Tuning Chemical Looping Steam Reforming of Methane Performance via Ni-Fe-Al Interaction in Spinel Ferrites
Abstract
1. Introduction
2. Materials and Methods
2.1. Oxygen Carrier Preparation
2.2. Experimental Setup
2.3. Oxygen Carrier Characterization
3. Results and Discussion
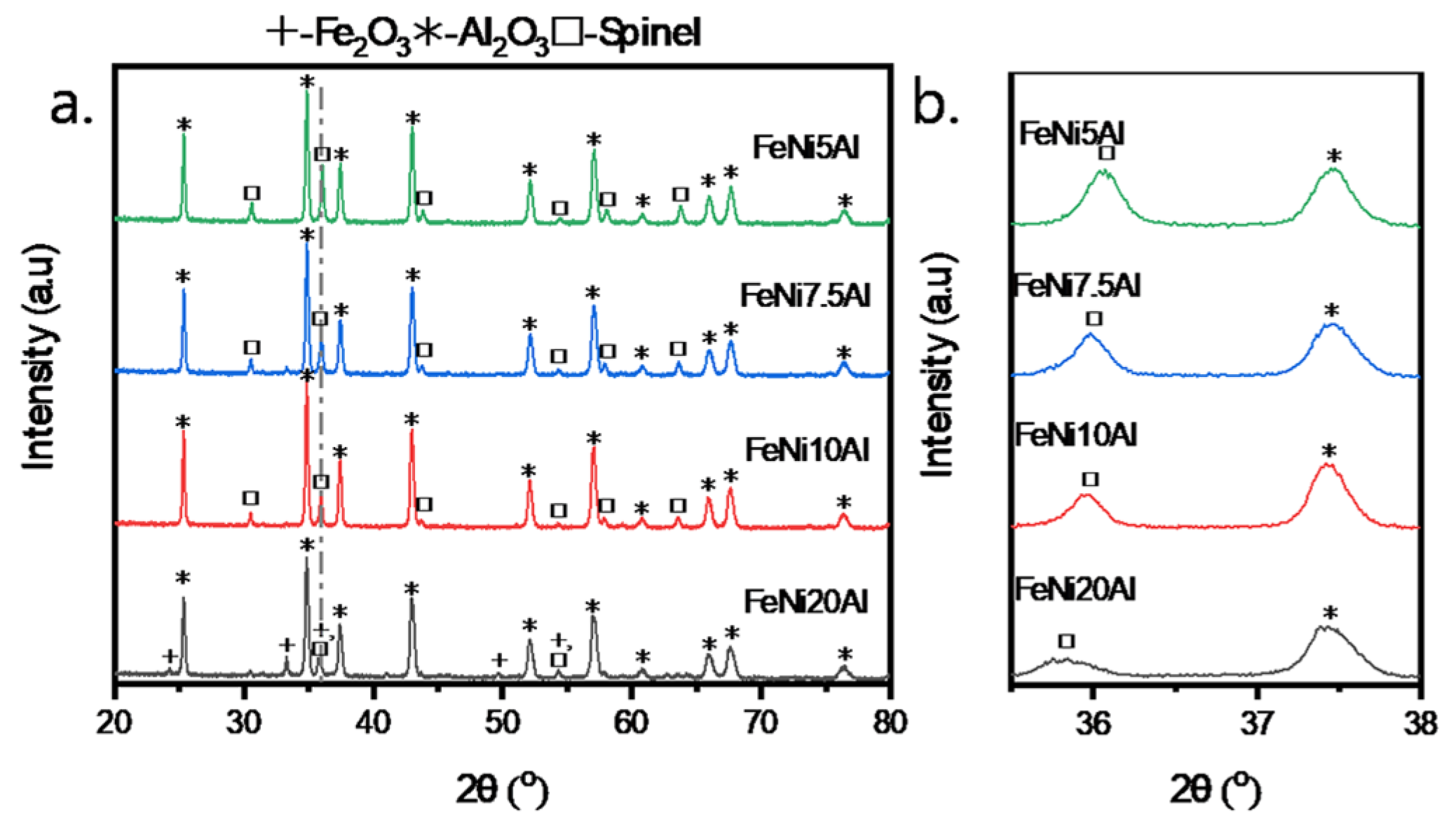
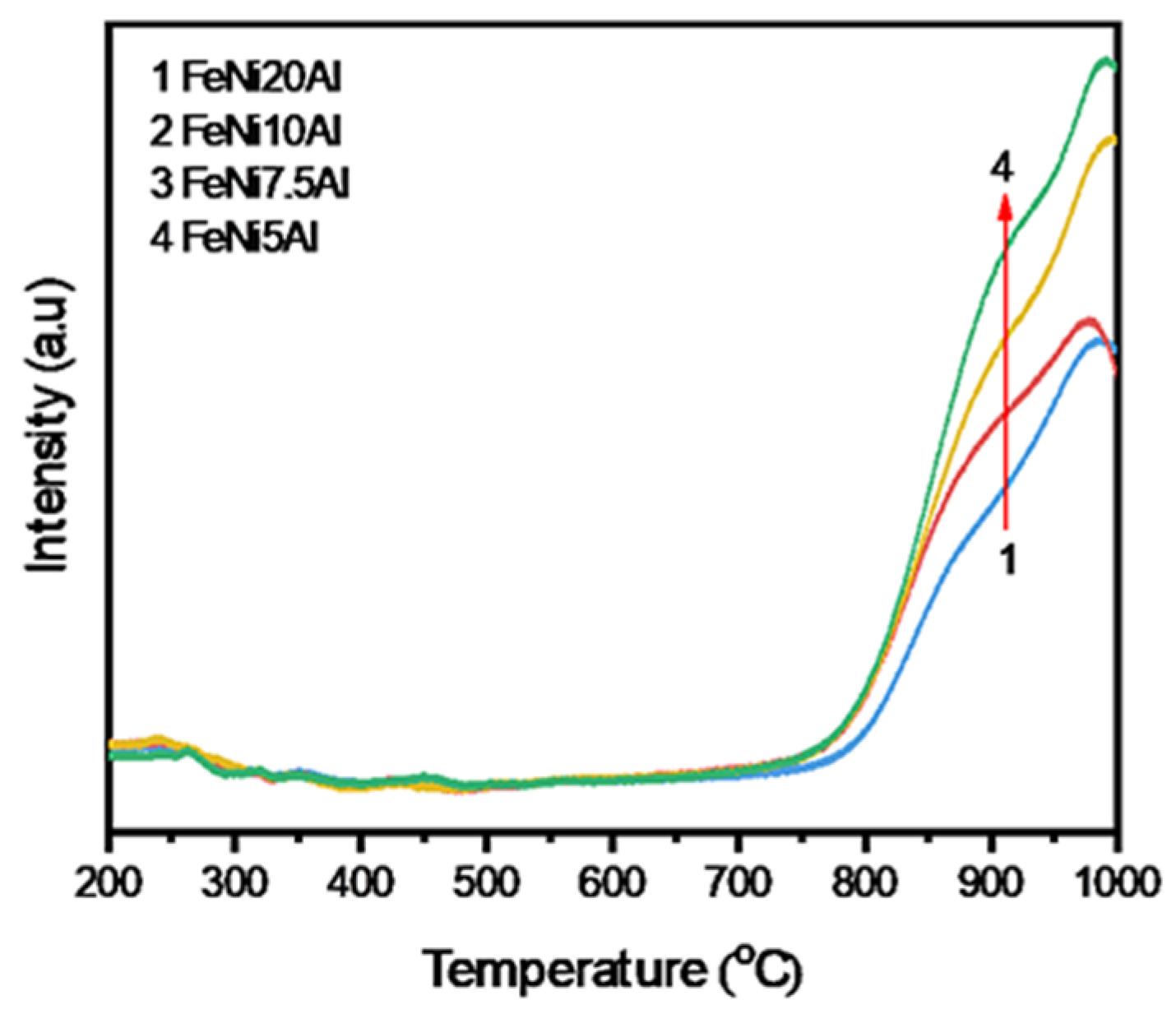
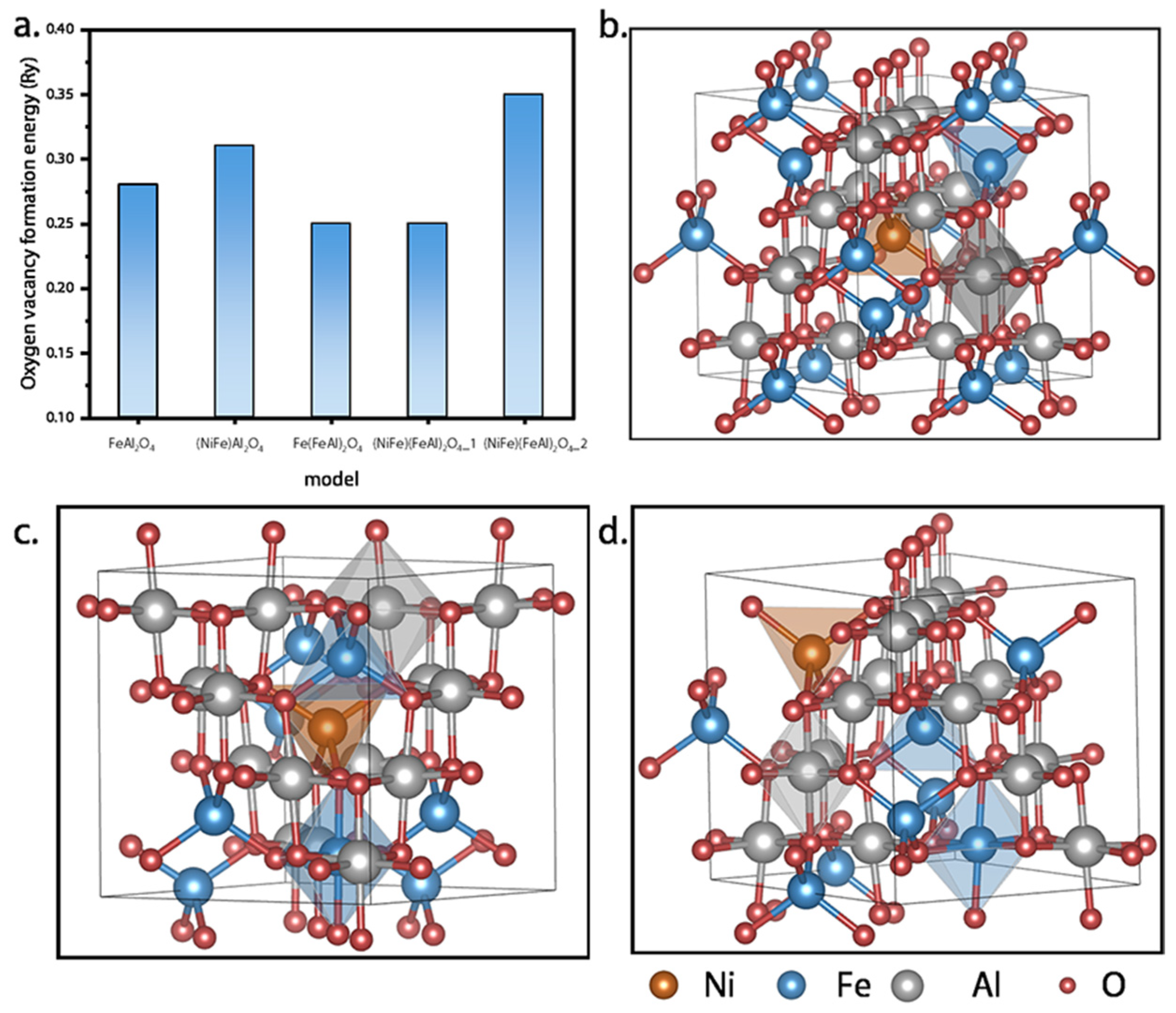
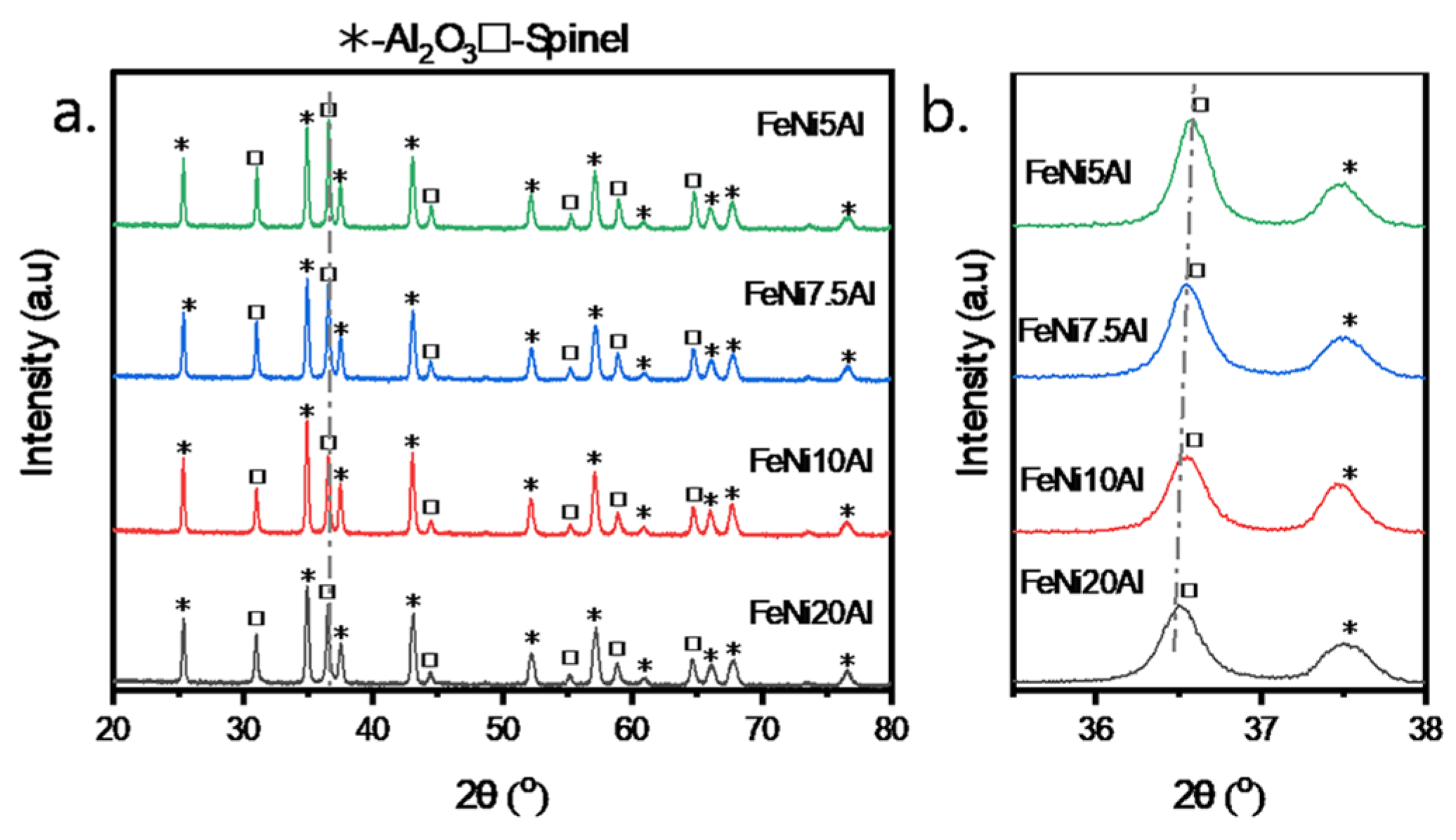
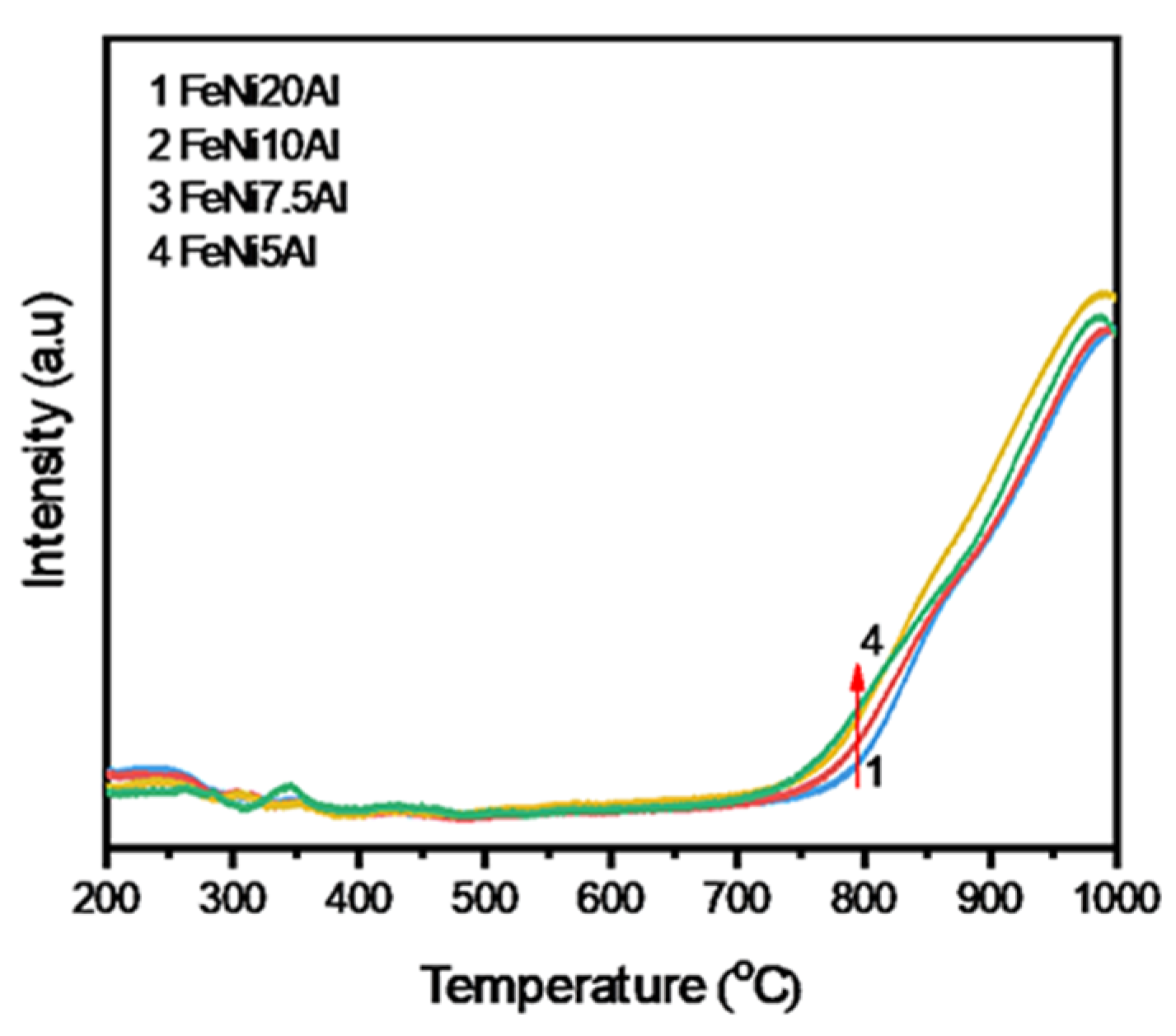
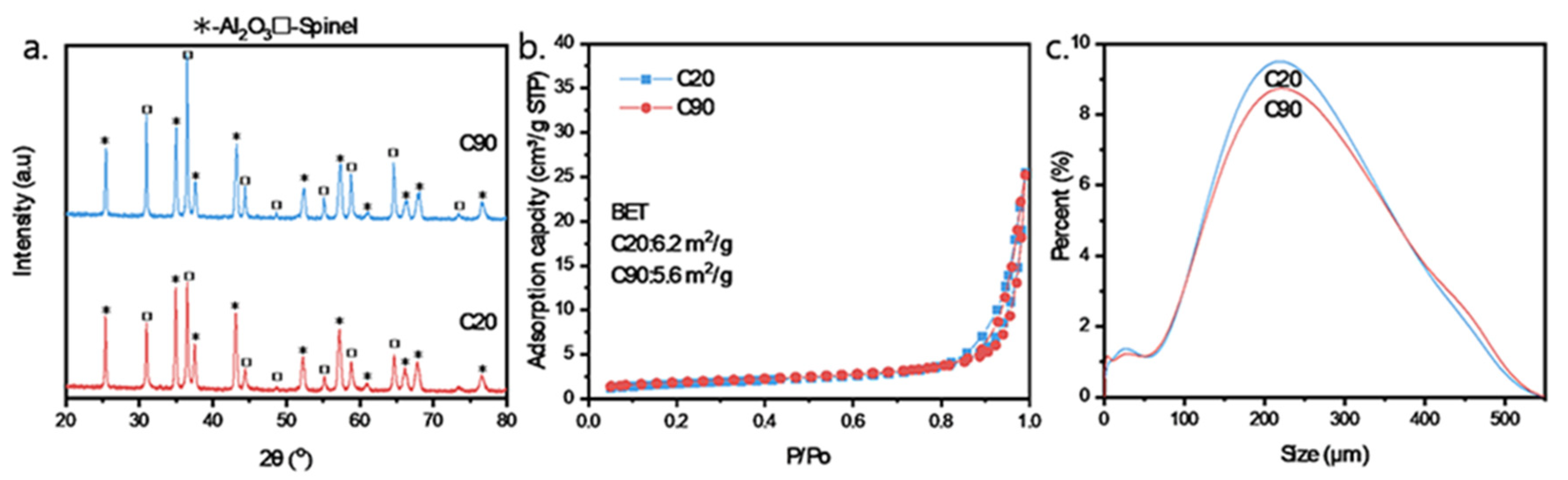
3.1. Interaction Between Ni, Fe, and Al
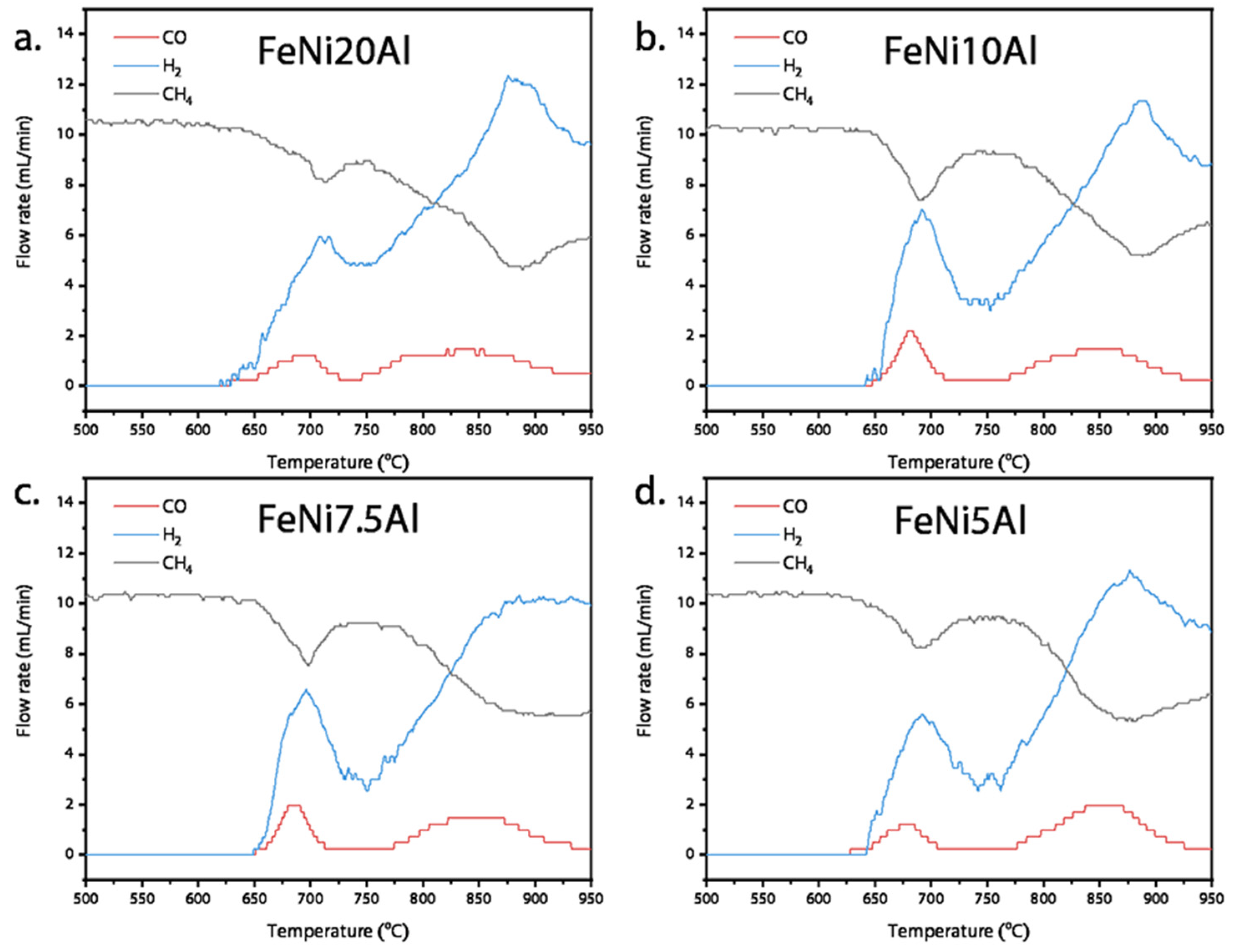
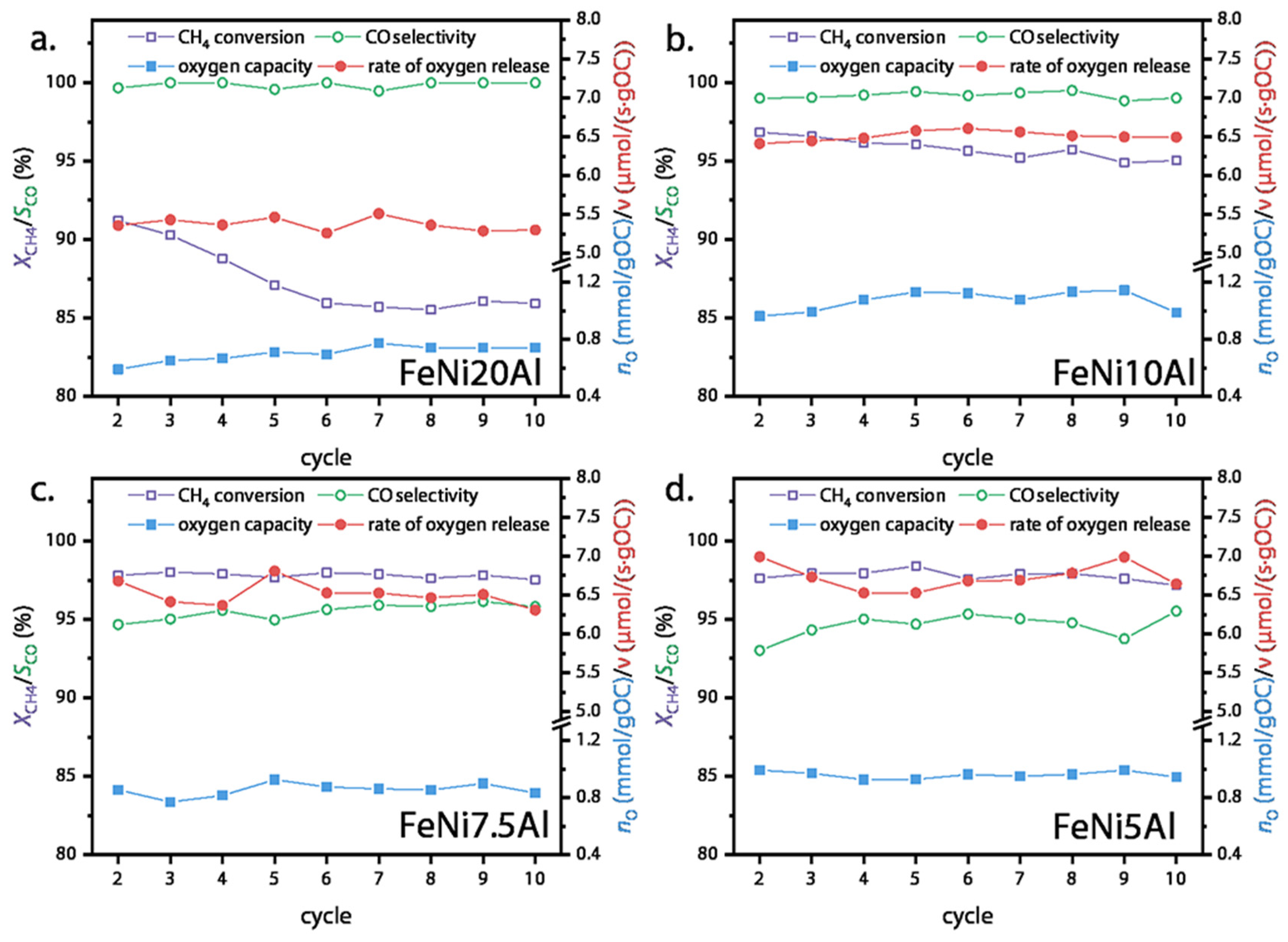
3.2. CLSR Performance of OCs
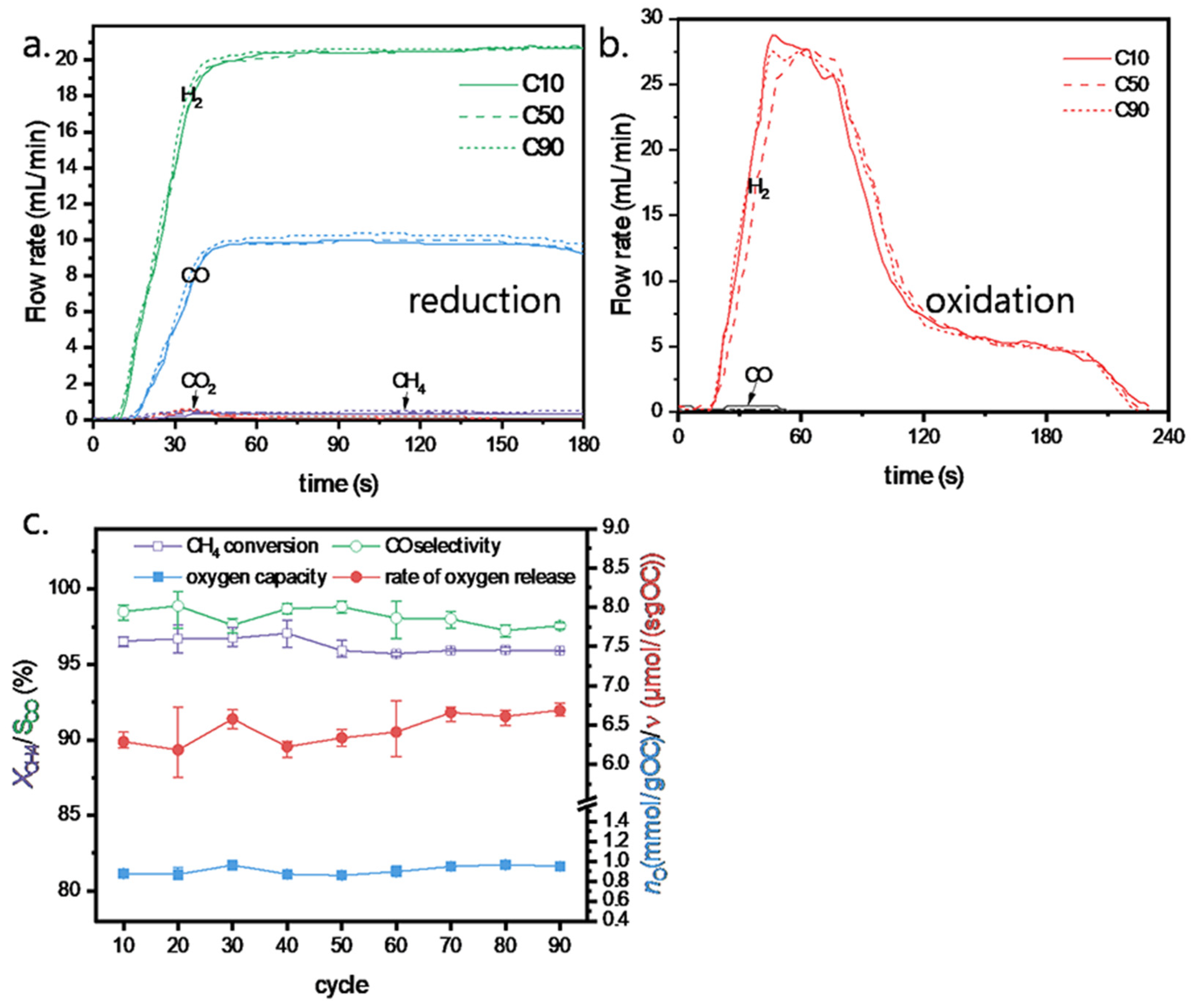
3.3. Cyclic Stability of FeNi10Al OC
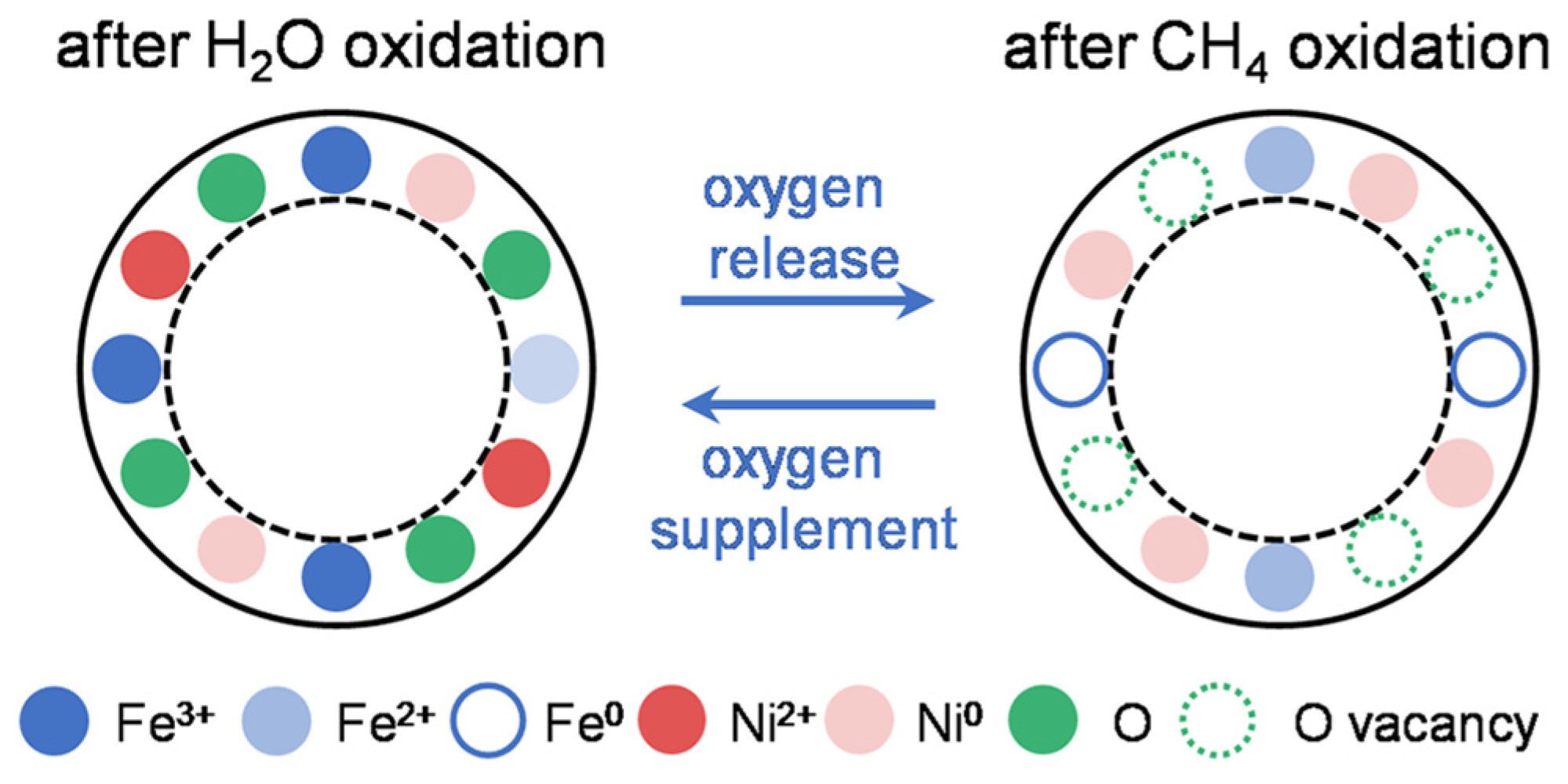
3.4. Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Jiang, Y.; Liu, X.; Xia, W.; Huang, A.; Zong, J.; Wang, Z.; Qian, B.; Donat, F. One-Step Synthesis of CaO/CuO Composite Pellets for Enhanced CO2 Capture Performance in a Combined Ca/Cu Looping Process via a Facile Gel-Casting Technique. Sep. Purif. Technol. 2024, 328, 125057. [Google Scholar] [CrossRef]
- Chen, J.; Duan, L.; Sun, Z. Review on the Development of Sorbents for Calcium Looping. Energy Fuels 2020, 34, 7806–7836. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhong, M.; Wang, C.; Cai, Y.; Xiao, B.; Xu, T.; Wang, X. Chemical Looping Reforming with Water Splitting (CLRWS) for Syngas and Hydrogen Coproduction Using the Ternary Metal-Oxides (Ca-Fe-Cu–O) as Oxygen Carriers. Fuel 2024, 368, 131640. [Google Scholar] [CrossRef]
- Zhong, M.; Xu, T.; Wang, C.; Teng, Y.; Cai, Y.; Zhang, Z.; Xiao, B.; Wang, X. Utilizing High Entropy Oxide (Ni0.2Co0.2Ca0.2Cu0.2Mg0.2)Fe2O4 in Chemical Looping Process for Highly Efficient and Stable Hydrogen Production. Chem. Eng. J. 2024, 487, 150521. [Google Scholar] [CrossRef]
- Wang, C.; Liu, T.; Xiao, R.; Zeng, D. High-Purity Hydrogen Obtained via a Plasma-Assisted Chemical Looping Process Using Perovskite-Supported Iron Oxides as Oxygen Carriers. Energy Fuels 2023, 37, 14141–14149. [Google Scholar] [CrossRef]
- Long, Y.; Yang, K.; Gu, Z.; Lin, S.; Li, D.; Zhu, X.; Wang, H.; Li, K. Hydrogen Generation from Water Splitting over Polyfunctional Perovskite Oxygen Carriers by Using Coke Oven Gas as Reducing Agent. Appl. Catal. B-Environ. 2022, 301, 120778. [Google Scholar] [CrossRef]
- Wen, Z.; Duan, N.; Zhang, R.; Li, H.; Wu, Y.; Sun, Z.; Sun, Z. Machine Learning-Based Deoxidizer Screening for Intensified Hydrogen Production from Steam Splitting. J. Cleaner Prod. 2024, 449, 141779. [Google Scholar] [CrossRef]
- Du, J.; Wu, M.; Chen, S.; Xiang, W. Interactions of Mg-Fe-Al-O Oxygen Carriers with Rare Earth Dopants (Ce, Y, Sm, La, and Pr) in Chemical Looping Steam Reforming. Fuel 2024, 361, 130606. [Google Scholar] [CrossRef]
- Sayyed, S.Z.; Vaidya, P.D. Chemical Looping-Steam Reforming of Biogas and Methane over Lanthanum-Based Perovskite for Improved Production of Syngas and Hydrogen. Energy Fuels 2023, 37, 19082–19091. [Google Scholar] [CrossRef]
- He, J.; Yang, Q.; Song, Z.; Chang, W.; Huang, C.; Zhu, Y.; Ma, X.; Wang, X. Improving the Carbon Resistance of Iron-Based Oxygen Carrier for Hydrogen Production via Chemical Looping Steam Methane Reforming: A Review. Fuel 2023, 351, 128864. [Google Scholar] [CrossRef]
- Song, D.; Long, T.; Li, C.; Li, Y.; Fan, M.; Lu, Y.; Zhou, Y.; Chen, H.; Lin, Y.; Huang, Z.; et al. Micro-Structure Change and Crystal-Structure Modulated of Oxygen Carriers for Chemical Looping: Controlling Local Chemical Environment of Lattice Oxygen. Fuel 2024, 364, 131087. [Google Scholar] [CrossRef]
- Sun, Z.; Russell, C.K.; Whitty, K.J.; Eddings, E.G.; Dai, J.; Zhang, Y.; Fan, M.; Sun, Z. Chemical Looping-Based Energy Transformation via Lattice Oxygen Modulated Selective Oxidation. Prog. Energy Combust. Sci. 2023, 96, 101045. [Google Scholar] [CrossRef]
- Yang, T.; Luo, R.; Shi, X.; Zhang, X.; Wu, S.; Pei, C.; Zhao, Z.-J.; Gong, J. Achieving Surface and Bulk Rate Matching for Chemical Looping Partial Oxidation of Methane by Modulating Oxygen Transport. Sci. China Mater. 2024, 67, 1217–1224. [Google Scholar] [CrossRef]
- Liu, F.; Liu, J.; Yang, Y. Review on the Theoretical Understanding of Oxygen Carrier Development for Chemical-Looping Technologies. Energy Fuels 2022, 36, 9373–9384. [Google Scholar] [CrossRef]
- Wu, S.; Ren, Z.; Hu, Q.; Yao, D.; Yang, H. Upcycling Plastic Waste into Syngas by Staged Chemical Looping Gasification with Modified Fe-Based Oxygen Carriers. Appl. Energy 2024, 353, 122105. [Google Scholar] [CrossRef]
- Wang, F.; Chen, S.; Duan, L.; Xiang, W. Carbon Dioxide Capture and Hydrogen Production with a Chemical Looping Concept: A Review on Oxygen Carrier and Reactor. Energy Fuels 2023, 37, 16245–16266. [Google Scholar] [CrossRef]
- Zhao, L.; Dou, B.; Zhang, H.; Wang, Z. Oxygen Carriers for Chemical-Looping Water Splitting to Hydrogen Production: A Critical Review. Carbon Capture Sci. Technol. 2021, 1, 100006. [Google Scholar] [CrossRef]
- Tian, M.; Wang, C.; Han, Y.; Wang, X. Recent Advances of Oxygen Carriers for Chemical Looping Reforming of Methane. ChemCatChem 2021, 13, 1615–1637. [Google Scholar] [CrossRef]
- Li, D.; Xu, R.; Gu, Z.; Zhu, X.; Qing, S.; Li, K. Chemical-Looping Conversion of Methane: A Review. Energy Technol. 2020, 8, 1900925. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, T.; Rao, Q.; Gai, Z.; Li, P.; Shen, Y.; Liu, M.; Pan, Y.; Jin, H. Enhancement of Fe/Ce Oxygen Carrier Performance in Chemical Looping Dry Reforming of Methane. Fuel 2024, 366, 131344. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, D.; Barakat, M.; Sun, L.; Gong, F.; Xiao, R. Improving the Reactivity and Stability of Fe2O3/Al2O3 in Chemical Looping Process by Optimizing the Al2O3 Precursor. Ind. Eng. Chem. Res. 2024, 63, 3092–3103. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, Y.; Han, H.; Zhang, Y.; Wang, H.; Gong, X.; Chen, Y. Characterization and Performance of Sr and Co Doped Fe2O3–LaFeO3 Quaternary Perovskite Composite Oxides for Efficient Realization of CH4 Chemical Looping Conversion. ACS Sustain. Chem. Eng. 2024, 12, 3265–3278. [Google Scholar] [CrossRef]
- Hu, J.; Chen, S.; Xiang, W. Ni, Co and Cu-Promoted Iron-Based Oxygen Carriers in Methane-Fueled Chemical Looping Hydrogen Generation Process. Fuel Process. Technol. 2021, 221, 106917. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Lin, Y.; Huang, Z.; Zhang, Y.; Xiong, Y.; Yi, Q.; Yan, L.; Li, J.; Deng, L.; et al. Effect of Nickel-Modified Hematite on the Conversion of Volatile and Char during Chemical Looping Gasification of Wood-Based Panel Waste. Energy Fuels 2024, 38, 8842–8852. [Google Scholar] [CrossRef]
- Samprón, I.; García-Labiano, F.; Izquierdo, M.T.; de Diego, L.F. Understanding the Structural Changes on Fe2O3/Al2O3 Oxygen Carriers under Chemical Looping Gasification Conditions. Fuel 2024, 355, 129326. [Google Scholar] [CrossRef]
- Kang, Y.; Tian, M.; Wang, Y.; Wang, Y.; Huang, C.; Zhu, Y.; Li, L.; Wang, G.; Wang, X. Silica Modified Alumina as Supports of Fe2O3 with High Performance in Chemical Looping Combustion of Methane. ACS Sustain. Chem. Eng. 2018, 6, 12884–12892. [Google Scholar] [CrossRef]
- Liu, W. Controlling Lattice Oxygen Activity of Oxygen Carrier Materials by Design: A Review and Perspective. React. Chem. Eng. 2021, 6, 1527–1537. [Google Scholar] [CrossRef]
- Rao, Q.; Zhang, J.; Yang, T.; Li, Y.; Gai, Z.; Li, P.; Wang, X.; Pan, Y.; Jin, H. A Nickel-Modified Perovskite-Supported Iron Oxide Oxygen Carrier for Chemical Looping Dry Reforming of Methane for Syngas Production. Chem. Eng. J. 2024, 485, 150033. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, G.; Huo, C.; Liu, J.; Zhang, B.; Yang, B.; Tian, X.; Wu, Z. Tailoring Catalytic and Oxygen Release Capability in LaFe1–xNixO3 to Intensify Chemical Looping Reactions at Medium Temperatures. ACS Catal. 2024, 14, 7771–7787. [Google Scholar] [CrossRef]
- Liang, S.; Liao, Y.; Yang, H.; Zhang, T.; Ma, X. Inhibition Strategies for Phase Separation of Iron-Based Oxygen Carriers in Chemical Looping Gasification- Enhanced Oxygen Transport Efficiency through Oxide-Support Interactions. Sep. Purif. Technol. 2024, 343, 127173. [Google Scholar] [CrossRef]
- Cui, D.; Qiu, Y.; Lv, Y.; Li, M.; Zhang, S.; Tippayawong, N.; Zeng, D.; Xiao, R. A High-Performance Oxygen Carrier with High Oxygen Transport Capacity and Redox Stability for Chemical Looping Combustion. Energy Convers. Manag. 2019, 202, 112209. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, H.S.; Lee, M.; Kim, M.; Kang, D.; Lee, J.W. Enhanced Morphological Preservation and Redox Activity in Al-Incorporated NiFe2O4 for Chemical Looping Hydrogen Production. ACS Sustain. Chem. Eng. 2021, 9, 14800–14810. [Google Scholar] [CrossRef]
- Chang, W.; Gao, Y.; He, J.; Xia, X.; Huang, C.; Hu, Y.; Xu, W.; Jiang, B.; Han, Y.; Zhu, Y.; et al. Asymmetric Coordination Activated Lattice Oxygen in Perovskite Ferrites for Selective Anaerobic Oxidation of Methane. J. Mater. Chem. A 2023, 11, 4651–4660. [Google Scholar] [CrossRef]
- Fan, Q.; Huang, C.; Xi, S.; Yan, Y.; Huang, J.; Saqline, S.; Tao, L.; Dai, Y.; Borgna, A.; Wang, X.; et al. Breaking the Stoichiometric Limit in Oxygen-Carrying Capacity of Fe-Based Oxygen Carriers for Chemical Looping Combustion Using the Mg-Fe-O Solid Solution System. ACS Sustain. Chem. Eng. 2022, 10, 7242–7252. [Google Scholar] [CrossRef]
- Zhang, X.; Pei, C.; Chang, X.; Chen, S.; Liu, R.; Zhao, Z.-J.; Mu, R.; Gong, J. FeO6 Octahedral Distortion Activates Lattice Oxygen in Perovskite Ferrite for Methane Partial Oxidation Coupled with CO2 Splitting. J. Am. Chem. Soc. 2020, 142, 11540–11549. [Google Scholar] [CrossRef]
- Xia, X.; Chang, W.; Cheng, S.; Huang, C.; Hu, Y.; Xu, W.; Zhang, L.; Jiang, B.; Sun, Z.; Zhu, Y.; et al. Oxygen Activity Tuning via FeO6 Octahedral Tilting in Perovskite Ferrites for Chemical Looping Dry Reforming of Methane. ACS Catal. 2022, 12, 7326–7335. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled Preparation, Oxygen Reduction/Evolution Reaction Application, and Beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef]
- Benrabaa, R.; Trentesaux, M.; Roussel, P.; Rubbens, A.; Vannier, R.-N.; Löfberg, A. NiAlxFe2−xO4 Mixed Oxide Catalysts for Methane Reforming with CO2: Effect of Al vs Fe Contents and Precursor Salts. J. CO2 Util. 2023, 67, 102319. [Google Scholar] [CrossRef]
- Hu, J.; Chen, S.; Xiang, W. Sintering and Agglomeration of Fe2O3-MgAl2O4 Oxygen Carriers with Different Fe2O3 Loadings in Chemical Looping Processes. Fuel 2020, 265, 116983. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, S.; Soomro, A.; Hu, J.; Sun, Z.; Ma, S.; Xiang, W. Effects of Supports on Reduction Activity and Carbon Deposition of Iron Oxide for Methane Chemical Looping Hydrogen Generation. Appl. Energy 2018, 225, 912–921. [Google Scholar] [CrossRef]
- Zhu, Y.; Jin, N.; Liu, R.; Sun, X.; Bai, L.; Tian, H.; Ma, X.; Wang, X. Bimetallic BaFe2MAl9O19 (M = Mn, Ni, and Co) Hexaaluminates as Oxygen Carriers for Chemical Looping Dry Reforming of Methane. Appl. Energy 2020, 258, 114070. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Cook, M.G. X-Ray Photoelectron Studies on Some Oxides and Hydroxides of Cobalt, Nickel, and Copper. Anal. Chem. 1975, 47, 2208–2213. [Google Scholar] [CrossRef]
- Salvati, L.; Makovsky, L.E.; Stencel, J.M.; Brown, F.R.; Hercules, D.M. Surface Spectroscopic Study of Tungsten-Alumina Catalysts Using x-Ray Photoelectron, Ion Scattering, and Raman Spectroscopies. J. Phys. Chem. 1981, 85, 3700–3707. [Google Scholar] [CrossRef]
- Huang, Z.; Gao, N.; Lin, Y.; Wei, G.; Zhao, K.; Zheng, A.; Zhao, Z.; Yuan, H.; Li, H. Exploring the Migration and Transformation of Lattice Oxygen during Chemical Looping with NiFe2O4 Oxygen Carrier. Chem. Eng. J. 2022, 429, 132064. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Yu, H.; Wang, Y. Tuning Chemical Looping Steam Reforming of Methane Performance via Ni-Fe-Al Interaction in Spinel Ferrites. Fuels 2025, 6, 76. https://doi.org/10.3390/fuels6040076
Hu J, Yu H, Wang Y. Tuning Chemical Looping Steam Reforming of Methane Performance via Ni-Fe-Al Interaction in Spinel Ferrites. Fuels. 2025; 6(4):76. https://doi.org/10.3390/fuels6040076
Chicago/Turabian StyleHu, Jun, Hongyang Yu, and Yanan Wang. 2025. "Tuning Chemical Looping Steam Reforming of Methane Performance via Ni-Fe-Al Interaction in Spinel Ferrites" Fuels 6, no. 4: 76. https://doi.org/10.3390/fuels6040076
APA StyleHu, J., Yu, H., & Wang, Y. (2025). Tuning Chemical Looping Steam Reforming of Methane Performance via Ni-Fe-Al Interaction in Spinel Ferrites. Fuels, 6(4), 76. https://doi.org/10.3390/fuels6040076




