Abstract
The regiochemistry of [3+2] cycloaddition (32CA) processes between benzonitrile N-oxide 1 and β-phosphorylated analogues of nitroethenes 2a–c has been studied using the Density Functional Theory (DFT) at the M062X/6-31+G(d) theory level. The obtained results of reactivity indices show that benzonitrile N-oxide 1 can be classified both as a moderate electrophile and moderate nucleophile, while β-phosphorylated analogues of nitroethenes 2a–c can be classified as strong electrophiles and marginal nucleophiles. Moreover, the analysis of CDFT shows that for [3+2] cycloadditions with the participation of β-phosphorylatednitroethene 2a and β-phosphorylated α-cyanonitroethene 2b, the more favored reaction path forms 4-nitro-substituted Δ2-isoxazolines 3a–b, while for a reaction with β-phosphorylated β-cyanonitroethene 2c, the more favored path forms 5-nitro-substituted Δ2-isoxazoline 4c. This is due to the presence of a cyano group in the alkene. The CDFT study correlates well with the analysis of the kinetic description of the considered reaction channels. Moreover, DFT calculations have proven the clearly polar nature of all analyzed [3+2] cycloaddition reactions according to the polar one-step mechanism.
1. Introduction
The [3+2] cycloadditions (32CA) are all-purpose strategiesto the regio- and stereoselective synthesis of five-membered heterocyclic molecular systems [1,2,3,4]. These types of reactions generally proceed under mild conditions, without the presence of catalysts, giving high yields of target compounds [2,3,4]. Furthermore, 32CA are realized with “full atomic economy.” It is particularly important for the green chemistry aspect [5]. Due to the listed advantages, 32CA are increasingly used by experimental chemists, mainly because of the possibility for using heterocyclic compounds in pharmacology [3,6,7]. The application of 32CA for synthesis has also increased interest among researchers in the theoretical sciences. Research of 32CA via the quantum-chemical calculations enables not only the design of appropriate reaction conditions, but also gives information on the selectivity of these processes [8,9].
As one of the many three atoms components (TACs) used in [3+2] cycloadditions, nitrile N-oxides have a wide application [10]. Reactions between nitrile N-oxides and alkenes obtain Δ2-isoxazolines, which are widely spread out as biological and pharmacological substances. They exhibit, for example, an antibacterial [11] and an antiviral [12] effect; therefore, they can be used in medicine as anti-inflammatory [13,14] and analgesic drugs [15]. The most popular medicines containing isoxazolines rings in their structures are Zonisamid and Risperidone [16]. They can also be successfully used as antipsychotic therapy drugs for the treatment of schizophrenia, bipolar disorder and irritability associated with autism [17]. Industrially, isoxazolines can also be successfully applied as dyes, agrochemical substances, electrical insulating oils and high temperature lubricants [18]. Moreover, isoxazolines are extremely valuable in organic synthesis. They can be valuable precursors for the synthesis of many versatile compounds, such as α,β-unsaturated ketones, β-hydroxyketones, nitriles, oximes and also β-amino acids and they are also substrates to obtain azaheterocycles [19,20,21].
In the present paper, we carried out theoretical experiments exploring the possibility of the synthesis of isoxazolines functionalized by a nitro and phosphate group. The possibility of attaching both of these groups in a one-step process provides the 32CA reactions between nitrile N-oxide and phosphorylated analogues of nitroethene. The presence in the molecular structures of nitro and phosphonate groups significantly increases the application spectrum of these compounds and also their further modification, which could be obtained in such reactions as: cycloaddition via Nef reaction [22], the Mukuiyama reaction [23] or the Henry reaction [22]. Moreover, the presence of a nitro group in the compound has the ability to stimulate biological properties of the molecule [24]. The phosphorus atom and phosphate group play a main role in the biochemistry of living, not only because 9% of nucleic acids consist of phosphorus, but also because they appeared to have a great impact on the enzyme activity. For instance, thanks to certain phosphate groups, proteins can carry out particular processes in cells [25,26].
We decided to use a benzonitrile N-oxide 1 as the model TAC. This TAC has been a very popular component in 32CAs, both in experimental research [27,28] and for theoretical research [29,30]. As a dipolarophile, we selected β-phosphoryl nitroethene 2a and its corresponding α- and β-cyano analogues 2b and 2c. The cyano group in 2a allows research into the effect of the electron-withdrawing characteristics of conjugated nitroalkenes and its influence on the activation of nitrovinyl moiety. For these defined addends, the [3+2] cycloaddition may theoretically proceed via two competitive channels, giving 4- and 5-nitro-substituted Δ2-isoxazolines (3a–c and 4a–c) (see Scheme 1).
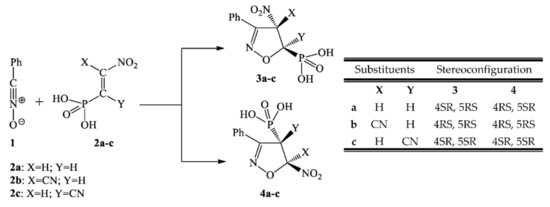
Scheme 1.
Theoretically possible reaction paths of [3+2] cycloaddition between benzonitrile N-oxide 1 and β-phosphorylated analogues of nitroethenes 2a–c.
It should be underlined that the molecular mechanism of the presented reaction cannot be defined a priori. For 32CAs, several more mechanisms are possible, e.g., non-polar mechanisms, including synchronous mechanisms, a biradical one-step mechanism and a stepwise biradical mechanism [31], as well as polar mechanisms, including synchronous mechanisms, a one-step-two-stage mechanism or a stepwise zwitterionic mechanism [32,33]. It is impossible to predict the relevant mechanism a priori without any data, which are obtained in the practice. We hope that our study will provide a better understanding of intermolecular interactions of addends and the probable selectivity and the mechanism created by these heterocyclic organic compounds, especially for synthetic chemists.
2. Computational Details
All calculations associated with the [3+2] cycloaddition were performed using the GAUSSIAN 09 package [34] in the Prometheus computer cluster of the CYFRONET regional computer center in Cracow. All stationary points were optimized using the M062X functional [35], together with the 6-31+G(d) basis set. This theory level has been commonly used for the mechanistic research aspects of 32CA reactions [8,36,37,38,39].
The optimizations of stationary points were carried out using vibrational analysis. For the structure optimization of the molecules, the Berny algorithm [40,41] was used. It was found that starting molecules as well as products had positive Hessian matrices. The transition states showed only one negative eigenvalue in their diagonalized Hessian matrices, and their associated eigenvectors were confirmed to correspond to the motion along the reaction coordinate under consideration. For all optimized transition states, intrinsic reaction coordinate (IRC) [42] calculations have been performed to verify if the located TSs are associated with the corresponding minimum stationary points connected to substrates and products.
Calculations of all critical structures were performed for a temperature T = 298 K and pressure p = 1 atm. The solvent effects were simulated using a relatively simple self-consistent reaction field (SCRF) [43,44] based on the polarizable continuum model (PCM) [45].
The global electron density transfer (GEDT) [46] values were designated based on the formula: GEDT = + ΣqA, where qA is the net Mulliken charge, and the sum is performed over all the atoms of benzonitrile N-oxide 1.
In turn, the σ-bond development (l) indices were designated based on the formula [47]:
where rTSX−Y is the distance between the reaction centers X and Y in the structure of transition state and rPX−Y is the same distance in the corresponding product.
Global electronic properties of used addends were estimated based on the equations defined on the basis of conceptual Density Functional Theory (CDFT) based on the equations recommended by Parr [48] and Domingo [49,50]. The calculation used the functional B3LYP on the basis set 6-31G(d) in the gas phase [49,50,51].
The electronic chemical potentials (μ) and chemical hardness (η) were evaluated in terms of one-electron energies of FMO (EHOMO and ELUMO) using the following equations [49,52]:
where EHOMO and ELUMO can be defined as terms of the one-electron energies of the frontier Mos, respectively HOMO and LUMO. The obtained values of μ and η were next used to approach a global electrophilicity ω based on the formula [48,49]:
In turn, the global nucleophilicity N can be expressed as [50]:
where EHOMO (TCE) is HOMO energy for tetracyanoethylene (TCE) is the reference value because it presents the lowest HOMO (EHOMO (TCE) = −9.368 eV).
The local electrophilicity (ωk) and the local nucleophilicity (Nk) focused on atom k was designated based on global properties and using the Parr function (Pk+ or Pk‾), based on the formulas [53]:
3. Results and Discussion
Our theoretical study was divided into three parts: (i) firstly, the analysis of the electronic properties of addends and their intermolecular interactions according to Conceptual Density Functional Theory (CDFT) reactivity indices was carried out; (ii) in the next paragraph, reaction profiles of [3+2] cycloaddition between benzonitrile N-oxide 1 and β-phosphorylated analogues of nitroethenes 2a–c are explored and characterized; (iii) at the end, a full diagnostic of all critical structures of reaction 1 with 2a–c are studied.
3.1. Analysis of the Electronic Properties of Addents and Their Intermolecular Interactions according to CDFT
The CDFT is a very important tool to understand the reactivity in polar cycloadditions. The CDFT indices were calculated in the gas phase according to the B3LYP/6-31G(d) theory level because it was used to define the electrophilicity and nucleophilicity scales. The global reactivity indices, namely, electronic chemical potential μ, chemical hardness η, global electrophilicity ω and global nucleophilicity N, for the reagents involved in these 32CA reactions are given in Table 1.

Table 1.
Electronic chemical potential μ, chemical hardness η, global electrophilicity ω and nucleophilicity N, in eV, for the studied reagents, calculated based onB3LYP/6-31G(d) theory level.
The electronic chemical potential µ of benzonitrile N-oxide 1, −3.83 eV, is significantly lower than for β-phosphorylated analogues of nitroethenes 2a–c (see Table 1). It means that while along the 32CA reactions between TAC 1 and nitroethenes 2a–c, the flux of the electron density will take place from benzonitrile N-oxide 1 to these nitroethenes 2a–c.
The global electrophilicity ω and the global nucleophilicity N indexes for benzonitrile N-oxide 1 are respectively 1.46 and 2.78 eV (see Table 1). Based on the electrophilicity [48,49] and nucleophilicity [50] scale, TAC 1 can be classified as a moderate electrophile and a moderate nucleophile. According to the same scale, β-phosphorylated analogues of nitroethenes 2a–c can be classified as strong electrophiles (ω = 2.98–3.87 eV) and marginal nucleophiles (N = 0.64–1.00 eV). What is more, it is worth noting that the introduction of a cyano group to nitroalkene 2a both directly at carbon atom of nitro group (Cα) and at the same atom as the phosphoryl group (Cβ) causes a significant increase in the values of global electrophilicity (by approximately 0.7–0.8 eV), while we can see a decrease of the values of global nucleophilicity (by approximately 0.3–0.4 eV) (Table 1).
To summarize, in the analyzed [3+2] cycloaddition reactions benzonitrile N-oxide 1 will participate as nucleophile (electron donor), while β-phosphorylated analogues of nitroethenes 2a–c will perform a role of electrophile (electron acceptor). Moreover, according to one of the fundamental MEDT rules, the interaction between the tested components should be evidently classified as polar processes [54]. Therefore, for analyzed processes non-polar mechanisms such as biradicaloidal one-step or stepwise biradical are not possible.
The regioselectivity of a polar reaction involving the participation of non-symmetric reagents can be specified through interaction between the most electrophilic centre for the electrophile component and the most nucleophilic centre for the nucleophile component. Due to this, the Parr functions were the most exact and discerning implements that enabled the study of the local parameters of the reaction [55]. Therefore, the electrophilic Pk+ and nucleophilic Pk‾ Parr functions of addends were analyzed to determine the most nucleophilic and the most electrophilic centres for the analyzed addends (see Figure 1).
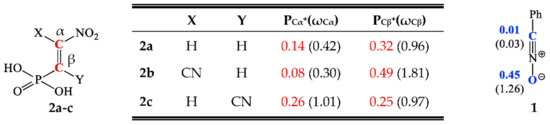
Figure 1.
The local electronic properties for benzonitrile N-oxide 1 and β-phosphorylated analogues of nitroethenes 2a–c. The nucleophilic Pk‾ is given in blue and the electrophilic Pk+ is given in red. The indexes of local nucleophilicity Nk and local electrophilicity ωk are given in brackets.
Studies of the nucleophilic Parr functions for benzonitrile N-oxide 1 show that the oxygen atom is the most nucleophilic centre of this species presenting the maximum value, PO‾ = 0.45 eV. For this atom, the value of the local nucleophilicity Nk index is NO = 1.26 eV (see Figure 1).
In turn, the analysis of the electrophilic Pk+ Parr functions of β-phosphorylated analogues of nitroethenes 2a and 2b shows that for these molecules the most electrophilic centre of this species are the Cβ carbons, presenting the maximum values in the range PCβ+ = 0.32–0.49 eV. At these atoms, the values of the local electrophilicity ωCβ indexes are in the range ωCβ = 0.96–1.81 eV (see Figure 1). In turn, the electrophilic Pk+ Parr functions for 2c are practically identical, both for the atom Cα, PCα+ = 0.26 eV, and Cβ, PCβ+ = 0.25 eV. The values of the local electrophilicity of these centres are, respectively, 1.01 (ωCα) and 0.97 (ωCβ) eV (see Figure 1).
According to CDFT theory, the described interactions show that for the [3+2] cycloadditions between 1 and 2a–b, the more preferred reaction channel is forming 4-nitro-substituted Δ2-isoxazolines 3a–b, while for the process of 1 with 2c both regioisomers of 4-nitro- and 5-nitro-substituted Δ2-isoxazolines (respectively 3c and 4c) can be formed with the similar probability.
3.2. Reaction Profiles of 32CAbetween Benzonitrile N-Oxide and β-Phosphorylated Analogues of Nitroethenes
Secondly, the 32CA processes between benzonitrile N-oxide 1 and β-phosphorylated nitroethenes 2a–c were studied. The quantum-chemical calculation was performed according to the functional M062X with the 6-31+G(d) basis set. Stationary points corresponding to the existence of molecular complexes (MCs) and transition complexes (TSs) were found on both reaction paths, between minimums for the addends and the products of reaction (see Scheme 2).
Scheme 2.
32CA reaction between benzonitrile N-oxide 1 and β-phosphoryl nitroethene 2a.
Quantum-chemical calculations have shown that the reaction between 1 and 2a in toluene (ε = 2.38) as a solvent leads to molecular complexes MC at the first reaction stage for both of the reaction channels. MCs are created without necessity of crossing an activation barrier. Due to this fact, we can observe a significant drop in an enthalpy, as well as a small reduction of entropy. This, in turn, eliminates the appearance of MC structures as stable intermediates due to thermodynamic rules considered at room temperature. A similar type of intermediate was also localized in the 32CAs containing cyano analogues β-phosphorylated nitroethenes 2b and 2c (see Table S1).
Further conversion of reaction system of 1 + 2a alongside the process path, irrespective of the 32CA channel, leads in the next stage to the transition state TS. It is confirmed by IRC analysis. The appearance of a transition state was established by the presence of one imaginary eigenvalue in the Hessian and it is associated with increase of the Gibbs free energy of the process by 23 kcal in a relation with the creating of 3a and about 24 kcal/mol in the case of creating of 4a (see Table S1). Based on the given values, it can be concluded that both of reaction channels lead to 4-nitro- and 5-substituted adducts (respectively 3a and 4a) and both should be considered as kinetically allowed (see Figure 2). Moreover, the more favoured reaction channel is a creation of 4-substituted Δ2-isoxazoline 3a. This conclusion corresponds well with the results of the intermolecular interactions of addends according to CDFT discussed above (see Figure 1).
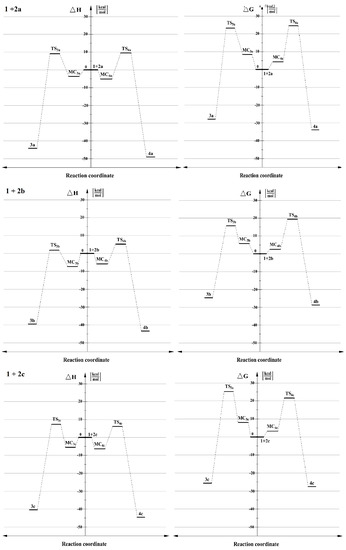
Figure 2.
Energy profiles for the 32CA between benzonitrile N-oxide 1 and β-phosphorylated analogues of nitroethenes 2a–c,in toluene, according to M062X/6-31+G(d) calculations (PCM).
When more polar solvents, such as nitromethane (ε = 36.56) solution (see Table S1), were included as dielectric media in DFT calculations, the reaction profiles did not change qualitatively, but they did change quantitatively to a small extent. In particular, the MCs in all the energy profiles were slightly flat and all activation barriers were significantly higher. Nevertheless, the order of the process channels priority based on kinetics, in nitromethane, which is characterized as a greatly polar solvent, was identic as analyzed once in toluene solution (see Figure 2).
When the structure of 2a is modified by replacement of α-hydrogen within nitrovinyl moiety by a cyano group, the profile of the considered reaction channel 1 + 2b does not change qualitatively. Even so, some transformations occur in a quantitative way (see Table S1). In particular, activation barriers in both channels are lower than analyzed above 32CA. It is caused by the presence of the two electron-withdrawing (EWG) groups—nitro and cyano—directly situated at the same α–carbon in nitroalkene 2b. These groups have an impact for molecule 2b, causing significant influence of electrophilicity on β-carbon (see Figure 1 and Table S1). Also, for the analyzed cycloadditon 1 + 2b, forming of adducts 3b and 4b should be considered as kinetically allowed. Moreover, the more favoured reaction channel is the formation of 4-substituted Δ2-isoxazoline 3b. The above observations are also similar for nitromethane as a solvent. All presented results correlate well with the analysis of the intermolecular interactions of addends according to CDFT (see Figure 1).
In turn, if the structure of 2a is altered by the replacement of β-hydrogen within nitrovinyl moiety by a cyano group, the profile of the considered reaction channel 1 + 2c does change both qualitatively as well as quantitatively (see Table S1). In particular, the values of the activation barrier are very similar in comparison with the model reaction 1 + 2a. Furthermore, for analyzed cycloaddition 1 + 2c, the forming of adducts 3c and 4c should be considered as kinetically allowed; however, in contrast to 32CA analyzed above, the more favoured reaction channel is a creation of 5-subsituted Δ2-isoxazoline 4c, independently of a solvent. The change of regioisomerism for the analyzed cycloaddition 1 + 2c is caused by the occurrence of a cyano group in a β-position of nitroalkene 2c. The substituent interacts on α-atom of carbon in 2c, increasing the value of electrophilicity (see Figure 1 and Table S1). These observations correlate well with the analysis of the intermolecular interaction of addends according to CDFT (see Figure 1).
3.3. Critical Structures for Reaction of 32CA between Benzonitrile N-Oxide and β-Phosphorylated Nitroethenes
3.3.1. Pre-Reaction Molecular Complexes (MC)
As noted in previous paragraphs, the first stage in each of the 32CA processes considered in toluene solution (ε = 2.38) is always the formation of a molecular complex MC (respectively MC3a and MC4a for competitive reaction channels) (see Figure 3 and Table S2). Analysis of the structural aspects of each MC have shown that the bond lengths for O1–N2, N2–C3 and C4–C5 were practically identical as analogous lengths in the individual molecules. What is more, within MCs distances between reaction centres (respectively C3–C4 and C5–O1) were about a few Å, still remaining far outside the range typical for r bonds in transition states. Also, in the MCs, the orientations of the reaction centres compared to each other are not the same as they are in the final products. Additionally, based on the GEDT values (0.0 e), none of the MCs are charge-transfer complexes. Change of the solvent to the slightly more polar nitromethane (ε = 36.56) does not cause significant changes in the nature of the molecular complexes (see Figure 3 and Table S2).
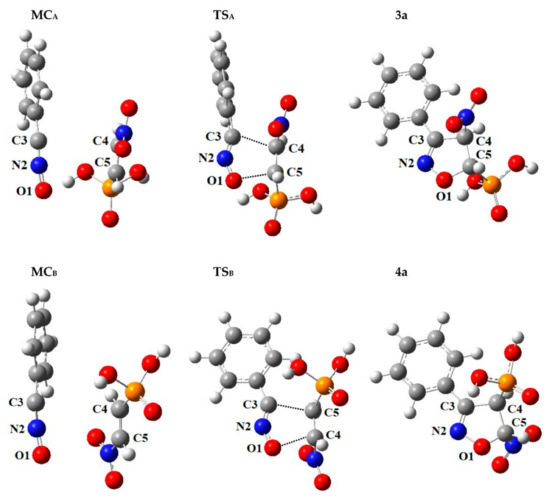
Figure 3.
Critical structures for the reaction of 32CA between benzonitrile N-oxide 1 and β-phosphoryl nitroethene 2a, in toluene, according to M062X/6-31+G(d) calculations (PCM).
3.3.2. Transition States (TS)
Conversion of the MC into the required adduct was always found to occur via a single TS (respectively TS3a and TS4a for competitive reaction channels) (see Figure 3 and Table S2). Within TSs, the C3–C4 and C5–O1 atomic distances have been significantly shortened, compared to the corresponding distances within the MC. The nature of the TSs depends to some extent on the relative orientations of its substructures. In particular, for cycloaddition with benzonitrile N-oxide 1 with β-phosphoryl nitroethene 2a in toluene solution (ε = 2.38), for the TS3a obtained in the reaction channel which ultimately yields 4-nitro-substituted Δ2-isoxazoline 3a, the extent of C3–C4 and C5–O1 forming new bonds is rather similar (l = 0.477 and 0.450, respectively). In turn, within the competitive TS4a, obtained in the reaction channel which ultimately yields 5-nitro-substituted Δ2-isoxazoline 4a, the stage of advancement C5–O1 sigma bond is 0.442, while the C3–C4 sigma bond is formed not much earlier, 0.551. It seems that, for the more favoured reaction channel in a kinetic point of view, TS3a is more synchronic. Likewise, both TSs exhibit evidently [54] polar nature, which is confirmed in the GEDT index value (see Table S1). In particular, more asynchronous TS4a is connected with the higher value of GEDT.
In the case of a similar cycloaddition between 1 and β-phosphorylated α-cyanonitroethene 2b, in the same reaction medium (toluene), the level of asynchronicity for both TSs changes significantly. In particular, for the TS3b received in the reaction channel which finally yields 3b, the stage of advancement C5–O1 sigma bond increases (l = 0.681), while for the C3–C4 sigma bond the stage of advancement is reduced (l = 0.426), in comparison to a model reaction 1 + 2a (see Table S2). Similarly, for the competitive TSB, obtained in the reaction channel which ultimately yields 4b, the stage of advancement C5–O1 sigma bond is reduced (l = 0.425), whilst for the C3–C4 sigma bond the stage of advancement increases (l = 0.682), in comparison to a model reaction 1 + 2a (see Table S2). A much higher stage of advancement of the C5–O1 sigma bond for reaction channel 3b and C3–C4 sigma bond for reaction channel 4b is caused by the presence of a cyano group in α–carbon in nitroalkene 2b, causing a significant influence of electrophilicity on carbon in β position. For reaction 1 + 2b, both TSs present a similar synchronic and are characterized by a more polar nature, in comparison to 32CA analyzed above (see GEDT in Table S2). The slightly higher values of GEDT are caused by the presence of an additional EWG and associated with it, increasing asynchronicity when creating new sigma bonds.
The clear influence of the existence of a cyano group on the structure of TS can be also observed for cycloaddition between 1 and β-phosphorylated β-cyanonitroethene 2c, in toluene. For the analyzed case, the asynchronicity level of both TSs is similar to above analyzed 32CA and simultaneously higher in comparison to a model reaction 1 + 2a (see Table S2). In particular, for the TS3c localized within the reaction channel which ultimately yields 3c, the stage of advancement the C3–C4 sigma bond (l = 0.579) is higher than for the C5–O1 sigma bond (l = 0.517); however, the difference is not very significant (see Table S2). On the other hand, for the competitive TS4c, obtained in the reaction channel which ultimately yields 4c, the higher stage of advancement is observed for the C5–O1 sigma bond (l = 0.609), than the C3–C4 sigma bond (l = 0.469). It seems that, for the more favoured reaction channel in a kinetic point of view, TS4c is more asynchronic. Also, both TS3c and TS4c are characterized by a similar polar nature, in comparison to 32CA analyzed above, except that for a more asynchronous TS4c the value of GEDT is significantly higher (see Table S2). In this case, a much higher stage of advancement of the C3–C4 sigma bond for reaction channel 3c and the C5–O1 sigma bond for reaction channel 4c is also caused by the presence of a cyano group in β-carbon in nitroalkene 2c. It causes an influence of electrophilicity on carbon in α position. However, this effect is smaller than in the previously analyzed 32CA due to the presence of a nitro group in α position.
Due to the use of a strong polar medium, such as nitromethane (ε = 36.56) in the reaction medium, the main parameters of all analyzed structures are not changed significantly (see Table S2). In particular, for the structure of all TSs the GEDT values increase, which exhibits their more polar nature.
4. Conclusions
The reactions of [3+2] cycloaddition between benzonitrile N-oxide 1 and β-phosphorylated analogues of nitroethenes 2a–c have been studied according to M062X/6-31+G(d) theory level. Received results are confirmed by the analysis combination of the Conceptual Density Functional Theory reactivity indices at the ground state of the addends and analyzed according to the reaction profiles and study of the critical structures located on the reaction paths.
The analysis of the reactivity shows that benzonitrile N-oxide 1 can be classified both as a moderate electrophile and moderate nucleophile. The mentioned analysis has also shown that β-phosphorylated analogues of nitroethenes 2a–c can be classified as a strong electrophiles and marginal nucleophiles. Moreover, for a model reaction 1 + 2a the more favored reaction path is forming 4-nitro-substituted Δ2-isoxazoline 3a. The same preference can be observed for cycloaddition 1 + 2b, while for reaction of 1 + 2c both reaction channels are possible to the same extent. It is caused by the presence of a cyano group in different positions of nitrovinyl moiety. What is more, the interaction between the tested components should be evidently classified as polar processes.
The analysis of the reaction profiles for all cycloadditions indicates that the processes between 1 and 2a–c should be regarded as polar, except one-step processes. All attempts for the localization of competitive channels leads to zwitterionic intermediates were not successful. It should be underlined at this point that estimated parameters of the activation suggest that the synthesis of the expected phosphorylated nitroisoxazolines is fully allowed with good regioselectivity even under mild conditions. Finally, the significant influence on the kinetic aspects as well as predicted regioselectivity exhibit the presence of EWG at the α-position at nitrovinyl moiety.
Supplementary Materials
The following are available online at https://www.mdpi.com/2673-401X/2/1/3/s1.
Author Contributions
Conceptualization, K.K. and K.Z.; methodology, K.K.; investigation, K.K., K.Z.; resources, K.K. and K.Z.; data curation, K.K.; writing—original draft preparation, K.K.; writing—review and editing, K.K. and K.Z.; visualization, K.K. and K.Z.; supervision, K.K; project administration, K.K. and K.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
All calculations reported in this paper were performed on “Prometheus” supercomputer cluster in the CYFRONET computational centre in Cracow. Support of this research is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huisgen, R. 1,3-Dipolare Cycloadditionen Rückschau und Ausblick. Angew. Chem. Int. Ed. 1963, 75, 604–637. [Google Scholar] [CrossRef]
- Jasiński, R.; Żmigrodzka, M.; Dresler, E.; Kula, K. A full regio- and stereoselective synthesis of 4-nitroisoxazolidines via stepwise [3+2] cycloaddition reactions between (Z)-C-(9-anthryl)-N-arylnitrones and (E)-3,3,3-trichloro-1-nitroprop-1-ene: Comprehensive experimental and theoretical study. J. Heterocyc. Chem. 2017, 54, 3314–3320. [Google Scholar] [CrossRef]
- Łapczuk-Krygier, A.; Kącka-Zych, A.; Kula, K. Recent progress in the field of cycloaddition reactions involving conjugated nitroalkenes. Curr. Chem. Lett. 2019, 8, 13–38. [Google Scholar] [CrossRef]
- Fryzlewicz, A.; Łapczuk-Krygier, A.; Kula, K.; Demchuk, O.M.; Dresler, E.; Jasiński, R. Regio- and stereoselective synthesis of nitro-functionalized analogs of nicotine. Chem. Heterocycl. Compd. 2020, 56, 120–122. [Google Scholar] [CrossRef]
- Martina, K.; Tagliapietra, S.; Veselov, V.V.; Cravotto, G. Green Protocols in Heterocycle Syntheses via 1,3-Dipolar Cycloadditions. Front. Chem. 2019, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.Z.; Li, X.L.; Chen, H.; Li, Y.N.; Wang, R. The synthesis and biological activity of novel spiro-isoxazoline C-disaccharides based on 1,3-dipolar cycloaddition of exo-glycals and sugar nitrile oxides. Tetrahedron Lett. 2007, 48, 7813–7816. [Google Scholar] [CrossRef]
- Jasinski, R.; Dresler, E. On the Question of Zwitterionic Intermediates in the [3+2] Cycloaddition Reactions: A Critical Review. Organics 2020, 7, 5. [Google Scholar] [CrossRef]
- Kula, K.; Łapczuk-Krygier, A. A DFT computational study on the [3+2] cycloaddition between parent thionitrone and nitroethene. Curr. Chem. Lett. 2018, 7, 27–34. [Google Scholar] [CrossRef]
- Kula, K.; Dobosz, J.; Jasiński, R.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Mirosław, B.; Demchuk, O.M. [3+2] Cycloaddition of diaryldiazomethanes with (E)-3,3,3-trichloro-1-nitroprop-1-ene: An experimental, theoretical and structural study. J. Mol. Struct. 2020, 1203, 127473. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Jeddeloh, M.R.; Holden, J.B.; Nouri, D.H.; Kurth, M.J. A Library of 3-aryl-4,5- dihydroisoxazole-5-carboxamides. J. Comb. Chem. 2007, 9, 1041–1045. [Google Scholar] [CrossRef]
- Quadrelli, P.; Martinez, N.V.; Scrocchi, R.; Corsaro, A.; Pistarà, V. Syntheses of Isoxazoline-Carbocyclic Nucleosides and Their Antiviral Evaluation: A Standard Protocol. Sci. World J. 2014, 2014, 492178. [Google Scholar] [CrossRef] [PubMed]
- Znati, M.; Debbabi, M.; Romdhane, A.; Ben Jannet, H.; Bouajila, J. Synthesis of new anticancer and anti-inflammatory isoxazolines and aziridines from the natural (-)-deltoin. J. Pharm. Pharmacol. 2018, 70, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Filial, I.; Bouajila, J.; Znati, M.; Bousejra-El Garah, F.; Ben Jannet, H. Synthesis of new isoxazoline derivatives from harmine and evaluation of their anti-Alzheimer, anti-cancer and anti-inflammatory activities. J. Enzyme Inhibit. Med. Chem. 2014, 30, 371–376. [Google Scholar] [CrossRef]
- Saravanan, G.; Alagarsamy, V.; Dineshkumar, P. Synthesis, analgesic, anti-inflammatory and in vitro antimicrobial activities of some novel isoxazole coupled quinazolin-4(3H)-one derivatives. Arch. Pharm. Res. 2013. [Google Scholar] [CrossRef]
- Sharifi, B.; Zade, B.G.; Zoladl, M.; Najafi, D.S.; Ghafarian, S.; Hamid, R.; Hashemi, M.; Abad, N. Side effects of risperidone. Life Sci. J. 2012, 9, 1463–1467. [Google Scholar] [CrossRef]
- Barceló, M.; Raviña, E.; Masaguer, C.F.; Domínguez, E.; Areias, F.M.; Brea, J.; Loza, M.I. Synthesis and binding affinity of new pyrazole and isoxazole derivatives as potential atypical antipsychotics. Bioorg. Med. Chem. Lett. 2007, 17, 4873–4877. [Google Scholar] [CrossRef]
- Pinho e Melo, T.M.V.D. Recent Advances on the Synthesis and Reactivity of Isoxazoles. Curr. Org. Chem. 2005, 9, 925–958. [Google Scholar] [CrossRef]
- Kanemasa, S.; Tsuge, O. Recent advances in synthetic applications of nitrile oxide cycloaddition. Heterocycles 1990, 30, 719–736. [Google Scholar] [CrossRef]
- Sewald, N. Synthetic Routes towards Enantiomerically Pure β-Amino Acids. Angew. Chem. Int. Ed. 2003, 42, 5794–5795. [Google Scholar] [CrossRef]
- Harada, K.; Kaji, E.; Takahashi, K.; Zen, S. Ring Transformation of 2-Isoxazoline 2-Oxides by Lewis Acids. Rev. Heteroatom Chem. 1997, 16, 171–195. [Google Scholar] [CrossRef]
- Ono, N. The Nitro Group in Organic Synthesis; Wiley-VSH: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Padwa, A.; Pearson, W.H. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Boguszewska-Czubara, A.; Kula, K.; Wnorowski, A.; Biernasiuk, A.; Popiolek, P.; Miodowski, D.; Demchuk, O.M.; Jasiński, R. Novel functionalized β-nitrostyrenes: Promising candidates for new antibacterial drugs. Saudi Pharm. J. 2019, 27, 593–601. [Google Scholar] [CrossRef]
- Yan-Mei, L.; Ying-Wu, Y.; Yu-Fen, Z. Phosphoryl group participation leads to peptide formation from N-phosphorylamino acids. Int. J. Peptide Protein Res. 1992, 39, 375–381. [Google Scholar] [CrossRef]
- Nalwa, H.S. Handbook of Surfaces and Interfaces of Materials; Academic Press: Los Angeles, CA, USA, 2001. [Google Scholar]
- Ram, V.J.; Sethi, A.; Nath, M.; Pratap, R. The Chemistry of Heterocycles; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Mersbergen, D.; Wijnen, J.W.; Engberts, J.B.F.N. 1,3-Dipolar Cycloadditions of Benzonitrile Oxide with Various Dipolarophiles in Aqueous Solutions. A Kinetic Study. J. Org. Chem. 1998, 63, 8801–8805. [Google Scholar] [CrossRef]
- Jasiński, R.; Jasińska, E.; Dresler, E. A DFT computational study of the molecular mechanism of [3+2] cycloaddition reactions between nitroethene and benzonitrile N-oxides. J. Mol. Model. 2017, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Emamian, S.; Salami, M.; Ríos-Gutiérrez, M. Understanding the molecular mechanism of the [3+2] cycloaddition reaction of benzonitrile oxide toward electron-rich N-vinylpyrrole: A DFT study. J. Phys. Org. Chem. 2016, 29, 368–376. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos Gutiérrez, M.; Castellanos Soriano, J. Understanding the Origin of the Regioselectivity in Non-Polar [3+2] Cycloaddition Reactions through the Molecular Electron Density Theory. Organics 2020, 1, 3. [Google Scholar] [CrossRef]
- Jasiński, R.; Ziółkowska, M.; Demchuk, O.M.; Maziarka, A. Regio- and stereoselectivity of polar [2+3] cycloaddition reactions between (Z)-C-(3,4,5-trimethoxyphenyl)-N-methylnitrone and selected (E)-2-substituted nitroethenes. Cent. Eur. J. Chem. 2014, 12, 586–593. [Google Scholar] [CrossRef]
- Jasiński, R. Competition between one-step and two-step mechanism in polar [3+2] cycloadditions of (Z)-C-(3,4,5-trimethoxyphenyl)-N-methyl-nitrone with (Z)-2-EWG-1-bromo-1-nitroethenes. Comput. Theor. Chemia. 2018, 1125, 77–85. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 Rev. A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Jasiński, R. A new insight on the molecular mechanism of the reaction between (Z)-C,N-diphenylnitrone and 1,2-bismethylene-3,3,4,4,5,5-hexamethylcyclopentane. J. Mol. Graph. Model. 2020, 94, 107461. [Google Scholar] [CrossRef]
- Kącka-Zych, A. Participation of Phosphorylated Analogues of Nitroethene in Diels–Alder Reactions with Anthracene: A Molecular Electron Density Theory Study and Mechanistic Aspect. Organics 2020, 1, 4. [Google Scholar] [CrossRef]
- Kula, K.; Zawadzińska, K. Local nucleophile-electrophile interactions in [3+2] cycloaddition reactions between benzonitrile N-oxide and selected conjugated nitroalkenes in the light of MEDT computational study. Curr. Chem. Lett. 2021, 10, 9–16. [Google Scholar] [CrossRef]
- Stephens, P.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Domingo, L.R.; Kula, K.; Ríos-Gutiérrez, M. Unveiling the Reactivity of Cyclic Azomethine Ylides in [3+2] Cycloaddition Reactions within the Molecular Electron Density Theory. Eur. J. Org. Chem. 2020, 5938–5948. [Google Scholar] [CrossRef]
- Schlegel, H.B. Optimization of equilibrium geometries and transition structures. J. Comput. Chem. 1982, 3, 214–218. [Google Scholar] [CrossRef]
- Schlegel, H.B. Modern Electronic Structure Theory; Yarkony, D.R., Ed.; World Scientific Publishing: Singapore, 1994. [Google Scholar]
- Fukui, K. Formulation of the reaction coordinate. J. Phys. Chem. 1970, 74, 4161–4163. [Google Scholar] [CrossRef]
- Tapia, O. Solvent Effect Theories: Quantum and Classical Formalism and their Applications in Chemistry and Biochemistry. J. Math. Chem. 1992, 10, 131–181. [Google Scholar] [CrossRef]
- Tomasi, J.; Perisco, M. Molecular Interactions in Solution: An Overview of Methods Based on Continuous Distributions of the Solvent. Chem. Rev. 1994, 94, 2017–2094. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Chem. 1996, 225, 327–335. [Google Scholar] [CrossRef]
- Domingo, L.R. A New C-C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Mloston, G.; Jasiński, R.; Kula, K.; Heimgartner, H. A DFT Study on the Barton-Kellogg Reaction—The Molecular Mechanism of the Formation of Thiiranes in the Reaction between Diphenyldiazomethane and Diaryl Thioketones. Eur. J. Org. Chem. 2020, 176–182. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef]
- Pérez, P.; Domingo, L.R.; Duque-Noreña, M.; Chamorro, E. A condensed-to-atom nucleophilicity index. An application to the director effects on the electrophilic aromatic substitutions. J. Mol. Struct. 2009, 895, 86–91. [Google Scholar] [CrossRef]
- Greelings, P.; De Proft, F.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef]
- Pérez, P.; Domingo, L.R.; Aurell, M.J.; Contreras, R. Quantitative characterization of the global electrophilicity pattern of some reagents involved in 1,3-dipolar cycloaddition reactions. Tetrahedron 2003, 59, 3117–3125. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P.; Saez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Domingo, L.R.; Saez, J.A. Understanding the mechanism of polar Diels-Alder reactions. Org. Biomol. Chem. 2009, 7, 3576–3583. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M. On the nature of organic electron density transfer complexes within molecular electron density theory. Org. Biomol. Chem. 2019, 17, 6478–6488. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).