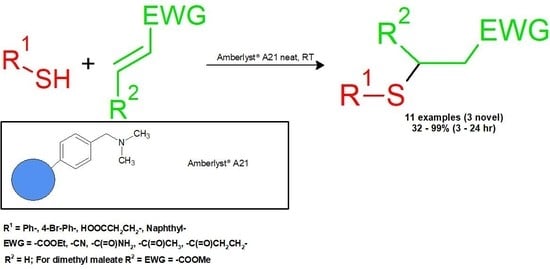
Thia-Michael Reaction under Heterogeneous Catalysis
Abstract
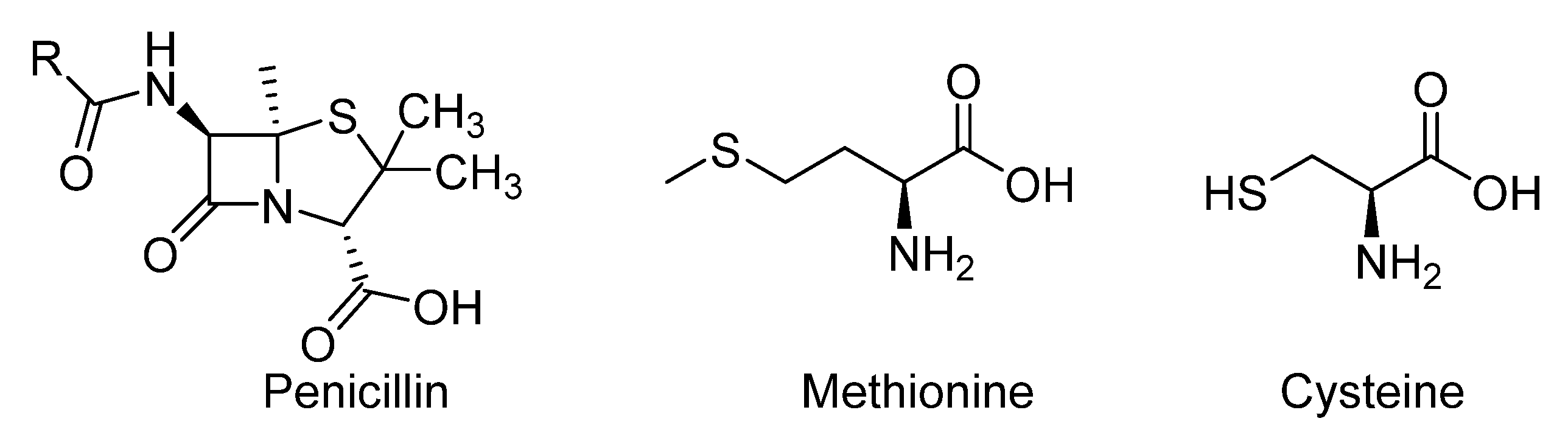
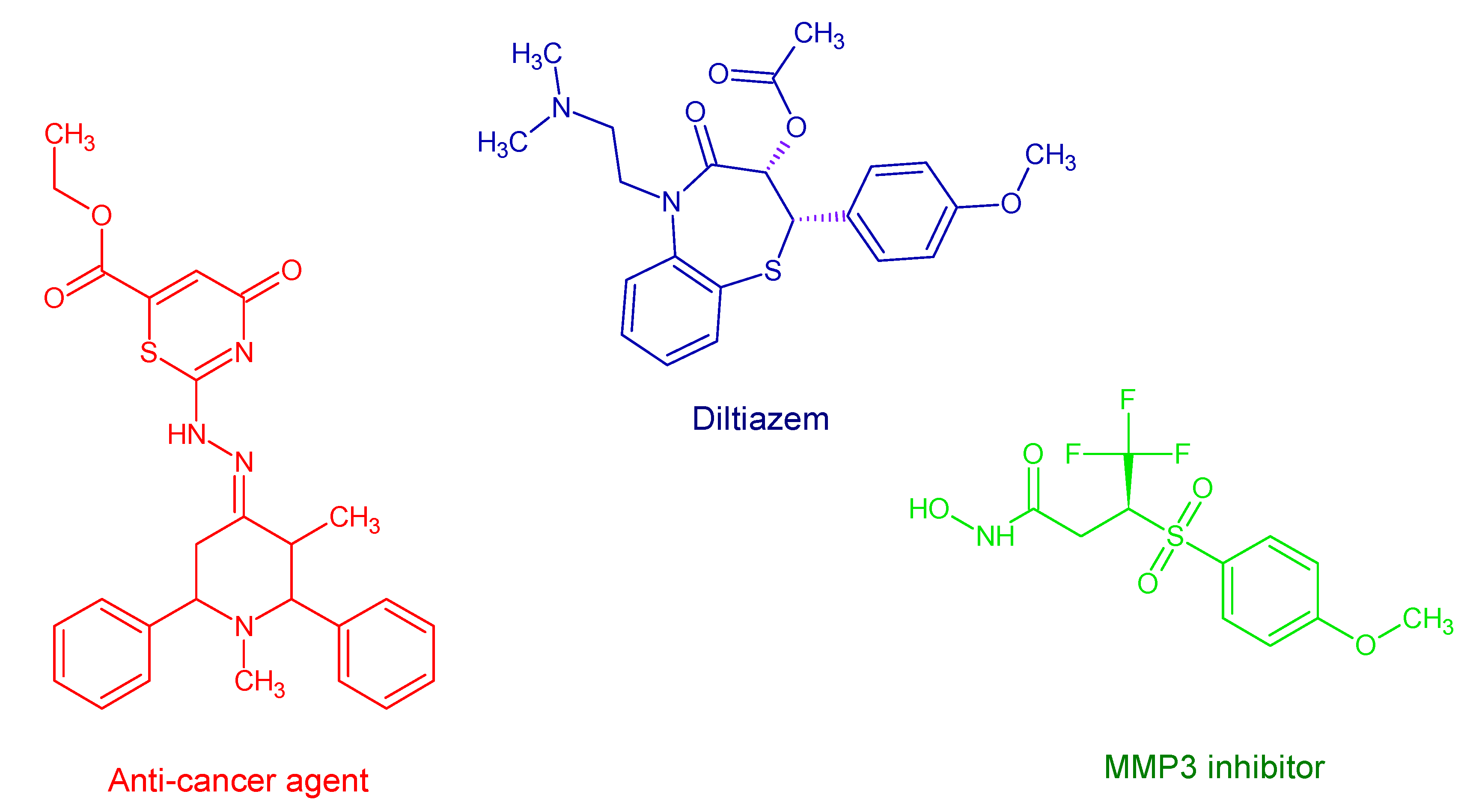
1. Introduction
2. Materials and Methods
2.1. General Information
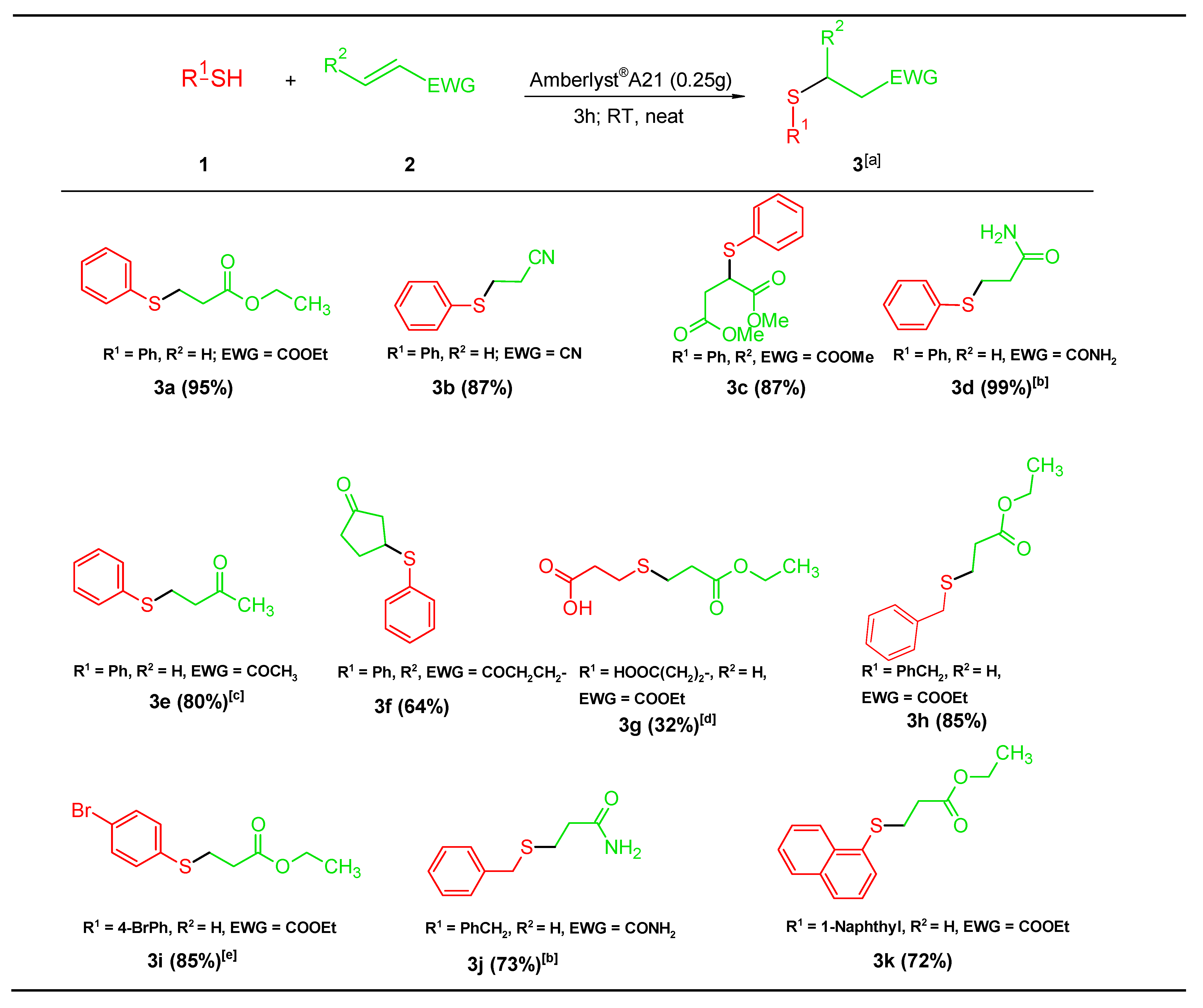
2.2. General Procedure
2.3. Product Identification
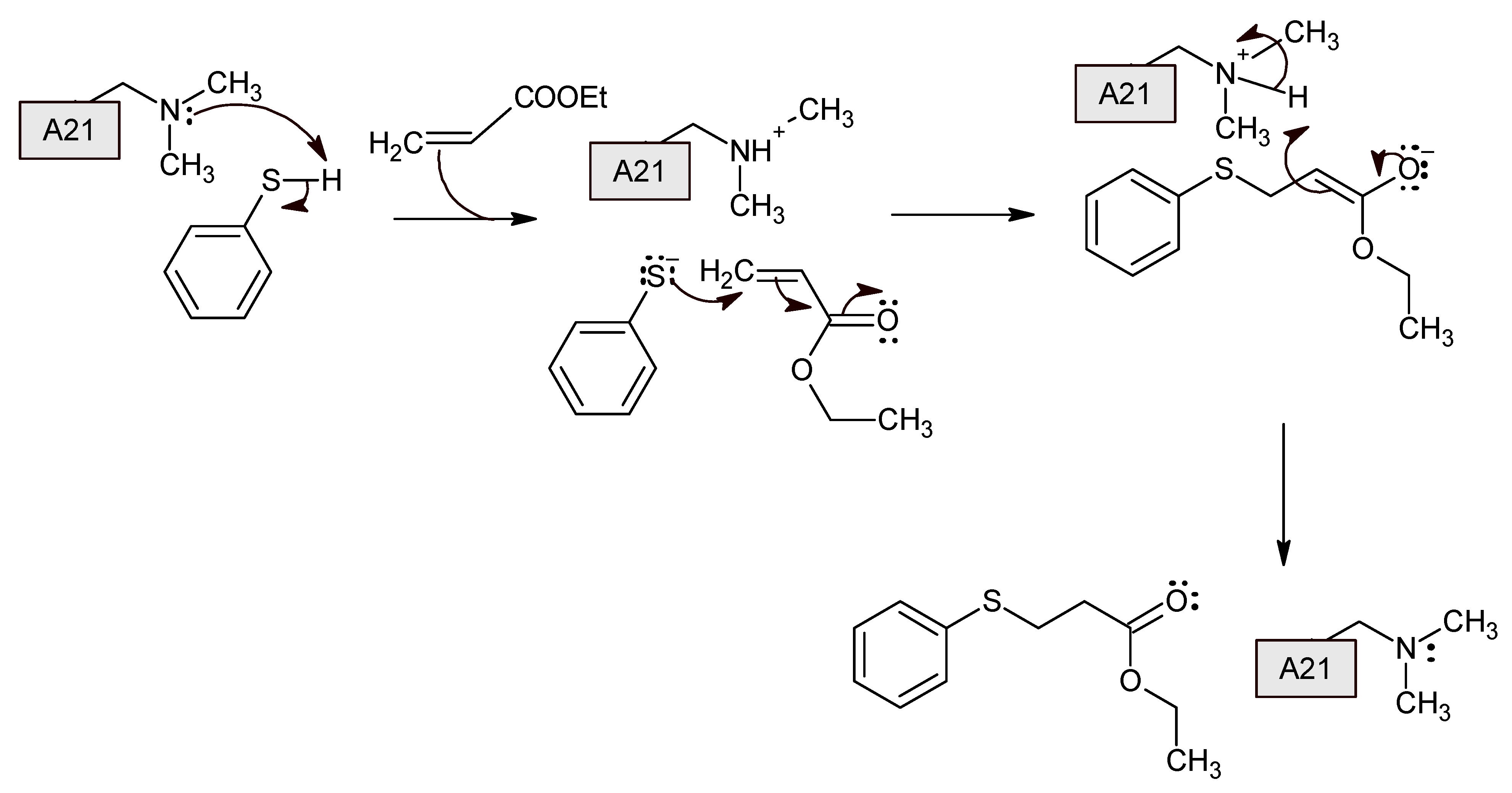
3. Results and Discussion
3.1. The Catalyst—Properties and Recyclability
3.2. E-Factor and Atom Economy of the Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Akanksha. Amino acid catalyzed thia-Michael addition reactions. Tetrahedron 2007, 63, 11086–11092. [Google Scholar] [CrossRef]
- Wadhwa, P.; Kharbanda, A.; Sharma, A. Thia-Michael addition: An emerging strategy in organic synthesis. Asian J. Org. Chem. 2018, 7, 634–661. [Google Scholar] [CrossRef]
- Feng, M.; Tang, B.; Lang, S.H.; Jiang, X. Sulphur containing scaffolds in drugs: Synthesis and application in medicinal chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. [Google Scholar] [CrossRef] [PubMed]
- Thiol, I.V.; Allen, A.C.F.H.; Fournier, J.O.; Humphlett, W.J. The thermal reversibility of the Michael reaction. Can. J. Chem. 1964, 42, 2616–2620. [Google Scholar]
- Nicponski, D.; Marchi, J. Selectivity reversal during thia-Michael additions using tetrabutylammonium hydroxide: Operationally simple and extremely high turnover. Synthesis 2014, 46, 1725–1730. [Google Scholar] [CrossRef]
- Bandini, M.; Cozzi, P.G.; Giacomini, M.; Melchiorre, P.; Selva, S.; Umani-Ronchi, A. Sequential one-pot InBr3-catalyzed 1,4-then 1,2-nucleophilic addition to enones. J. Org. Chem. 2002, 67, 3700–3704. [Google Scholar] [CrossRef]
- Pore, D.M.; Soudagar, M.S.; Desai, U.V.; Thopate, T.S.; Wadagaonkar, P.P. Potassium phosphate or silica sulphuric acid catalyzed conjugate addition of thiols to α,β-unsaturated ketones at room temperature under solvent-free conditions. Tetrahedron Lett. 2006, 47, 9325–9328. [Google Scholar] [CrossRef]
- Wadhwa, P.; Bagchi, S.; Sharma, A. A Regioselective multicomponent cascade to access thiosemicarbazone-fused thiazinones: Scope, structure elucidation and gram scale synthesis. ChemistrySelect 2017, 2, 1386–1391. [Google Scholar] [CrossRef]
- Bosica, G.; Abdilla, R. Aza-Michael Mono-addition Using Acidic Alumina under Solventless Conditions. Molecules 2016, 21, 815. [Google Scholar] [CrossRef]
- Bosica, G.; Saliba, R. Aza-Michael Mono- and Bis-Addition of Primary and Secondary Amines Promoted by Silica-Supported Polyphosphoric Acid, PPA/SiO2. ChemistrySelect 2022, 7, e202104359. [Google Scholar] [CrossRef]
- Park, C.; Lee, S. One-pot sulfa-Michael addition reactions of disulfides using a pyridine-borane complex under blue light irradiation. Bull. Korean Chem. Soc. 2022, 43, 951–954. [Google Scholar] [CrossRef]
- Xu, X.B.; Yin, X.H.; Zhu, Y.Y.; Xu, X.H.; Luo, T.; Li, Y.H.; Lu, X.; Shao, L.L.; Pan, J.G.; Yang, R.H. Reduction cleavage of S-S bond by Zn/Cp2TiCl2: Application for the synthesis of β-arylthiocarbonyl compounds. J. Chem. Res. 2009, 12, 750–752. [Google Scholar] [CrossRef]
- Abbasi, M.; Nowrouzi, N.; Khezri, R. CuI-catalyzed tandem synthesis of thioethers using aryl halides, electron-deficient alkenes, and sodium iso-propyl xanthogenate. Appl. Organomet. Chem. 2020, 34, 1–9. [Google Scholar] [CrossRef]
- Hans, M.; Delaude, L.; Rodriguez, J.; Coquerel, Y. N-heterocyclic carbene catalyzed carba-, sulfa-, and phospha-Michael additions with NHC·CO2 adducts as precatalysts. J. Org. Chem. 2014, 79, 2758–2764. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Tarasenko, E.A.; Khokhlov, A.R.; Tyurin, V.S. Poly(N-vinylimidazole) as efficient and recyclable catalyst for the addition of thiols to Michael acceptors in aqueous medium. Russ. J. Org. Chem. 2007, 43, 1733–1736. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Bardajee, G.R.; Veranlou, R.O.C. KF/Al2O3-mediated Michael addition of thiols to electron-deficient olefins. Synth. Commun. 2005, 35, 2427–2433. [Google Scholar] [CrossRef]
- Payra, S.; Saha, A.; Banerjee, S. On-water magnetic NiFe2O 4 nanoparticle-catalyzed Michael additions of active methylene compounds, aromatic/aliphatic amines, alcohols and thiols to conjugated alkenes. RSC Adv. 2016, 6, 95951–95956. [Google Scholar] [CrossRef]
- Civit, M.G.; Sanz, X.; Vogels, C.M.; Webb, J.D.; Geier, S.J.; Decken, A.; Bo, C.; Westcott, S.A.; Fernández, E. Thioboration of α,β-unsaturated ketones and aldehydes toward the synthesis of β-sulfido carbonyl compounds. J. Org. Chem. 2015, 80, 2148–2154. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Gorginpour, F.; Samadi, A. Dithiooxamide as an effective sulphur surrogate for odourless high-yielding carbon-sulphur bond formation in wet PEG200 as an eco-friendly, safe, and recoverable solvent. Eur. J. Org. Chem. 2015, 2015, 2914–2920. [Google Scholar] [CrossRef]
- Barbero, M.; Cadamuro, S.; Dughera, S. O-Benzenedisulfonimide as a reusable Brønsted acid catalyst for hetero-Michael reactions. Synth. Commun. 2013, 43, 758–767. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Krishnakumar, V.; Gunanathan, C. KOtBu-catalyzed Michael addition reactions under mild and solvent-free conditions. Chem. Asian J. 2020, 15, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Liu, Y.; Chai, L.; Lai, Z.; Fang, X.; Liu, B.; Zhang, W.; Lu, M.; Xu, Y.; Xu, H. Nmp-based ionic liquids: Recyclable catalysts for both hetero-Michael addition and Knoevenagel condensation in water. Synth. Commun. 2018, 48, 1060–1067. [Google Scholar] [CrossRef]
- Sigma, A. Amberlyst(R) A21 Free Base. Available online: https://www.sigmaaldrich.com/MT/en/product/aldrich/216410 (accessed on 6 February 2023).
- Bonfils, F.; Cazaux, I.; Hodge, P.; Caze, C. Michael reactions carried out using a bench-top flow system. Org. Biomol. Chem. 2006, 4, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Bosica, G.; Zammit, R. One-pot multicomponent nitro-Mannich reaction using a heterogeneous catalyst under solvent-free conditions. PeerJ 2018, 6, 1–29. [Google Scholar] [CrossRef]
- Khan, A.T.; Ghosh, S.; Choudhury, L.H. Perchloric acid impregnated on silica gel: A versatile catalyst for Michael addition of thiols to the electron-deficient alkenes. Eur. J. Org. Chem. 2006, 2006, 2226–2231. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Liu, L.; Xu, H.; Wang, Y. RuCl3 in poly(ethylene glycol): A highly efficient and recyclable catalyst for the conjugate addition of nitrogen and sulphur nucleophiles. Synthesis 2005, 13, 2129–2136. [Google Scholar] [CrossRef]



 | ||||
| Entry [a] | Catalyst [b] | T (°C) | Time (h) | Yield (%) of 3a [c] |
| 1 | Acid alumina | 50 | 2 | 94 |
| 2 | Neutral alumina | 50 | 2 | 95 |
| 3 | No | 50 | 2 | 47 |
| 4 | Basic alumina | 50 | 2 | 95 |
| 5 | Basic alumina | r.t | 3 | 90 |
| 6 | Amberlyst® A21 | r.t | 3 | 95 |
| 7 | Amberlyst® A15 | r.t | 3 | 40 |
| 8 | Montmorillonite K-10 | r.t | 3 | 55 |
| Catalyst Used | Solvent | Temperature/Time | Yield | Reusability | Reference |
|---|---|---|---|---|---|
| 0.5 mol% HClO4-SiO2 | CH2Cl2 | RT/10–15 min | 95% | 4 times (11% yield drop) | [26] |
| 0.5 mol% RuCl3 | PEG 2000 | 50 °C/8 h | 92% | 5 times (4% yield drop) | [27] |
| Amberlyst® A21 | Neat | RT/2 h | 95% | 5 times (12% yield drop) | - (this study) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosica, G.; Abdilla, R.; Petrellini, A. Thia-Michael Reaction under Heterogeneous Catalysis. Organics 2023, 4, 86-96. https://doi.org/10.3390/org4010007
Bosica G, Abdilla R, Petrellini A. Thia-Michael Reaction under Heterogeneous Catalysis. Organics. 2023; 4(1):86-96. https://doi.org/10.3390/org4010007
Chicago/Turabian StyleBosica, Giovanna, Roderick Abdilla, and Alessio Petrellini. 2023. "Thia-Michael Reaction under Heterogeneous Catalysis" Organics 4, no. 1: 86-96. https://doi.org/10.3390/org4010007
APA StyleBosica, G., Abdilla, R., & Petrellini, A. (2023). Thia-Michael Reaction under Heterogeneous Catalysis. Organics, 4(1), 86-96. https://doi.org/10.3390/org4010007