Abstract
The conversion of polycyclic aromatic hydrocarbons (PAHs) to monocyclic aromatic hydrocarbons holds significant importance in the petrochemical and coal chemical industries, as it enables the production of high-value-added chemicals. In this study, we investigated the methane-assisted hydroconversion of PAHs to monocyclic aromatic hydrocarbons with methyl side chains over Zn-based catalysts from Hβ zeolites treated with citric acid (CA) at different concentrations. The CA-modified Hβ catalysts were characterized using X-ray diffraction (XRD), N2 adsorption–desorption, pyridine–Fourier transform infrared spectroscopy (Py-FTIR), and ammonia temperature-programmed desorption (NH3-TPD). The results show that low CA concentrations facilitate the removal of amorphous aluminum from the zeolite framework, thereby increasing the specific surface area, pore volume, and pore diameter of the Zn/Hβ catalyst, as well as improving its Lewis/Brønsted (L/B) acid ratio. In contrast, excessive CA treatment causes the undesirable removal of framework aluminum and leads to structural collapse in the mesoporous regions formed at the interfaces between certain crystal aggregates. This, in turn, has a negative impact on the catalyst’s specific surface area, pore volume, pore size distribution, total acidity, and L/B ratio. Experimental data further indicate that the optimal Zn/Hβ catalyst, prepared using Hβ treated with 0.08 M CA, achieves a naphthalene conversion rate of up to 99% and a benzene–toluene–xylene (BTX) selectivity of 60% in the liquid product over a 10 h reaction period. These findings confirm that CA treatment not only enhances the catalytic activity of Zn/Hβ but also significantly improves its operational stability. This work provides new insights into the rational design of catalysts for the efficient conversion of PAHs to monocyclic aromatic hydrocarbons and the utilization of methane resources.
1. Introduction
Heavy oil is abundant in polycyclic aromatic hydrocarbons (PAHs), which can undergo hydrocracking to form monocyclic aromatic hydrocarbons. The hydrocracking process typically demands large quantities of hydrogen (H2), yet H2 is not naturally abundant. Usually, H2 is obtained from refining byproducts like catalytic reforming units or produced via steam reforming of coal or methane (CH4) [1]. This high cost of hydrogen can drive up the overall production costs of the hydrocracking process. Hence, strategies to cut down H2 consumption and boost the yield of high-value products are vital for heavy oil processing. Among natural hydrocarbons, CH4 has the highest atomic hydrogen content. So, replacing part or all of the H2 with methane in the PAH hydrogenation process could notably reduce production costs.
Choudhary et al. [2] were the first to show that methane can be highly activated, achieving high conversion rates to higher hydrocarbons and aromatics at low temperatures (400° to 600 °C), through its reaction over H-galloaluminosilicate ZSM-5 type (MFI) zeolite in the presence of alkenes or higher alkanes. He et al. [3] studied a thermal overcracking process for heavy crude oil over an Ag/ZSM-5 catalyst (Ag supported on ZSM-5 zeolite) under CH4 or N2 atmospheres and emphasized the technical benefits of upgrading heavy oil in a methane environment as a more cost-effective and eco-friendly alternative to traditional hydrotreating methods. Moreover, He et al. [4] examined the catalytic performance for partial upgrading of bitumen over Ag-Ga/ZSM-5 in a CH4 atmosphere and used NMR spectroscopy to confirm the incorporation of CH4 into product molecules. Shen et al. [5,6] studied the hydrocracking reaction of polycyclic aromatic hydrocarbons in a hydrogen and methane mixed atmosphere. The results showed that the presence of methane not only improved the overall selectivity of BTX but also significantly increased the selectivity of monocyclic aromatic hydrocarbons with methyl side chains. The aforementioned relevant research results collectively suggest that CH4 can be activated during reactions to provide methyl groups and increase the C/H ratio, laying a foundation for its role in converting polycyclic aromatics into monocyclic aromatics via hydrogenation under methane or mixed methane and hydrogen atmospheres.
However, when converting polycyclic aromatics to monocyclic aromatics over catalysts like Zn/Hβ in relevant atmospheres, there are key unresolved challenges. Reaction temperatures often exceed 400 °C, and strong acid catalysts from zeolites are prone to coking and deactivation. Minimizing coke deposition, ensuring long-term stability, and addressing the limitations when using CH4 instead of H2 are critical.
Acid treatment methods for catalysts can effectively modify both the textural and acidic properties of catalysts without damaging their structural integrity, thus ensuring sustained stability. Wei et al. [7] showed a decrease in strong acid sites within zeolite structures, probably due to aluminum (Al) dissolution from Y zeolite frameworks. Wang et al. [8] demonstrated that citric acid (CA) treatment can expand pore sizes and regulate acid distribution within zeolites, which enhances mass transfer for macromolecules while also increasing acidity, thereby strengthening the catalyst’s stability. Compared to other dealumination agents or zeolite modification methods (such as oxalic acid, sulfuric acid, or alkaline treatments), CA has unique advantages. It can moderately remove framework aluminum and adjust the acid sites more precisely, and its chelating ability helps in a more controlled modification process, which is beneficial for tailoring the zeolite’s properties to suit the PAH hydrocracking reaction. So, to further enhance the catalytic stability of zeolite-supported Zn-based catalysts in a methane/hydrogen mixed atmosphere for the hydrocracking of polycyclic aromatic hydrocarbons to monocyclic aromatic hydrocarbons, CA-treated Zn/Hβ catalysts are employed, as they significantly improve both the pore structure and acidity of zeolites, thereby enhancing overall catalytic stability.
This study aims to explore how to improve catalytic stability in the process of methane-assisted hydroconversion of polycyclic aromatics. Specifically, it investigates how treating Hβ with CA solutions of different molar concentrations affects the pore structure and acidity of Zn/Hβ catalysts, with the goal of establishing the structure–function relationship between the catalytic activity of Zn/Hβ and its pore structure/acidity after modification with CA solutions of varying concentrations.
2. Materials and Methods
2.1. Materials
Materials were sourced as follows: ammonia (NH3) type β zeolite (Si/Al = 25, sourced from Nankai University Catalyst Factory, Tianjin, China); zinc nitrate hexahydrate (Zn(NO3)2·6H2O AR, obtained from Xiya Reagent, Linyi, China); n-Hexane (C6H14 95%, supplied by Tianjin Damao Chemical Reagent Factory, Tianjin, China); naphthalene (C10H8 AR, provided by Aladdin Chemical Reagent Company, Shanghai, China); and citric acid (C6H8O7 AR, acquired from Aladdin Chemical Reagent Company, Shanghai, China).
2.2. Preparation of Catalysts
The NH3 type β zeolite (Si:Al ratio of 23) [5] was transformed into Hβ through calcination at 550 °C in an air atmosphere for 5 h. Hβ zeolites were treated with CA solutions of varying concentrations (0 M, 0.03 M, 0.05 M, 0.08 M, 0.10 M, 0.15 M) and stirred at 100 °C for 3 h, followed by drying, grinding, and calcination. The CA-modified Hβ zeolites were then impregnated using a Zn(NO3)2·6H2O solution to prepare Zn/Hβ catalyst precursors with the same Zn loading content. Subsequently, the catalyst precursors were maintained in an oven at 110 °C for 10 h, calcined at 550 °C for 4 h, and finally ground into particles sized between 20 to 40 mesh. Ultimately, the Zn loading in all catalysts was maintained at 5% of the total catalyst weight. The catalysts were designated according to the various concentrations of the CA solution as Zn/Hβ-CA-0M, Zn/Hβ-CA-0.03M, Zn/Hβ-CA-0.05M, Zn/Hβ-CA-0.08M, Zn/Hβ-CA-0.10M, and Zn/Hβ-CA-0.15M (where M denotes molarity of CA). To ensure statistical validity, each catalyst sample treated with a specific CA concentration was tested in triplicate to monitor changes in its structural properties and improvements in catalytic activity.
2.3. Catalyst Characterization
The catalysts were subjected to powder X-ray diffraction (XRD) analysis using an X-ray diffractometer from the company Bruker AXS GmbH (Karlsruhe, Germany) with Cu Kα radiation. The XRD patterns were collected over a 2θ range of 5–80° with a step size of 0.02° and a scanning speed of 2°/min.
The specific surface area, pore volume, and pore size of the catalyst samples were assessed utilizing an ASAP2020 nitrogen adsorption/desorption system from Micromeritics (Norcross, GA, USA). The Brunauer–Emmett–Teller (BET) method was used to calculate the specific surface area, and the Barrett–Joyner–Halenda (BJH) model was employed for pore size distribution analysis [9,10].
Acidity measurements were conducted via ammonia temperature-programmed desorption (NH3-TPD) employing a ChemiSorb 2750 multifunctional adsorber from Micrometrics (Norcross, GA, USA). The procedure was as follows: 200 mg of sample was placed in a U-tube for gas tightness testing. The sample was purged with helium gas (50 mL/min), and the programmed temperature was increased to 350 °C and kept for 90 min for moisture removal and impurity removal. The sample was then cooled down to 50 °C, and NH3 (50 mL/min) was adsorbed for 120 min. Argon (50 mL/min) was introduced to purge the sample for 90 min to eliminate residual NH3 on the surface, and then the temperature was ramped up to 800 °C at 10 °C/min for online inspection using the Chemisoft TPx system V1.03.
The Brønsted and Lewis acidic characteristics of the samples were derived from Fourier transform infrared spectrometer (Nicolet 6700, Thermo Fisher Scientific Inc., Waltham, MA, USA) and in situ transmission cell and high vacuum system (Xiamen Tuozheng Instrument Development Co., Ltd. Xiamen, China). The spectra were recorded in the range of 1400–1700 cm−1 with a resolution of 4 cm−1 at 150 °C and 350 °C.
The coke content in the catalysts was evaluated through thermogravimetric analysis (TGA), performed with a simultaneous thermal analyzer (TGA/DSC 1 METTLER TOLEDO, Greifensee, Switzerland). The samples were heated from room temperature to 800 °C at a rate of 10 °C/min under air flow. The experimental error for coke yield determination was within ±3%.
2.4. Catalyst Performance Evaluation
The catalysts were assessed for their performance in converting polycyclic aromatics to monocyclic aromatics within a self-constructed fixed-bed reactor (inner diameter = 10 mm, length = 500 mm). A total of 0.8 g (approximately 1.6 mL) of catalyst was charged into the constant temperature zone of the reactor with quartz cotton placed at both the ends. The temperature distribution along the catalyst bed was controlled by a three-zone furnace with a temperature control precision of ±1 °C, ensuring a uniform temperature within the reaction zone. To ensure the absence of diffusion gradients, the catalyst particles were sized from 20–40 mesh, and the gas and liquid flow rates were optimized to operate in the kinetic regime.
The catalyst was activated at a hydrogen flow rate of 20 mL/min, under a pressure of 3.5 MPa, with a programmed temperature ramping up to 400 °C. After stabilizing for 30 min, an n-hexane solution containing 5% (by mass) naphthalene was introduced as the feedstock using a high-precision pump. The reaction conditions were as follows: reaction pressure at 3.5 MPa, temperature at 400 °C, liquid hourly space velocity (LHSV) set to 4 h−1, a CH4/H2 mixing volume ratio of 1, and a gas/oil volume ratio maintained at 800. The product samples were collected and analyzed every two hours using gas chromatography with an HP-5 column (30 m × 0.32 mm × 0.25 μm) equipped with a flame ionization detector (FID) (GC-7890B, Agilent Technologies, Santa Clara, CA, USA).
Naphthalene conversion (x, %), BTX selectivity (si, %), gas-phase yield (y1, %), liquid-phase yield (y2, %), and coke yield (y3, %) were used as evaluation metrics for the reactions as defined in Equations (1)–(5).
Equations (1)–(5) illustrate the relationship between m1 and m2, which represent the mass of naphthalene prior to and following the reaction, respectively. The variable mi denotes the mass of raw material naphthalene consumed in producing each component of the reaction. The i component includes BTX, benzene, toluene, and xylene; m3 signifies the feed mass of reacted feedstock; m4 indicates the mass of liquid-phase products that have undergone reaction; and m5 represents the total mass of coke deposited on the catalyst.
3. Results and Discussions
3.1. Characterization of Zn/Hβ Catalysts Treated with Different Concentrations of CA
3.1.1. The XRD Analysis
The XRD spectra of catalyst samples subjected to varying concentrations of CA treatment are presented in Figure 1. As illustrated in the figure, all catalyst samples exhibit characteristic diffraction peaks at 2θ = 7.60°, 21.2°, and 22.4°, corresponding to the diffraction peaks of Hβ zeolite [11]. The XRD diffraction patterns for all catalysts align perfectly with that of Hβ zeolite, indicating that no significant crystalline transformation or deformation occurs during the CA treatment process [12]. Furthermore, the impregnation of active metal and calcination processes ensure that Zn species are uniformly dispersed across the catalyst surface [13].
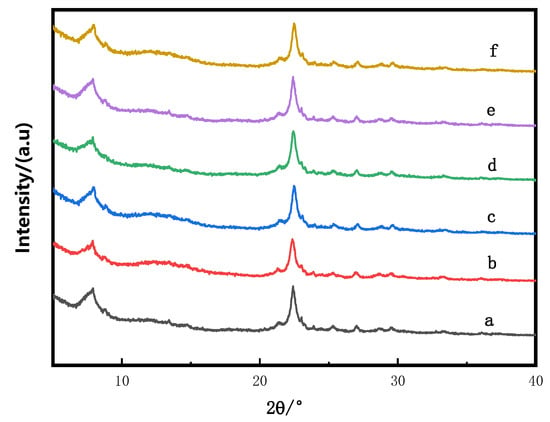
Figure 1.
XRD patterns of Zn/Hβ catalysts treated with different concentrations of CA. (a) Zn/Hβ-CA-0M; (b) Zn/Hβ-CA-0.03M; (c) Zn/Hβ- CA-0.05M; (d) Zn/Hβ- CA-0.08M; (e) Zn/Hβ-CA-0.10M; (f) Zn/Hβ-CA-0.15M.
3.1.2. Nitrogen Adsorption–Desorption
The characteristics of the pore structure of the catalysts are presented in Table 1. It is shown that with the increase in CA treatment concentrations, both the pore volumes and pore sizes of the catalysts were augmented, especially in terms of average pore sizes [14]. However, the BET surface area (SBET), pore volume, and pore size all exhibited a trend of first increasing and then decreasing as the concentration of the CA solution varied. Specifically, for the SBET, it rose from 428.910 m2·g−1 for Zn/Hβ-CA-0M to a peak of 452.511 m2·g−1 for Zn/Hβ-CA-0.08M and then declined to 418.123 m2·g−1 for Zn/Hβ-CA-0.15M. The total pore volume (VTotal) followed a similar pattern, increasing from 0.315 m2·g−1 (Zn/Hβ-CA-0M) to 0.444 mL·g−1 (Zn/Hβ-CA-0.08M) and then decreasing to 0.428 mL·g−1 (Zn/Hβ-CA-0.15M). Regarding the average pore size (Daverage), it went up from 2.383 nm (Zn/Hβ-CA-0M) to 4.434 nm (Zn/Hβ-CA-0.08M) and subsequently dropped to 4.187 nm (Zn/Hβ-CA-0.15M). Notably, when the CA concentration was elevated from 0 M to 0.08 M, DBJH rose significantly from an initial value of 3.482 nm to 8.345 nm, thereby confirming that CA treatment can markedly enhance the pore size [15]. Combined with XRD results, this enhancement is attributed to the fact that CA treatment does not compromise the zeolite structure but rather increases the diameter of the microchannels of the zeolite [16]. It can be inferred that amorphous or extra-framework Al species obstructing surface micropore channels could be effectively removed through CA treatment, resulting in an increase in pore size and total surface area [17]. However, when the concentration of CA treatment exceeds 0.08 M, the specific surface area and pore size of the catalysts start to diminish. This phenomenon arises from the potential dissolution of certain aluminum atoms embedded within the skeletal structure of zeolite crystals, leading to a compromised pore integrity of Hβ zeolite. Consequently, as the concentration of CA was increased beyond 0.08 M, the BET surface area, pore volume, and pore size of the catalysts were all adversely affected [18].

Table 1.
Pore structure properties of Zn/Hβ catalysts treated with different concentrations of CA.
In summary, a controlled application of CA treatment can effectively eliminate certain amorphous species from zeolites, thereby enhancing both the specific surface area and the number of mesopores present. However, excessive CA treatment may compromise the structural integrity of zeolites and yield detrimental effects [19]. Therefore, the concentration of CA treatment emerges as a critical factor influencing BET surface area, pore volume, and pore size in catalysts.
3.1.3. The NH3-TPD Analysis
The acidic strength distribution of catalysts supported on CA-treated Hβ samples is investigated using NH3-TPD, and the results are presented in Figure 2. As shown in Figure 2, NH3-desorption peaks were observed at temperature ranges of 150–300 °C, 350–450 °C, and 450–550 °C, corresponding to weak acidic sites, medium-strength acidic sites, and strong acidic sites [6,16], respectively. With increasing concentrations of CA treatment, there was a noticeable shift in peak positions for each sample towards lower temperatures [20]. Additionally, the center of medium-strong acid initially increased and then decreased. When the concentration of CA solutions was 0.08 M, the acidity reached its highest level. This phenomenon indicates that when Hβ is treated with lower-concentration solutions of CA (less than 0.08 M), the CA facilitates amorphous or extra-framework Al species obstructing surface micropore channels of Hβ and removes some cover shielding from the acidic center [21], leading to an increase in acidity. However, when the concentration of CA solutions exceeds 0.08 mol/L, some aluminum atoms within the skeletal structure may be dissolved, which alters the silicon-to-aluminum atomic ratio in zeolite crystals and consequently affects their acidic content and strength [20,22]. These findings are consistent with results obtained from XRD analysis and nitrogen adsorption–desorption studies.
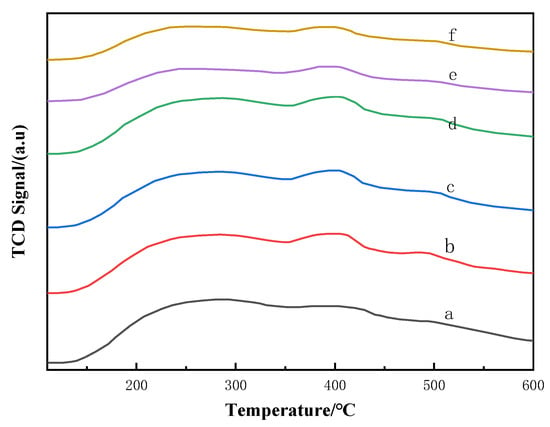
Figure 2.
Temperature-programmed desorption of ammonia spectra of Zn/Hβ catalysts treated with different concentrations of CA. (a) Zn/Hβ-CA-0M; (b) Zn/Hβ-CA-0.03M; (c) Zn/Hβ-CA-0.05M; (d) Zn/Hβ-CA-0.08M; (e) Zn/Hβ-CA-0.10M; (f) Zn/Hβ-CA-0.15M.
3.1.4. The Pyridine Infrared Characterization
To further differentiate the types of acid sites on the catalysts subjected to varying concentrations of CA treatment, pyridine-infrared spectroscopy was employed for acidic property analysis in Figure 3, and the calculated results are presented in Table 2. It is indicated that there is a decrease in overall acidity of the catalysts following CA treatment, particularly showing a significant reduction in both Lewis (L) acidity and total acidity at CA concentrations exceeding 0.08 mol/L [23]. The removal of non-framework aluminum contributes to a slight decrease in L acidity of Hβ zeolite treated with a lower CA concentration, while partial removal of framework aluminum from Hβ zeolite treated with a higher CA concentration leads to structural collapse and a subsequent substantial reduction in L acid sites [24]. Within the low-concentration region, varying concentrations of CA have minimal impact on Brønsted (B) acid sites; however, the elution of framework aluminum from the zeolite could expose and enhance B acidity [25], potentially resulting in a decreased L/B ratio [26]. Therefore, based on these characterizations, we conclude that treatment with an appropriate concentration of citric acid not only induces a pore-expanding effect but also adjusts the acidic properties of β zeolite [27].
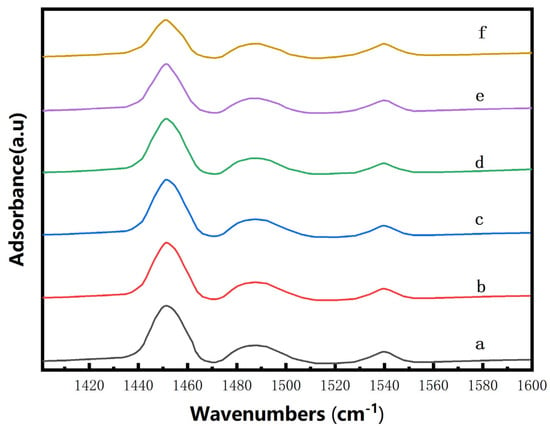
Figure 3.
Pyridine infrared spectra of Zn/Hβ catalysts treated with different concentrations of CA (a) Zn/Hβ-CA-0M; (b) Zn/Hβ-CA-0.03M; (c) Zn/Hβ-CA-0.05M; (d) Zn/Hβ-CA-0.08M; (e) Zn/Hβ-CA-0.10M; (f) Zn/Hβ-CA-0.15M.

Table 2.
Acidic properties of Zn/Hβ catalysts with different concentrations of CA treatment.
3.2. Catalytic Performance of the Zn/Hβ Catalysts from Different Concentrations of CA
In this study, naphthalene—a representative polycyclic aromatic hydrocarbon commonly found in diesel and light cycle oil from fluidized catalytic cracking units and coal tar—was utilized as a model compound to evaluate the methane-assisted hydrocracking of PAHs into benzene–toluene–xylene (BTX) over Zn-based catalysts supported on citric acid-treated Hβ zeolite [28]. Figure 4 and Figure 5 respectively depict naphthalene conversion and BTX selectivity as functions of reaction time. As shown in Figure 4, the naphthalene conversion of the untreated Zn/Hβ catalyst decreases significantly with prolonged reaction time. In contrast, modifying the Zn/Hβ supports with CA solutions of varying concentrations notably enhances their catalytic performance [29]. For instance, the Zn/Hβ catalysts treated with 0.03 mol/L, 0.05 mol/L, and 0.08 mol/L CA all exhibit substantially improved hydrocracking efficiency and catalytic stability. Notably, for the Zn/Hβ catalyst treated with 0.08 mol/L CA, the naphthalene conversion maintained a stable value of 98.8% after eight hours of reaction time. However, when the concentration of CA was further increased beyond 0.08 mol/L, the benefits of acid treatment began to diminish, leading to lower initial naphthalene conversion and reduced catalytic stability. These results indicate that citric acid concentration plays a crucial role in influencing the catalytic performance of methane-assisted hydrocracking of PAHs.
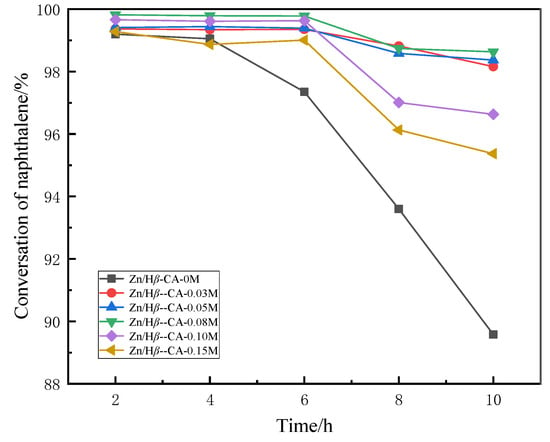
Figure 4.
Naphthalene conversion ratio of Zn/Hβ catalysts treated with different concentrations of CA during the methane-assisted hydrocracking of polycyclic aromatics.
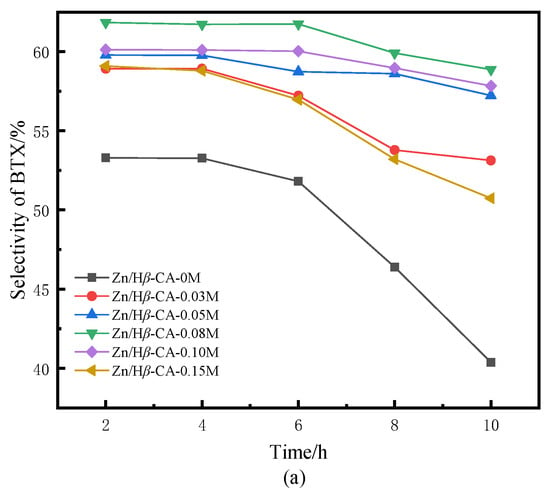
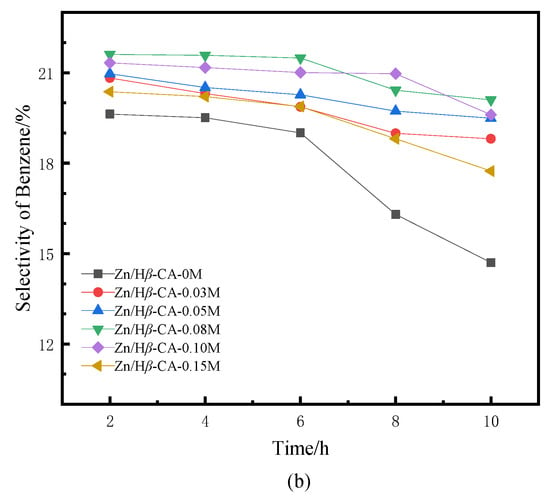
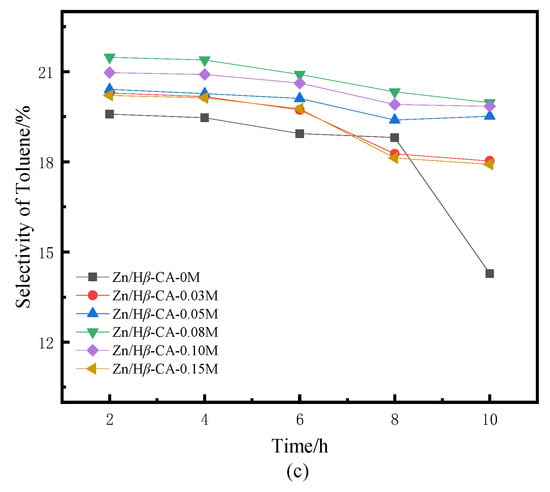
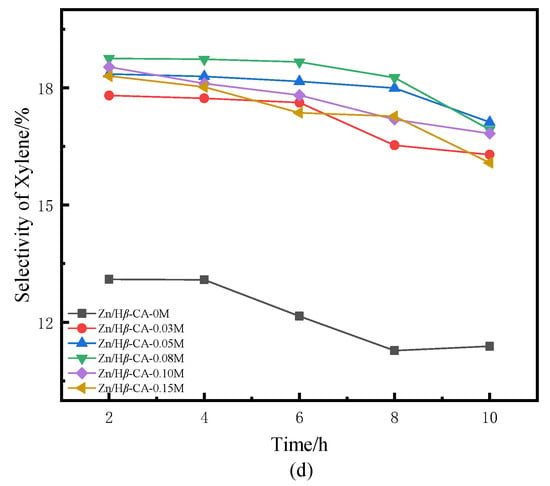
Figure 5.
BTX selectivity of Zn/Hβ catalysts treated with different concentrations of CA during the methane-assisted hydrocracking of polycyclic aromatics: (a) selectivity of BTX, (b) selectivity of benzene, (c) selectivity of toluene, (d) selectivity of xylene.
Figure 5 illustrates the selectivity towards BTX products over the Zn/Hβ-CA-x catalysts as a function of reaction time. In contrast to traditional hydrocracking under an H2 atmosphere, where benzene is typically the major product [30], our study demonstrates a significant enhancement in the selectivity of toluene and xylene. Compared to the untreated Zn/Hβ catalyst, both the total BTX selectivity and the individual selectivities for benzene, toluene, and particularly xylene are markedly increased in the CA-treated Zn/Hβ catalysts [31]. Correspondingly, it is evident that when Hβ is treated with a 0.08 mol/L CA solution, the product distribution and BTX selectivity are optimized relative to varying CA concentrations. This is in line with the aforementioned naphthalene conversion results. The findings indicate that the CA-treated Hβ zeolite is well-suited for the hydrocracking of polycyclic aromatics into BTX under H2 and CH4 atmospheres, which contributes to maintaining the catalysts’ initial activity and enhancing their catalytic stability [32]. Overall, the selectivity for BTX products aligns with the findings from previous studies [5,6].
When the Hβ zeolite support is treated with 0.08 mol/L CA, the catalyst also exhibits optimal BTX selectivity. Higher concentrations of CA treatment result in a concurrent decrease in both naphthalene conversion and selectivity. Consequently, the catalytic performance of a sample is typically influenced by the combined effects of textural and acidic properties, which are readily affected by treatments involving varying concentrations of CA [33]. It is noteworthy that at low CA concentrations, the conversion of naphthalene exhibits a positive correlation with the increase in average pore diameter and pore volume of mesoporous structures in CA-treated samples (Table 1). This phenomenon may be attributed to the relatively small micropores present in non-CA-treated β zeolite, which restricts accessibility to active sites or allows them to be easily covered or blocked by coke, resulting in lower conversion rates and yields. A larger pore size is crucial for enhancing access to active sites; this accessibility directly influences the overall reaction rate, making the pore-expansion effect highly beneficial for accelerating the mass transfer efficiency of macromolecules. However, when the concentration of CA exceeded 0.08 M, both the average pore diameter and pore volume of mesoporous structures continued to increase, while the acidity decreased sharply. Accompanied by changes in structure and properties, the conversion of naphthalene and product selectivity began to decline. These results indicate that the mass transfer effect diminishes with increasing CA concentration, suggesting that acidity plays a critical role in catalytic activity. In this context, catalytic activity decreases alongside a reduction in L acidity as well as medium-strong and strong acidic sites [34]. Previous experiments have confirmed that L/B ratio, total acid amount, and medium-strong or strong acidic sites contribute significantly to methane activation [8]. Table 2 indicates that all acidic properties decreased when Zn/Hβ was treated with CA. However, the effect of lower concentrations of CA was insufficient to significantly alter the acidic properties of the catalysts. Therefore, it is speculated that an appropriate pore structure and acidity are essential for maintaining catalyst activity and stability during the methane-assisted hydrocracking of polycyclic aromatics into BTX.
In order to further detect the effect of the CA treatment of Hβ on catalytic performance, the gas–liquid–coke yields are displayed in Figure 6. When Hβ supports were modified with lower concentrations of CA, especially for Zn/Hβ-CA-0.03M, the product distribution showed almost no change compared to the untreated catalyst. As the concentration of citric acid was increased from 0.03 M to 0.08 M, both the gas and coke yields gradually decreased, while the liquid yield slightly increased. This is attributed to increases in pore volume, specific surface area, and L/B ratio of the catalysts following CA treatment. Notably, the enhancement in specific surface areas, pore volumes, and pore diameters of the catalysts will improve the mass transfer of reactants and boost the volumetric carbon capacity of catalyst pores to a certain extent, ultimately leading to enhanced stability of the catalyst. When the concentration of CA was further increased, both the gas and coke yields began to rise while the liquid yield decreased. This phenomenon is attributed to the significant reduction in L acid amount and the increase of B acidic sites following treatment with higher concentrations of CA [35]. The former can diminish the activation of methane, and the latter can enhance cracking reactions and condensation reactions of naphthalene. At a CA treatment concentration of 0.08 M, both the gas and coke yields were at their minimum, and the liquid yield reached its peak. At this concentration, the pore volume, specific surface area, and L/B ratio of the catalyst were optimal, resulting in superior catalytic performance. These results further demonstrate that the catalytic performance of the catalyst is significantly influenced by both textural and acidic properties [7].
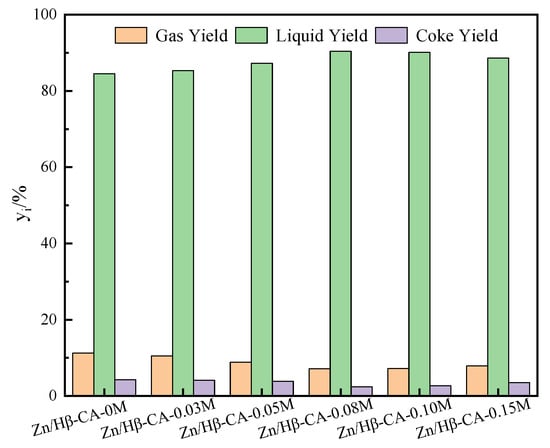
Figure 6.
The gas–liquid–coke yields of Zn/Hβ catalysts treated with different concentrations of CA during the methane-assisted hydrocracking of polycyclic aromatics.
4. Conclusions
The textural and acidic properties of Zn/Hβ catalysts could be modulated via treatments with different concentrations of CA, and their performance in the methane-assisted hydrocracking of PAHs could also be improved.
CA treatment was found to serve dual purposes: enlarging pore size and regulating acid distribution. This effectively enhanced mass transfer and diffusion capacity, as well as catalytic performance, particularly the catalytic stability of Zn/Hβ, during methane-assisted PAH hydrocracking. The concentration of CA used for treatment emerged as a critical factor influencing the structural characteristics and catalytic stability of Zn/Hβ. When the CA concentration was below 0.08 M, the acidic solution mainly enhanced the pore structure by dissolving amorphous aluminum of Hβ, with minimal impact on the acidity of Hβ. However, when the concentration exceeded 0.08 M, the acidic solution started extracting aluminum atoms from the zeolite framework, thereby compromising its crystal structure and properties, and ultimately affecting catalytic activity.
The Zn/Hβ catalyst obtained by treating Hβ with 0.08 mol/L CA exhibited excellent catalytic stability in the methane-assisted hydrocracking of PAHs. The naphthalene conversion rate reached up to 99%, and the BTX selectivity in the liquid product attained 60% over a 10 h reaction duration.
Author Contributions
Conceptualization, Z.S. and J.Z.; methodology, S.L. and L.L.; validation, L.L. and Z.S.; formal analysis, S.Z. and R.T.; investigation, Z.S.; data curation, L.L. and S.Z.; writing—original draft preparation, Z.S., L.L. and J.Z.; project administration, Z.S.; funding acquisition, Z.S. and R.T. All authors have read and agreed to the published version of the manuscript.
Funding
Natural Science Basic Research Plan in Shaanxi Province, China (Program No. 2024JC-YBMS-085), Xi’an Science and Technology Plan Project (No. 24GXFW0072), Opening Fund of the State Key Laboratory of Heavy Oil (No. SKLHOP202402008 and No. SKLHOP202403015), and CNPC Innovation Fund (No. 2022DQ02-0402).
Data Availability Statement
The authors will provide the raw data supporting the conclusions of this article upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Z.; Li, Y.; Xu, H.; Jarvis, J.; Meng, S.; Song, H. Effect of methane presence on catalytic heavy oil partial upgrading. Fuel 2021, 297, 120733. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Kinage, A.K.; Choudhary, T.V. Low-temperature nonoxidative activation of methane over H-galloaluminosilicate (MFI) zeolite. Science 1997, 275, 1286–1288. [Google Scholar] [CrossRef]
- He, P.; Song, H. Catalytic conversion of biomass by natural gas for oil quality upgrading. Ind. Eng. Chem. Res. 2014, 53, 15862–15870. [Google Scholar] [CrossRef]
- He, P.; Luan, Y.; Zhao, L.; Cheng, W.; Wu, C.; Chen, S.; Song, H. Catalytic bitumen partial upgrading over Ag-Ga/ZSM-5 under methane environment. Fuel Process. Technol. 2017, 156, 290–297. [Google Scholar] [CrossRef]
- Shen, Z.; He, P.; Wang, A.; Harrhy, J.; Meng, S.; Peng, H.; Song, H. Conversion of naphthalene as model compound of polyaromatics to mono-aromatic hydrocarbons under the mixed hydrogen and methane atmosphere. Fuel 2019, 243, 469–477. [Google Scholar] [CrossRef]
- Shen, Z.; Fu, R.; Zhang, S.; Wang, S.; Wu, Z.; Tang, R.; Liang, S.; Zhang, J.; Yuan, S.; Jiang, H. Selective Hydrogenation of Polycyclic Aromatics to Monocyclic Aromatics over NiMoC/H beta Catalysts in a Methane and Hydrogen Environment. China Pet. Process. Petrochem. Technol. 2023, 25, 92–100. [Google Scholar]
- Wei, Q.; Zhang, J.; Liu, X.; Zhang, P.; Wang, S.; Wang, Y.; Zhang, Z.; Zhang, T.; Zhou, Y. Citric acid-treated zeolite Y (CY)/Zeolite beta composites as supports for vacuum gas oil hydrocracking catalysts: High yield production of highly-aromatic heavy naphtha and low-BMCI value tail oil. Front. Chem. 2019, 7, 705. [Google Scholar] [CrossRef]
- Wang, B.; Yan, X.; Zhang, X.; Zhang, H.; Li, F. Citric acid-modified beta zeolite for polyoxymethylene dimethyl ethers synthesis: The textural and acidic properties regulation. Appl. Catal. B Environ. 2020, 266, 118645. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmet, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.V.; Toktarev, A.V.; Kazakov, M.O.; Kodenev, E.G.; Pereyma, V.Y.; Gabrienko, A.A.; Bukhtiyarov, A.V.; Echevsky, G.V. Effect of sulfosalicylic acid treatment on the properties of Beta zeolite and performance of NiW/Beta-based catalysts in hexadecane hydrocracking. Appl. Catal. A Gen. 2020, 598, 117573. [Google Scholar] [CrossRef]
- Zhu, M.; Muhammad, Y.; Hu, P.; Wang, B.; Wu, Y.; Sun, X.; Tong, Z.; Zhao, Z. Enhanced interfacial contact of dopamine bridged melamine-graphene/TiO2 nano-capsules for efficient photocatalytic degradation of gaseous formaldehyde. Appl. Catal. B Environ. 2018, 232, 182–193. [Google Scholar] [CrossRef]
- Imai, H.; Abe, M.; Terasaka, K.; Yamazaki, H.; Osuga, R.; Kondo, J.N.; Yokoi, T. Hydroconversion of methyl laurate over beta-zeolite-supported Ni–Mo catalysts: Effect of acid and base treatments of beta zeolite. Fuel Process. Technol. 2020, 197, 106182. [Google Scholar] [CrossRef]
- Halgeri, A.B.; Das, J. Recent advances in selectivation of zeolites for para-disubstituted aromatics. Catal. Today 2002, 73, 65–73. [Google Scholar] [CrossRef]
- Feng, C.; Huang, T.; Chen, H.M.; Yang, J.H.; Zhang, N.; Wang, Y.; Zhang, C.L.; Zhou, Z.W. Carbon nanotubes induced poly (vinylidene fluoride) crystallization from a miscible poly (vinylidene fluoride)/poly (methyl methacrylate) blend. Colloid Polym. Sci. 2014, 292, 3279–3290. [Google Scholar] [CrossRef]
- Bai, G.; Ma, Z.; Shi, L.; Lan, X.; Wang, Y.; Han, J.; Qiu, M.; Fu, H.; Liu, P. Continuous synthesis of bis (indolyl) phenylmethane over acid modified Hβ zeolite. Appl. Catal. A Gen. 2012, 427, 114–118. [Google Scholar] [CrossRef]
- Ateş, A.; Ozkan, I.; Canbaz, G.T. Role of modification of natural zeolite in removal of arsenic from aqueous solutions. Acta Chim. Slov. 2018, 65, 586–598. [Google Scholar] [CrossRef]
- Gac, W.; Greluk, M.; Słowik, G.; Millot, Y.; Valentin, L.; Dzwigaj, S. Effects of dealumination on the performance of Ni-containing BEA catalysts in bioethanol steam reforming. Appl. Catal. B Environ. 2018, 237, 94–109. [Google Scholar] [CrossRef]
- He, P.; Gatip, R.; Yung, M.; Zeng, H.; Song, H. Co-aromatization of olefin and methane over Ag-Ga/ZSM-5 catalyst at low temperature. Appl. Catal. B Environ. 2017, 211, 275–288. [Google Scholar] [CrossRef]
- de Lucas, A.; Ramos, M.J.; Dorado, F.; Sánchez, P.; Valverde, J.L. Influence of the Si/Al ratio in the hydroisomerization of n-octane over platinum and palladium beta zeolite-based catalysts with or without binder. Appl. Catal. A Gen. 2005, 289, 205–213. [Google Scholar] [CrossRef]
- Barzetti, T.; Selli, E.; Moscotti, D.; Forni, L. Pyridine and ammonia as probes for FTIR analysis of solid acid catalysts. J. Chem. Soc. Faraday Trans. 1996, 92, 1401–1407. [Google Scholar] [CrossRef]
- Austin, D.; Wang, A.; He, P.; Qian, H.; Zeng, H.; Song, H. Catalytic valorization of biomass derived glycerol under methane: Effect of catalyst synthesis method. Fuel 2018, 216, 218–226. [Google Scholar] [CrossRef]
- Garbarino, G.; Vijayakumar, R.P.P.; Riani, P.; Finocchio, E.; Busca, G. Ethanol and diethyl ether catalytic conversion over commercial alumina and lanthanum-doped alumina: Reaction paths, catalyst structure and coking. Appl. Catal. B Environ. 2018, 236, 490–500. [Google Scholar] [CrossRef]
- Wang, C.; Leng, S.; Guo, H.; Cao, L.; Huang, J. Acid and alkali treatments for regulation of hydrophilicity/hydrophobicity of natural zeolite. Appl. Surf. Sci. 2019, 478, 319–326. [Google Scholar] [CrossRef]
- Wang, A.; Austin, D.; Qian, H.; Zeng, H.; Song, H. Catalytic valorization of furfural under methane environment. ACS Sustain. Chem. Eng. 2018, 6, 8891–8903. [Google Scholar] [CrossRef]
- Anis, S.F.; Singaravel, G.; Hashaikeh, R. Hierarchical nano zeolite-Y hydrocracking composite fibers with highly efficient hydrocracking capability. RSC Adv. 2018, 8, 16703–16715. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Z.; Pryde, R.L.; Li, Y.; Song, H. Participation of methane in an economically and environmentally favorable catalytic asphaltene upgrading process. Chem. Commun. 2020, 56, 5492–5495. [Google Scholar] [CrossRef]
- Wang, A.; Song, H. Maximizing the production of aromatic hydrocarbons from lignin conversion by coupling methane activation. Bioresour. Technol. 2018, 268, 505–513. [Google Scholar] [CrossRef]
- Tong, Y.; Ke, M. Study on the Acidic Modification of Mesoporous HZSM-5 Zeolite and Its Catalytic Cracking Performance. Catalysts 2024, 14, 713. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yun, G.N.; Lee, Y.K. Novel Ni2P/zeolite catalysts for naphthalene hydrocracking to BTX. Catal. Commun. 2014, 45, 133–138. [Google Scholar] [CrossRef]
- Penth, B.; Hying, C.; Hoerpel, G.; Schmidt, F.G. Permeable Composite Material, Method for Producing Said Composite Material, and Use of the Same. U.S. Patent 6,841,075, 11 January 2005. [Google Scholar]
- Fan, Y.; Lin, X.; Shi, G.; Liu, H.; Bao, X. Realumination of dealuminated HZSM-5 zeolite by citric acid treatment and its application in preparing FCC gasoline hydro-upgrading catalyst. Microporous Mesoporous Mater. 2007, 98, 174–181. [Google Scholar] [CrossRef]
- Pan, H.J.; Hu, X. Biomimetic hydrogenation catalyzed by a manganese model of [Fe]-hydrogenase. Angew. Chem. Int. Ed. 2020, 59, 4942–4946. [Google Scholar] [CrossRef]
- Fang, D.; Wang, G.; Sheng, Q.; Ge, S.; Gao, C.; Gao, J. Preparation of hydrogen donor solvent for asphaltenes efficient liquid-phase conversion via heavy cycle oil selective hydrogenation. Fuel 2019, 257, 115886. [Google Scholar] [CrossRef]
- Wang, F.; Zhong, M.; Li, J.; Yalkun, T.; Jin, L.J. Preparation of mesoporous NiMo catalyst by mechanical ball milling for hydrogenation of phenanthrene. J. Fuel Chem. Technol. 2023, 51, 165–174. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).