A Global Review of Blue and Green Hydrogen Fuel Production Technologies, Trends and Future Outlook to 2050
Abstract
1. Introduction
Properties of Hydrogen
| Properties | Normal Hydrogen | Deuterium |
|---|---|---|
| Atomic number | 1 | 1 [34] |
| Atomic weight | 1.0080 | 2.0141 |
| Electron affinity | 0.7542 electron volts | 0.754 electron volts |
| Nuclear spin | ½ | 1 |
| Properties | Molecular Hydrogen | Deuterium |
|---|---|---|
| Bond distance | 0.7416 Angstrom | 0.7416 Angstrom |
| Dissociation energy (25 °C) | 104.19 kilocalories/mol | 105.97 kilocalories/mol |
| Ionisation potential | 15.427 electron volts | 15.457 electron volts |
| Density of solid | 0.08671 g/cm3 | 0.1967 g/cm3 |
| Melting point | −259.20 °C | −254.43 °C |
| Heat of fusion | 28 calories/mol | 47 calories/mol |
| Density of liquid | 0.07099 g/cm3 (−252.78 °C) | 0.1630 g/cm3 (−249.75 °C) |
| Boiling point | −252.77 °C [33] | −249.49 °C |
| Heat of vaporisation | 216 calories/mol | 293 calories/mol |
| Critical temperature | −240.0 °C | −243.8 °C |
| Critical pressure | 13.0 atmospheres | 16.4 atmospheres |
| Critical density | 0.0310 g/cm3 | 0.0668 g/cm3 |
2. Technologies of Blue and Green Hydrogen Fuel Production
2.1. Blue Hydrogen
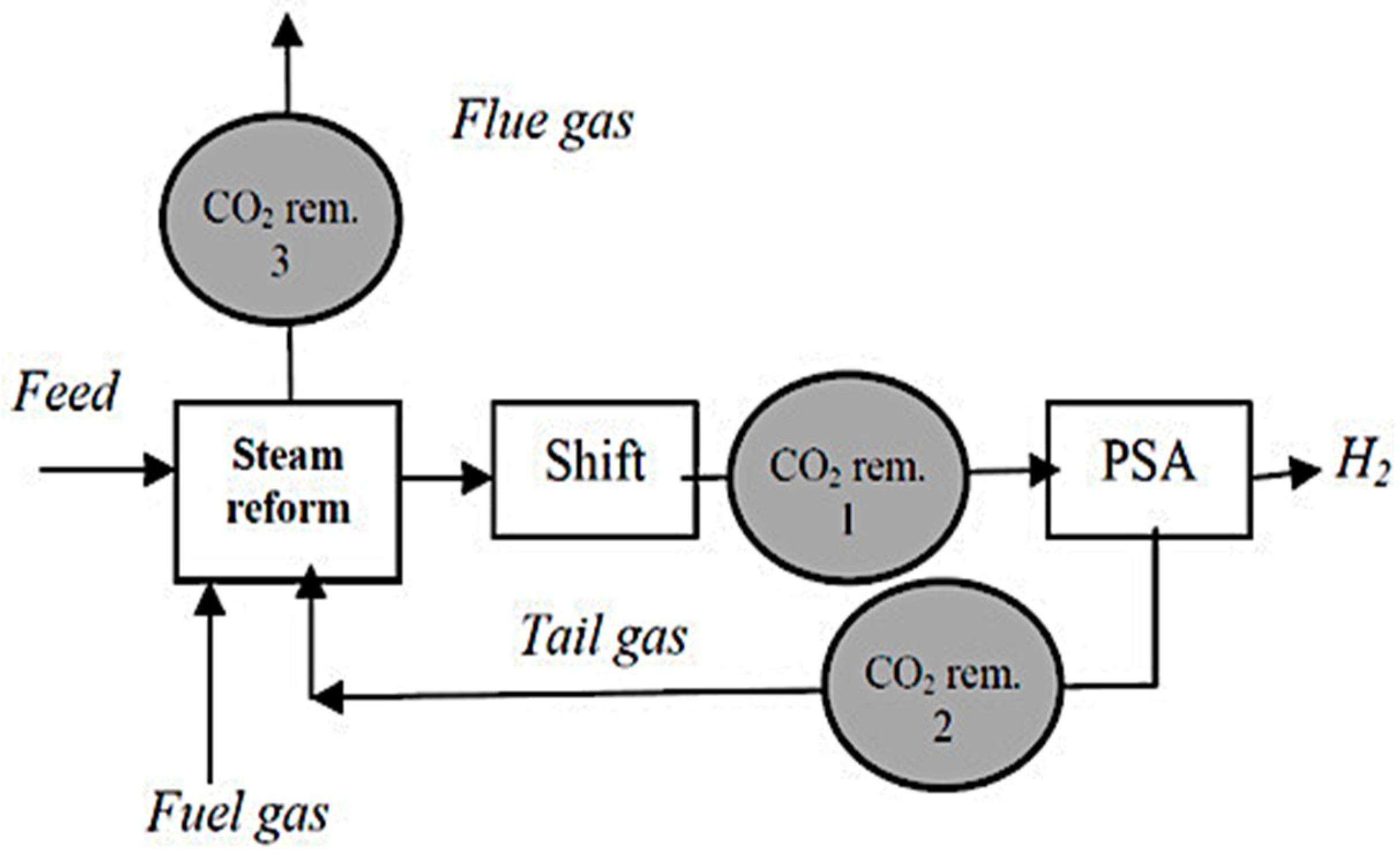
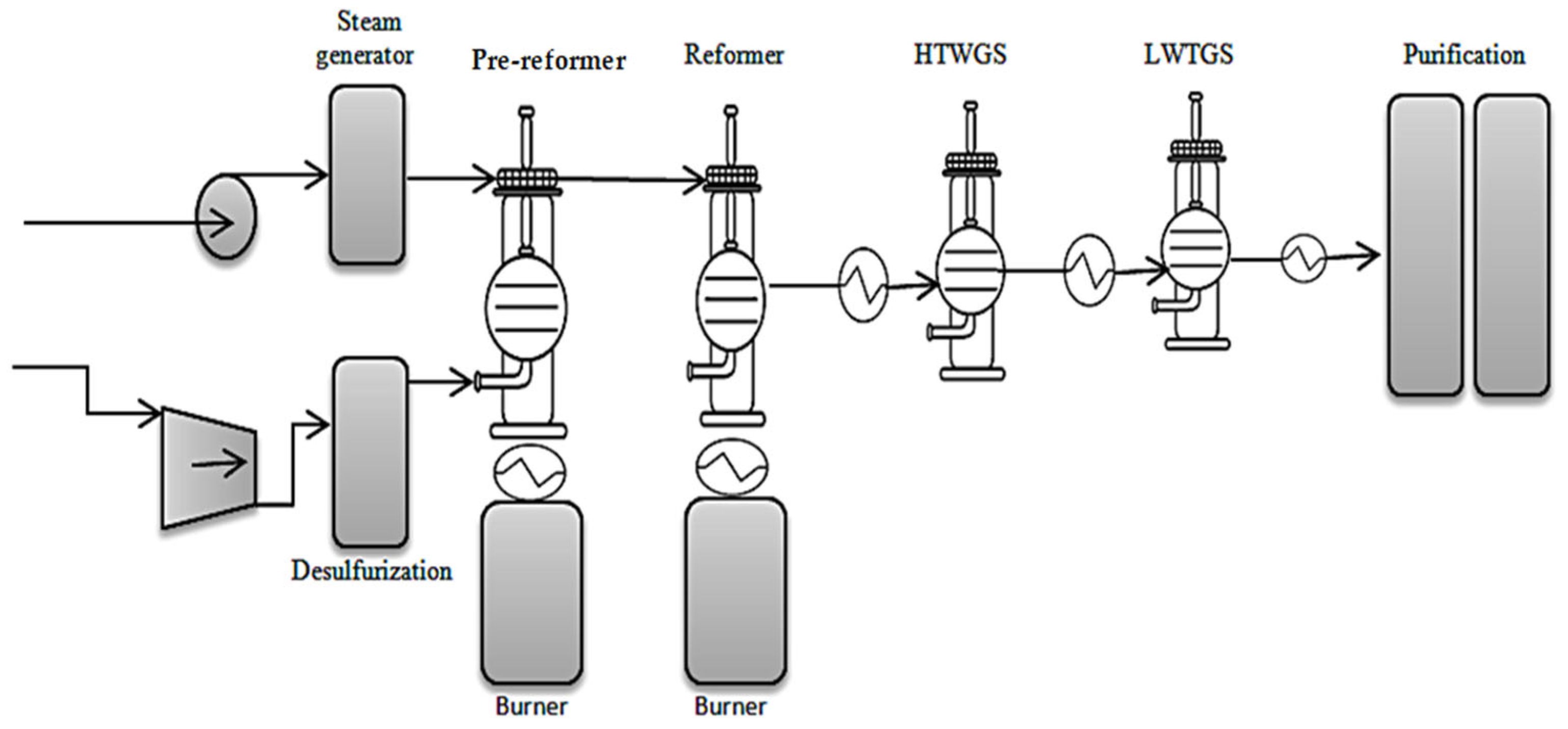
2.1.1. Production of Blue Hydrogen by Steam Reforming of Methane and Light Hydrocarbons
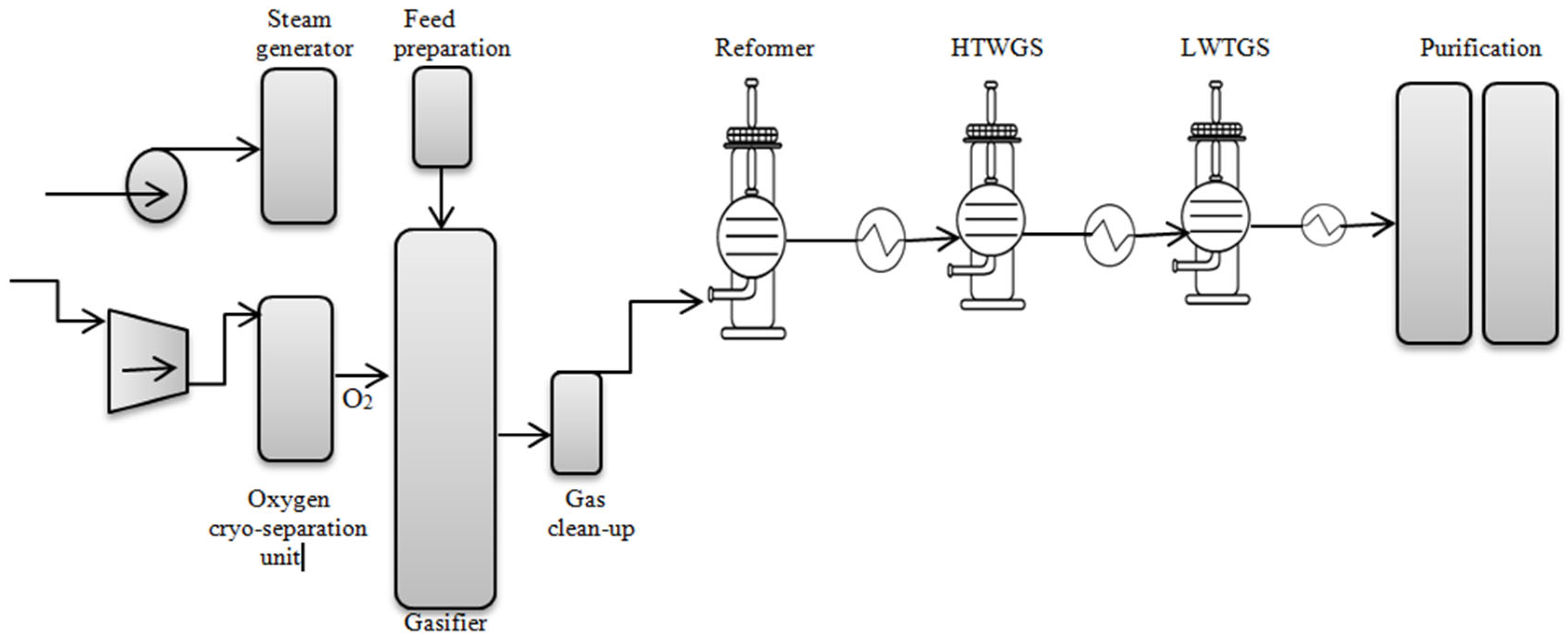
2.1.2. Blue Hydrogen Production by Gasification of Coal and Biomass
2.2. Green Hydrogen
2.2.1. Green Hydrogen Production by Water Electrolysis
2.2.2. Thermochemical Conversion Method of Producing Green Hydrogen
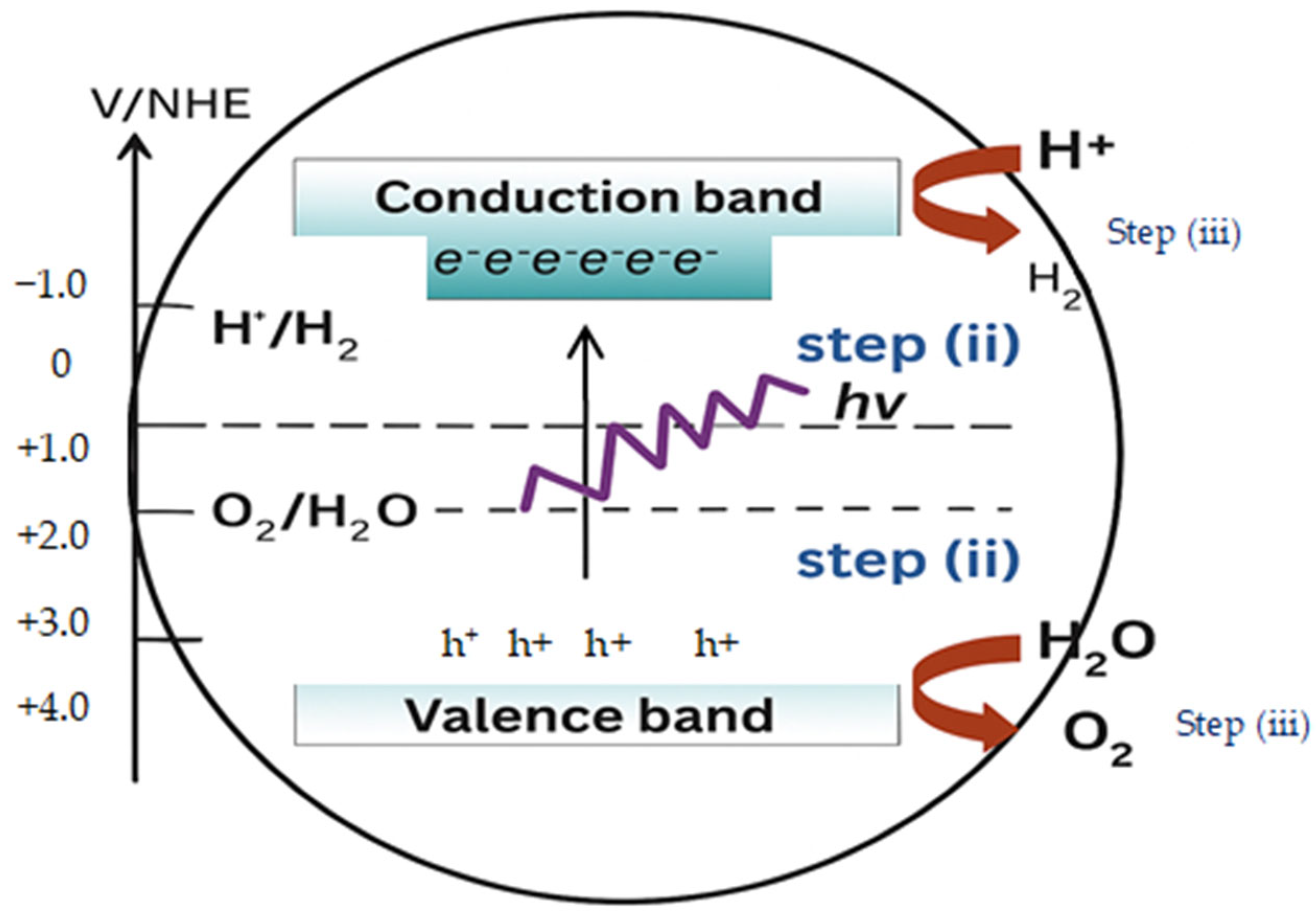
2.2.3. Photochemical Conversion Method of Green Hydrogen Production
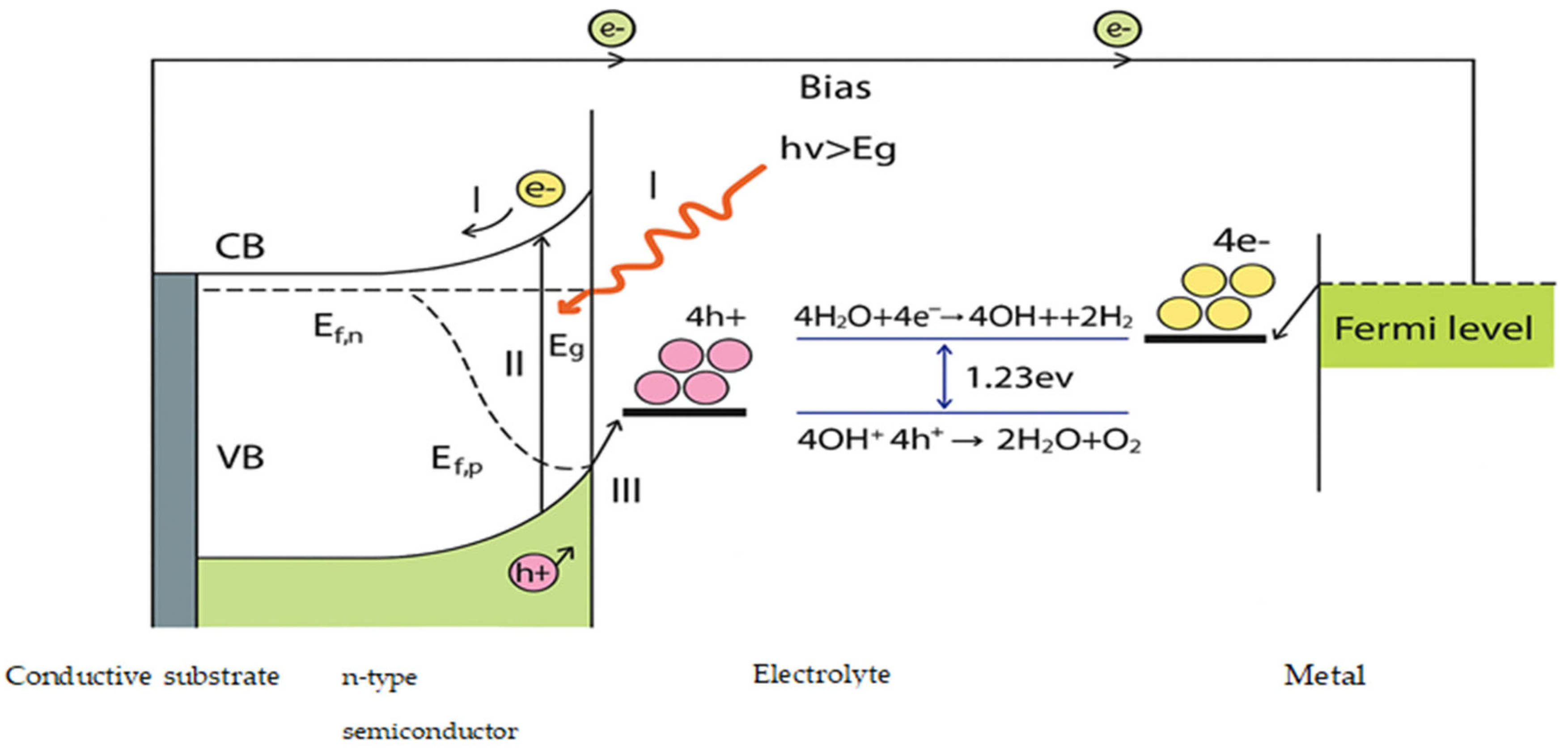
2.2.4. Photoelectrochemical Conversion
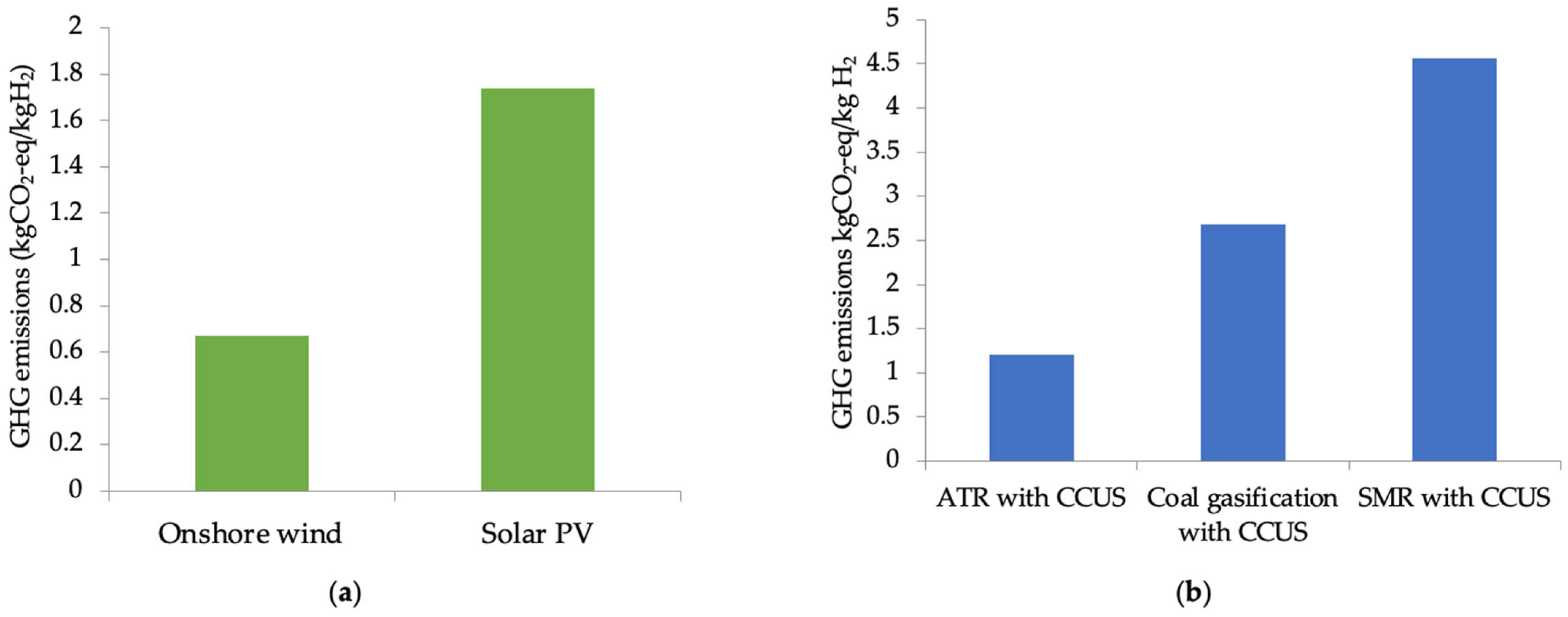
2.3. Comparison of Green and Blue Hydrogen Emissions
3. Global Production of Hydrogen
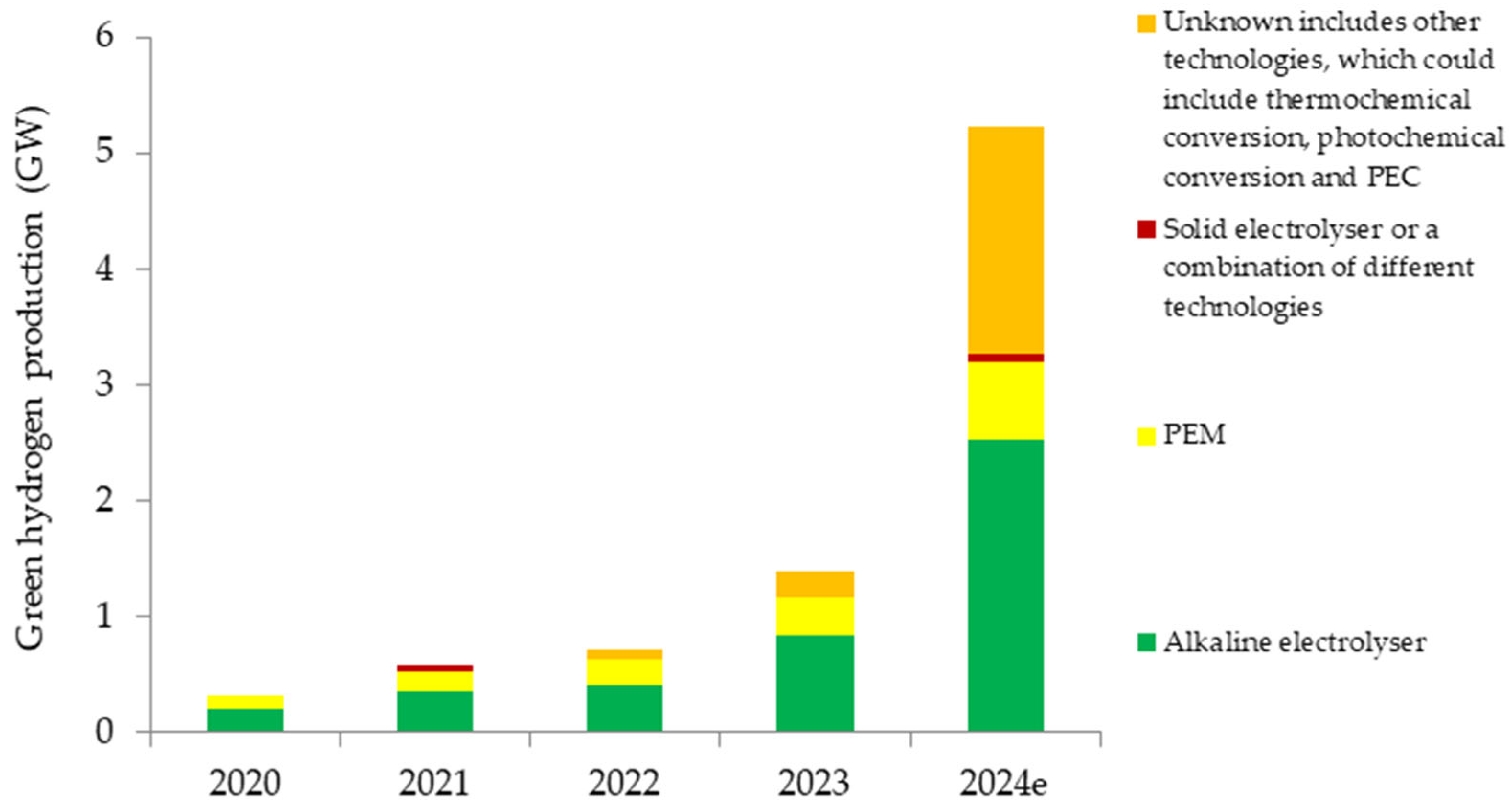
3.1. Global Production of Green and Blue Hydrogen
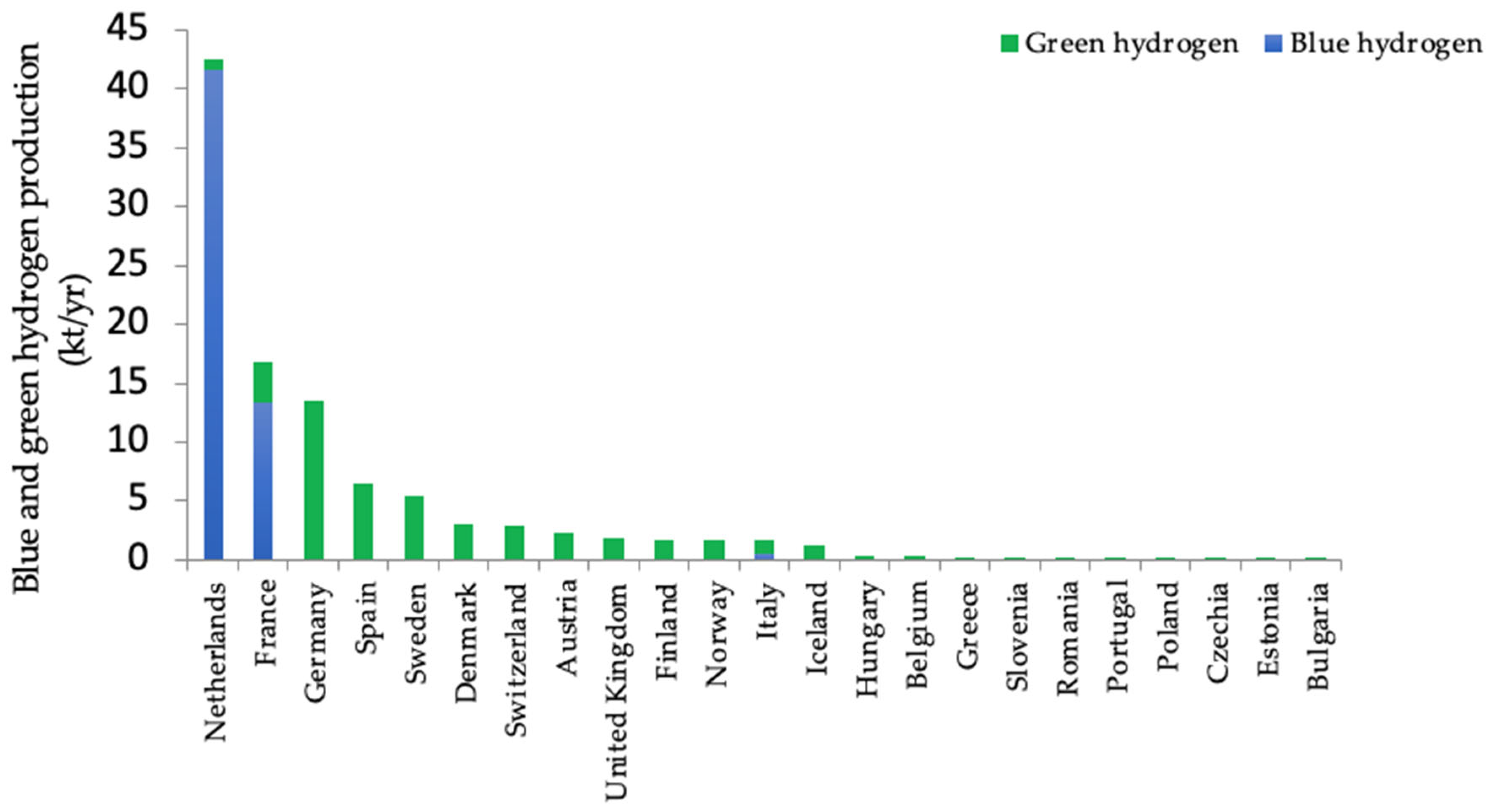
3.2. Blue and Green Hydrogen Production in Europe
4. Future Scope of Blue and Green Hydrogen Production and Demand
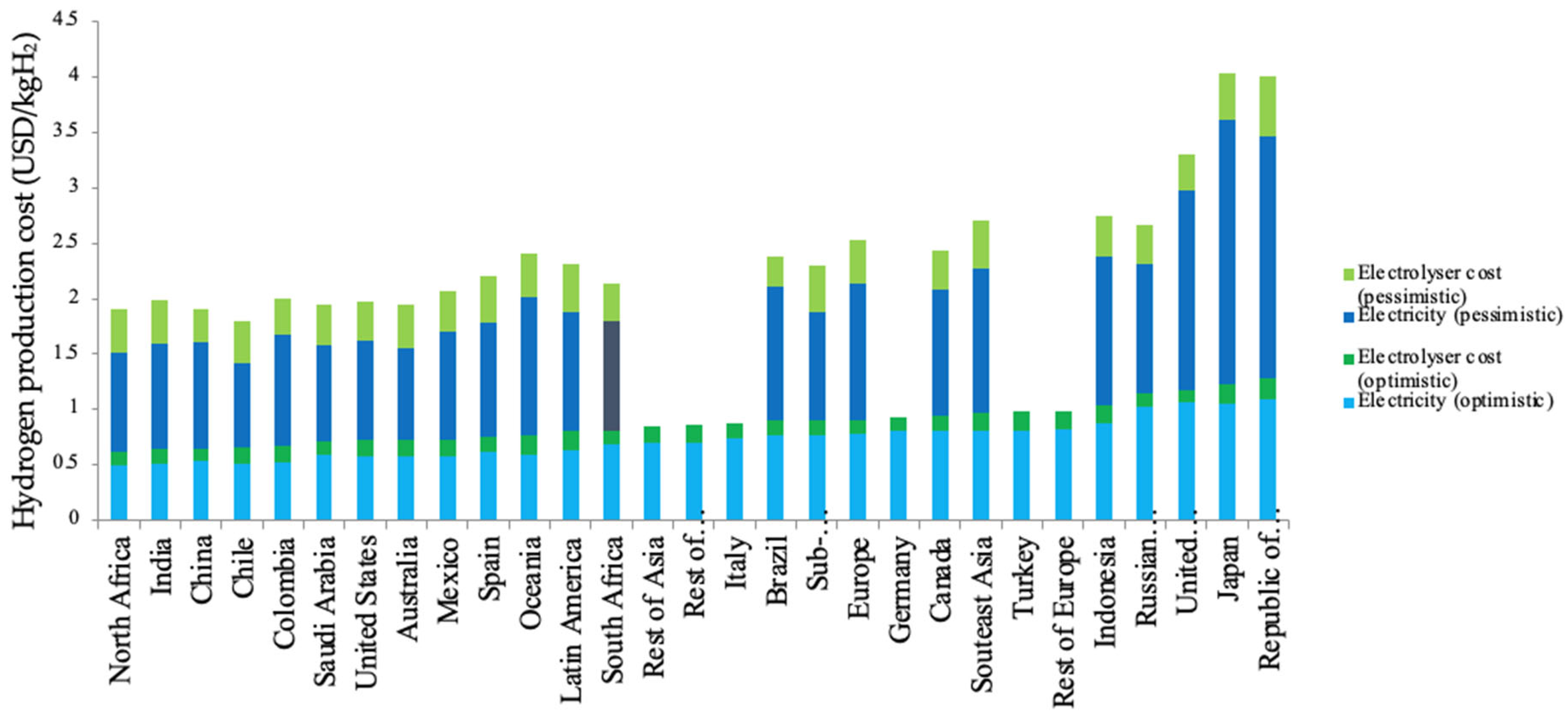
4.1. Future Scope for Blue and Green Hydrogen as a Fuel
4.2. Comparative Analysis for Both Blue and Green Hydrogen
4.3. Reduction in CO2 Emissions Using Hydrogen Energy
4.4. Long Short-Term Memory (LSTM) Prediction Model for Blue and Green Hydrogen Production Until 2050
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| import numpy as np import pandas as pd import matplotlib.pyplot as plt from sklearn.preprocessing import MinMaxScaler from tensorflow.keras.models import Sequential from tensorflow.keras.layers import LSTM, Dense # Data years = np.arange(2010, 2023) blue_h2 = [1.2, 1.3, 1.4, 1.6, 1.7, 1.9, 2.0, 2.1, 2.3, 2.5, 2.6, 2.8, 3.0] green_h2 = [0.2, 0.25, 0.3, 0.35, 0.45, 0.5, 0.55, 0.6, 0.7, 0.8, 0.9, 1.1, 1.3] data = pd.DataFrame({“Blue”: blue_h2, “Green”: green_h2}) # Normalize scaler = MinMaxScaler() scaled = scaler.fit_transform(data) # Sequences seq_len = 3 X, y = [], [] for i in range(len(scaled) - seq_len): X.append(scaled[i:i+seq_len]) y.append(scaled[i+seq_len]) X, y = np.array(X), np.array(y) # Model model = Sequential([ LSTM(50, activation=‘relu’, input_shape=(seq_len, 2)), Dense(2) ]) model.compile(optimizer=‘adam’, loss=‘mse’) model.fit(X, y, epochs=300, verbose=0) # Forecast future_years = np.arange(2023, 2051) last_seq = scaled[-seq_len:] preds = [] for _ in future_years: pred = model.predict(last_seq[np.newaxis, :, :], verbose=0)[0] preds.append(pred) last_seq = np.vstack([last_seq [1:], pred]) forecast = scaler.inverse_transform(preds) # Combine forecast_df = pd.DataFrame(forecast, columns=[“Blue”, “Green”]) forecast_df[“Year”] = future_years all_df = pd.concat([data.assign(Year=years), forecast_df], ignore_index=True) # Plot plt.figure(figsize=(10,5)) plt.plot(all_df[“Year”], all_df[“Blue”], label=“Blue H2 (LSTM)”) plt.plot(all_df[“Year”], all_df[“Green”], label=“Green H2 (LSTM)”) plt.xlabel(“Year”); plt.ylabel(“Million Tonnes”); plt.title(“LSTM Forecast of Hydrogen”) plt.legend(); plt.grid(); plt.tight_layout() plt.show() # Save if needed all_df.to_excel(“Hydrogen_Production_LSTM_Forecast_2023_2050.xlsx”, index=False) |
References
- Zhang, J. Energy Access Challenge and the Role of Fossil Fuels in Meeting Electricity Demand: Promoting Renewable Energy Capacity for Sustainable Development. Geosci. Front. 2024, 15, 101873. [Google Scholar] [CrossRef]
- Kabir, M.; Habiba, U.E.; Khan, W.; Shah, A.; Rahim, S.; De los Rios-Escalante, P.R.; Farooqi, Z.U.R.; Ali, L. Climate Change Due to Increasing Concentration of Carbon Dioxide and Its Impacts on Environment in 21st Century; a Mini Review. J. King Saud Univ. Sci. 2023, 35, 102693. [Google Scholar] [CrossRef]
- Kabir, M.; Habiba, U.; Iqbal, M.Z.; Shafiq, M.; Farooqi, Z.R.; Shah, A.; Khan, W. Impacts of Anthropogenic Activities & Climate Change Resulting from Increasing Concentration of Carbon Dioxide on Environment in 21st Century; A Critical Review. IOP Conf. Ser. Earth Environ. Sci. 2023, 1194, 012010. [Google Scholar] [CrossRef]
- Höhne, N.; Gidden, M.J.; den Elzen, M.; Hans, F.; Fyson, C.; Geiges, A.; Jeffery, M.L.; Gonzales-Zuñiga, S.; Mooldijk, S.; Hare, W.; et al. Wave of Net Zero Emission Targets Opens Window to Meeting the Paris Agreement. Nat. Clim. Change 2021, 11, 820–822. [Google Scholar] [CrossRef]
- Muchiri, M.K.; Erdei-Gally, S.; Fekete-Farkas, M.; Lakner, Z. Bibliometric Analysis of Green Finance and Climate Change in Post-Paris Agreement Era. J. Risk Financ. Manag. 2022, 15, 561. [Google Scholar] [CrossRef]
- King, L.C.; van den Bergh, J.C.J.M. Potential Carbon Leakage under the Paris Agreement. Clim. Change 2021, 165, 52. [Google Scholar] [CrossRef]
- Hassan, Q.; Algburi, S.; Sameen, A.Z.; Jaszczur, M.; Salman, H.M. Hydrogen as an Energy Carrier: Properties, Storage Methods, Challenges, and Future Implications. Environ. Syst. Decis. 2024, 44, 327–350. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, C.; Bai, F.; Wang, W.; An, S.; Zhao, K.; Li, Z.; Li, J.; Sun, H. A Comprehensive Review of the Promising Clean Energy Carrier: Hydrogen Production, Transportation, Storage, and Utilisation (HPTSU) Technologies. Fuel 2024, 355, 129455. [Google Scholar] [CrossRef]
- Geopolitics of the Energy Transformation: The Hydrogen Factor; The International Renewable Energy Agency (IRENA): Abu Dhabi, United Arab Emirates, 2022; p. 117.
- Yu, M.; Wang, K.; Vredenburg, H. Insights into Low-Carbon Hydrogen Production Methods: Green, Blue and Aqua Hydrogen. Int. J. Hydrogen Energy 2021, 46, 21261–21273. [Google Scholar] [CrossRef]
- Rosenow, J.; Lowes, R. Will Blue Hydrogen Lock Us into Fossil Fuels Forever? One Earth 2021, 4, 1527–1529. [Google Scholar] [CrossRef]
- Cloete, S.; Arnaiz del Pozo, C.; Jiménez Álvaro, Á. System-Friendly Process Design: Optimizing Blue Hydrogen Production for Future Energy Systems. Energy 2022, 259, 124954. [Google Scholar] [CrossRef]
- Hassan, Q.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. Large-Scale Green Hydrogen Production via Alkaline Water Electrolysis Using Solar and Wind Energy. Int. J. Hydrogen Energy 2023, 48, 34299–34315. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A.; Rajendran, S.; Khusnun, N.F.; Bahari, M.B.; Johari, A.; Kamaruddin, M.J.; Ismail, M. Recent Review and Evaluation of Green Hydrogen Production via Water Electrolysis for a Sustainable and Clean Energy Society. Int. J. Hydrogen Energy 2024, 52, 420–441. [Google Scholar] [CrossRef]
- Shin, H.; Jang, D.; Lee, S.; Cho, H.S.; Kim, K.H.; Kang, S. Techno-Economic Evaluation of Green Hydrogen Production with Low-Temperature Water Electrolysis Technologies Directly Coupled with Renewable Power Sources. Energy Convers. Manag. 2023, 286, 117083. [Google Scholar] [CrossRef]
- Arcos, J.M.M.; Santos, D.M.F. The Hydrogen Color Spectrum: Techno-Economic Analysis of the Available Technologies for Hydrogen Production. Gases 2023, 3, 25–46. [Google Scholar] [CrossRef]
- Hordeski, M.F. Alternative Fuels: The Future of Hydrogen, 3rd ed.; Taylor & Francis Group: Oxford, UK, 2020. [Google Scholar] [CrossRef]
- Zohuri, B. Hydrogen Energy: Challenges and Solutions for a Cleaner Future; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 1–283. [Google Scholar] [CrossRef]
- Hoffmann, P. The Forever Fuel: The Story of Hydrogen; Westview Press: Boulder, CO, USA, 2019; pp. 1–271. [Google Scholar] [CrossRef]
- Nouni, M.R.; Manna, J. Green Hydrogen: An Introduction. In Challenges and Opportunities in Green Hydrogen Production. Energy, Environment, and Sustainability; Part F2879; Springer: Singapore, 2024; pp. 9–38. [Google Scholar] [CrossRef]
- Bin Navaid, H.; Emadi, H.; Watson, M. A Comprehensive Literature Review on the Challenges Associated with Underground Hydrogen Storage. Int. J. Hydrogen Energy 2023, 48, 10603–10635. [Google Scholar] [CrossRef]
- Ince, M.; Ince, O.K. Hydrocarbon Pollution and Its Effect on the Environment; IntechOpen: London, UK, 2019. [Google Scholar]
- Panigrahi, P.K.; Chandu, B.; Motapothula, M.R.; Puvvada, N. Potential Benefits, Challenges and Perspectives of Various Methods and Materials Used for Hydrogen Storage. Energy Fuels 2024, 38, 2630–2653. [Google Scholar] [CrossRef]
- Lin, Z.J.; Mahammed, S.A.R.; Liu, T.F.; Cao, R. Multifunctional Porous Hydrogen-Bonded Organic Frameworks: Current Status and Future Perspectives. ACS Cent. Sci. 2022, 8, 1589–1608. [Google Scholar] [CrossRef] [PubMed]
- Peng, S. Storage and Application of Hydrogen Energy. In Electrochemical Hydrogen Production from Water Splitting; Springer: Singapore, 2023; pp. 203–235. [Google Scholar] [CrossRef]
- Climate Change: Science and Solutions Hydrogen and Ammonia 1 Climate Change: Science and Solutions|Briefing 4. Available online: https://pdf4pro.com/view/climate-change-science-and-solutions-briefing-4-663daa.html (accessed on 5 November 2025).
- Orlova, S.; Mezeckis, N.; Vasudev, V.P.K. Compression of Hydrogen Gas for Energy Storage: A Review. Latv. J. Phys. Tech. Sci. 2023, 60, 4–16. [Google Scholar] [CrossRef]
- Sakoda, N.; Shindo, K.; Shinzato, K.; Kohno, M.; Takata, Y.; Fujii, M. Review of the Thermodynamic Properties of Hydrogen Based on Existing Equations of State. Int. J. Thermophys. 2010, 31, 276–296. [Google Scholar] [CrossRef]
- Hoschke, J.; Chowdhury, M.F.W.; Venezuela, J.; Atrens, A. A Review of Hydrogen Embrittlement in Gas Transmission Pipeline Steels. Corros. Rev. 2023, 41, 277–317. [Google Scholar] [CrossRef]
- Li, H.; Cao, X.; Liu, Y.; Shao, Y.; Nan, Z.; Teng, L.; Peng, W.; Bian, J. Safety of Hydrogen Storage and Transportation: An Overview on Mechanisms, Techniques, and Challenges. Energy Rep. 2022, 8, 6258–6269. [Google Scholar] [CrossRef]
- Hydrogen Pipelines|Department of Energy. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-pipelines (accessed on 19 August 2025).
- Ausfelder, F.; Beilmann, C.; Bertau, M.; Bräuninger, S.; Heinzel, A.; Hoer, R.; Koch, W.; Mahlendorf, F.; Metzelthin, A.; Peuckert, M.; et al. Energy Storage as Part of a Secure Energy Supply. ChemBioEng Rev. 2017, 4, 144–210. [Google Scholar] [CrossRef]
- Chatterjee, S.; Parsapur, R.K.; Huang, K.W. Limitations of Ammonia as a Hydrogen Energy Carrier for the Transportation Sector. ACS Energy Lett. 2021, 6, 4390–4394. [Google Scholar] [CrossRef]
- Di Martino, R.M.C.; Maxwell, B.D.; Pirali, T. Deuterium in Drug Discovery: Progress, Opportunities and Challenges. Nat. Rev. Drug Discov. 2023, 22, 562–584. [Google Scholar] [CrossRef] [PubMed]
- Incer-Valverde, J.; Korayem, A.; Tsatsaronis, G.; Morosuk, T. “Colors” of Hydrogen: Definitions and Carbon Intensity. Energy Convers. Manag. 2023, 291, 117294. [Google Scholar] [CrossRef]
- El-Adawy, M.; Dalha, I.B.; Ismael, M.A.; Al-Absi, Z.A.; Nemitallah, M.A. Review of Sustainable Hydrogen Energy Processes: Production, Storage, Transportation, and Color-Coded Classifications. Energy Fuels 2024, 38, 22686–22718. [Google Scholar] [CrossRef]
- Franco, A.; Giovannini, C. Recent and Future Advances in Water Electrolysis for Green Hydrogen Generation: Critical Analysis and Perspectives. Sustainability 2023, 15, 16917. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rasul, M.G.; Jahirul, M.I.; Sattar, M.A.; Hassan, N.M.S. Hydrogen Horizons: Navigating Challenges and Opportunities for a Sustainable Energy Future. In Proceedings of the 2023 International Conference on Sustainable Technology and Engineering (i-COSTE), Nadi, Fiji, 4–6 December 2023. [Google Scholar] [CrossRef]
- Lubbe, F.; Rongé, J.; Bosserez, T.; Martens, J.A. Golden Hydrogen. Curr. Opin. Green Sustain. Chem. 2023, 39, 100732. [Google Scholar] [CrossRef]
- Ingale, G.U.; Kwon, H.M.; Jeong, S.; Park, D.; Kim, W.; Bang, B.; Lim, Y., II; Kim, S.W.; Kang, Y.B.; Mun, J.; et al. Assessment of Greenhouse Gas Emissions from Hydrogen Production Processes: Turquoise Hydrogen vs. Steam Methane Reforming. Energies 2022, 15, 8679. [Google Scholar] [CrossRef]
- Boretti, D.A. Advances in Sustainable Turquoise Hydrogen Production via Methane Pyrolysis in Molten Metals. Clean. Chem. Eng. 2025, 11, 100139. [Google Scholar] [CrossRef]
- The Hydrogen Colour Spectrum|National Grid. Available online: https://www.nationalgrid.com/stories/energy-explained/hydrogen-colour-spectrum (accessed on 5 November 2025).
- Joseph, V.; Oghenegare, E. Article No.JENRR.99222 Review Article Joseph and Oghenegare. J. Energy Res. Rev. 2023, 13, 64–79. [Google Scholar] [CrossRef]
- AlHumaidan, F.S.; Absi Halabi, M.; Rana, M.S.; Vinoba, M. Blue Hydrogen: Current Status and Future Technologies. Energy Convers. Manag. 2023, 283, 116840. [Google Scholar] [CrossRef]
- Massarweh, O.; Al-khuzaei, M.; Al-Shafi, M.; Bicer, Y.; Abushaikha, A.S. Blue Hydrogen Production from Natural Gas Reservoirs: A Review of Application and Feasibility. J. CO2 Util. 2023, 70, 102438. [Google Scholar] [CrossRef]
- Sovacool, B.K.; Del Rio, D.F.; Herman, K.; Iskandarova, M.; Uratani, J.M.; Griffiths, S. Reconfiguring European Industry for Net-Zero: A Qualitative Review of Hydrogen and Carbon Capture Utilisation and Storage Benefits and Implementation Challenges. Energy Environ. Sci. 2024, 17, 3523–3569. [Google Scholar] [CrossRef]
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The Role of Green and Blue Hydrogen in the Energy Transition—A Technological and Geopolitical Perspective. Sustainability 2020, 13, 298. [Google Scholar] [CrossRef]
- Página No Encontrada—CERTIFHY. Available online: https://www.certifhy.eu/images/media/files/CertifHy_2_deliverables/CertifHy_H2-criteria-definition_V1-1_2019-03-13_clean_endorsed.pdf (accessed on 20 August 2025).
- Simoes, S.G.; Catarino, J.; Picado, A.; Lopes, T.F.; di Berardino, S.; Amorim, F.; Gírio, F.; Rangel, C.M.; Ponce de Leão, T. Water Availability and Water Usage Solutions for Electrolysis in Hydrogen Production. J. Clean. Prod. 2021, 315, 128124. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, A. Life Cycle Assessment of Hydrogen Production from Underground Coal Gasification. Appl. Energy 2015, 147, 556–568. [Google Scholar] [CrossRef]
- Al-Qahtani, A.; Parkinson, B.; Hellgardt, K.; Shah, N.; Guillen-Gosalbez, G. Uncovering the True Cost of Hydrogen Production Routes Using Life Cycle Monetisation. Appl. Energy 2021, 281, 115958. [Google Scholar] [CrossRef]
- Rafique, N.; Khalil, F.K.; Ullah, I.U.I.; Abbas, M.T.; Safdar, M.; Aziz, U. Hydrogen fuel cell technology: A sustainable future for mechanical power systems. Spectr. Eng. Sci. 2025, 3, 214–224. [Google Scholar] [CrossRef]
- Wismann, S.T. Electrically Heated Steam Methane Reforming; Technical University of Denmark: Lyngby, Denmark, 2019. [Google Scholar]
- Wang, N.; Otor, H.O.; Rivera-Castro, G.; Hicks, J.C. Plasma Catalysis for Hydrogen Production: A Bright Future for Decarbonisation. ACS Catal. 2024, 14, 6749–6798. [Google Scholar] [CrossRef]
- Yusuf, B.O.; Umar, M.; Kotob, E.; Abdulhakam, A.; Taialla, O.A.; Awad, M.M.; Hussain, I.; Alhooshani, K.R.; Ganiyu, S.A. Recent Advances in Bimetallic Catalysts for Methane Steam Reforming in Hydrogen Production: Current Trends, Challenges, and Future Prospects. Chem. An Asian J. 2024, 19, e202300641. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.M.; Guil, R.; Fierro, J.L.G. Introduction to Hydrogen Production. In Compendium of Hydrogen Energy; Woodhead Publishing: London, UK, 2015; pp. 21–61. [Google Scholar] [CrossRef]
- Supported Metals in Catalysis (2nd Edition)—Google Books. Available online: https://books.google.co.uk/books?hl=en&lr=&id=Bk67CgAAQBAJ&oi=fnd&pg=PA301&dq=A+typical+nickel+(Ni)+catalyst+speeds+the+turnover+frequency+of+approximately+0.5s-1+at+723+K+under+industrial+conditions,+leading+to+CH4+conversion+of+about+10%25&ots=NYtVVjPi6b&sig=x0Xnv4UEm0BRlIHca7pi0RIjmpk&redir_esc=y#v=onepage&q&f=false (accessed on 20 August 2025).
- Pashchenko, D. Intra-Particle Diffusion Limitation for Steam Methane Reforming over a Ni-Based Catalyst. Fuel 2023, 353, 129205. [Google Scholar] [CrossRef]
- Deng, M.; Liu, J.; Zhang, X.; Li, J.; Fu, L. Energy and Parameter Analysis of SOFC System for Hydrogen Production from Methane Steam Reforming. J. Therm. Sci. 2022, 31, 2088–2110. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, B.; Gao, Y.; Yu, K.; Lv, Z.; Si-ma, W.; Yuan, D.; Tang, D. A Compact and High-Efficiency Electrified Reactor for Hydrogen Production by Methane Steam Reforming. Int. J. Hydrogen Energy 2022, 47, 41421–41431. [Google Scholar] [CrossRef]
- Didenko, L.P.; Babak, V.N.; Sementsova, L.A.; Dorofeeva, T.V.; Chizhov, P.E.; Gorbunov, S.V. Steam Conversion of Ethane and Methane–Ethane Mixtures in a Membrane Reactor with a Foil Made of a Pd–Ru Alloy. Membr. Membr. Technol. 2023, 5, 69–79. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, R.; Sharma, J.P.; Ahmadi, M.H.; Najser, J.; Blazek, V.; Prokop, L. The Role of Catalyst in Hydrogen Production: A Critical Review. J. Therm. Anal. Calorim. 2024, 149, 14517–14534. [Google Scholar] [CrossRef]
- Fowles, M.; Carlsson, M. Steam Reforming of Hydrocarbons for Synthesis Gas Production. Top. Catal. 2021, 64, 856–875. [Google Scholar] [CrossRef]
- Jeong, D.W.; Subramanian, V.; Shim, J.O.; Jang, W.J.; Seo, Y.C.; Roh, H.S.; Gu, J.H.; Lim, Y.T. High-Temperature Water Gas Shift Reaction over Fe/Al/Cu Oxide Based Catalysts Using Simulated Waste-Derived Synthesis Gas. Catal. Lett. 2013, 143, 438–444. [Google Scholar] [CrossRef]
- Chakrabarti, D.; De Klerk, A.; Prasad, V.; Gnanamani, M.K.; Shafer, W.D.; Jacobs, G.; Sparks, D.E.; Davis, B.H. Conversion of CO2over a Co-Based Fischer-Tropsch Catalyst. Ind. Eng. Chem. Res. 2015, 54, 1189–1196. [Google Scholar] [CrossRef]
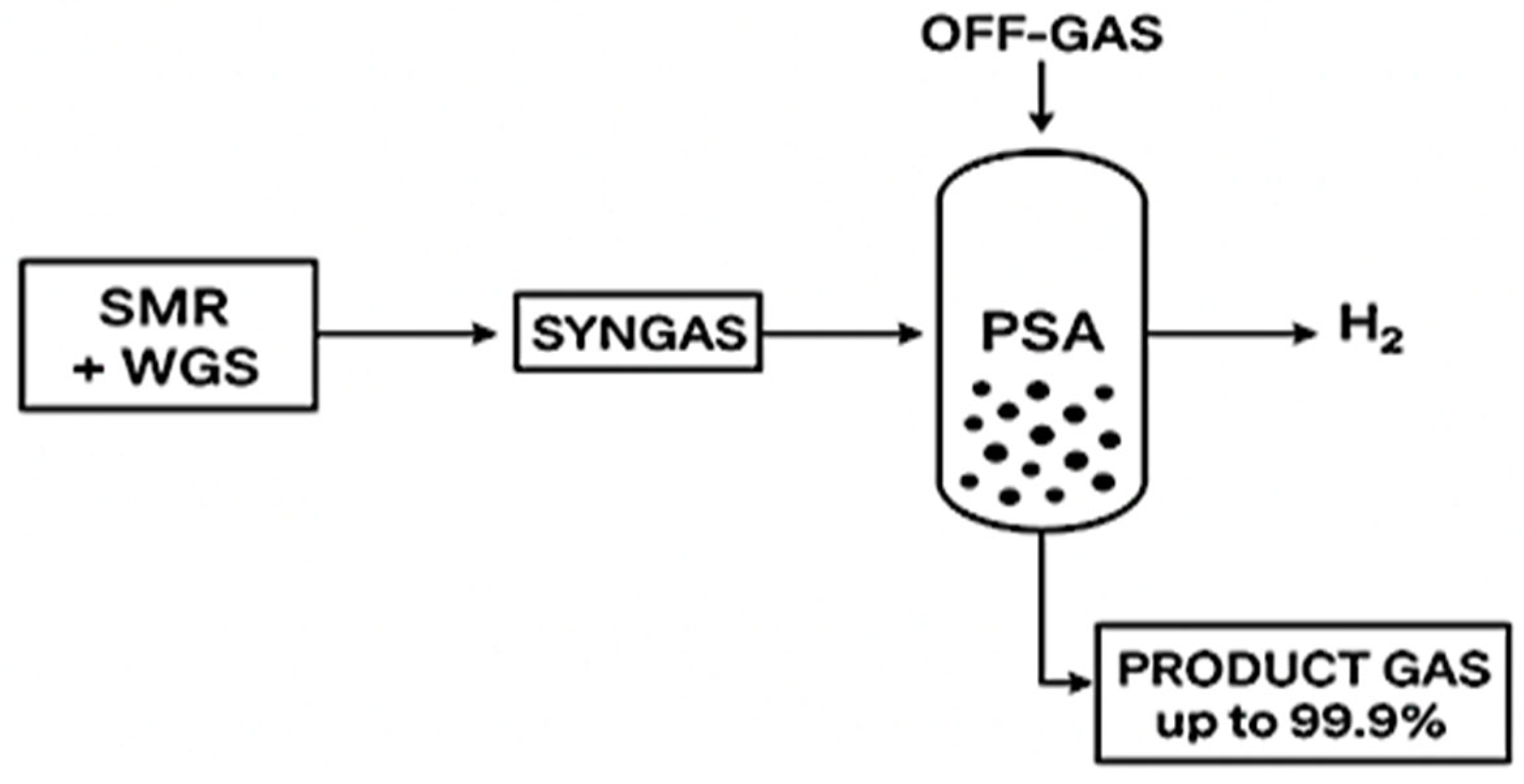
- Krótki, A.; Bigda, J.; Spietz, T.; Ignasiak, K.; Matusiak, P.; Kowol, D. Performance Evaluation of Pressure Swing Adsorption for Hydrogen Separation from Syngas and Water–Gas Shift Syngas. Energies 2025, 18, 1887. [Google Scholar] [CrossRef]
- Kim, Y.; Yang, H. Hydrogen Purity: Influence of Production Methods, Purification Techniques, and Analytical Approaches. Energies 2025, 18, 741. [Google Scholar] [CrossRef]
- Mohd Yunus, S.; Yusup, S.; Johari, S.S.; Mohd Afandi, N.; Manap, A.; Mohamed, H. Comparative Hydrogen Production Routes via Steam Methane Reforming and Chemical Looping Reforming of Natural Gas as Feedstock. Hydrogen 2024, 5, 761–775. [Google Scholar] [CrossRef]
- Pangestu, M.R.G.; Malaibari, Z.; Muhammad, A.; Al-Rowaili, F.N.; Zahid, U. Comprehensive Review on Methane Pyrolysis for Sustainable Hydrogen Production. Energy Fuels 2024, 38, 13514–13538. [Google Scholar] [CrossRef]
- Oh, T.; Lee, S. Optimisation of Steam-Methane Reforming Process Using PSA off Gas. Int. J. Hydrogen Energy 2024, 95, 902–915. [Google Scholar] [CrossRef]
- Bauer, C.; Treyer, K.; Antonini, C.; Bergerson, J.; Gazzani, M.; Gencer, E.; Gibbins, J.; Mazzotti, M.; McCoy, S.T.; McKenna, R.; et al. On the Climate Impacts of Blue Hydrogen Production. Sustain. Energy Fuels 2021, 6, 66–75. [Google Scholar] [CrossRef]
- Davids, D.; Grant, N.; Mittal, S.; Hawkes, A.; Oluleye, G. Impact of Methane Leakage Rate and Carbon Capture Rate on Blue Hydrogen Sustainability Using Combined Warming Index. Appl. Energy 2025, 394, 125888. [Google Scholar] [CrossRef]
- Wu, W.; Zhai, H.; Holubnyak, E. Technological Evolution of Large-Scale Blue Hydrogen Production toward the U.S. Hydrogen Energy Earthshot. Nat. Commun. 2024, 15, 5684. [Google Scholar] [CrossRef]
- Novotny, V. Blue Hydrogen Can Be a Source of Green Energy in the Period of Decarbonisation. Int. J. Hydrogen Energy 2023, 48, 7202–7218. [Google Scholar] [CrossRef]
- Shirizadeh, B.; Villavicencio, M.; Douguet, S.; Trüby, J.; Bou Issa, C.; Seck, G.S.; D’herbemont, V.; Hache, E.; Malbec, L.M.; Sabathier, J.; et al. The Impact of Methane Leakage on the Role of Natural Gas in the European Energy Transition. Nat. Commun. 2023, 14, 5756. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.A.; Barchyn, T.E.; Risk, D.; Ravikumar, A.P.; Hugenholtz, C.H. A Review of Close-Range and Screening Technologies for Mitigating Fugitive Methane Emissions in Upstream Oil and Gas. Environ. Res. Lett. 2019, 14, 053002. [Google Scholar] [CrossRef]
- Financing Solutions to Reduce Natural Gas Flaring and Methane Emissions—Gianni Lorenzato, Silvana Tordo, Berend van Den Berg, Huw Martyn Howells—Google Books. Available online: https://books.google.co.uk/books?hl=en&lr=&id=TU9uEAAAQBAJ&oi=fnd&pg=PP1&dq=replacing+outdated+equipment+such+as+high-bleed+pneumatic+controllers,+improving+pipeline+integrity+and+implementing+best+practices+in+natural+gas+production+sites+can+minimize+methane+leakage&ots=rixXngyumO&sig=f9Y38Ba3iiemeEM2Wr6TEKe3MJg&redir_esc=y#v=onepage&q&f=false (accessed on 27 August 2025).
- Ruokonen, J.; Nieminen, H.; Dahiru, A.R.; Laari, A.; Koiranen, T.; Laaksonen, P.; Vuokila, A.; Huuhtanen, M. Modelling and Cost Estimation for Conversion of Green Methanol to Renewable Liquid Transport Fuels via Olefin Oligomerisation. Processes 2021, 9, 1046. [Google Scholar] [CrossRef]
- Olczak, M.; Piebalgs, A.; Balcombe, P. A Global Review of Methane Policies Reveals That Only 13% of Emissions Are Covered with Unclear Effectiveness. One Earth 2023, 6, 519–535. [Google Scholar] [CrossRef]
- Pettersen, J.; Steeneveldt, R.; Grainger, D.; Scott, T.; Holst, L.M.; Hamborg, E.S. Blue Hydrogen Must Be Done Properly. Energy Sci. Eng. 2022, 10, 3220–3236. [Google Scholar] [CrossRef]
- Sharma, G.; Dewangan, A.K.; Yadav, A.K.; Ahmad, A. Feasibility of Waste-to-Hydrogen Generation System Based on Gasification/Pyrolysis: A Comprehensive Review of Experimental Studies. J. Therm. Anal. Calorim. 2024, 149, 13629–13651. [Google Scholar] [CrossRef]
- Wang, B.; Li, W.; Ma, C.; Yang, W.; Pudasainee, D.; Gupta, R.; Sun, L. Synergistic Effect on the Co-Gasification of Petroleum Coke and Carbon-Based Feedstocks: A State-of-the-Art Review. J. Energy Inst. 2022, 102, 1–13. [Google Scholar] [CrossRef]
- Rauch, R.; Kiros, Y.; Engvall, K.; Kantarelis, E.; Brito, P.; Nobre, C.; Santos, S.M.; Graefe, P.A. Hydrogen from Waste Gasification. Hydrogen 2024, 5, 70–101. [Google Scholar] [CrossRef]
- Singh, A.; Shivapuji, A.M.; Dasappa, S. Hydrogen Production through Agro-Residue Gasification and Adsorptive Separation. Appl. Therm. Eng. 2023, 234, 121247. [Google Scholar] [CrossRef]
- Dada, T.K.; Sheehan, M.; Murugavelh, S.; Antunes, E. A Review on Catalytic Pyrolysis for High-Quality Bio-Oil Production from Biomass. Biomass Convers. Biorefinery 2021, 13, 2595–2614. [Google Scholar] [CrossRef]
- Brar, J.S.; Singh, K.; Wang, J.; Kumar, S. Cogasification of Coal and Biomass: A Review. Int. J. For. Res. 2012, 2012, 363058. [Google Scholar] [CrossRef]
- Istiqamah, D.A.; Boedoyo, M.S.; Thamrin, S. Coal Gasification Technology to Support the Energy Transition: Opportunities and Challenges. Int. J. Humanit. Educ. Soc. Sci. 2024, 3, 2007–2015. [Google Scholar] [CrossRef]
- Smith, K.H.; Stanislowski, J.J.; Swanson, M.L.; Siefert, N.S. Biomass and Coal Cofiring Gasification with Pre-Combustion Carbon Capture: Impact of Mixed Feedstocks on CO2 Absorption Using a Physical Solvent. Int. J. Greenh. Gas Control 2025, 141, 104300. [Google Scholar] [CrossRef]
- Emery Energy Company, LLC. Executive Summary Extended Operational Runs on Emery Hybrid Gasifier to Accelerate Commercial Adoption. 2012. Available online: https://www.uwyo.edu/ser/_files/docs/cleancoal/executive-summary-reports/2009/final-executive-summary-phillips-2009.pdf (accessed on 20 August 2025).
- Romero, M.; German, B.; Rivadeneira, D.; Chulde, D.; Chico-Proano, A.; Narvaez, R.A.; Martinez-Gomez, J. Assessment of Pilot Scale Pyrolysis Gas Production as Fuel for Cogeneration. Chem. Eng. Trans. 2022, 92, 211–216. [Google Scholar] [CrossRef]
- State, R.N.; Volceanov, A.; Muley, P.; Boldor, D. A Review of Catalysts Used in Microwave Assisted Pyrolysis and Gasification. Bioresour. Technol. 2019, 277, 179–194. [Google Scholar] [CrossRef]
- Miroshnychenko, D.; Korchak, B.; Ungureanu, N.; Vlădut, N.-V.; Tefan, B.; Ionescu, M.; Gheorghiță, N.-E. Municipal Solid Waste Gasification: Technologies, Process Parameters, and Sustainable Valorisation of By-Products in a Circular Economy. Sustainability 2025, 17, 6704. [Google Scholar] [CrossRef]
- Frolov, S.M. Organic Waste Gasification: A Selective Review. Fuels 2021, 2, 556–650. [Google Scholar] [CrossRef]
- Kun, U.H.; Ksepko, E. Advancing Municipal Solid Waste Management Through Gasification Technology. Process 2025, 13, 2000. [Google Scholar] [CrossRef]
- Kelley, M.A.; Jakulewicz, M.S.; Dreyer, C.B.; Parker, T.E.; Porter, J.M. System Overview and Characterisation of a High-Temperature, High-Pressure, Entrained-Flow, Laboratory-Scale Gasifier. Rev. Sci. Instrum. 2015, 86, 55106. [Google Scholar] [CrossRef] [PubMed]
- Dossow, M.; Klüh, D.; Umeki, K.; Gaderer, M.; Spliethoff, H.; Fendt, S. Electrification of Gasification-Based Biomass-to-X Processes—A Critical Review and in-Depth Assessment. Energy Environ. Sci. 2024, 17, 925–973. [Google Scholar] [CrossRef]
- Zisopoulos, G.; Detsios, N.; Atsonios, K.; Nikolopoulos, N.; Grammelis, P. Process Analysis and Design Considerations of a Low Carbon Methanol Synthesis Plant from Lignite/Waste Gasification. Fuels 2022, 3, 245–274. [Google Scholar] [CrossRef]
- Baraj, E.; Ciahotný, K.; Hlinčík, T. The Water Gas Shift Reaction: Catalysts and Reaction Mechanism. Fuel 2021, 288, 119817. [Google Scholar] [CrossRef]
- Cui, X.; Su, H.Y.; Chen, R.; Yu, L.; Dong, J.; Ma, C.; Wang, S.; Li, J.; Yang, F.; Xiao, J.; et al. Room-Temperature Electrochemical Water–Gas Shift Reaction for High Purity Hydrogen Production. Nat. Commun. 2019, 10, 86. [Google Scholar] [CrossRef]
- Boruah, A.; Phukan, A.; Singh, S. Utilisation of Coal for Hydrogen Generation. Int. J. Coal Prep. Util. 2024, 45, 1412–1433. [Google Scholar] [CrossRef]
- Oviroh, P.O.; Oyinbo, S.T.; Karimzadeh, S.; Imoisili, P.E.; Jen, T.-C. Technologies of Hydrogen Generation and Purification. Emerg. At. Layer Depos. Hydrog. Energy 2025, 348, 27–76. [Google Scholar] [CrossRef]
- Porshnov, D. Evolution of Pyrolysis and Gasification as Waste to Energy Tools for Low Carbon Economy. Wiley Interdiscip. Rev. Energy Environ. 2022, 11, e421. [Google Scholar] [CrossRef]
- Mishra, A.; Gautam, S.; Sharma, T. Effect of Operating Parameters on Coal Gasification. Int. J. Coal Sci. Technol. 2018, 5, 113–125. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Li, H.; Han, X.; Zhang, M.; Sun, Y.; Fan, X.; Tu, R.; Zeng, Y.; Xu, C.C.; et al. Applications of Catalysts in Thermochemical Conversion of Biomass (Pyrolysis, Hydrothermal Liquefaction and Gasification): A Critical Review. Renew. Energy 2022, 196, 462–481. [Google Scholar] [CrossRef]
- Bashir, M.A.; Ji, T.; Weidman, J.; Soong, Y.; Gray, M.; Shi, F.; Wang, P. Plastic Waste Gasification for Low-Carbon Hydrogen Production: A Comprehensive Review. Energy Adv. 2025, 4, 330–363. [Google Scholar] [CrossRef]
- Jayanarasimhan, A.; Pathak, R.M.; Shivapuji, A.M.; Rao, L. Tar Formation in Gasification Systems: A Holistic Review of Remediation Approaches and Removal Methods. ACS Omega 2024, 9, 2060–2079. [Google Scholar] [CrossRef]
- Lysne, A.; Saxrud, I.; Snidaro, R.L.G.; Blekkan, E.A. Noble Metal (Pt, Pd and Rh) Promoted Ni-Co/Mg(Al)O Catalysts for Steam Reforming of Tar Impurities from Biomass Gasification. J. Catal. 2024, 436, 115567. [Google Scholar] [CrossRef]
- Xu, M.-X.; Liu, Z.-S.; Zhang, X.-Y.; Di, J.-Y.; Meng, X.-X.; Zhao, L.; Lu, Q. Steam Reforming of Biomass Gasification Tar over Ni-Based Catalyst Supported by TiO2-SiO2 Composite. Fuel 2023, 343, 127934. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Arshad, F.; Hassan, I.U.; Tabook, M.A.; Pedram, M.Z.; Mustaqeem, M.; Tabassum, H.; Ahmed, W.; Rezakazemi, M. Thermocatalytic Hydrogen Production Through Decomposition of Methane-A Review. Front. Chem. 2021, 9, 736801. [Google Scholar] [CrossRef]
- Panigrahy, B.; Narayan, K.; Ramachandra Rao, B. Green Hydrogen Production by Water Electrolysis: A Renewable Energy Perspective. Mater. Today Proc. 2022, 67, 1310–1314. [Google Scholar] [CrossRef]
- Hassan, Q.; Tabar, V.S.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. A Review of Green Hydrogen Production Based on Solar Energy; Techniques and Methods. Energy Harvest. Syst. 2024, 11, 20220134. [Google Scholar] [CrossRef]
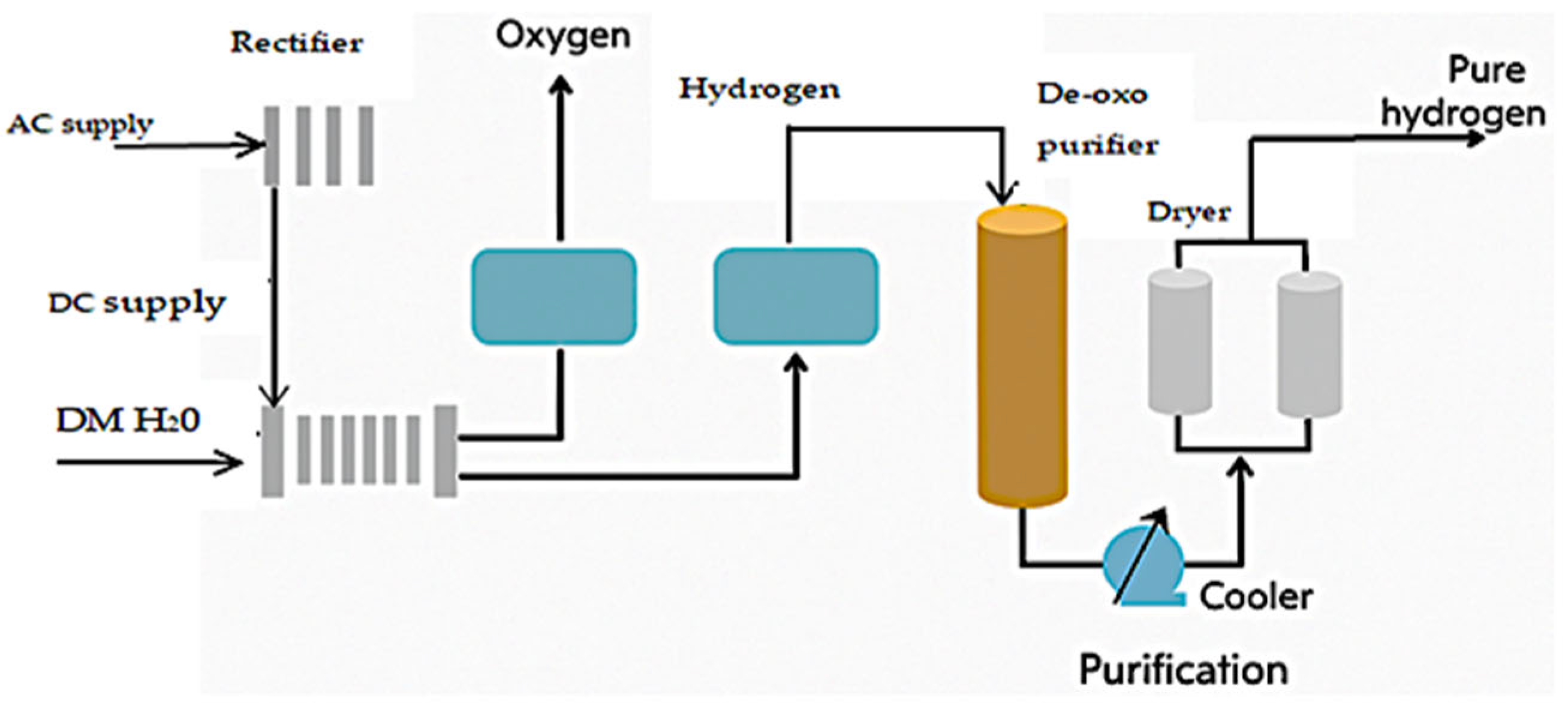
- Noor Azam, A.M.I.; Li, N.K.; Zulkefli, N.N.; Masdar, M.S.; Majlan, E.H.; Baharuddin, N.A.; Mohd Zainoodin, A.; Mohamad Yunus, R.; Shamsul, N.S.; Husaini, T.; et al. Parametric Study and Electrocatalyst of Polymer Electrolyte Membrane (PEM) Electrolysis Performance. Polymers 2023, 15, 560. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.Z.; Shaigan, N.; Song, C.; Aujla, M.; Neburchilov, V.; Kwan, J.T.H.; Wilkinson, D.P.; Bazylak, A.; Fatih, K. The Porous Transport Layer in Proton Exchange Membrane Water Electrolysis: Perspectives on a Complex Component. Sustain. Energy Fuels 2022, 6, 1824–1853. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, Y.; Xu, H.; Martinez, A.; Chen, K.S. PEM Fuel Cell and Electrolysis Cell Technologies and Hydrogen Infrastructure Development—A Review. Energy Environ. Sci. 2022, 15, 2288–2328. [Google Scholar] [CrossRef]
- Ahmed, K.W.; Jang, M.J.; Park, M.G.; Chen, Z.; Fowler, M. Effect of Components and Operating Conditions on the Performance of PEM Electrolyzers: A Review. Electrochem 2022, 3, 581–612. [Google Scholar] [CrossRef]
- Liao, Y.; Deng, G.; Ding, L.; Wang, H. A Thin and Flexible Composite Membrane with Low Area Resistance and High Bubble Point Pressure for Advanced Alkaline Water Electrolysis. J. Memb. Sci. 2024, 689, 122182. [Google Scholar] [CrossRef]
- Tüysüz, H. Alkaline Water Electrolysis for Green Hydrogen Production. Acc. Chem. Res. 2023, 57, 558–567. [Google Scholar] [CrossRef]
- Sher, F.; Ziani, I.; Al-Shara, N.K.; Chupin, A.; Horo, N.; Wang, B.; Rahman, S.; Fareed, B.; Nemţanu, M.R. Critical Insights into Eutectic Molten Hydroxide Electrolysis for Sustainable Green Hydrogen Production. Sustain. Energy Fuels 2024, 8, 4429–4452. [Google Scholar] [CrossRef]
- Cavaliere, P. Alkaline Liquid Electrolyte Water Electrolysis. In Water Electrolysis for Hydrogen Production; Springer: Cham, Switzerland, 2023; pp. 203–232. [Google Scholar] [CrossRef]
- Tello, A.; Cataño, F.A.; Arunachalam, A.; Oyarzún, D.; Henriquez, R.; Valdivia, P.; Ramalinga Viswanathan, M.; Gómez, H. Green Hydrogen Production by Photovoltaic-Assisted Alkaline Water Electrolysis: A Review on the Conceptualisation and Advancements. Int. J. Hydrogen Energy 2025, 107, 378–395. [Google Scholar] [CrossRef]
- Lopes, F.D.C.; Watanabe, E.H. Experimental and Theoretical Development of a PEM Electrolyzer Model Applied to Energy Storage Systems. In Proceedings of the 2009 Brazilian Power Electronics Conference, Bonito, Mato Grosso do Sul, Brazil, 27 September–1 October 2009; pp. 775–782. [Google Scholar] [CrossRef]
- Li, Y.; Feng, A.; Dai, L.; Xi, B.; An, X.; Xiong, S.; An, C. Progress on the Design of Electrocatalysts for Large-Current Hydrogen Production by Tuning Thermodynamic and Kinetic Factors. Adv. Funct. Mater. 2024, 34, 2316296. [Google Scholar] [CrossRef]
- Bashkatov, A.; Park, S.; Demirkır, Ç.; Wood, J.A.; Koper, M.T.M.; Lohse, D.; Krug, D. Performance Enhancement of Electrocatalytic Hydrogen Evolution through Coalescence-Induced Bubble Dynamics. J. Am. Chem. Soc. 2024, 146, 10177–10186. [Google Scholar] [CrossRef]
- Sangtam, B.T.; Park, H. Review on Bubble Dynamics in Proton Exchange Membrane Water Electrolysis: Towards Optimal Green Hydrogen Yield. Micromachines 2023, 14, 2234. [Google Scholar] [CrossRef]
- Riaz, M.A.; Trogadas, P.; Aymé-Perrot, D.; Sachs, C.; Dubouis, N.; Girault, H.; Coppens, M.O. Water Electrolysis Technologies: The Importance of New Cell Designs and Fundamental Modelling to Guide Industrial-Scale Development. Energy Environ. Sci. 2025, 18, 5190–5214. [Google Scholar] [CrossRef]
- Le, T.T.; Sharma, P.; Bora, B.J.; Tran, V.D.; Truong, T.H.; Le, H.C.; Nguyen, P.Q.P. Fueling the Future: A Comprehensive Review of Hydrogen Energy Systems and Their Challenges. Int. J. Hydrogen Energy 2024, 54, 791–816. [Google Scholar] [CrossRef]
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I.; et al. Water Electrolysis: From Textbook Knowledge to the Latest Scientific Strategies and Industrial Developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar] [CrossRef]
- Henkensmeier, D.; Cho, W.C.; Jannasch, P.; Stojadinovic, J.; Li, Q.; Aili, D.; Jensen, J.O. Separators and Membranes for Advanced Alkaline Water Electrolysis. Chem. Rev. 2024, 124, 6393–6443. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Koning, V.; Theodorus de Groot, M.; de Groot, A.; Mendoza, P.G.; Junginger, M.; Kramer, G.J. Present and Future Cost of Alkaline and PEM Electrolyser Stacks. Int. J. Hydrogen Energy 2023, 48, 32313–32330. [Google Scholar] [CrossRef]
- Paulec, T.; Tvarožek, J.; Resutík, P.; Špánik, P.; Praženica, M. Review of the Dynamic Response of Water Electrolyzer. Electr. Eng. 2025, 107, 10499–10506. [Google Scholar] [CrossRef]
- Ahmad Kamaroddin, M.F.; Sabli, N.; Tuan Abdullah, T.A.; Siajam, S.I.; Abdullah, L.C.; Abdul Jalil, A.; Ahmad, A. Membrane-Based Electrolysis for Hydrogen Production: A Review. Membranes 2021, 11, 810. [Google Scholar] [CrossRef] [PubMed]
- Nagao, Y. Proton-Conducting Polymers: Key to Next-Generation Fuel Cells, Electrolyzers, Batteries, Actuators, and Sensors. ChemElectroChem 2024, 11, e202300846. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Lysova, A.A.; Voropaeva, D.Y.; Yaroslavtsev, A.B. Approaches to the Modification of Perfluorosulfonic Acid Membranes. Membranes 2023, 13, 721. [Google Scholar] [CrossRef]
- Alam Rimon, S.T.; Mourshed, M.; Kibria, M.G. Proton Exchange Membrane Fuel Cells: Advances in Materials Development, Performance Optimisation, and Future Outlook. Energy Convers. Manag. X 2025, 27, 101102. [Google Scholar] [CrossRef]
- Hancke, R.; Bujlo, P.; Holm, T.; Ulleberg, Ø. High-Pressure PEM Water Electrolyser Performance up to 180 Bar Differential Pressure. J. Power Sources 2024, 601, 234271. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L. Analysis of Proton Exchange Membranes for Fuel Cells Based on Statistical Theory and Data Mining. iScience 2024, 27, 109360. [Google Scholar] [CrossRef]
- Hou, X.; Jiang, Y.; Wei, K.; Jiang, C.; Jen, T.C.; Yao, Y.; Liu, X.; Ma, J.; Irvine, J.T.S. Syngas Production from CO2 and H2O via Solid-Oxide Electrolyzer Cells: Fundamentals, Materials, Degradation, Operating Conditions, and Applications. Chem. Rev. 2024, 124, 5119–5166. [Google Scholar] [CrossRef]
- Matkin, D.E.; Starostina, I.A.; Hanif, M.B.; Medvedev, D.A. Revisiting the Ionic Conductivity of Solid Oxide Electrolytes: A Technical Review. J. Mater. Chem. A 2024, 12, 25696–25714. [Google Scholar] [CrossRef]
- Li, J.; Cai, Q.; Amini Horri, B. Highly Conductive and Stable Electrolytes for Solid Oxide Electrolysis and Fuel Cells: Fabrication, Characterisation, Recent Progress and Challenges. Mater. Adv. 2025, 6, 39–83. [Google Scholar] [CrossRef]
- Yoon, K.J.; Lee, S.; Park, S.Y.; Minh, N.Q. Advances in High-Temperature Solid Oxide Electrolysis Technology for Clean Hydrogen and Chemical Production: Materials, Cells, Stacks, Systems and Economics. Prog. Mater. Sci. 2025, 154, 101520. [Google Scholar] [CrossRef]
- Reznicek, E.P.; Braun, R.J. Reversible Solid Oxide Cell Systems for Integration with Natural Gas Pipeline and Carbon Capture Infrastructure for Grid Energy Management. Appl. Energy 2020, 259, 114118. [Google Scholar] [CrossRef]
- Naeini, M.; Cotton, J.S.; Adams, T.A. An Eco-Technoeconomic Analysis of Hydrogen Production Using Solid Oxide Electrolysis Cells That Accounts for Long-Term Degradation. Front. Energy Res. 2022, 10, 1015465. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Habibi, R. A Review on Hydrogen Production Thermochemical Water-Splitting Cycles. J. Clean. Prod. 2020, 275, 123836. [Google Scholar] [CrossRef]
- Peng, S. Electrochemical Hydrogen Production from Water Splitting: Basic, Materials and Progress; Springer Nature: Singapore, 2023; pp. 1–243. [Google Scholar] [CrossRef]
- Sameer; Havilah, P.R.; Yadav, U.S.; Sharma, A.K. Hydrogen Production and Utilisation Through Electrochemical Techniques. In Challenges and Opportunities in Green Hydrogen Production. Energy, Environment, and Sustainability; Part F2879; Springer: Singapore, 2024; pp. 177–209. [Google Scholar] [CrossRef]
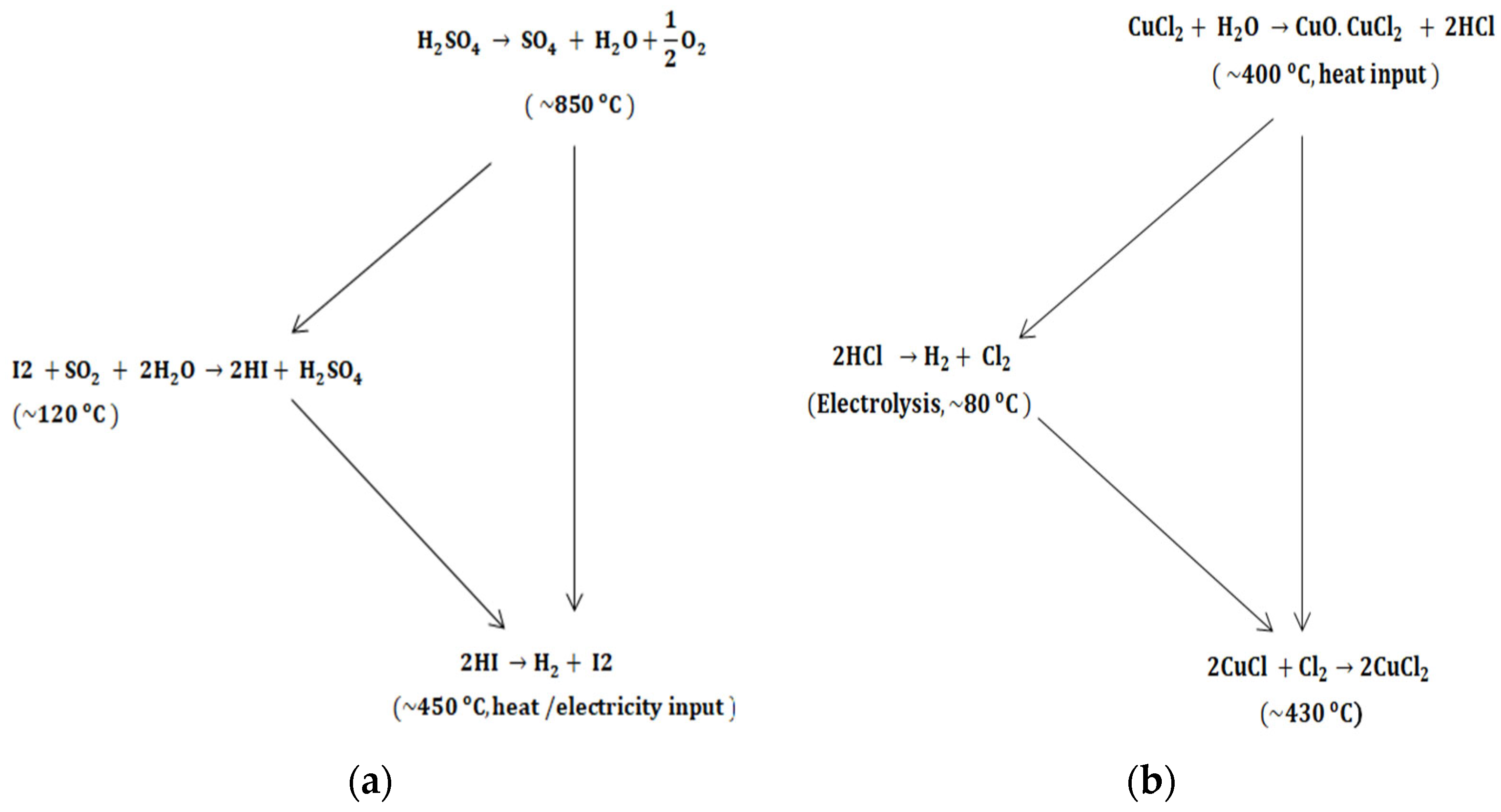
- Safari, F.; Dincer, I. A Review and Comparative Evaluation of Thermochemical Water Splitting Cycles for Hydrogen Production. Energy Convers. Manag. 2020, 205, 112182. [Google Scholar] [CrossRef]
- Farsi, A.; Zamfirescu, C.; Dincer, I.; Naterer, G.F. Thermodynamic Assessment of a Lab-Scale Experimental Copper-Chlorine Cycle for Sustainable Hydrogen Production. Int. J. Hydrogen Energy 2019, 44, 17595–17610. [Google Scholar] [CrossRef]
- Boretti, A. Concentrated Solar Energy with Thermal Energy Storage for Hydrogen Production by Three-Step Thermochemical Water-Splitting Cycles. Energy Fuels 2021, 35, 10832–10840. [Google Scholar] [CrossRef]
- Araya, S.S.; Liso, V.; Venizelou, V.; Poullikkas, A. Comprehensive Overview of Recent Research and Industrial Advancements in Nuclear Hydrogen Production. Energies 2024, 17, 2836. [Google Scholar] [CrossRef]
- Temiz, M.; Dincer, I. Concentrated Solar Driven Thermochemical Hydrogen Production Plant with Thermal Energy Storage and Geothermal Systems. Energy 2021, 219, 119554. [Google Scholar] [CrossRef]
- Lee, J.E.; Shafiq, I.; Hussain, M.; Lam, S.S.; Rhee, G.H.; Park, Y.K. A Review on Integrated Thermochemical Hydrogen Production from Water. Int. J. Hydrogen Energy 2022, 47, 4346–4356. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Ghorbani, B.; Khodaverdi, M. Hydrogen Production by Thermochemical Water Splitting Cycle Using Low-Grade Solar Heat and Phase Change Material Energy Storage System. Int. J. Energy Res. 2022, 46, 7590–7609. [Google Scholar] [CrossRef]
- Kumar, G.; Eswari, A.P.; Kavitha, S.; Kumar, M.D.; Kannah, R.Y.; How, L.C.; Muthukaruppan, G.; Banu, J.R. Thermochemical Conversion Routes of Hydrogen Production from Organic Biomass: Processes, Challenges and Limitations. Biomass Convers. Biorefinery 2023, 13, 8509–8534. [Google Scholar] [CrossRef]
- Jolaoso, L.A.; Bello, I.T.; Ojelade, O.A.; Yousuf, A.; Duan, C.; Kazempoor, P. Operational and Scaling-up Barriers of SOEC and Mitigation Strategies to Boost H2 Production- a Comprehensive Review. Int. J. Hydrogen Energy 2023, 48, 33017–33041. [Google Scholar] [CrossRef]
- Zhang, J.; He, Y.; Zeng, J.; Song, W.; Weng, W.; Wang, Z. Updating and 24 H Testing of State Key Laboratory of Clean Energy Utilisation’s Thermochemical Iodine–Sulfur Cycle Water-Splitting Hydrogen Production System. Appl. Sci. 2025, 15, 5183. [Google Scholar] [CrossRef]
- Molaei, M.J. Recent Advances in Hydrogen Production through Photocatalytic Water Splitting: A Review. Fuel 2024, 365, 131159. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, N.K.; Kumar, R.S.; Singh, R. Production of Green Hydrogen through Photocatalysis. ACS Symp. Ser. 2024, 1468, 1–24. [Google Scholar] [CrossRef]
- Sahu, P.K.; Champati, A.; Pradhan, A.; Naik, B. Design and Development of Nanostructured Photocatalysts for Large-Scale Solar Green Hydrogen Generation. Sustain. Energy Fuels 2024, 8, 1872–1917. [Google Scholar] [CrossRef]
- Navalón, S.; Dhakshinamoorthy, A.; Álvaro, M.; Ferrer, B.; García, H. Metal-Organic Frameworks as Photocatalysts for Solar-Driven Overall Water Splitting. Chem. Rev. 2023, 123, 445–490. [Google Scholar] [CrossRef]
- Nasir, J.A.; Rehman, Z.U.; Shah, S.N.A.; Khan, A.; Butler, I.S.; Catlow, C.R.A. Recent Developments and Perspectives in CdS-Based Photocatalysts for Water Splitting. J. Mater. Chem. A 2020, 8, 20752–20780. [Google Scholar] [CrossRef]
- Pei, J.; Li, H.; Yu, D.; Zhang, D. G-C3N4-Based Heterojunction for Enhanced Photocatalytic Performance: A Review of Fabrications, Applications, and Perspectives. Catalysts 2024, 14, 825. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. Updates on the Roadmap for Photocatalysis. ACS Catal. 2020, 10, 5493–5501. [Google Scholar] [CrossRef]
- Li, R.; Luan, J.; Zhang, Y.; Jiang, L.; Yan, H.; Chi, Q.; Yan, Z. A Review of Efficient Photocatalytic Water Splitting for Hydrogen Production. Renew. Sustain. Energy Rev. 2024, 206, 114863. [Google Scholar] [CrossRef]
- Srinivasu, K.; Ghosh, S.K. Photocatalytic Splitting of Water on S-Triazine Based Graphitic Carbon Nitride: An Ab Initio Investigation. J. Mater. Chem. A 2015, 3, 23011–23016. [Google Scholar] [CrossRef]
- Zong, X.; Li, C. 7-Photocatalytic Water Splitting on Metal Oxide-Based Semiconductor Photocatalysts. In Metal Oxides in Heterogeneous Catalysis; Elsevier: Amsterdam, The Netherlands, 2018; pp. 355–399. ISBN 978-0-12-811631-9. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I. Artificial Photosynthesis: Powering a Green New Deal for Sustainable Energy. Int. J. Hydrogen Energy 2024, 90, 199–209. [Google Scholar] [CrossRef]
- Lakhera, S.K.; Rajan, A.; Bernaurdshaw, N. A Review on Particulate Photocatalytic Hydrogen Production System: Progress Made in Achieving High Energy Conversion Efficiency and Key Challenges Ahead. Renew. Sustain. Energy Rev. 2021, 152, 111694. [Google Scholar] [CrossRef]
- Bahnemann, D.; Robertson, P.; Wang, C.; Choi, W.; Daly, H.; Danish, M.; de Lasa, H.; Escobedo, S.; Hardacre, C.; Jeon, T.H.; et al. 2023 Roadmap on Photocatalytic Water Splitting. J. Phys. Energy 2023, 5, 012004. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Ding, S.; Wawrzy´nczak, A.; Wawrzy´nczak, W.; Feliczak-Guzik, A. Hydrogen Production Using Modern Photocatalysts. Coatings 2024, 14, 366. [Google Scholar] [CrossRef]
- Zhao, Y.; Niu, Z.; Zhao, J.; Xue, L.; Fu, X.; Long, J. Recent Advancements in Photoelectrochemical Water Splitting for Hydrogen Production. Electrochem. Energy Rev. 2023, 6, 14. [Google Scholar] [CrossRef]
- Khan, H.; Bera, S.; Jung, M.J.; Kwon, S.H. Rational Design of Photoanodes to Produce Value-Added Chemicals Coupled with Hydrogen. ChemElectroChem 2024, 11, e202400239. [Google Scholar] [CrossRef]
- Li, S.; Xu, W.; Meng, L.; Tian, W.; Li, L. Recent Progress on Semiconductor Heterojunction-Based Photoanodes for Photoelectrochemical Water Splitting. Small Sci. 2022, 2, 2100112. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, M.; Huang, Z.; Zhu, W.; Zheng, J.; Jiang, Q.; Wang, Z.; Liang, H. Surface Reconstruction of Water Splitting Electrocatalysts. Adv. Energy Mater. 2022, 12, 2201713. [Google Scholar] [CrossRef]
- Liu, D.; Kuang, Y. Particle-Based Photoelectrodes for PEC Water Splitting: Concepts and Perspectives. Adv. Mater. 2024, 36, 2311692. [Google Scholar] [CrossRef]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical Devices for Solar Water Splitting—Materials and Challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef]
- Zabara, M.A.; Ölmez, B.; Buldu-Akturk, M.; Yarar Kaplan, B.; Kırlıoğlu, A.C.; Alkan Gürsel, S.; Ozkan, M.; Ozkan, C.S.; Yürüm, A. Photoelectrocatalytic Hydrogen Generation: Current Advances in Materials and Operando Characterisation. Glob. Chall. 2024, 8, 2400011. [Google Scholar] [CrossRef]
- Moss, B.; Babacan, O.; Kafizas, A.; Hankin, A. A Review of Inorganic Photoelectrode Developments and Reactor Scale-Up Challenges for Solar Hydrogen Production. Adv. Energy Mater. 2021, 11, 2003286. [Google Scholar] [CrossRef]
- Thangamuthu, M.; Ruan, Q.; Ohemeng, P.O.; Luo, B.; Jing, D.; Godin, R.; Tang, J. Polymer Photoelectrodes for Solar Fuel Production: Progress and Challenges. Chem. Rev. 2022, 122, 11778–11829. [Google Scholar] [CrossRef] [PubMed]
- Suri, D.; Das, S.; Choudhary, S.; Venkanna, G.; Sharma, B.; Afroz, M.A.; Tailor, N.K.; Joshi, R.; Satapathi, S.; Tripathi, K. Enigma of Sustainable CO2 Conversion to Renewable Fuels and Chemicals Through Photocatalysis, Electrocatalysis, and Photoelectrocatalysis: Design Strategies and Atomic Level Insights. Small 2025, 21, 2408981. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.Y.; Jiang, J.C.; Meng, L.Y. Carbon-Based Photoelectrochemical Sensors: Recent Developments and Future Prospects. Dalt. Trans. 2024, 53, 11192–11215. [Google Scholar] [CrossRef]
- Garcia, G.M.; Oliva, H.S. Technical, Economic, and CO2 Emissions Assessment of Green Hydrogen Production from Solar/Wind Energy: The Case of Chile. Energy 2023, 278, 127981. [Google Scholar] [CrossRef]
- de Kleijne, K.; Huijbregts, M.A.J.; Knobloch, F.; van Zelm, R.; Hilbers, J.P.; de Coninck, H.; Hanssen, S.V. Worldwide Greenhouse Gas Emissions of Green Hydrogen Production and Transport. Nat. Energy 2024, 9, 1139–1152. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Dickson, R.; Liu, J.J. Life Cycle Assessment of Inland Green Hydrogen Supply Chain Networks with Current Challenges and Future Prospects. ACS Sustain. Chem. Eng. 2021, 9, 17152–17163. [Google Scholar] [CrossRef]
- Franco, A. Green Hydrogen and the Energy Transition: Hopes, Challenges, and Realistic Opportunities. Hydrogen 2025, 6, 28. [Google Scholar] [CrossRef]
- Romano, M.C.; Antonini, C.; Bardow, A.; Bertsch, V.; Brandon, N.P.; Brouwer, J.; Campanari, S.; Crema, L.; Dodds, P.E.; Gardarsdottir, S.; et al. Comment on “How Green Is Blue Hydrogen? ” Energy Sci. Eng. 2022, 10, 1944–1954. [Google Scholar] [CrossRef]
- Ameli, H.; Strbac, G.; Pudjianto, D.; Ameli, M.T. A Review of the Role of Hydrogen in the Heat Decarbonisation of Future Energy Systems: Insights and Perspectives. Energies 2024, 17, 1688. [Google Scholar] [CrossRef]
- Angelico, R.; Giametta, F.; Bianchi, B.; Catalano, P. Green Hydrogen for Energy Transition: A Critical Perspective. Energies 2025, 18, 404. [Google Scholar] [CrossRef]
- Hydrogen Production—Global Hydrogen Review 2024—Analysis—IEA. Available online: https://www.iea.org/reports/global-hydrogen-review-2024/hydrogen-production (accessed on 28 August 2025).
- Global Hydrogen Review 2023—Analysis—IEA. Available online: https://www.iea.org/reports/global-hydrogen-review-2023 (accessed on 16 July 2025).
- Bal, S. Global Trends in Innovation across Hydrogen Production, Supply and Demand Chains. Int. J. Thermofluids 2025, 29, 101365. [Google Scholar] [CrossRef]
- Ishaq, H.; Crawford, C. Towards Less Carbon-Intensive Blue Hydrogen: Integrated Natural Gas Reforming and CO2 Capture Approach. J. Environ. Chem. Eng. 2025, 13, 115043. [Google Scholar] [CrossRef]
- Vallejo, V.; Nguyen, Q.; Ravikumar, A.P. Geospatial Variation in Carbon Accounting of Hydrogen Production and Implications for the US Inflation Reduction Act. Nat. Energy 2024, 9, 1571–1582. [Google Scholar] [CrossRef]
- Patonia, A.; Rahmatallah, P. Cost-Competitive Green Hydrogen: How to Lower the Cost of Electrolysers? The Oxford Institute for Energy Studies: Oxford, UK, 2022. [Google Scholar]
- Odenweller, A.; Ueckerdt, F. The Green Hydrogen Ambition and Implementation Gap. Nat. Energy 2025, 10, 110–123. [Google Scholar] [CrossRef]
- Spilleir, D.; Castañeda-Ayarza, J.; de Mello-Ayres, R.; Brucieri, A. Characteristics and Influence of Macroenvironment in the Brazilian Hydrogen Energy Sector. Clean. Prod. Lett. 2024, 6, 100059. [Google Scholar] [CrossRef]
- Saha, P.; Akash, F.A.; Shovon, S.M.; Monir, M.U.; Ahmed, M.T.; Khan, M.F.H.; Sarkar, S.M.; Islam, M.K.; Hasan, M.M.; Vo, D.V.N.; et al. Grey, Blue, and Green Hydrogen: A Comprehensive Review of Production Methods and Prospects for Zero-Emission Energy. Int. J. Green Energy 2024, 21, 1383–1397. [Google Scholar] [CrossRef]
- Nuttall, W.J.; Powell, J.B.; Anaya-Stucchi, K.L.; Bakenne, A.T.; Wilson, A. Hydrogen Around the World. In Insights into the New Hydrogen Economy; Springer: Cham, Switzerland, 2025; pp. 85–112. [Google Scholar] [CrossRef]
- U.S. Refinery Hydrogen Production Capacity as of January 1 (Million Cubic Feet per Day). Available online: https://www.eia.gov/dnav/pet/hist/LeafHandler.ashx?n=PET&s=8_NA_8PH_NUS_6&f=A (accessed on 29 August 2025).
- Bolscher, H.; Brownsort, P.; Guevara Opinska, L.; Jordal, K.; Kraemer, D.; Mikunda, T.; Parmiter, P.; Rycroft, L.; Yearwood, J. High Level Report: CCUS in Europe. 2019. Available online: https://www.ccusnetwork.eu/?wsidchk=17844817 (accessed on 27 August 2025).
- Akerboom, S.; Waldmann, S.; Mukherjee, A.; Agaton, C.; Sanders, M.; Kramer, G.J. Different This Time? The Prospects of CCS in the Netherlands in the 2020s. Front. Energy Res. 2021, 9, 644796. [Google Scholar] [CrossRef]
- Kollar, D.J.; Qin, S.; Xiao, Y. Executive Summary 2 China Hydrogen Economy Report. Available online: https://www.s-ge.com/sites/default/files/publication/free/2024_e_campaign_clean_website_gatedcontent_text_ct5_en_china-hyd.pdf (accessed on 29 August 2025).
- Ghorbani, B.; Zendehboudi, S.; Saady, N.M.C.; Naterer, G.F. Canada’s Hydrogen Future: Innovations, Policies, and Global Perspectives. Energy Fuels 2025, 39, 7605–7648. [Google Scholar] [CrossRef]
- ATCO EnPower to Proceed with Atlas Carbon Storage Hub: “A Significant Step Forward in Alberta’s Energy Transition”. Available online: https://www.atco.com/en-ca/about-us/news/2024/123013-atco-enpower-to-proceed-with-atlas-carbon-storage-hub-a-signific.html (accessed on 29 August 2025).
- Friedmann, J. About Qamar Energy. 2021. Available online: https://www.qamarenergy.com/sites/default/files/The%20UAE%27s%20Role%20in%20the%20Global%20Hydrogen%20Economy.pdf (accessed on 29 August 2025).
- IOGP. CCUS Projects in Europe. Available online: https://iogpeurope.org/wp-content/uploads/2022/11/Map-of-EU-CCUS-Projects.pdf (accessed on 29 August 2025).
- Riera, J.A.; Lima, R.M.; Knio, O.M. A Review of Hydrogen Production and Supply Chain Modeling and Optimisation. Int. J. Hydrogen Energy 2023, 48, 13731–13755. [Google Scholar] [CrossRef]
- Khatiwada, D.; Vasudevan, R.A.; Santos, B.H. Decarbonisation of Natural Gas Systems in the EU—Costs, Barriers, and Constraints of Hydrogen Production with a Case Study in Portugal. Renew. Sustain. Energy Rev. 2022, 168, 112775. [Google Scholar] [CrossRef]
- Algburi, S.; Munther, H.; Al-Dulaimi, O.; Fakhruldeen, H.F.; Sapaev, I.B.; Al Seedi, K.F.K.; Khalaf, D.H.; Jabbar, F.I.; Hassan, Q.; Khudhair, A.; et al. Green Hydrogen Role in Sustainable Energy Transformations: A Review. Results Eng. 2025, 26, 105109. [Google Scholar] [CrossRef]
- Nyangon, J.; Darekar, A. Advancements in Hydrogen Energy Systems: A Review of Levelized Costs, Financial Incentives and Technological Innovations. Innov. Green Dev. 2024, 3, 100149. [Google Scholar] [CrossRef]
- Hydrogen—IEA. Available online: https://www.iea.org/energy-system/low-emissions-fuels/hydrogen (accessed on 16 July 2025).
- Merchant Hydrogen Plant Capacities in Europe|H2tools|Hydrogen Tools. Available online: https://h2tools.org/hyarc/hydrogen-data/merchant-hydrogen-plant-capacities-europe (accessed on 18 July 2025).
- Hydrogen Production Capacity|Tableau Public. Available online: https://public.tableau.com/app/profile/h2observatory/viz/Hydrogenproductioncapacity/Productioncostsdashboard (accessed on 27 August 2025).
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen Production, Storage, Utilisation and Environmental Impacts: A Review. Environ. Chem. Lett. 2021, 20, 153–188. [Google Scholar] [CrossRef]
- Ramezani, R.; Di Felice, L.; Gallucci, F. A Review of Chemical Looping Reforming Technologies for Hydrogen Production: Recent Advances and Future Challenges. J. Phys. Energy 2023, 5, 024010. [Google Scholar] [CrossRef]
- Howarth, R.W.; Jacobson, M.Z. Reply to Comment on “How Green Is Blue Hydrogen?". Energy Sci. Eng. 2022, 10, 1955–1960. [Google Scholar] [CrossRef]
- Riemer, M.; Duscha, V. Carbon Capture in Blue Hydrogen Production Is Not Where It Is Supposed to Be—Evaluating the Gap between Practical Experience and Literature Estimates. Appl. Energy 2023, 349, 121622. [Google Scholar] [CrossRef]
- Ocko, I.B.; Hamburg, S.P. Climate Consequences of Hydrogen Emissions. Atmos. Chem. Phys. 2022, 22, 9349–9368. [Google Scholar] [CrossRef]
- Raganati, F.; Ammendola, P. CO2 Post-Combustion Capture: A Critical Review of Current Technologies and Future Directions. Energy Fuels 2024, 38, 13858–13905. [Google Scholar] [CrossRef]
- Williams Rioux, B. Reducing Methane Emissions. Lect. Notes Energy 2023, 98, 145–182. [Google Scholar] [CrossRef]
- Hancock, L.; Ralph, N. A Framework for Assessing Fossil Fuel ‘Retrofit’ Hydrogen Exports: Security-Justice Implications of Australia’s Coal-Generated Hydrogen Exports to Japan. Energy 2021, 223, 119938. [Google Scholar] [CrossRef]
- Jesse, B.J.; Kramer, G.J.; Koning, V.; Vögele, S.; Kuckshinrichs, W. Stakeholder Perspectives on the Scale-up of Green Hydrogen and Electrolyzers. Energy Rep. 2024, 11, 208–217. [Google Scholar] [CrossRef]
- Xie, Z.; Jin, Q.; Su, G.; Lu, W. A Review of Hydrogen Storage and Transportation: Progresses and Challenges. Energies 2024, 17, 4070. [Google Scholar] [CrossRef]
- Osman, A.I.; Hefny, M.; Abdel Maksoud, M.I.A.; Elgarahy, A.M.; Rooney, D.W. Recent Advances in Carbon Capture Storage and Utilisation Technologies: A Review. Environ. Chem. Lett. 2020, 19, 797–849. [Google Scholar] [CrossRef]
- Chari, S.; Sebastiani, A.; Paulillo, A.; Materazzi, M. The Environmental Performance of Mixed Plastic Waste Gasification with Carbon Capture and Storage to Produce Hydrogen in the U.K. ACS Sustain. Chem. Eng. 2023, 11, 3248–3259. [Google Scholar] [CrossRef]
- Ueckerdt, F.; Verpoort, P.C.; Anantharaman, R.; Bauer, C.; Beck, F.; Longden, T.; Roussanaly, S. On the Cost Competitiveness of Blue and Green Hydrogen. Joule 2024, 8, 104–128. [Google Scholar] [CrossRef]
- George, J.F.; Müller, V.P.; Winkler, J.; Ragwitz, M. Is Blue Hydrogen a Bridging Technology? The Limits of a CO2 Price and the Role of State-Induced Price Components for Green Hydrogen Production in Germany. Energy Policy 2022, 167, 113072. [Google Scholar] [CrossRef]
- Jumah, A. bin A Comprehensive Review of Production, Applications, and the Path to a Sustainable Energy Future with Hydrogen. RSC Adv. 2024, 14, 26400–26423. [Google Scholar] [CrossRef]
- Boettcher, S.W. Introduction to Green Hydrogen. Chem. Rev. 2024, 124, 13095–13098. [Google Scholar] [CrossRef]
- Southall, E.; Lukashuk, L. Hydrogen Storage and Transportation Technologies to Enable the Hydrogen Economy: Liquid Organic Hydrogen Carriers: Overview and Perspectives on Liquid Organic Hydrogen Carriers Technology. Johnson Matthey Technol. Rev. 2022, 66, 246–258. [Google Scholar] [CrossRef]
- Athia, N.; Pandey, M.; Sen, M.; Saxena, S. Factors Affecting the Production Cost of Green Hydrogen and Its Challenge for Sustainable Development. Environ. Dev. Sustain. 2024, 1–45. [Google Scholar] [CrossRef]
- Liponi, A.; Pasini, G.; Baccioli, A.; Ferrari, L. Evaluating the Potential and Limits of Green Electrolysis in Future Energy Scenarios with High Renewable Share. Energies 2025, 18, 1654. [Google Scholar] [CrossRef]
- Ikuerowo, T.; Bade, S.O.; Akinmoladun, A.; Oni, B.A. The Integration of Wind and Solar Power to Water Electrolyzer for Green Hydrogen Production. Int. J. Hydrogen Energy 2024, 76, 75–96. [Google Scholar] [CrossRef]
- Global Planned Electrolyser Capacity and Estimation of Additional Renewable Capacity by Country/Region—Charts—Data & Statistics—IEA. Available online: https://www.iea.org/data-and-statistics/charts/global-planned-electrolyser-capacity-and-estimation-of-additional-renewable-capacity-by-country-region (accessed on 13 July 2025).
- IRENA (International Renewable Energy Agency). Global Hydrogen Trade to Meet the 1.5 °C Climate Goal: Green Hydrogen Cost and Potential; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2022; pp. 1–114. [Google Scholar]
- European Commission; Joint Research Center; Bolard, J.; Dolci, F.; Gryc, K.; Eynard, U.; Georgakaki, A.; Letout, S.; Mountraki, A.; Ince, E.; et al. Clean Energy Technology Observatory: Water Electrolysis and Hydrogen in the European Union—2024 Status Report on Technology Development, Trends, Value Chains and Markets; Publications Office of the European Union: Luxembourg, 2024. [Google Scholar] [CrossRef]
- Madadi Avargani, V.; Habibzadeh, M.; Abdlla Maarof, H.; Zendehboudi, S.; Duan, X. Harnessing Renewable Energy for Hydrogen Production: Advances, Challenges, and Opportunities. Ind. Eng. Chem. Res. 2025, 64, 12368–12418. [Google Scholar] [CrossRef]
- Koeva, D.; Rachev, S. Integrating Hydrogen Production Capacity in the Energy Transition: Energy Efficiency and Challenges. In Proceedings of the 2023 4th International Conference on Communications, Information, Electronic and Energy Systems (CIEES), Plovdiv, Bulgaria, 23–25 November 2023. [Google Scholar] [CrossRef]
- Curcio, E. Techno-Economic Analysis of Hydrogen Production: Costs, Policies, and Scalability in the Transition to Net-Zero. Int. J. Hydrogen Energy 2025, 128, 473–487. [Google Scholar] [CrossRef]
- Frieden, F.; Leker, J. Future Costs of Hydrogen: A Quantitative Review. Sustain. Energy Fuels 2024, 8, 1806–1822. [Google Scholar] [CrossRef]
- Zun, M.T.; McLellan, B.C. Cost Projection of Global Green Hydrogen Production Scenarios. Hydrogen 2023, 4, 932–960. [Google Scholar] [CrossRef]
- Durakovic, G.; del Granado, P.C.; Tomasgard, A. Are Green and Blue Hydrogen Competitive or Complementary? Insights from a Decarbonized European Power System Analysis. Energy 2023, 282, 128282. [Google Scholar] [CrossRef]
- Benalcazar, P.; Komorowska, A. Techno-Economics of Green Hydrogen: Present Trends and Future Prospects. In Challenges and Opportunities in Green Hydrogen Production. Energy, Environment, and Sustainability; Part F2879; Springer: Singapore, 2024; pp. 569–597. [Google Scholar] [CrossRef]
- Dincer, I.; Aydin, M.I. New Paradigms in Sustainable Energy Systems with Hydrogen. Energy Convers. Manag. 2023, 283, 116950. [Google Scholar] [CrossRef]
- Borup, R.; Krause, T.; Brouwer, J. Hydrogen Is Essential for Industry and Transportation Decarbonisation. Electrochem. Soc. Interface 2021, 30, 79–83. [Google Scholar] [CrossRef]
- Shaterabadi, M.; Sadeghi, S.; Jirdehi, M.A. The Role of Green Hydrogen in Achieving Low and Net-Zero Carbon Emissions: Climate Change and Global Warming. In Green Hydrogen in Power Systems. Green Energy and Technology; Part F2414; Springer: Cham, Switzerland, 2024; pp. 141–153. [Google Scholar] [CrossRef]
- Sakthimurugan, V.; Lakshmikanth, G.; Balaji, N.; Roopashree, R.; Kumar, D.; Devarajan, Y. Green Hydrogen Revolution: Advancing Electrolysis, Market Integration, and Sustainable Energy Transitions towards a Net-Zero Future. Results Eng. 2025, 26, 104849. [Google Scholar] [CrossRef]
- Bataille, C.; Nilsson, L.J.; Jotzo, F. Industry in a Net-Zero Emissions World: New Mitigation Pathways, New Supply Chains, Modelling Needs and Policy Implications. Energy Clim. Change 2021, 2, 100059. [Google Scholar] [CrossRef]
- Marouani, I.; Guesmi, T.; Alshammari, B.M.; Alqunun, K.; Alzamil, A.; Alturki, M.; Hadj Abdallah, H. Integration of Renewable-Energy-Based Green Hydrogen into the Energy Future. Process 2023, 11, 2685. [Google Scholar] [CrossRef]
- Patnaik, D.; Pattanaik, A.K.; Bagal, D.K.; Rath, A. Reducing CO2 Emissions in the Iron Industry with Green Hydrogen. Int. J. Hydrogen Energy 2023, 48, 23449–23458. [Google Scholar] [CrossRef]
- Dash, S.K.; Chakraborty, S.; Roccotelli, M.; Sahu, U.K. Hydrogen Fuel for Future Mobility: Challenges and Future Aspects. Sustainability 2022, 14, 8285. [Google Scholar] [CrossRef]
- Kyriakopoulos, G.L.; Aravossis, K.G. Literature Review of Hydrogen Energy Systems and Renewable Energy Sources. Energies 2023, 16, 7493. [Google Scholar] [CrossRef]
- Brown, A.L.; Sperling, D.; Austin, B.; DeShazo, J.; Fulton, L.; Lipman, T.; Murphy, C.W.; Saphores, J.D.; Tal, G.; Abrams, C.; et al. Driving California’s Transportation Emissions to Zero. 2021. Available online: https://escholarship.org/uc/item/3np3p2t0 (accessed on 22 August 2025).
- Jeffers, M.; Kelly, K.; Lipman, T.; Fernandes Tomon Avelino, A.; Johnson, C.; Li, M.; Post, M.; Zhang, Y. Comprehensive Review of California’s Innovative Clean Transit Regulation: Phase I Summary Report. 2022. Available online: https://research-hub.nrel.gov/en/publications/comprehensive-review-of-californiaaposs-innovative-clean-transit-/ (accessed on 20 August 2025).
- Dou, Y.; Sun, L.; Ren, J.; Dong, L. Opportunities and Future Challenges in Hydrogen Economy for Sustainable Development. In Hydrogen Economy; Academic Press: Berlin, Germany, 2023; pp. 537–569. [Google Scholar] [CrossRef]
- Jaradat, M.; Almashaileh, S.; Bendea, C.; Juaidi, A.; Bendea, G.; Bungau, T. Green Hydrogen in Focus: A Review of Production Technologies, Policy Impact, and Market Developments. Energies 2024, 17, 3992. [Google Scholar] [CrossRef]
- Zaheer, S.; Anjum, N.; Hussain, S.; Algarni, A.D.; Iqbal, J.; Bourouis, S.; Ullah, S.S. A Multi Parameter Forecasting for Stock Time Series Data Using LSTM and Deep Learning Model. Mathematics 2023, 11, 590. [Google Scholar] [CrossRef]
- Malashin, I.; Tynchenko, V.; Gantimurov, A.; Nelyub, V.; Borodulin, A. Applications of Long Short-Term Memory (LSTM) Networks in Polymeric Sciences: A Review. Polymers 2024, 16, 2607. [Google Scholar] [CrossRef]
- Bui, V.; Le, N.T.; Nguyen, V.H.; Kim, J.; Jang, Y.M. Multi-Behavior with Bottleneck Features LSTM for Load Forecasting in Building Energy Management System. Electronics 2021, 10, 1026. [Google Scholar] [CrossRef]
- Elegbeleye, I.; Oguntona, O.; Elegbeleye, F. Green Hydrogen: Pathway to Net Zero Green House Gas Emission and Global Climate Change Mitigation. Hydrogen 2025, 6, 29. [Google Scholar] [CrossRef]
- Li, X.; Raorane, C.J.; Xia, C.; Wu, Y.; Tran, T.K.N.; Khademi, T. Latest Approaches on Green Hydrogen as a Potential Source of Renewable Energy towards Sustainable Energy: Spotlighting of Recent Innovations, Challenges, and Future Insights. Fuel 2023, 334, 126684. [Google Scholar] [CrossRef]
- Segovia-Hernández, J.G.; Hernández, S.; Cossío-Vargas, E.; Juarez-García, M.; Sánchez-Ramírez, E. Green Hydrogen Production for Sustainable Development: A Critical Examination of Barriers and Strategic Opportunities. RSC Sustain. 2025, 3, 134–157. [Google Scholar] [CrossRef]
- Dudley, H.; Jordan, A.; Lorenzoni, I. Advising National Climate Policy Makers: A Longitudinal Analysis of the UK Climate Change Committee. Glob. Environ. Change 2022, 76, 102589. [Google Scholar] [CrossRef]
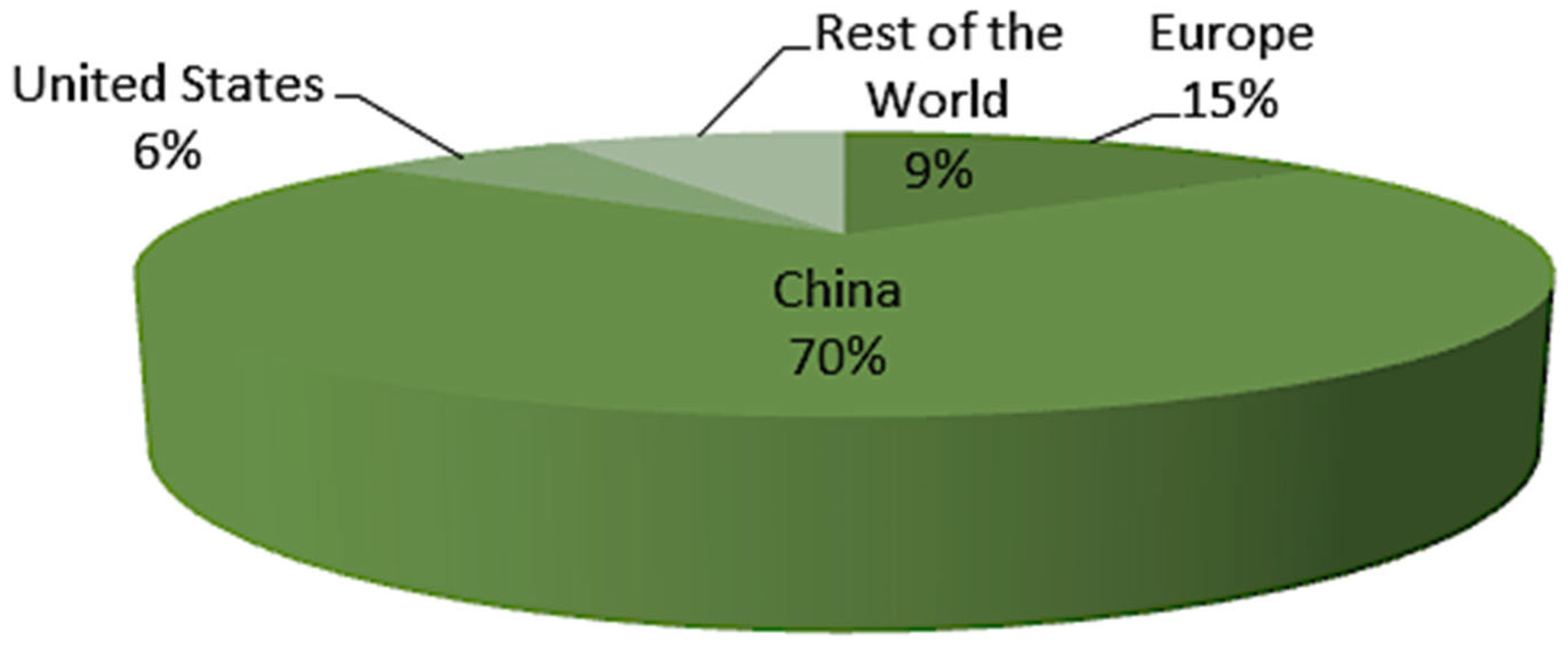
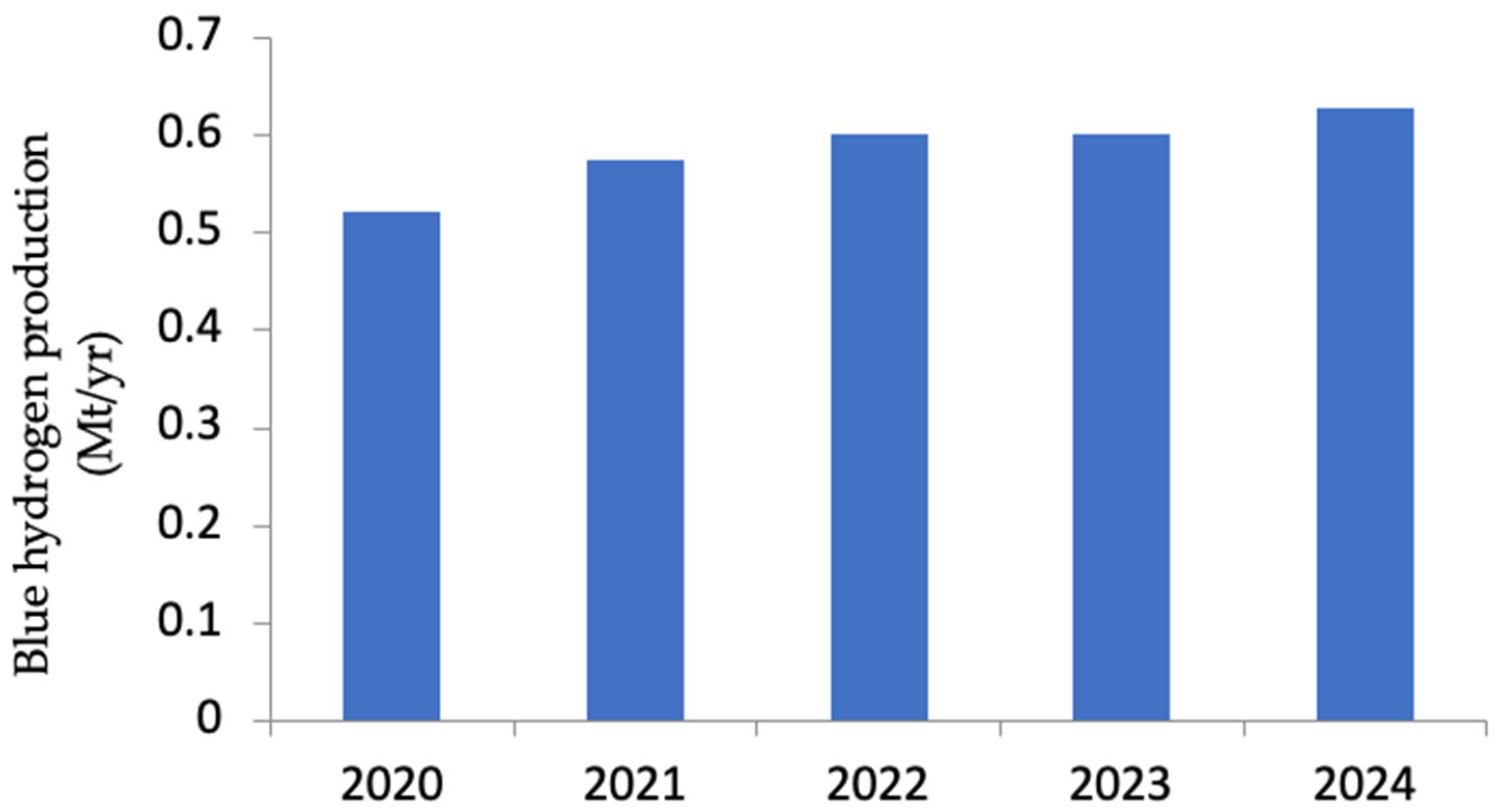
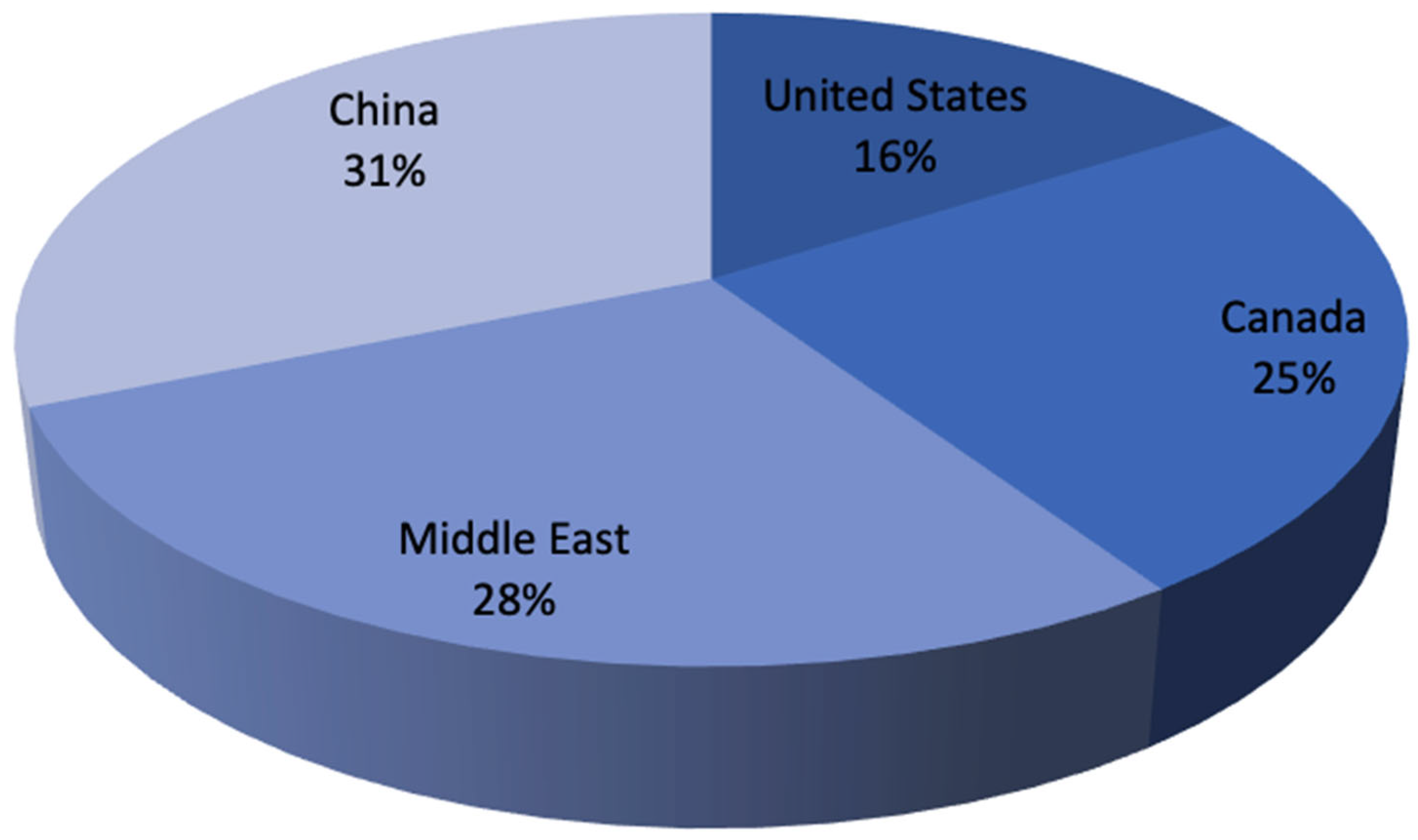
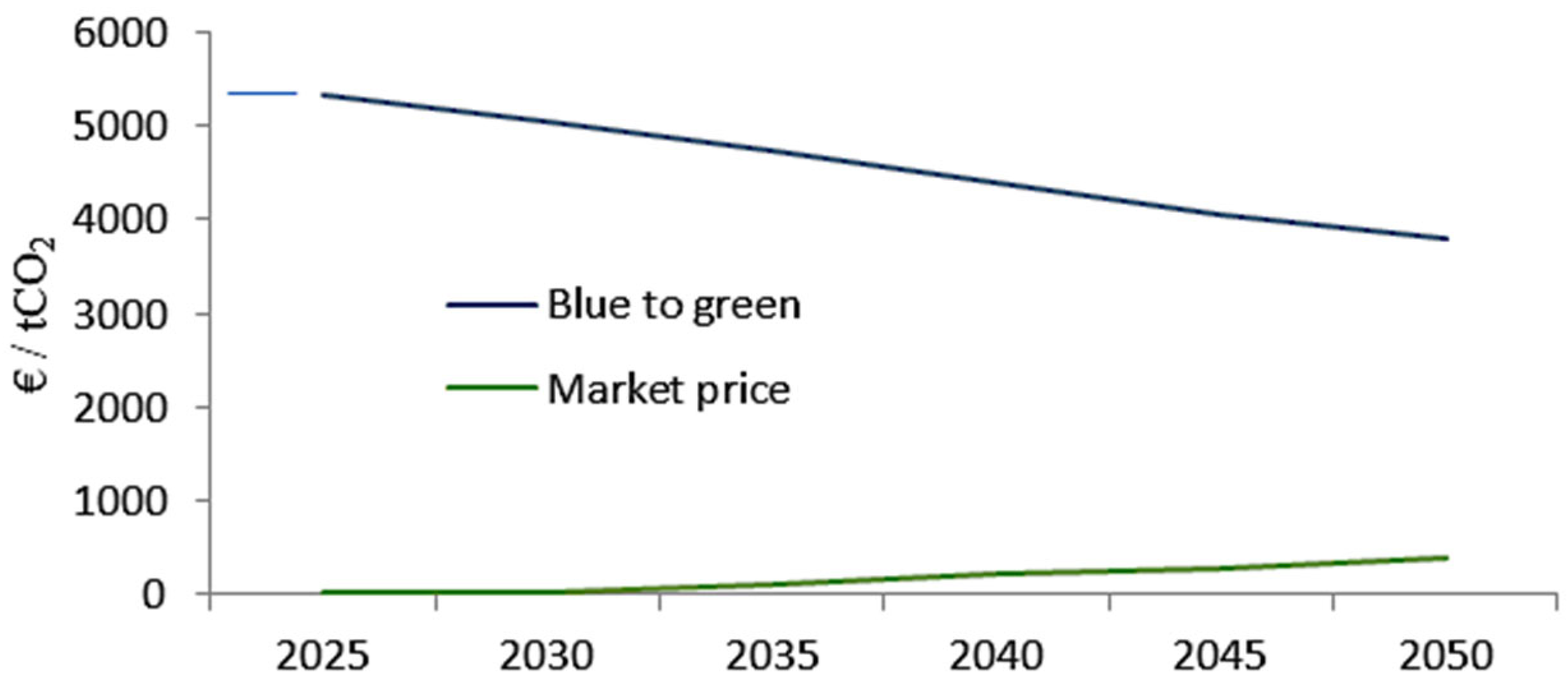
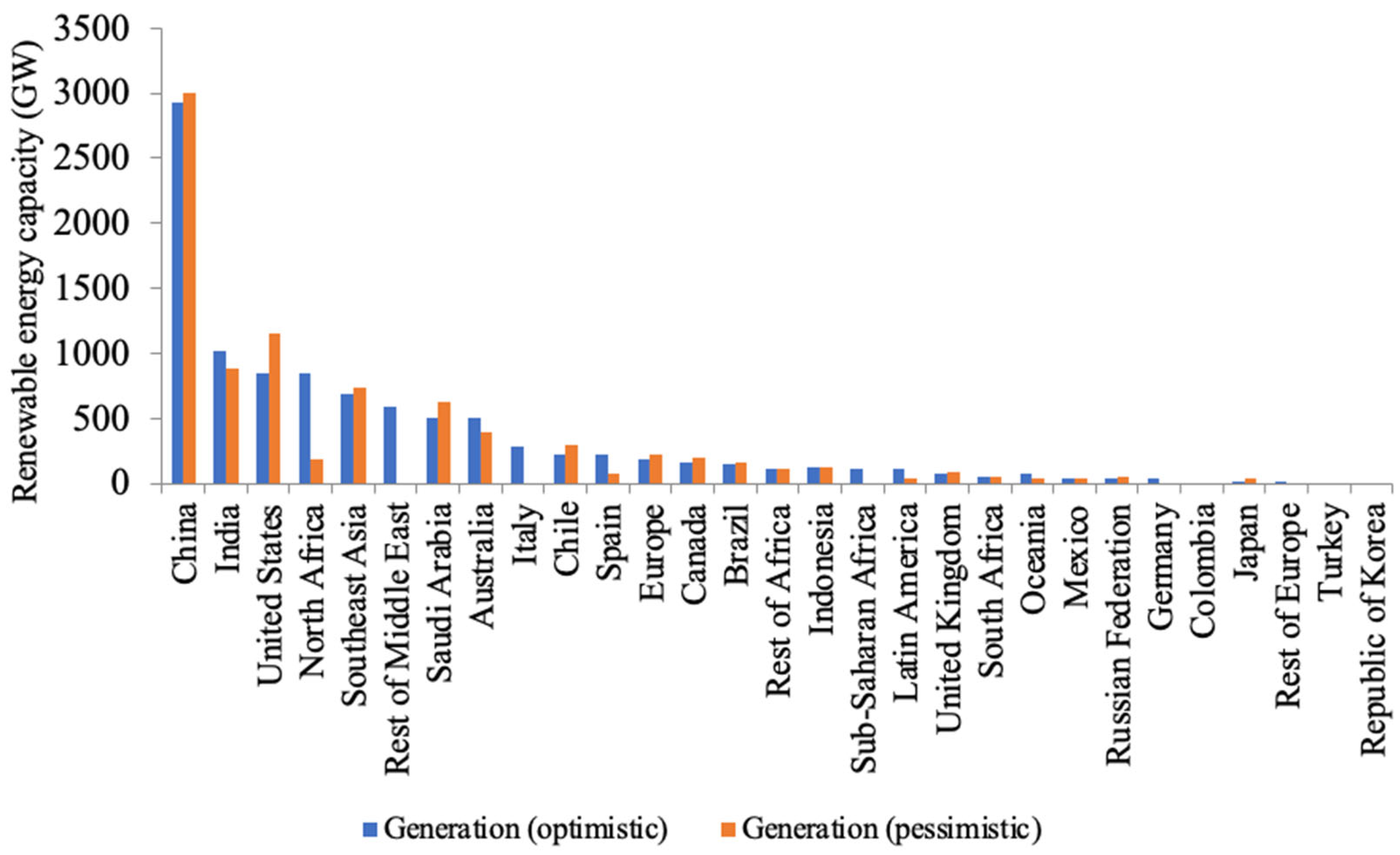
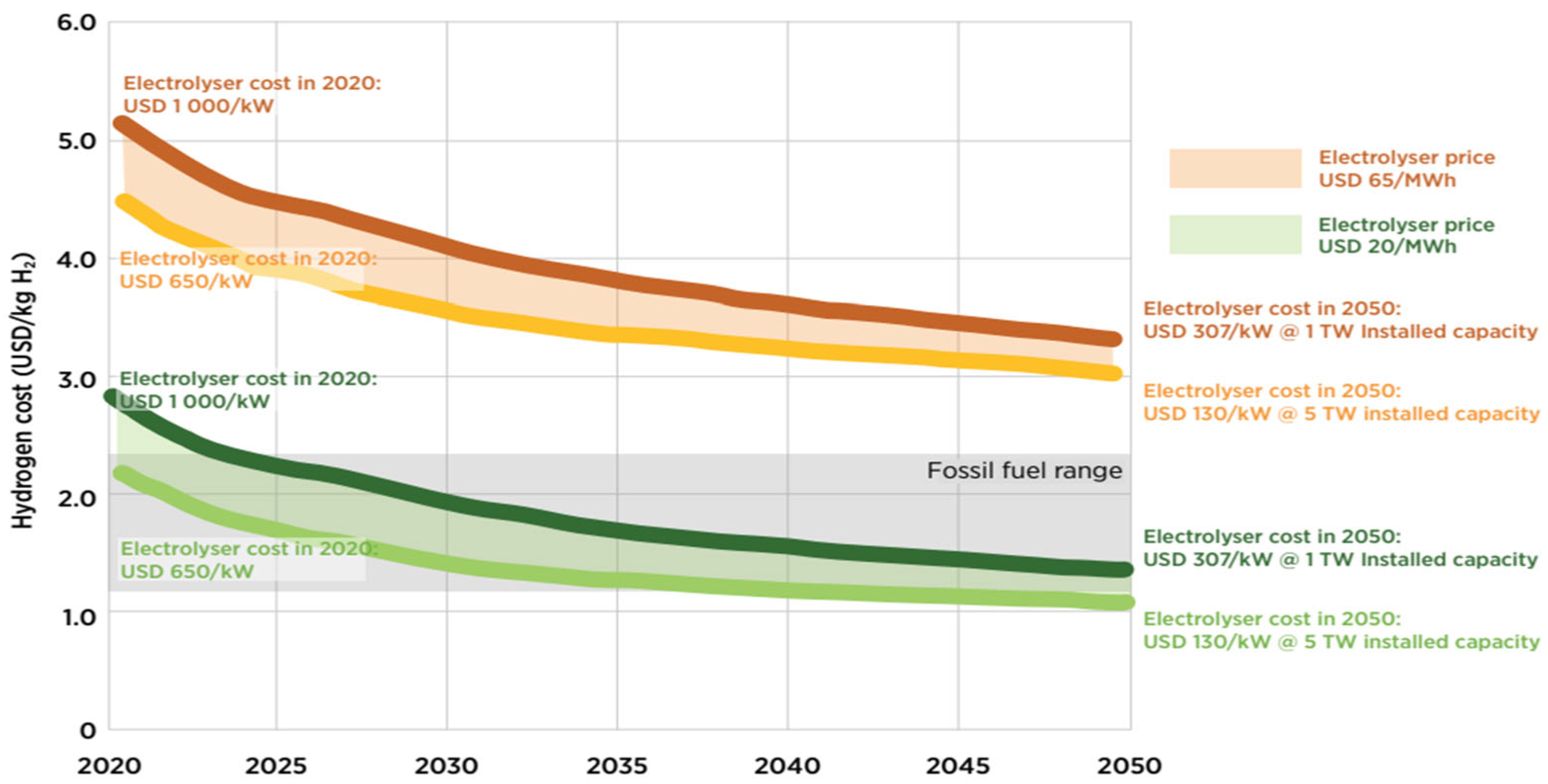
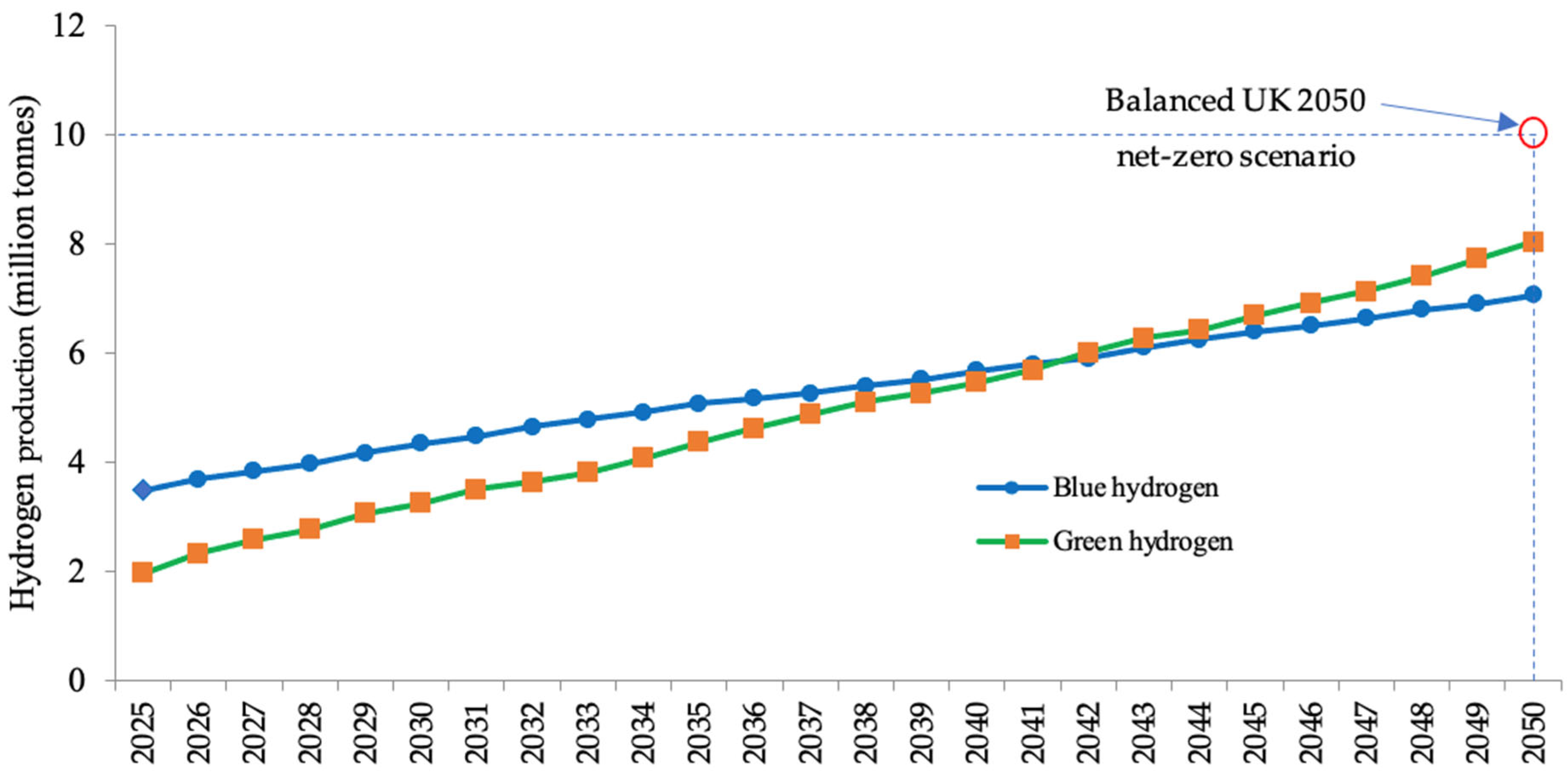
| Parameter | Blue Hydrogen | Green Hydrogen |
|---|---|---|
| Feedstock | Natural gas | Water and renewable electricity |
| Emission profile | Low (10–30 gCO2/MJ) | Very low (<5 gCO2/MJ) |
| CAPEX | USD 800–$1400/Kw | USD 1100–1800/kW |
| Levelised cost | USD 2.0–$3.5/kg H2 | USD 3.5–6.0/kg H2 |
| Maturity | High (commercial) | Emerging (pilot-scale to low-scale) |
| Key barrier | CH4 leakage, CCS cost | High electricity demand/cost |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammar, M.; Oyewale, B.O.; Elseragy, A.; Albayati, I.M.; Aliyu, A.M. A Global Review of Blue and Green Hydrogen Fuel Production Technologies, Trends and Future Outlook to 2050. Fuels 2025, 6, 88. https://doi.org/10.3390/fuels6040088
Ammar M, Oyewale BO, Elseragy A, Albayati IM, Aliyu AM. A Global Review of Blue and Green Hydrogen Fuel Production Technologies, Trends and Future Outlook to 2050. Fuels. 2025; 6(4):88. https://doi.org/10.3390/fuels6040088
Chicago/Turabian StyleAmmar, Muhammad, Babatunde Oyeleke Oyewale, Ahmed Elseragy, Ibrahim M. Albayati, and Aliyu M. Aliyu. 2025. "A Global Review of Blue and Green Hydrogen Fuel Production Technologies, Trends and Future Outlook to 2050" Fuels 6, no. 4: 88. https://doi.org/10.3390/fuels6040088
APA StyleAmmar, M., Oyewale, B. O., Elseragy, A., Albayati, I. M., & Aliyu, A. M. (2025). A Global Review of Blue and Green Hydrogen Fuel Production Technologies, Trends and Future Outlook to 2050. Fuels, 6(4), 88. https://doi.org/10.3390/fuels6040088