl3) δ (ppm): 7.60 (s, 1H, NH), 7.26–7.21 (m, 1H), 7.18–7.12 (m, 2H), 6.92–6.87 (m, 3H), 6.85–6.81 (m, 1H), 6.70–6.67 (m, 1H), 4.38 (s, 2H, CH2), 4.03 (s, 1H, OH); 13C NMR (100 MHz, CDCl3): δ (ppm): 156.1, 148.5, 135.1, 130.4, 129.4, 128.9, 122.7, 120.4, 120.3, 116.6, 115.4, 113.7, 47.8.
2-(((2-Fluorophenyl)amino)methyl)phenol (4)
Compound 4 was obtained as a white solid (83%). M.p: 50 °C; FTIR (νmax, cm−1): 3314 (NH); 1H NMR (400 MHz, CDCl3) δ (ppm): 7.93 (s, 1H, NH), 7.25 (t, J = 8Hz, 1H), 7.19 (d, J = 8Hz, 1H), 7.13–7.01 (m, 2H), 6.98–6.89 (m, 3H), 6.86–6.78 (m, 1H), 4.43 (s, 2H, CH2), 4.26 (s, 1H, OH); 13C NMR (100 MHz, CDCl3): δ (ppm): 156.4, 154.0, 151.6, 135.7, 129.3, 128.6, 124.7, 122.8, 120.3, 116.7, 115.5, 114.9, 50.0.
2-((o-Tolylamino)methyl)phenol (5)
Compound 5 was obtained as a white solid (86%). M.p: 84 °C; FTIR (νmax, cm−1): 3360 (NH); 1H NMR (400 MHz, CDCl3) δ (ppm): 8.31 (s, 1H, NH), 7.19–7.05 (m, 4H), 6.87–6.77 (m, 4H), 4.36 (s, 2H, CH2), 3.71 (s, 1H, OH), 2.13 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ (ppm): 156.8, 145.0, 130.4, 129.2, 128.9, 127.3, 125.2, 122.9, 120.6, 120.1, 116.7, 113.4, 48.3, 17.7.
2-((Phenylamino)methyl)phenol (6)
Compound 6 was obtained as a white solid (48%). M.p: 118 °C; FTIR (νmax, cm−1): 3262 (NH); 1H NMR (400 MHz, CDCl3) δ (ppm): 8.25 (s, 1H, NH), 7.20–7.13 (m, 3H), 7.15 (dd, J = 8.0, 2.0 Hz, 1H), 6.87–6.75 (m, 5H), 4.34 (s, 2H, CH2), 3.55 (s, 1H, OH); 13C NMR (100 MHz, CDCl3): δ (ppm): 156.8, 147.2, 129.4, 129.2, 128.7, 122.9, 120.9, 120.1, 116.7, 115.9, 48.8.
2.3. Biological Evaluation
2.3.1. Antioxidant Activity
ABTS Assay
The ABTS•+ scavenging activity was evaluated following the method described by Re et al. [23], with results expressed as IC50 values. This assay is based on the reduction of the ABTS radical cation (ABTS•+) in the presence of an antioxidant, which causes a color change from transparent to green. Positive controls, such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT), were used for comparison. The ABTS•+ radical was generated by dissolving 19.2 mg (7 mM) of ABTS in 5 mL of distilled water, followed by the addition of 3.3 mg (2.45 mM) of potassium persulfate (K2S2O8) and an additional 5 mL of water. The solution was incubated in the dark for 16 h. Before use, the solution’s absorbance was adjusted to 0.700 ± 0.020 at 734 nm using ethanol. For the assay, 40 µL of the ethanolic test solution was mixed with 160 µL of ABTS•+ solution in a microplate well. The reaction mixture was incubated for 10 min at room temperature, and the absorbance was measured at 734 nm using a spectrophotometer. The percentage of inhibition was calculated to determine antioxidant capacity.
Phenanthroline Assay
The phenanthroline assay was performed according to the protocol by Szydlowska-Czerniak [24], and the results were expressed as A0.5 values. This method evaluates the reduction of Fe3+ ions to Fe2+ in the presence of antioxidants, leading to the formation of a red-orange [Fe2+-phenanthroline] complex. Positive standards, such as BHA and BHT, were included for comparison. The phenanthroline solution (0.5%) was prepared by dissolving 0.05 g of 1,10-phenanthroline in 10 mL of methanol, while the iron chloride solution (0.2%) was prepared by dissolving 0.02 g of FeCl3 in 10 mL of ultra-pure water. For the assay, 10 µL of the test solution was combined with 50 µL of the FeCl3 solution, 30 µL of the phenanthroline solution, and 110 µL of methanol in a microplate well. The reaction mixture was incubated at 30 °C for 20 min, and the absorbance was measured at 510 nm using a spectrophotometer.
2.3.2. Anti-Inflammatory Activity
The in vitro anti-inflammatory activity was assessed using the BSA denaturation inhibition method as described by Kandikattu et al. [25]. This assay measures the ability of test compounds to prevent the heat-induced denaturation of bovine serum albumin (BSA) at 72 °C. To prepare the assay solutions, a 0.05 M Tris-HCl buffer (pH 6.6) was made by dissolving 1.21 g of Tris-HCl in 200 mL of double-distilled water and adjusting the pH to 6.6 with HCl. A 0.2% BSA solution was prepared by dissolving 0.2 g of BSA in 100 mL of the Tris-HCl buffer. Different concentrations of the test compounds were prepared from a stock solution of 10,000 ppm, and diclofenac sodium (500 ppm stock) was used as the positive control, dissolved in distilled water. For blanks, compound blanks were prepared by adding 1 mL of the test solution to 1 mL of Tris-HCl buffer to account for the absorbance of the compounds, and BSA blanks were prepared by adding 1 mL of the BSA solution to 1 mL of the solvent used for the compounds. In the procedure, 1 mL of each compound or standard was mixed with 1 mL of BSA solution and incubated at 37 °C for 15 min, followed by heating at 72 °C for 5 min in a water bath. After cooling, turbidity was measured at 660 nm using a spectrophotometer.
2.3.3. Insecticidal Activity
Test Insect
Tribolium castaneum (Coleoptera: Tenebrionidae) used in this study were obtained from laboratory cultures maintained on wheat flour. A plastic bottle (25 cm in height and 12 cm in width) was filled with 200 g of wheat flour containing 5% (w/w) brewer’s yeast. Adult insects, measuring 4 mm × 1.5 mm, were transferred to new bottles after 5 days of oviposition. To ensure a uniform age of adults, the cultures were regularly monitored. The T. castaneum cultures were kept at 30 ± 1 °C and 80 ± 5% relative humidity. Two-week-old adults were used in all insect bioassays. All tests were conducted under conditions identical to those of the cultures. In all bioassays, insects were considered dead when no movement of legs or antennae was observed.
Contact Toxicity Assay
The contact toxicity of six compounds (1 to 6) was assessed on adult T. castaneum following the method described by Preetha et al. [26]. A single concentration (20 mM) of each compound was prepared in 1 mL acetone. One microliter (1 μL) of each compound solution was applied directly to the thorax of each insect. The control group was treated with acetone only. Ten insects were used per treatment and control group, and the experiment was repeated three times. Both treated and control insects were placed in Petri dishes (90 mm diameter) containing 10 g of crushed sterile wheat seeds. Mortality was monitored over a 7-day period following treatment. The percentage of mortality was calculated using the Abbott correction formula to account for natural mortality in the untreated control group [27].
where n = number of insects, T = treated, and Co = control.
Statistical Analysis
The results were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s HSD post hoc test for multiple comparisons, using SPSS software version 25.0. Differences were considered significant at p < 0.05.
2.4. Quantum Chemistry Calculations
The computational studies in this work were performed using Gaussian 09 software, utilizing the B3LYP functional paired with the 6-311G(d,p) basis set for all calculations [28]. This approach was selected to ensure an optimal balance of precision and computational efficiency. The stability of the optimized ground states was confirmed by verifying the absence of imaginary frequencies. To account for solvent effects, the ethanol solvation environment was modeled using the SMD solvation model [29]. Key molecular properties, including frontier molecular orbitals (FMOs), electrostatic potential maps (ESPs), and noncovalent interactions (analyzed through the reduced density gradient method, NCI-RDG), were examined using Multiwfn 3.8 software and visualized with VMD 1.9.3 [30,31]. Thermodynamic parameters related to the antioxidant mechanisms were computed as described in our previous investigations [32,33].
3. Results and Discussion
3.1. Synthesis
The target compounds (1–6) were synthesized in two steps following the classical protocol established by Iskander et al. [34], as illustrated in Scheme 1. The final yields ranged from 48% to 86%. Compounds that lacked substituents (6) or contained electron-withdrawing groups, like chlorine (2, 3), displayed the lowest yields, ranging from 48% to 53%, except for fluorine (4), which achieved a yield of 83%. On the other hand, compounds with electron-donating groups, such as methyl (1, 5), produced the highest yields, from 70% to 86%. This trend can be attributed to the enhanced reactivity of the aniline derivatives facilitated by the electron-donating groups. The molecular structures of the synthesized compounds were confirmed using 1H NMR, 13C NMR, and FTIR analyses, with results aligning well with previously reported data [35,36,37].
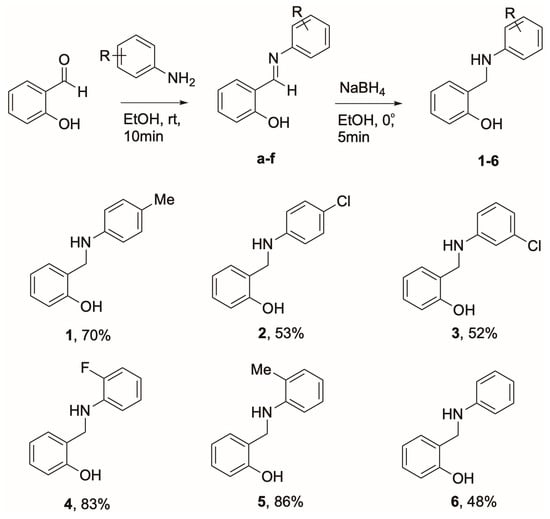
Scheme 1.
Synthesis of salicylaldehyde-derived secondary amines 1–6.
3.2. Biological Activity Evaluation
3.2.1. Antioxidant Activity
The antioxidant activity of compounds 1–6 was assessed using ABTS and phenanthroline assays. The ABTS assay evaluates antioxidant potential through direct scavenging via hydrogen or electron donation, while the phenanthroline assay measures electron-donating capacity by monitoring the reduction of Fe(III) to Fe(II) ions. BHT and BHA were used as positive controls, and the results, expressed as IC50 or A0.5 values, are presented in Table 1.

Table 1.
Antioxidant and anti-inflammatory activity of compounds 1–6.
In the ABTS assay, all compounds demonstrated significant antioxidant activity. Except for compounds 1 and 3, the remaining compounds exhibited IC50 values (5.14 ± 0.11 to 9.91 ± 0.19 µM) comparable to or lower than those of the reference antioxidants BHT (8.22 ± 0.45 µM) and BHA (7.16 ± 1.66 µM). Among them, compound 2 showed the highest activity with an IC50 of 5.14 ± 0.11 µM, surpassing both BHT and BHA.
In the phenanthroline assay, the tested compounds exhibited A0.5 values ranging from 9.42 to 31.73 µM, indicating strong electron-donating capabilities. Compound 5 displayed the lowest A0.5 value (9.42 ± 1.02 µM), slightly higher than that of BHT (7.32 ± 0.83 µM) and BHA (6.31 ± 0.83 µM). These results highlight the compounds’ strong antioxidant potential.
Notably, the para-chloro-substituted derivative (compound 2) exhibited the highest activity in the ABTS assay, while the ortho-methyl-substituted derivative (compound 5) demonstrated the best performance in the phenanthroline assay. Compared to BHT and BHA, these two compounds showed comparable or superior activity, underscoring their potential as promising antioxidants.
3.2.2. Anti-Inflammatory Activity
The anti-inflammatory activity of compounds 1–6, along with the standard diclofenac, measured via BSA denaturation, is summarized in Table 1. As shown, compounds 2 (IC50 = 839.64 ± 11.13 µM) and 5 (IC50 = 699.72 ± 7.36 µM) exhibited the lowest IC50 values among the tested compounds, although their values were higher than that of the standard diclofenac (IC50 = 128.83 ± 0.08 µM). This suggests that 2 and 5 may possess moderate anti-inflammatory activity. Notably, these compounds also demonstrated the highest antioxidant potential, highlighting their promise as dual antioxidant and anti-inflammatory candidates. In contrast, 1 (IC50 = 1734.72 ± 17.66 µM) and 3 (IC50 = 1287.09 ± 4.70 µM) displayed significantly higher IC50 values compared to the standard, indicating a lower anti-inflammatory potential. Compounds 4 and 6 showed no measurable anti-inflammatory activity.
3.2.3. Insecticidal Activity
The insecticidal activity of the prepared molecules 1–6 was evaluated against T. castaneum, commonly known as the red flour beetle, over a seven-day period, with mortality rates (%) recorded daily. T. castaneum was selected for this study due to its status as a major pest of stored cereal products, causing significant economic losses globally [38]. Its high reproductive rate, adaptability to various storage conditions, and resistance to conventional chemical treatments make it a persistent threat in storage facilities [39]. Investigating this pest is essential for developing effective and sustainable management strategies to protect cereal quality and minimize post-harvest losses.
The results revealed significant differences in mortality among the molecules, confirmed by one-way ANOVA tests (p < 0.001 across all days), indicating statistically significant variation in insect mortality (Table 2). Molecules 1 and 6 demonstrated the highest efficacy against T. castaneum, showing substantial mortality from day 1. By day 7, 6 achieved a maximum mortality rate of 76.67%, while 1 reached 73.31%. Both molecules consistently produced significantly higher mortality rates than the other treatments (p < 0.05). The strong activity of 6 can be attributed to the absence of substituents on the benzene ring. Similarly, 1, with a methyl group at position 4, showed activity similar to 6. In contrast, 2 (with a chlorine group at position 4), 3 (with chlorine at position 3), 4 (with a fluorine group at position 2), and 5 (with a substituent at position 2) displayed moderate insecticidal activity, with gradually increasing mortality rates over the seven days. By day 7, 2 achieved a moderate mortality rate of 50%, while 3, 4, and 5 reached 56.67%, 63%, and 56.60%, respectively. These molecules were statistically less effective than 1 and 6, especially during the initial days (p < 0.05), though their efficacy improved over time.

Table 2.
Mortality rates (%) of T. castaneum exposed to molecules 1–6 over a 7-day period at a concentration of 20 mM.
These findings suggest that 1 and 6 possess strong insecticidal potential against T. castaneum, with rapid and sustained mortality effects. Both molecules show promise as candidates for sustainable management of stored-product pests, aligning well with integrated pest control strategies.
3.3. Quantum Chemistry Calculations
DFT calculations at the B3LYP/6-311G(d,p) level of theory were performed for compounds 1–6 to investigate their molecular geometry, electronic properties, and antioxidant mechanisms. The calculations were performed in ethanol. The results are summarized in Figure 1, Figure 2, Figure 3 and Figure 4.
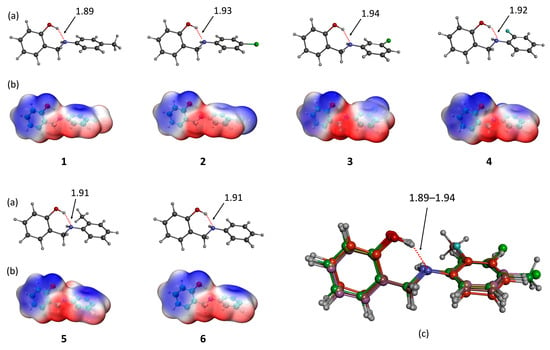
Figure 1.
Optimized molecular structures (a), electrostatic potential maps (b), and atom-by-atom superpositions (c) of compounds 1–6. Distances are reported in Å.
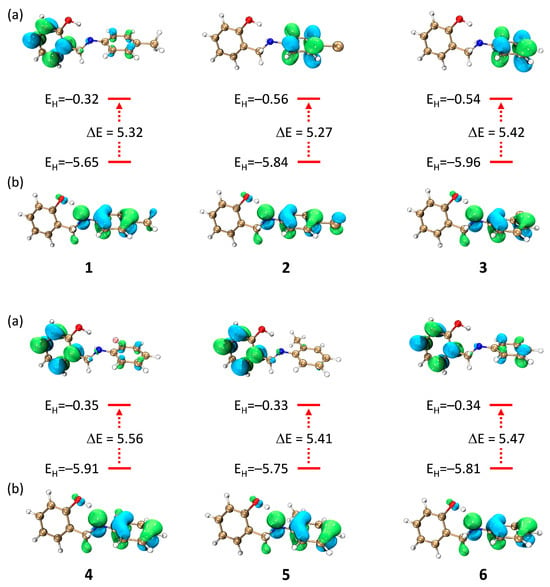
Figure 2.
Distribution of the frontier molecular orbitals [LUMOs (a) and HOMOs (b)], along with their energies and energy gaps, for compounds 1–6.
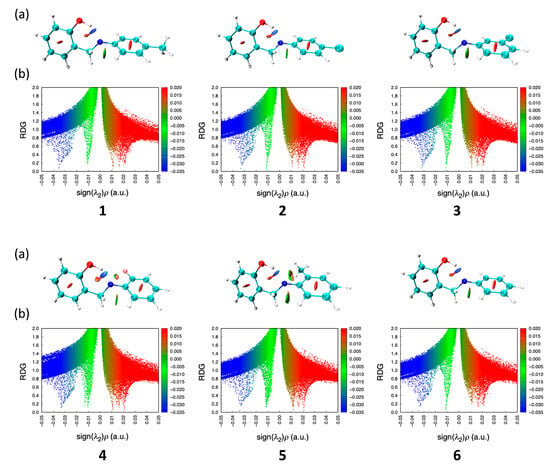
Figure 3.
The RDG isosurface maps (a) and scatter plots (b) of compounds 1–6.
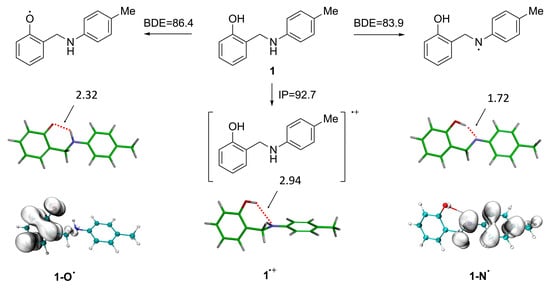
Figure 4.
Computed BDE and IP values of compound 1, the molecular geometry of the radical species of 1, and the spin density distribution of the radical formed through NH and OH abstraction. BDE and IP are in kcal/mol, and distances are reported in Å.
The molecular geometries and electronic charge distributions of compounds 1–6, shown in Figure 1, reveal non-planar structures stabilized by intramolecular hydrogen bonding between the hydroxyl (OH) group and the secondary amine (NH), with bond distances ranging from 1.89 to 1.94 Å. This interaction enhances the rigidity and stability of the molecules. Electrostatic potential (ESP) maps highlight regions of high electron density (blue) around the hydroxyl oxygen, amine nitrogen, and phenyl rings, indicating nucleophilic sites. Conversely, low-electron-density (red) regions correspond to electrophilic areas influenced by substituents. Substituent effects are significant: electron-donating groups (e.g., the methyl group in compound 5) and electron-withdrawing groups (e.g., chlorine in compounds 3 and 4) modulate the charge distribution and reactivity. Structural overlays show consistent geometry in the salicylaldehyde core, with slight variations in substituent orientation.
The frontier molecular orbitals (FMOs) of the compounds, illustrated in Figure 2, indicate that the HOMOs are primarily localized on the aniline fragment and partially on the hydroxyl group, marking these regions as key electron-donating sites. The LUMOs, in contrast, are generally delocalized over the salicylaldehyde core and the aromatic rings, except in compounds 2 and 3, where they extend more significantly onto the aniline aromatic ring. HOMO energy levels range from −5.65 to −5.96 eV, and LUMO levels from −0.32 to −0.56 eV, resulting in energy gaps (∆E) between 5.27 and 5.56 eV. Compound 2 has the smallest energy gap (∆E = 5.27 eV), indicating higher electronic reactivity, while compound 4 exhibits the largest gap (∆E = 5.56 eV), suggesting lower reactivity. These variations underscore the influence of substituents on electronic properties. The HOMO energy levels of the compounds are comparable to those of standard antioxidants such as BHT (−5.74 eV) and Trolox (−5.39 eV), supporting their potential antioxidant activity [40].
The RDG isosurface maps and scatter plots, presented in Figure 3, provide insights into intramolecular interactions of compounds 1–6. The isosurface maps highlight significant hydrogen-bonding interactions, particularly between the hydroxyl (OH) group of the salicylaldehyde moiety and adjacent nitrogen atom, visualized as blue regions. Weak π–π stacking interactions and steric repulsion are also evident, with red regions denoting repulsive forces. The scatter plots confirm these interactions: peaks in the negative-sign(λ2)ρ region indicate strong hydrogen bonding, more pronounced in compounds 1 and 4, while positive-sign(λ2)ρ regions reflect steric repulsion present across all compounds. Neutral van der Waals forces, located near the zero-sign(λ2)ρ axis, are observed primarily between the aniline moiety and the benzylic CH2 group.
The thermodynamic parameters associated with the antioxidant mechanism of compound 1, selected as a representative example, were evaluated. This study focused on the bond dissociation enthalpy (BDE) of the OH and NH bonds and the ionization potential (IP). The obtained results are summarized in Figure 4. The BDE of the NH bond was calculated to be 83.9 kcal/mol, approximately 3 kcal/mol lower than that of the OH bond, identifying the NH bond as the most reactive site for free radical attack. However, the small difference in BDE values suggests that both the NH and OH bonds may contribute to the radical scavenging activity of compound 1. This difference in reactivity can be explained by two factors. First, the intramolecular hydrogen bond between the NH and OH groups is greater in the NH radical (1.72 Å) compared to the OH radical (2.32 Å). Second, the spin density distribution is more delocalized in the NH radical than in the OH radical, as shown in Figure 4. Furthermore, the computed IP value (92.7 kcal/mol) exceeds the BDE values for both the NH and OH bonds (83.9–86.4 kcal/mol), indicating that hydrogen abstraction is thermodynamically more favorable than electron transfer.
4. Conclusions
This study presents an efficient synthesis of biologically active salicylaldehyde-derived secondary amines (1–6). Antioxidant activity evaluations demonstrated that compounds 2 and 5 exhibited significant activity in the ABTS and phenanthroline assays, with IC50 or A0.5 values comparable to or lower than those of the standard antioxidants BHT and BHA. Both compounds also displayed moderate anti-inflammatory activity, as measured by the BSA denaturation assay. In contrast, compounds 1 and 6 showed strong insecticidal activity against Tribolium castaneum, achieving maximum mortality rates of 73.31% and 76.67%, respectively, over a seven-day treatment period. DFT calculations highlighted robust intramolecular interactions between the OH and NH groups in all compounds, contributing to their stability. Additionally, these calculations underscored the pivotal role of hydrogen atom abstraction, particularly from the NH bond, in the antioxidant activity of the synthesized compounds.
While these findings are promising, they are based solely on in vitro studies. Further investigations, including studies using animal models, are needed to fully explore the biological potential of these salicylaldehyde-derived secondary amines.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/org6010011/s1: Table S1. The Cartesian coordinates of compounds 1–6 in ethanol media at the B3LYP/6-311G(d,p) level; Figure S1. 1H and 13C NMR spectra of compounds 1–6.
Author Contributions
Conceptualization, C.D. and H.B.; methodology, C.D. and H.B.; formal analysis, H.B.; investigation, C.D., N.R., R.D. and S.A.; resources, C.B. and S.H.; writing—original draft preparation, N.R. and H.B.; writing—review and editing, N.R., S.H. and H.B.; visualization, H.B.; supervision, C.D. and H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors. Data are contained within the article and Supplementary Materials.
Acknowledgments
The supercomputing resources used in this work were supported by the HPC of UCI-UFMC (Unité de Calcul Intesif of the University Fréres Mentouri Constantine 1).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Krátký, M.; Dzurková, M.; Janoušek, J.; Konečná, K.; Trejtnar, F.; Stolaříková, J.; Vinšová, J. Sulfadiazine Salicylaldehyde-Based Schiff Bases: Synthesis, Antimicrobial Activity and Cytotoxicity. Molecules 2017, 22, 1573. [Google Scholar] [CrossRef] [PubMed]
- Montaser, A.S.; Wassel, A.R.; Al-Shaye’a, O.N. Synthesis, characterization and antimicrobial activity of Schiff bases from chitosan and salicylaldehyde/TiO2 nanocomposite membrane. Int. J. Biol. Macromol. 2019, 124, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Yorur-Goreci, C.; Altas-Kiymaz, N.; Peksel, A.; Bilgin-Eran, B.; Sonmez, M. New p-Substituted Salicylaldehyde Phenylhydrazone Derivatives: Synthesis, Characterization, and Antioxidant Activities. Sci. Pharm. 2014, 82, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Selaković, S.; Rodić, M.V.; Novaković, I.; Matić, I.Z.; Stanojković, T.; Pirković, A.; Živković, L.; Spremo-Potparević, B.; Milčić, M.; Medaković, V.; et al. Cu(ii) complexes with a salicylaldehyde derivative and α-diimines as co-ligands: Synthesis, characterization, biological activity. Experimental and theoretical approach. Dalton Trans. 2024, 53, 2770–2788. [Google Scholar] [CrossRef]
- Koschier, E.H.; Hoffmann, D.; Riefler, J. Influence of salicylaldehyde and methyl salicylate on post-landing behaviour of Frankliniella occidentalis Pergande. J. Appl. Entomol. 2007, 131, 362–367. [Google Scholar] [CrossRef]
- Kim, J.H.; Campbell, B.C.; Mahoney, N.; Chan, K.L.; Molyneux, R.J. Chemosensitization of Aflatoxigenic Fungi to Antimycin A and Strobilurin Using Salicylaldehyde, a Volatile Natural Compound Targeting Cellular Antioxidation System. Mycopathologia 2011, 171, 291–298. [Google Scholar] [CrossRef] [PubMed]
- He, H.-W.; Xu, D.; Wu, K.-H.; Lu, Z.-Y.; Liu, X.; Xu, G. Discovery of novel salicylaldehyde derivatives incorporating an α-methylene-γ-butyrolactone moiety as fungicidal agents. Pest Manag. Sci. 2023, 79, 5015–5028. [Google Scholar] [CrossRef]
- Pelttari, E.; Karhumäki, E.; Langshaw, J.; Peräkylä, H.; Elo, H. Antimicrobial Properties of Substituted Salicylaldehydes and Related Compounds. Z. Naturforschung C 2007, 62, 487–497. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Wu, Y.; Song, S.; Feng, J.; Zhang, X. Natural α-methylenelactam analogues: Design, synthesis and evaluation of α-alkenyl-γ and δ-lactams as potential antifungal agents against Colletotrichum orbiculare. Eur. J. Med. Chem. 2017, 130, 286–307. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Durães, F.; Maia, M.; Sousa, E.; Pinto, M.M.M. Recent advances in the synthesis of xanthones and azaxanthones. Org. Chem. Front. 2020, 7, 3027–3066. [Google Scholar] [CrossRef]
- Kirilmis, C.; Ahmedzade, M.; Servi, S.; Koca, M.; Kizirgil, A.; Kazaz, C. Synthesis and antimicrobial activity of some novel derivatives of benzofuran: Part 2. The synthesis and antimicrobial activity of some novel 1-(1-benzofuran-2-yl)-2-mesitylethanone derivatives. Eur. J. Med. Chem. 2008, 43, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, M.; Shen, S.; Ghiviriga, I.; Miller, S.A. Sustainable polyvinyl acetals from bioaromatic aldehydes. Polym. Chem. 2017, 8, 5049–5059. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, H.; Liang, H.; Lu, J. Salicylaldehyde-functionalized block copolymer nano-objects: One-pot synthesis via polymerization-induced self-assembly and their simultaneous cross-linking and fluorescence modification. Polym. Chem. 2016, 7, 4761–4770. [Google Scholar] [CrossRef]
- Wu, W.; Ouyang, Q.; He, L.; Huang, Q. Optical and thermal properties of polymethyl methacrylate (PMMA) bearing phenyl and adamantyl substituents. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 130018. [Google Scholar] [CrossRef]
- Anju, V.P.; Narayanankutty, S.K. Impact of Bis-(3-triethoxysilylpropyl)tetrasulphide on the properties of PMMA/Cellulose composite. Polymer 2017, 119, 224–237. [Google Scholar] [CrossRef]
- Wong, K.W.; Teh, S.S.; Law, K.P.; Ismail, I.S.; Sato, K.; Mase, N.; Mah, S.H. Synthesis of benzylated amine-substituted xanthone derivatives and their antioxidant and anti-inflammatory activities. Arch. Der Pharm. 2023, 356, 2200418. [Google Scholar] [CrossRef] [PubMed]
- Lambert, W.T.; Buysse, A.M.; Wessels, F.J. Discovery of novel insecticidal 3-aminopyridyl ureas. Pest Manag. Sci. 2020, 76, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Djafarou, S.; Mermer, A.; Barut, B.; Yılmaz, G.T.; Amine khodja, I.; Boulebd, H. Synthesis and evaluation of the antioxidant and anti-tyrosinase activities of thiazolyl hydrazone derivatives and their application in the anti-browning of fresh-cut potato. Food Chem. 2023, 414, 135745. [Google Scholar] [CrossRef] [PubMed]
- Boulebd, H.; Tam, N.M.; Mechler, A.; Vo, Q.V. Substitution effects on the antiradical activity of hydralazine: A DFT analysis. New J. Chem. 2020, 44, 16577–16583. [Google Scholar] [CrossRef]
- Boulebd, H.; Khodja, I.A.; Bay, M.V.; Hoa, N.T.; Mechler, A.; Vo, Q.V. Thermodynamic and Kinetic Studies of the Radical Scavenging Behavior of Hydralazine and Dihydralazine: Theoretical Insights. J. Phys. Chem. B 2020, 124, 4123–4131. [Google Scholar] [CrossRef]
- Hamel, D.; Rozman, V.; Liška, A. Storage of Cereals in Warehouses with or without Pesticides. Insects 2020, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Magro, A.; Barros, G.; Carvalho, M.O. Stored products insects in Portugal—New data and overview. J. Stored Prod. Res. 2024, 105, 102230. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, L.C.G. Einstein–Cartan non-supersymmetric spin-polarised nucleons wall dynamos. Ann. Phys. 2021, 431, 168558. [Google Scholar] [CrossRef]
- Kandikattu Karthik, K.K.; Kumar, P.B.R.; Priya, R.V.; Kumar, K.S.; Rathore, R.S.B. Evaluation of anti-inflammatory activity of Canthium parviflorum by in-vitro method. Indian J. Res. Pharm. Biotechnol. 2013, 1, 729–731. [Google Scholar]
- Preetha, G.; Stanley, J.; Suresh, S. Toxicity of insecticides to wolf spider (Pardosa pseudoannulata) and rice leaf folder (Cnaphalocrocis medinalis): Assessing the risk of insecticides on spiders in the rice ecosystem. Int. J. Pest Manag. 2023, 1–10. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Boulebd, H. Is cannabidiolic acid an overlooked natural antioxidant? Insights from quantum chemistry calculations. New J. Chem. 2022, 46, 162–168. [Google Scholar] [CrossRef]
- Boulebd, H.; Pereira, D.M.; Amine Khodja, I.; Hoa, N.T.; Mechler, A.; Vo, Q.V. Assessment of the free radical scavenging potential of cannabidiol under physiological conditions: Theoretical and experimental investigations. J. Mol. Liq. 2022, 346, 118277. [Google Scholar] [CrossRef]
- Iskander, M.N.; Andrews, P.R. Synthesis of 3,4-dihydro-3-(p-methylphenyl)-1,3,2H-benzoxazine: An undergraduate organic chemistry experiment. J. Chem. Educ. 1985, 62, 913. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, W.; Zhu, Z.; Liu, H. Synthesis of 2,3-diaryl-3,4-dihydro-2H-1,3-benzoxazines and their fungicidal activities. J. Heterocycl. Chem. 2011, 48, 255–260. [Google Scholar] [CrossRef]
- Waltz, F.; Pillette, L.; Verhaeghe, E.; Ambroise, Y. Synthesis and Structure—Activity Relationships of a Class of Sodium Iodide Symporter Function Inhibitors. ChemMedChem 2011, 6, 1775–1777. [Google Scholar] [CrossRef] [PubMed]
- Kamble, R.D.; Hese, S.V.; Meshram, R.J.; Kote, J.R.; Gacche, R.N.; Dawane, B.S. Green synthesis and in silico investigation of dihydro-2H-benzo[1,3]oxazine derivatives as inhibitors of Mycobacterium tuberculosis. Med. Chem. Res. 2015, 24, 1077–1088. [Google Scholar] [CrossRef]
- Jung, J.-M.; Byeon, D.-h.; Kim, S.-H.; Sunghoon, J.; Lee, W.-H. Estimating economic damage to cocoa bean production with changes in the spatial distribution of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) in response to climate change. J. Stored Prod. Res. 2020, 89, 101681. [Google Scholar] [CrossRef]
- Wakil, W.; Kavallieratos, N.G.; Usman, M.; Gulzar, S.; El-Shafie, H.A.F. Detection of Phosphine Resistance in Field Populations of Four Key Stored-Grain Insect Pests in Pakistan. Insects 2021, 12, 288. [Google Scholar] [CrossRef]
- Boulebd, H. Comparative study of the radical scavenging behavior of ascorbic acid, BHT, BHA and Trolox: Experimental and theoretical study. J. Mol. Struct. 2020, 1201, 127210. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).