Theoretical Insight into the Reversal of Chemoselectivity in Diels-Alder Reactions of α,β-Unsaturated Aldehydes and Ketones Catalyzed by Brønsted and Lewis Acids
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
- (i)
- 1 + Cp in the absence and presence of the BA/LA catalysts; and
- (ii)
- 2 + Cp in the absence and presence of the BA/LA catalysts.
3.1. Analysis of the Global CDFT-Based Reactivity Indexes
3.2. Analysis of the Potential Energy Surface of the Uncatalyzed and Catalyzed DA Reactions of 1 and 2
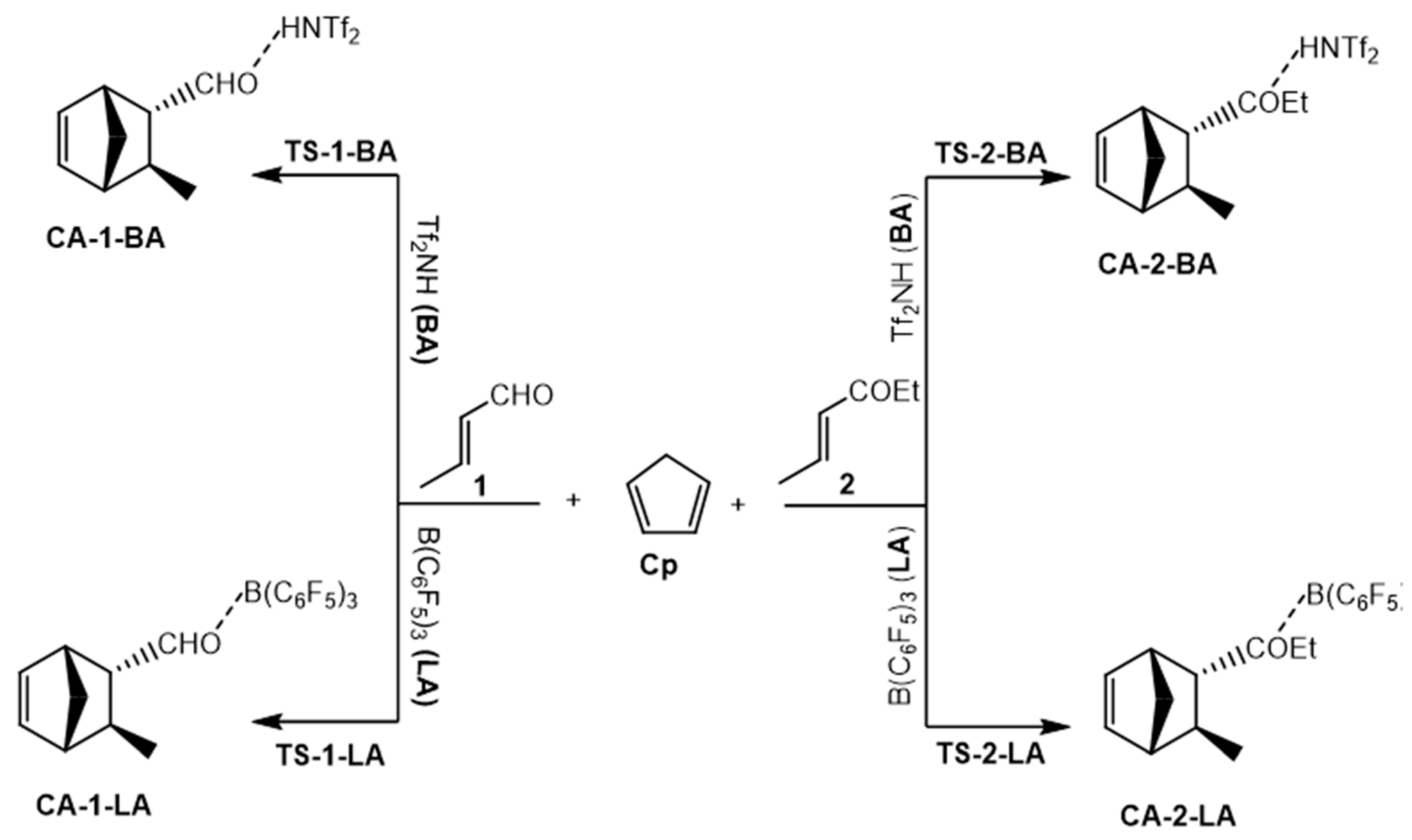
3.2.1. Competitive Uncatalyzed DA Reactions of 1 and 2 with Cp
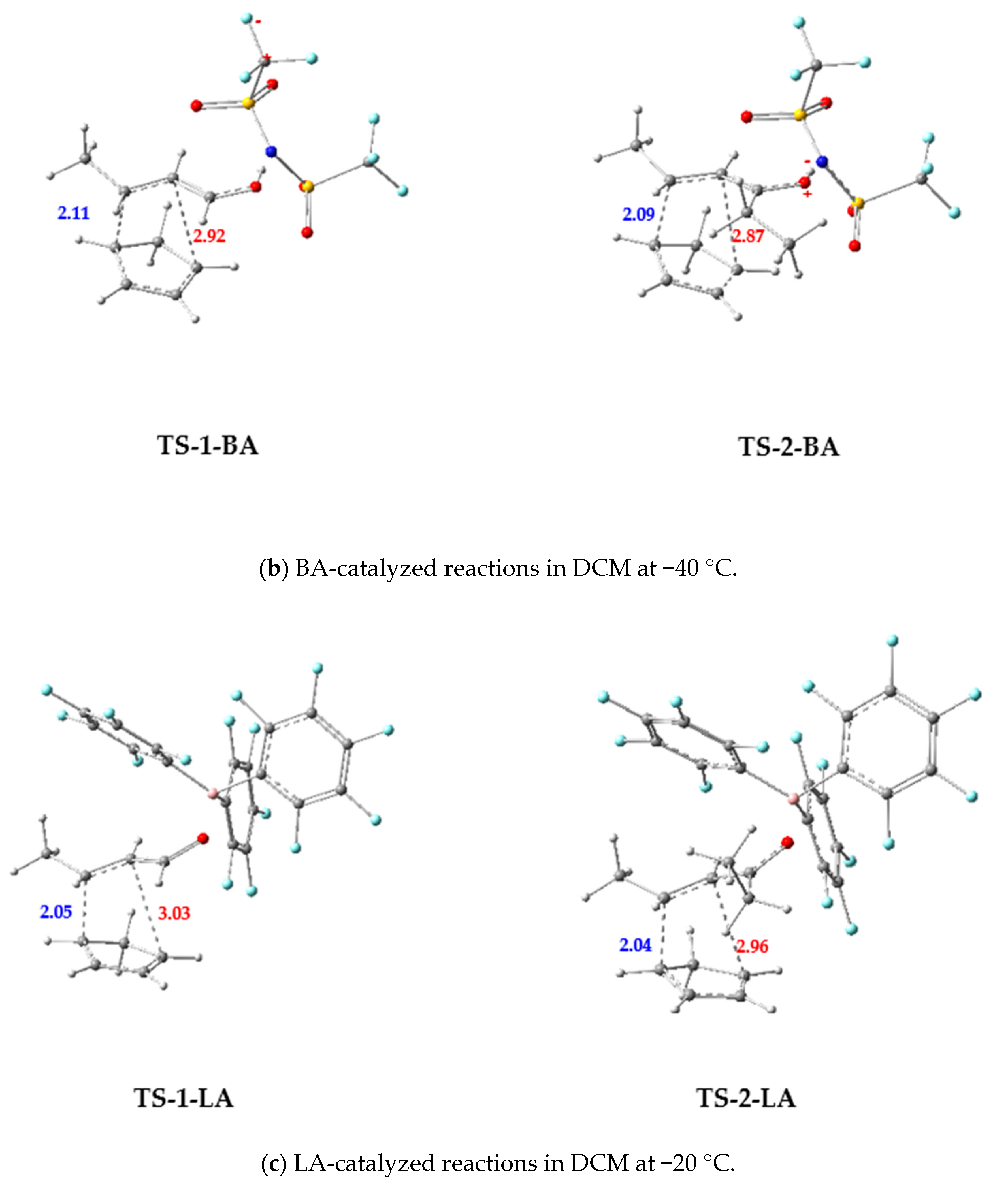
3.2.2. BA-Catalyzed DA Reactions of 1-BA and 2-BA with Cp
3.2.3. LA-Catalyzed Reaction of 1-LA and 2-LA with Cp
3.3. Relative Activation Free Energies and Boltzmann–Maxwell Populations
3.4. GEDT Analysis and Polarity
4. Conclusions
- (i)
- In the case of the uncatalyzed DA reactions, the 2 + Cp reaction was found to be kinetically more favored than the 1 + Cp reaction both in gas phase and in DCM.
- (ii)
- In the case of the DA reactions catalyzed by BA, the 2-BA + Cp reaction was found to be kinetically more favored than the 1-BA + Cp reaction both in gas phase and in DCM at −40 °C. Moreover, the calculated activation barriers, GEDTs at TSs, and electrophilicity differences (Δω) indicated that the BA-catalyzed reactions were predicted to be more polar and faster compared to the uncatalyzed reactions.
- (iii)
- In the case of the DA reactions catalyzed by LA, the 1-LA + Cp reaction was found to be kinetically more favored than the 2-LA + Cp reaction in DCM at −20 °C. In addition, the LA-catalyzed reactions were predicted to be more polar and faster compared to the uncatalyzed and BA-catalyzed reactions.
- (iv)
- The relative free energies and Maxwell–Boltzmann populations of the competitive TSs, calculated in DCM, put in evidence the reversal of the chemoselectivity when the BA catalyst Tf2NH -was replaced by the bulky LA catalyst B(C6F5)3, in agreement with the experimental findings.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huisgen, R. Cycloadditions—Definition, Classification, and Characterization. Angew. Chem. Int. Ed. 1968, 7, 321–328. [Google Scholar] [CrossRef]
- Padwa, A. 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; John Wiley and Sons: New York, NY, USA, 1984. [Google Scholar]
- Frühauf, H.-W. Metal-Assisted Cycloaddition Reactions in Organotransition Metal Chemistry. Chem. Rev. 1997, 97, 523–596. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Jørgensen, K.A. Frontmatter and Index. In Cycloaddition Reactions in Organic Synthesis; Kobayashi, S., Jørgensen, K.A., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2001. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels-Alder Reaction in Total Synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Fringuelli, F.; Taticchi, A. The Diels-Alder Reaction: Selected Practical Methods; Fringuelli, F., Taticchi, A., Eds.; Wiley: Chichester, UK; New York, NY, USA, 2002. [Google Scholar]
- Konovalov, A.I.; Kiselev, V.D. Diels-Alder Reaction. Effect of Internal and External Factors on the Reactivity of Diene—Dienophile Systems. Russ. Chem. Bull. 2003, 52, 293–311. [Google Scholar] [CrossRef]
- Hodgson, D.M.; Labande, A.H.; Muthusamy, S. Cycloadditions of Carbonyl Ylides Derived from Diazocarbonyl Compounds. In Organic Reactions; Denmark, S.E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; Chapter 2; pp. 133–496. [Google Scholar] [CrossRef]
- Ylijoki, K.E.O.; Stryker, J.M. [5+2] Cycloaddition Reactions in Organic and Natural Product Synthesis. Chem. Rev. 2013, 113, 2244–2266. [Google Scholar] [CrossRef]
- Martel, A.; Dhall, R.; Gaulon, C.; Laurent, M.Y.; Dujardin, G. Dihydrofurans by cycloadditions of oxadienes. In Organic Reactions, 1st ed.; Weinreb, S.M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; Chapter 1; pp. 1–916. [Google Scholar] [CrossRef]
- Boger, D.L. Diels-Alder reactions of heterocyclic aza dienes. Scope and applications. Chem. Rev. 1986, 86, 781–793. [Google Scholar] [CrossRef]
- Hayashi, Y. Catalytic Asymmetric Diels-Alder Reactions. In Cycloaddition Reactions in Organic Synthesis; Kobayashi, S., Jørgensen, K.A., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2001; Chapter 1; pp. 5–55. [Google Scholar] [CrossRef]
- Houk, K.N.; Loncharich, R.J.; Blake, J.F.; Jorgensen, W.L. Substituent effects and transition structures for Diels-Alder reactions of butadiene and cyclopentadiene with cyanoalkenes. J. Am. Chem. Soc. 1989, 111, 9172–9176. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Lim, D.; Blake, J.F. Ab initio study of Diels-Alder reactions of cyclopentadiene with ethylene, isoprene, cyclopentadiene, acrylonitrile, and methyl vinyl ketone. J. Am. Chem. Soc. 1993, 115, 2936–2942. [Google Scholar] [CrossRef]
- Domingo, L.R.; Sáez, J.A. Understanding the mechanism of polar Diels-Alder reactions. Org. Biomol. Chem. 2009, 7, 3576–3585. [Google Scholar] [CrossRef] [PubMed]
- Morales-Bayuelo, A.; Vivas-Reyes, R. Understanding the Polar Character Trend in a Series of Diels-Alder Reactions Using Molecular Quantum Similarity and Chemical Reactivity Descriptors. J. Quantum Chem. 2014, 2014. [Google Scholar] [CrossRef]
- Cui, C.-X.; Liu, Y.-J. A thorough understanding of the Diels-Alder reaction of 1,3-butadiene and ethylene: Diels-Alder reaction of 1,3-butadiene and ethylene. J. Phys. Org. Chem. 2014, 27, 652–660. [Google Scholar] [CrossRef]
- Levandowski, B.J.; Houk, K.N. Theoretical Analysis of Reactivity Patterns in Diels-Alder Reactions of Cyclopentadiene, Cyclohexadiene, and Cycloheptadiene with Symmetrical and Unsymmetrical Dienophiles. J. Org. Chem. 2015, 80, 3530–3537. [Google Scholar] [CrossRef]
- Jasiński, R. A reexamination of the molecular mechanism of the Diels-Alder reaction between tetrafluoroethene and cyclopentadiene. React. Kinet. Mech. Catal. 2016, 119, 49–57. [Google Scholar] [CrossRef]
- Chen, S.; Yu, P.; Houk, K.N. Ambimodal Dipolar/Diels-Alder Cycloaddition Transition States Involving Proton Transfers. J. Am. Chem. Soc. 2018, 140, 18124–18131. [Google Scholar] [CrossRef]
- Jasiński, R.; Kwiatkowska, M.; Barański, A. Stereoselectivity and kinetics of [4+2] cycloaddition reaction of cyclopentadiene to para-substituted E-2-arylnitroethenes. J. Phys. Org. Chem. 2011, 24, 843–853. [Google Scholar] [CrossRef]
- Domingo, L. Molecular Electron Density Theory: A Modern View of Reactivity in Organic Chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A Molecular Electron Density Theory Study of the Competitiveness of Polar Diels-Alder and Polar Alder-ene Reactions. Molecules 2018, 23, 1913. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Acharjee, N. Molecular Electron Density Theory: A New Theoretical Outlook on Organic Chemistry. In Frontiers in Computational Chemistry; Ul-Haq, Z., Wilson, A.K., Eds.; Bentham Science Publishers: Sharjah, UAE, 2020; Chapter 2; pp. 174–227. [Google Scholar] [CrossRef]
- Acharjee, N.; Banerji, A. A molecular electron density theory study to understand the interplay of theory and experiment in nitrone-enone cycloaddition. J. Chem. Sci. 2020, 132, 65. [Google Scholar] [CrossRef]
- Benhamed, L.; Mekelleche, S.M.; Benchouk, W.; Charif, I.E.; Ríos-Gutiérrez, M.; Domingo, L.R. Understanding the Influence of the Trifluoromethyl Group on the Selectivities of the [3+2] Cycloadditions of Thiocarbonyl S -methanides with α, β-Unsaturated Ketones. A MEDT study. ChemistrySelect 2020, 5, 12791–12806. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M. On the nature of organic electron density transfer complexes within molecular electron density theory. Org. Biomol. Chem. 2019, 17, 6478–6488. [Google Scholar] [CrossRef]
- Emamian, S.; Domingo, L.R.; Javad Hosseini, S.; Ali-Asgari, S. A Study of the Effects of the Lewis Acid Catalysts on Oxa-Diels-Alder Reactions through Molecular Electron Density Theory. ChemistrySelect 2020, 5, 5341–5348. [Google Scholar] [CrossRef]
- Hosseini, S.J. Exploring effects of the trifluoromethyl substituent on the chemoselectivity and regioselectivity of [3+2] cycloadditions of thiocarbonyl S-methanides with α, β-unsaturated ketones. J. Chin. Chem. Soc. 2020, 67, 703–710. [Google Scholar] [CrossRef]
- Jalali, H.; Hosseini, S.J.; Ali-Asgari, S.; Izadi Nia, J. Stereoselective aziridination of imines via ammonium ylides: A molecular electron density theory study. J. Heterocycl. Chem. 2020, 57, 419–427. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Nasri, L.; Khorief Nacereddine, A.; Djerourou, A.; Domingo, L.R. A molecular electron density theory study of the [3+2] cycloaddition reaction between an azomethine imine and electron deficient ethylenes. J. Phys. Org. Chem. 2018, 31, e3830. [Google Scholar] [CrossRef]
- Barama, L.; Bayoud, B.; Chafaa, F.; Nacereddine, A.K.; Djerourou, A. A mechanistic MEDT study of the competitive catalysed [4+2] and [2+2] cycloaddition reactions between 1-methyl-1-phenylallene and methyl acrylate: The role of Lewis acid on the mechanism and selectivity. Struct. Chem. 2018, 29, 1709–1721. [Google Scholar] [CrossRef]
- Fukui, K. Molecular Orbitals in Chemistry, Physics, and Biology. In Index to Reviews, Symposia Volumes and Monographs in Organic Chemistry; Lowdin, P.O., Ed.; Academic Press: New York, NY, USA; London, UK, 1966. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon Press: Oxford, UK; New York, NY, USA, 1990. [Google Scholar] [CrossRef]
- Carruthers, W.; Coldham, I. Modern Methods of Organic Synthesis; Carruthers, W., Coldham, I., Eds.; Cambridge University Press: Cambridge, UK, 2004; pp. 159–264. [Google Scholar]
- Yamamoto, H.; Boxer, M.B. Super Brønsted Acid Catalysis in Organic Synthesis. CHIMIA Int. J. Chem. 2007, 61, 279–281. [Google Scholar] [CrossRef]
- Cheon, C.H.; Yamamoto, H. Super Brønsted acid catalysis. Chem. Commun. 2011, 47, 3043. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H. Acid Catalysis in Organic Synthesis. In Inventing Reactions; Gooßen, L.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 315–334. [Google Scholar] [CrossRef]
- Ishihara, K. Chiral B (III) Lewis Acids. In Lewis Acids in Organic Synthesis; Yamamoto, H., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2000; pp. 135–190. [Google Scholar] [CrossRef]
- Fringuelli, F.; Piermatti, O.; Pizzo, F.; Vaccaro, L. Recent Advances in Lewis Acid Catalyzed Diels-Alder Reactions in Aqueous Media. Eur. J. Org. Chem. 2001, 17, 439–455. [Google Scholar] [CrossRef]
- Kagan, H.B.; Riant, O. Catalytic asymmetric Diels-Alder reactions. Chem. Rev. 1992, 92, 1007–1019. [Google Scholar] [CrossRef]
- Li, P.; Yamamoto, H. Lewis Acid Catalyzed Inverse-Electron-Demand Diels-Alder Reaction of Tropones. J. Am. Chem. Soc. 2009, 131, 16628–16629. [Google Scholar] [CrossRef]
- Shen, J.; Tan, C.-H. Brønsted-acid and Brønsted-base catalyzed Diels-Alder reactions. Org. Biomol. Chem. 2008, 6, 3229. [Google Scholar] [CrossRef]
- Pindur, U.; Lutz, G.; Otto, C. Acceleration and Selectivity Enhancement of Dlels-Alder Reactions by Special and Catalytic Methods. Chem. Rev. 1993, 93, 741–761. [Google Scholar] [CrossRef]
- Futatsugi, K.; Yamamoto, H. Oxazaborolidine-Derived Lewis Acid Assisted Lewis Acid as a Moisture-Tolerant Catalyst for Enantioselective Diels-Alder Reactions. Angew. Chem. Int. Ed. 2005, 44, 1484–1487. [Google Scholar] [CrossRef]
- Yamamoto, H. From designer Lewis acid to designer Brnsted acid towards more reactive and selective acid catalysis. Proc. Jpn. Acad. Ser. B 2008, 84, 134–146. [Google Scholar] [CrossRef]
- Jung, M.E.; Guzaev, M. Trimethylaluminum-Triflimide Complexes for the Catalysis of Highly Hindered Diels-Alder Reactions. Org. Lett. 2012, 14, 5169–5171. [Google Scholar] [CrossRef]
- Liu, L.; Kim, H.; Xie, Y.; Farès, C.; Kaib, P.S.J.; Goddard, R.; List, B. Catalytic Asymmetric [4+2]-Cycloaddition of Dienes with Aldehydes. J. Am. Chem. Soc. 2017, 139, 13656–13659. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, J. Triflimide (HNTf2) in Organic Synthesis. Chem. Rev. 2018, 118, 10349–10392. [Google Scholar] [CrossRef] [PubMed]
- Held, F.; Grau, D.; Tsogoeva, S. Enantioselective Cycloaddition Reactions Catalyzed by BINOL-Derived Phosphoric Acids and N-Triflyl Phosphoramides: Recent Advances. Molecules 2015, 20, 16103–16126. [Google Scholar] [CrossRef]
- Dodziuk, H. Strained Hydrocarbons: Beyond the van-t Hoff and Le Bel Hypothesis, 1st ed.; Dodziuk, H., Ed.; Wiley-VCH Verlag Gmbh & Co. KGaA: Weinheim, Germany, 2009. [Google Scholar] [CrossRef]
- Park, S.; Sugiyama, H. DNA-Based Hybrid Catalysts for Asymmetric Organic Synthesis. Angew. Chem. Int. Ed. 2010, 49, 3870–3878. [Google Scholar] [CrossRef]
- Megens, R.P.; Roelfes, G. Asymmetric Catalysis with Helical Polymers. Chem. A Eur. J. 2011, 17, 8514–8523. [Google Scholar] [CrossRef]
- Rioz-Martínez, A.; Roelfes, G. DNA-based hybrid catalysis. Curr. Opin. Chem. Biol. 2015, 25, 80–87. [Google Scholar] [CrossRef]
- Raynal, M.; Ballester, P.; Vidal-Ferran, A.; van Leeuwen, P.W.N.M. Supramolecular catalysis. Part 1: Non-covalent interactions as a tool for building and modifying homogeneous catalysts. Chem. Soc. Rev. 2014, 43, 1660–1733. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, D.; Yamamoto, H. Reversal of Chemoselectivity in Diels-Alder Reaction with α, β-Unsaturated Aldehydes and Ketones Catalyzed by Brønsted Acid or Lewis Acid. Org. Lett. 2005, 7, 1251–1253. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, K.B. Ab Initio Molecular Orbital Theory. J. Comput. Chem. 1986, 7, 379. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. Available online: https://gaussian.com/g09citation/ (accessed on 1 November 2016).
- Li, Y.; Fang, D.-C. DFT calculations on kinetic data for some [4+2] reactions in solution. Phys. Chem. Chem. Phys. 2014, 16, 15224. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Chen, Y. Theoretical Studies on the Mechanism of Cycloaddition Reaction between 1,2,4,5-Tetrazine and Cycloolefines. Acta Chim. Sin. 2014, 72, 253–256. [Google Scholar] [CrossRef][Green Version]
- Schlegel, H.B. Optimization of equilibrium geometries and transition structures. J. Comput. Chem. 1982, 3, 214–218. [Google Scholar] [CrossRef]
- Schlegel, H.B. Some thoughts on reaction-path following. J. Chem. Soc. Faraday Trans. 1994, 90, 1569. [Google Scholar] [CrossRef]
- Tomasi, J.; Persico, M. Molecular Interactions in Solution: An Overview of Methods Based on Continuous Distributions of the Solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. Geometry optimization of molecular structures in solution by the polarizable continuum model. J. Comput. Chem. 1998, 19, 404–417. [Google Scholar] [CrossRef]
- Domingo, L.R. A new C-C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Domingo, L.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef]
- Parr, R.G. Density-Functional Theory of the Electronic Structure of Molecules. Annu. Rev. Phys. Chem. 1995, 46, 701–728. [Google Scholar] [CrossRef]
- Chermette, H. Chemical reactivity indexes in density functional theory. J. Comput. Chem. 1999, 20, 129–154. [Google Scholar] [CrossRef]
- de Proft, F.; Geerlings, P. Conceptual and Computational DFT in the Study of Aromaticity. Chem. Rev. 2001, 101, 1451–1464. [Google Scholar] [CrossRef]
- Geerlings, P.; Proft, F.D.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793–1873. [Google Scholar] [CrossRef]
- Ayers, P.W.; Anderson, J.S.M.; Bartolotti, L.J. Perturbative perspectives on the chemical reaction prediction problem. Int. J. Quantum Chem. 2005, 101, 520–534. [Google Scholar] [CrossRef]
- Gázquez, J.L. Perspectives on the Density Functional Theory of Chemical Reactivity. J. Mex. Chem. Soc. 2008, 52, 3–10. [Google Scholar]
- Geerlings, P.; Fias, S.; Boisdenghien, Z.; de Proft, F. Conceptual DFT: Chemistry from the linear response function. Chem. Soc. Rev. 2014, 43, 4989–5008. [Google Scholar] [CrossRef]
- Calais, J.-L. Density-functional theory of atoms and molecules. Int. J. Quantum Chem. 1993, 47, 101. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A molecular electron density theory study of the participation of tetrazines in aza-Diels-Alder reactions. RSC Adv. 2020, 10, 15394–15405. [Google Scholar] [CrossRef]
- Jaramillo, P.; Domingo, L.R.; Chamorro, E.; Pérez, P. A further exploration of a nucleophilicity index based on the gas-phase ionization potentials. J. Mol. Struct. THEOCHEM 2008, 865, 68–72. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P. The nucleophilicity N index in organic chemistry. Org. Biomol. Chem. 2011, 9, 7168–7176. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.; Keith, T.; Millam, J. GaussView, Version 5.0.8; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]


| Global Properties (in eV) | |||||
|---|---|---|---|---|---|
| µ | η | ω | N | Δω 1 | |
| Cp | −0.123 | 0.352 | 0.58 | 3.63 | 0.00 |
| 1 | −0.162 | 0.351 | 1.02 | 2.56 | 0.44 |
| 2 | −0.156 | 0.350 | 0.94 | 2.76 | 0.36 |
| 1-BA | −0.201 | 0.362 | 1.52 | 1.36 | 0.94 |
| 2-BA | −0.193 | 0.367 | 1.40 | 1.60 | 0.82 |
| 1-LA | −0.197 | 0.256 | 2.05 | 2.91 | 1.47 |
| 2-LA | −0.192 | 0.265 | 1.89 | 2.94 | 1.31 |
| Gas Phase | In DCM at −40 °C | DCM at −20 °C | ||||
|---|---|---|---|---|---|---|
| E (in a.u.) | ΔE (in kcal/mol) | G° (in a.u.) | ΔG° (in kcal/mol) | G° (in a.u.) | ΔG° (in kcal/mol) | |
| Cp | −194.081227 | −194.010442 | −194.012466 | |||
| 1 | −231.214513 | −231.151897 | −231.154155 | |||
| 2 | −309.848932 | −309.732115 | −309.734767 | |||
| 1 + Cp | −425.295740 | 0.0 | −425.162339 | 0.0 | −425.166621 | 0.0 |
| 2 + Cp | −503.930160 | 0.0 | −503.742557 | 0.0 | −503.747233 | 0.0 |
| TS-1 | −425.268971 | 16.8 1 | −425.115299 | 29.5 1 | −425.118053 | 30.5 1 |
| TS-2 | −503.904754 | 15.92 | −503.695977 | 29.22 | −503.699094 | 30.22 |
| CA-1 | −425.337504 | −26.2 1 | −425.178925 | −10.4 1 | −425.176228 | −6.0 1 |
| CA-2 | −503.975693 | −28.6 2 | −503.762314 | −12.4 2 | −503.759272 | −7.5 2 |
| Gas Phase | In DCM at −40 °C | |||||
|---|---|---|---|---|---|---|
| E (in a.u.) | ΔE (in kcal/mol) | G° (in a.u.) | ΔG° (in kcal/mol) | ΔH° (in kcal/mol) | −TΔS° (in kcal/mol) | |
| Cp | −194.081227 | −194.010442 | ||||
| 1-BA | −2058.968156 | −2058.860635 | ||||
| 2-BA | −2137.604500 | −2137.442545 | ||||
| 1-BA + Cp | −2253.049384 | 0.0 | −2252.871077 | 0.0 | ||
| 2-BA + Cp | −2331.685728 | 0.0 | −2331.452987 | 0.0 | ||
| TS-1-BA | −2253.033186 | 10.2 1 | −2252.847153 | 15.0 1 | 2.6 (17.5%) | 12.4 (82.5%) |
| TS-2-BA | −2331.670205 | 9.72 | −2331.430226 | 14.32 | 2.1 (14.9%) | 12.2 (85.1%) |
| CA-1-BA | −2253.094037 | −28.0 1 | −2252.885020 | −8.7 1 | ||
| CA-2-BA | −2331.733508 | −30.0 2 | −2331.471355 | −11.5 2 | ||
| Gas Phase | In DCM at −20 °C | |||||
|---|---|---|---|---|---|---|
| E (in a.u.) | ΔE (in kcal/mol) | G° (in a.u.) | ΔG° (in kcal/mol) | ΔH° (in kcal/mol) | −TΔS° (in kcal/mol) | |
| Cp | −194.081227 | −194.010442 | ||||
| 1-LA | −2439.454482 | −2439.272585 | ||||
| 2-LA | −2518.095162 | −2517.856115 | ||||
| 1-LA + Cp | −2633.535709 | 0.0 | −2633.283027 | 0.0 | ||
| 2-LA + Cp | −2712.176389 | 0.0 | −2711.866557 | 0.0 | ||
| TS-1-LA | −2633.525405 | 6.5 1 | −2633.258044 | 15.71 | 5.2 (33.3%) | 10.5 (66.7%) |
| TS-2-LA | −2712.169099 | 4.62 | −2711.840294 | 16.5 2 | 5.0 (30.4%) | 11.5 (69.6%) |
| CA-1-LA | −2633.576707 | −25.7 1 | −2633.295055 | −7.5 1 | ||
| CA-2- LA | −2712.223191 | −29.4 2 | −2711.882229 | −9.8 2 | ||
| In DCM at −40 °C | in DCM at −20 °C | |||
|---|---|---|---|---|
| ΔΔG° pop(%) | ΔΔG° pop(%) | |||
| TS-1-BA | 0.7 | 17.14 | ||
| TS-2-BA | 0.0 | 82.86 | ||
| TS-1-LA | 0.0 | 83.07 | ||
| TS-2-LA | 0.8 | 16.93 | ||
| Uncatalyzed DA Reactions | BA-Catalyzed DA Reactions | LA-Catalyzed DA Reactions | ||||
|---|---|---|---|---|---|---|
| TS-1 | TS-2 | TS-1-BA | TS-2-BA | TS-1-LA | TS-2-LA | |
| GEDT | 0.13 | 0.12 | 0.26 | 0.23 | 0.38 | 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benhamed, L.; Mekelleche, S.M.; Benchouk, W. Theoretical Insight into the Reversal of Chemoselectivity in Diels-Alder Reactions of α,β-Unsaturated Aldehydes and Ketones Catalyzed by Brønsted and Lewis Acids. Organics 2021, 2, 38-49. https://doi.org/10.3390/org2010004
Benhamed L, Mekelleche SM, Benchouk W. Theoretical Insight into the Reversal of Chemoselectivity in Diels-Alder Reactions of α,β-Unsaturated Aldehydes and Ketones Catalyzed by Brønsted and Lewis Acids. Organics. 2021; 2(1):38-49. https://doi.org/10.3390/org2010004
Chicago/Turabian StyleBenhamed, Lakhdar, Sidi Mohamed Mekelleche, and Wafaa Benchouk. 2021. "Theoretical Insight into the Reversal of Chemoselectivity in Diels-Alder Reactions of α,β-Unsaturated Aldehydes and Ketones Catalyzed by Brønsted and Lewis Acids" Organics 2, no. 1: 38-49. https://doi.org/10.3390/org2010004
APA StyleBenhamed, L., Mekelleche, S. M., & Benchouk, W. (2021). Theoretical Insight into the Reversal of Chemoselectivity in Diels-Alder Reactions of α,β-Unsaturated Aldehydes and Ketones Catalyzed by Brønsted and Lewis Acids. Organics, 2(1), 38-49. https://doi.org/10.3390/org2010004






