2. Methodology
A compositional numerical model of a sector area of a limestone reservoir—meeting enhanced oil recovery (EOR) screening criteria for miscible CO
2 flooding—was developed using the CMG-GEM simulator (Computer Modelling Group, Version 2023) [
31]. Prior to simulation, fluid characterization and equation-of-state tuning were conducted using CMG-WinProp (Computer Modelling Group, Version 2023) [
32].
The compositional model incorporates complex fluid flow and CO2 retention mechanisms, including wettability alteration, miscibility, aqueous solubility of CO2, residual oil saturation, injected pore volume tracking, and the water-alternating-gas (WAG) injection scheme. EOR and sequestration efficiencies are evaluated through key metrics such as the CO2 utilization ratio, retention capacity, and sensitivity to both reservoir and operational parameters.
2.1. Overview
A single five-spot pattern is used to simulate the EOR/CCUS application for a limestone reservoir at a depth of 13,000 ft containing a light, 38° API oil. Such depth ensures pressure and temperature that are favorable for miscibility and storage efficiency. The facies are dominated by a clean limestone (calcite), which is suitable for CO
2 EOR injection, and an open strike-slip fault constitutes heterogeneity. The negligible vertical and horizontal permeability of the overlying and underlying formations ensure containment and no cap-rock leakage. The model’s general details are shown in
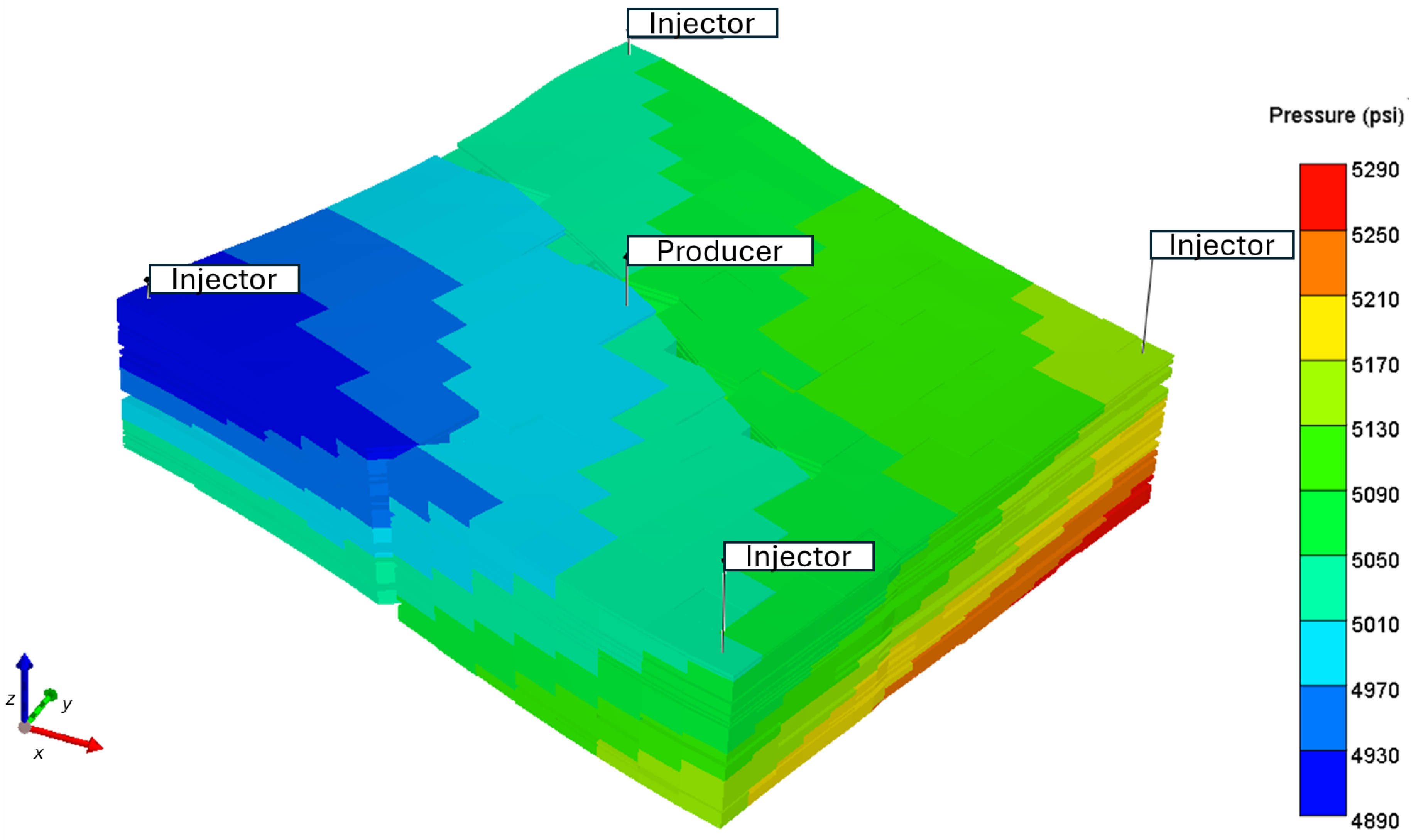
Table 2. A sector, 144 acres in size, is used where the four injectors are placed on the corner of the model, and the producer is centered as shown in
Figure 2. The injector to producer distance is 1773 ft. The oil in place is 8 MMSTB, with a Hydrocarbon Pore Volume (HCPV) of 66 MMSTB.
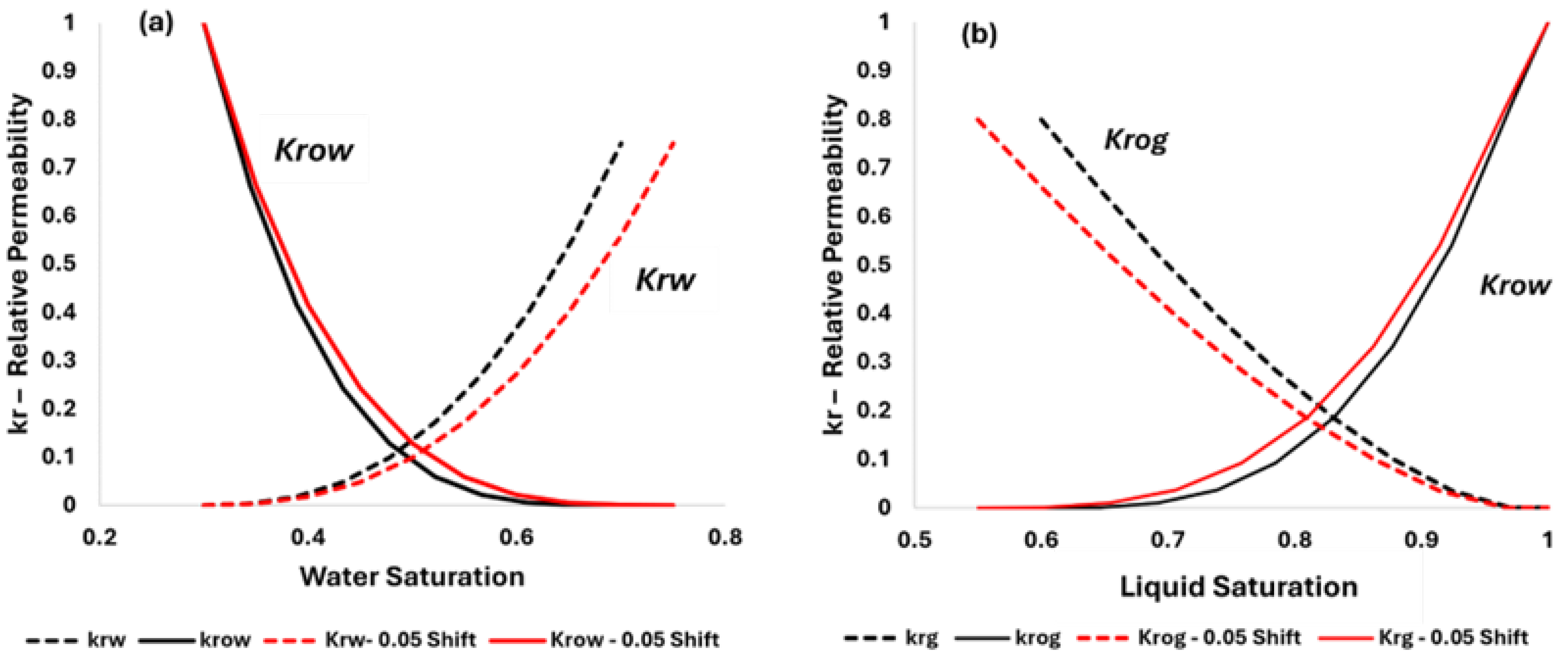
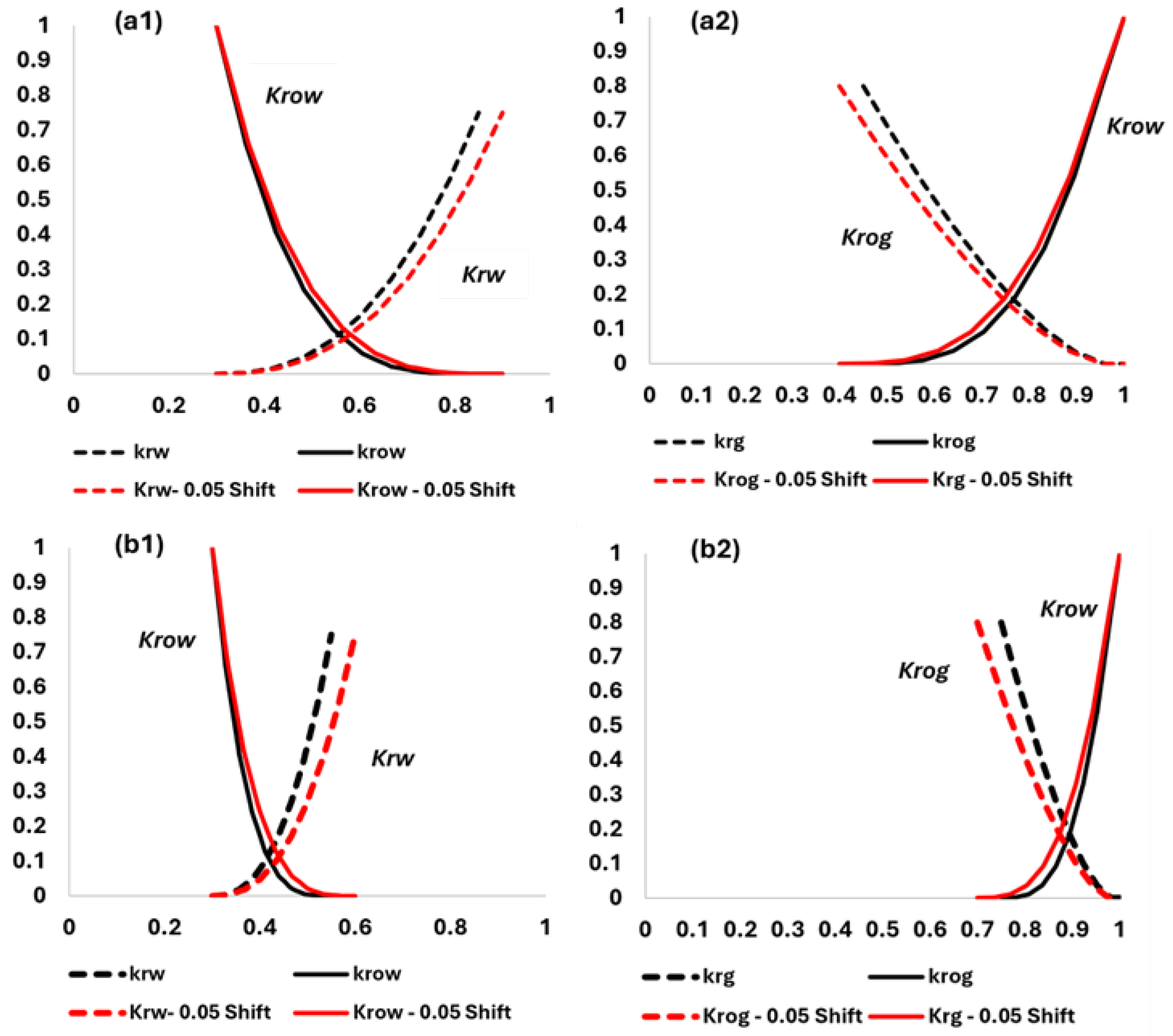
The relative permeability relations shown in
Figure 3 assumed a mixed-wet system (30% for both residual oil and irreducible water saturations). The following figure illustrates the impact of the CO
2 effect on relative permeability endpoints for oil–water and liquid–gas systems. The residual oil saturation is assumed to drop from the original 30% to 25% when CO
2 concentration exceeds 50%. Relative permeability curves are comparable to those presented by Alam et al. [
19] with small variations attributed to differences in rock characteristics.
2.2. CO2 Emission Source
This project considers the potential of eliminating a significant portion of the CO
2 emitted from the boilers used in a steam-injection EOR project in a heavy oil reservoir. Considering that the capacity of a single steam boiler is 58 tons/h [
33] and a total of 10 boilers are used, the CO
2 emission from the thermal EOR project amounts to 119,000 and 2,370,000 metric tons per year and over a project period of 20 years, respectively.
By the recent SCC estimate of USD 185 per metric ton [
9], this translates to a minimum social cost of carbon of USD 440 million for the proposed emission source over a 20-year period. Using this large CO
2 source to increase hydrocarbon recovery in a nearby oil field can offer an effective means of eliminating CO
2 from the atmosphere while improving the economics of the capture and sequestration process.
A typical example of thermal oil recovery with steam boilers is the oil field located in Kern County, California. Thermal recovery is the predominant enhanced oil recovery method in California, accounting for 96.5% of thermal applications in the United States [
34]. The capacity of steam boilers utilized for thermal applications in the United States begins at 127,500 lb/h [
33]. This is equal to 58 tons every hour. Another example is Petrofac, which created one of the largest projects in the Middle East, aiming for heavy oil production via steam flooding in the lower Fars Formation in Kuwait. Towering steam generators are utilized to supply the requisite steam for injection during heavy oil production [
35].
2.3. Fluid Characterization
A compositional model is used to assess the performances of improved oil recovery (IOR) and EOR techniques in a 5-spot pattern. To quantify the EOR and sequestration efficiencies, the CO2 utilization ratio (UR), CO2 retention capacity (RC), and recovery factors (RF) will be used. The technical evaluations assume the availability of a sufficient amount of CO2 at a favorable price as well as incentive structures to make project economics viable.
The reservoir fluid is characterized by fitting the available PVT data using the Peng–Robinson (PR) equation of state (EOS) [
36] with available PVT data, along with regression techniques to fit the data. The solubility of CO
2 in the aqueous phase and in oil will be validated later in this section to ensure reasonable input. The viscosity and specific gravity of the fluid are 0.5 cP and 38° API, respectively. The component lumping of fluid composition shown in
Table 3 is used to match the PVT data. The additional PVT properties are provided in
Table 4.
Fluid characterization and equation-of-state (EOS) tuning were conducted in CMG-WinProp, Version 2023 [
32] to generate the compositional input required for simulation. WinProp is a tool to align the equation-of-state (EOS) parameters with laboratory data and produce phase behavior predictions for compositional reservoir simulation [
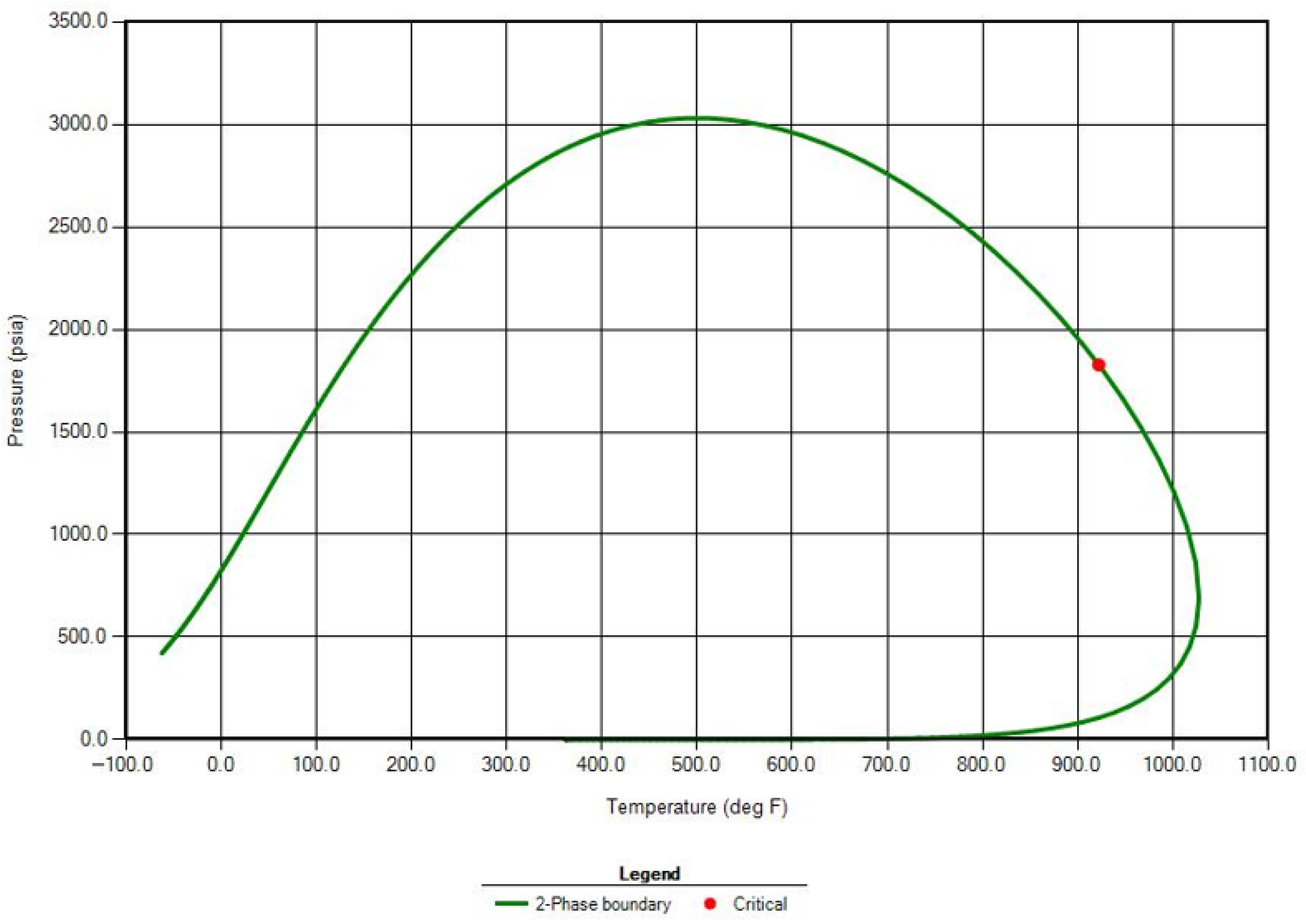
32]. Regression was performed to match saturation pressure, constant composition expansion (CCE), differential liberation, and viscosity data using the Peng–Robinson EOS. Greater weight factors were applied to saturation pressure and key fluid properties such as critical pressure and temperature, acentric factor, and the molecular weight of heavy pseudo-components. An example of the general phase behavior of the tuned fluid system generated in WinProp is shown in
Figure 4.
The minimum miscibility pressure (MMP) was calculated numerically via the multiple mixing cell method and verified by comparison to published correlations. The multiple mixing cell method divides the reservoir into segments with different compositions and generates key tie-lines for displacement [
37]. The MMP to achieve multiple contact miscibility was calculated as 3680 psi by the multiple mixing cell method, and a phase envelope similar to that in
Figure 4. This estimate of the MMP is in reasonable agreement with the empirical estimate of 3630 psia by Dindoruk Correlation [
38] for pure CO
2 injection.
2.4. Aqueous and Oil Solubility of CO2
The solubility of CO
2 is influenced by temperature, salinity, and pressure. The findings from Duan and Sun (2003) [
39] indicate that the CO
2 solubility in 1 mol aqueous NaCl solution is 1.1129 mol/kg (140 SCF/STB), while in 1 mol aqueous CaCl
2 solution and pure water, this value is 0.94 mol/kg (120 SCF/STB) and 1.3088 mol/kg (164 SCF/STB), respectively. The experimental work of Wang et al. (2024) [
40] showed that CO
2 solubility in live formations is less than that in dead water under reservoir conditions due to brine salinity. For example, the CO
2 solubility in distilled water is 1.4 mol/kg (190 SCF/STB) at 6000 psia [
40].
Figure 5 shows two trends of CO
2 aqueous solubility versus pressure from an experimental data set [
39] and Harvey correlation [
31]. The correlation estimate of CO
2 aqueous solubility was optimistic in comparison to the experimental range and literature data. The experimental range selected for this study was 1 m aqueous salinity = 50,000 ppm. For the correlation that was used in the CMG simulator, Henry’s constant under reservoir conditions (5000 psia and 240 °F) was calculated as
, which results in 203 SCF/STB once embedded in the Krichevsky–Kasarnovsky equation [
41]. The Harvey correlation [
31] was initially used in the numerical model to calculate the CO
2 solubility with temperature dependency. Therefore, the experimental range corresponding to the reservoir conditions in this work, 143 SCF/STB. This range was used for the CO
2 aqueous solubility and embedded in the numerical model.
As for the CO
2 solubility in oil, the CMG-Gems simulator numerically calculated the CO
2–oil mole fraction as 0.6. The experimental data (106 data points) compiled by Emera [
42], on the other hand, showed increasing solubility with pressure. Interpolating for the reservoir condition (5000 psia = 34 MPa, 240 °F = 116 °C) led to an approximately 0.63 mole fraction (solubility), which was reasonably aligned with the estimation of the simulator (CMG-Gems).
2.5. Case Scenarios
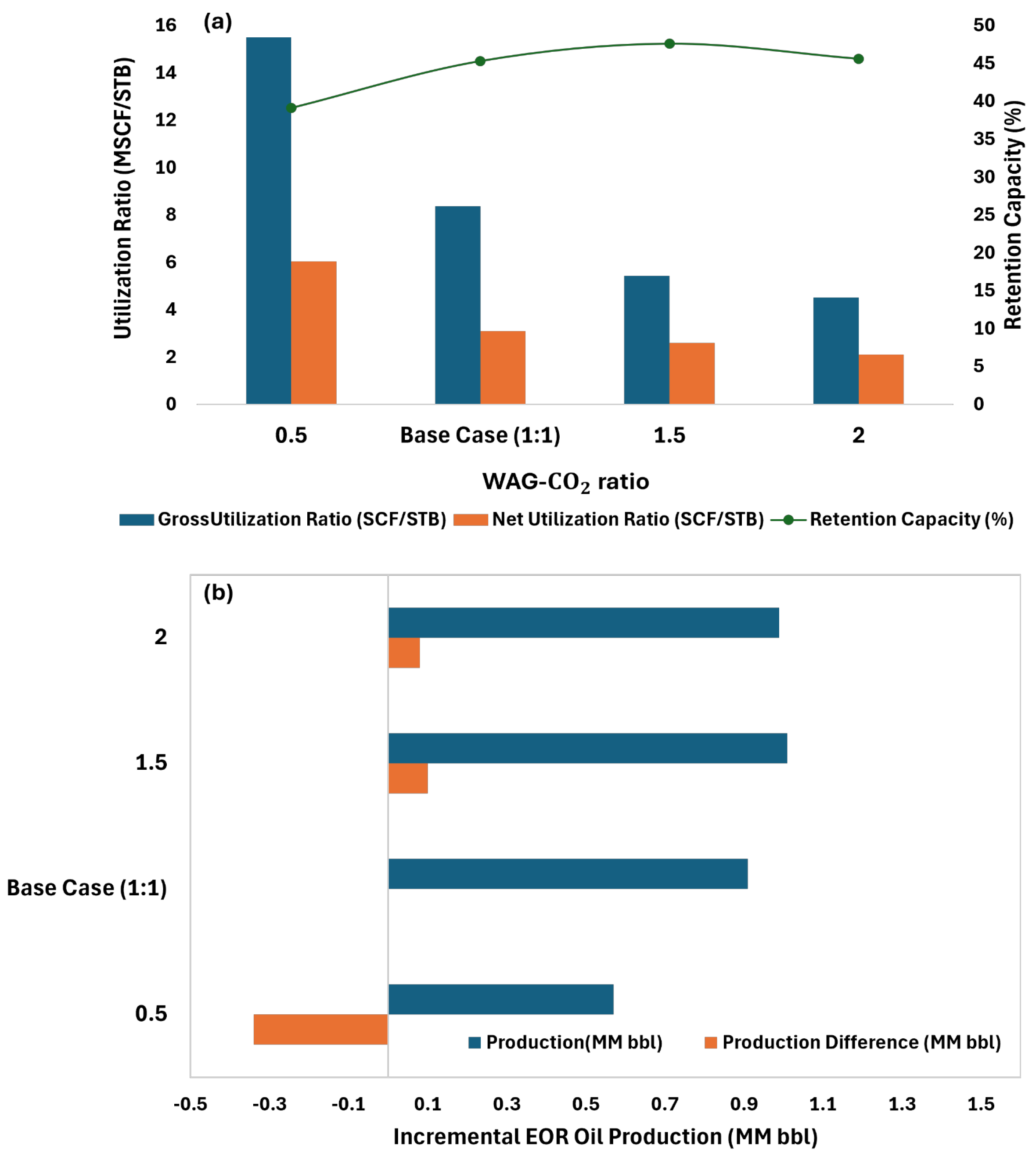
This study considers multiple recovery mechanisms: waterflooding, continuous miscible CO
2 flooding, and WAG-CO
2. In the WAG scenario, the cycle comprised one year of water injection followed by one year of CO
2 flooding. All scenarios were evaluated over a forecast duration of 20 years, with an annual injection rate of 5% HCPV; further details are shown in
Table 5.
Injected HCPV ranges are taken from practical applications [
38]. The producer constraint is based on the MMP, and the injected HCPV matches reservoir conditions. The injector pressure is controlled to ensure that the pressure gradient does not exceed 0.55 psi/ft, preventing fractures.
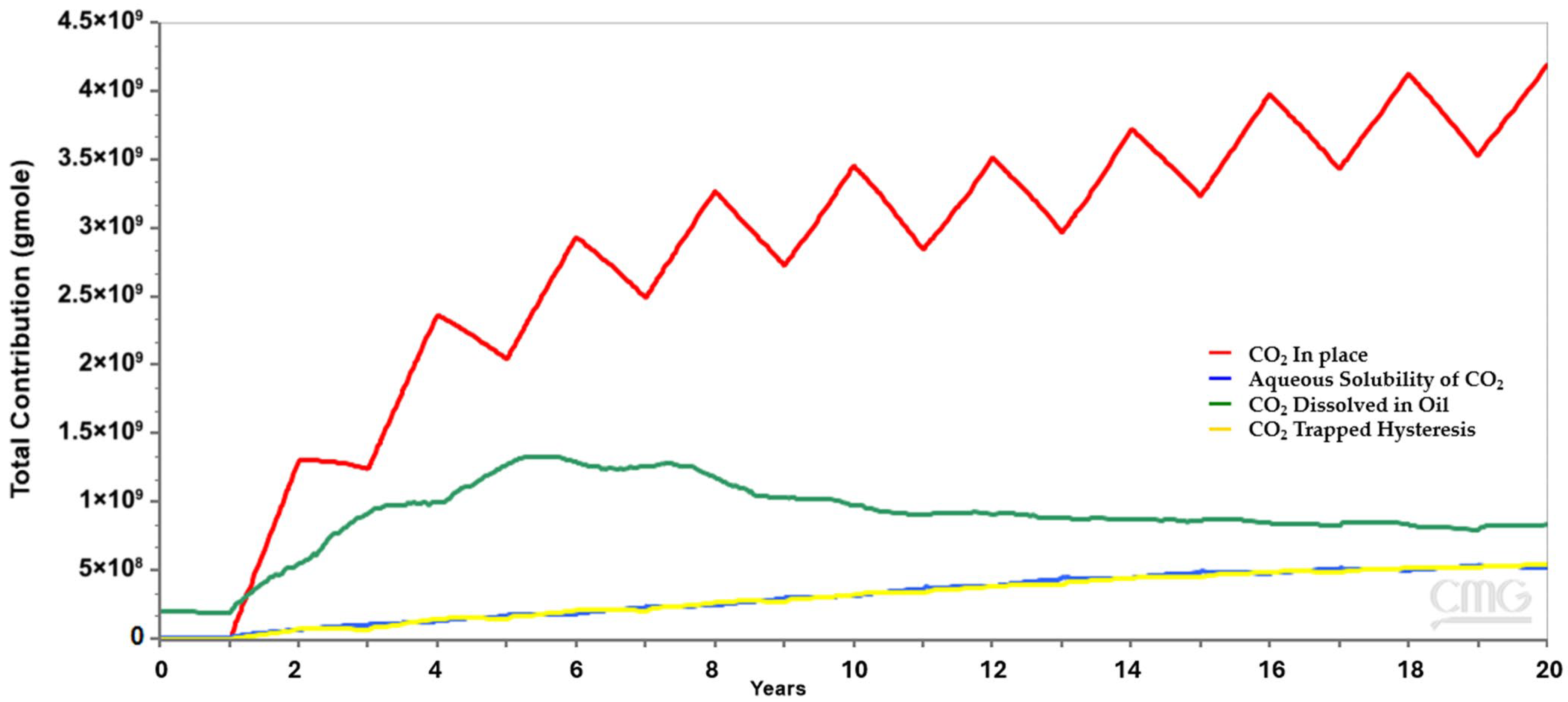
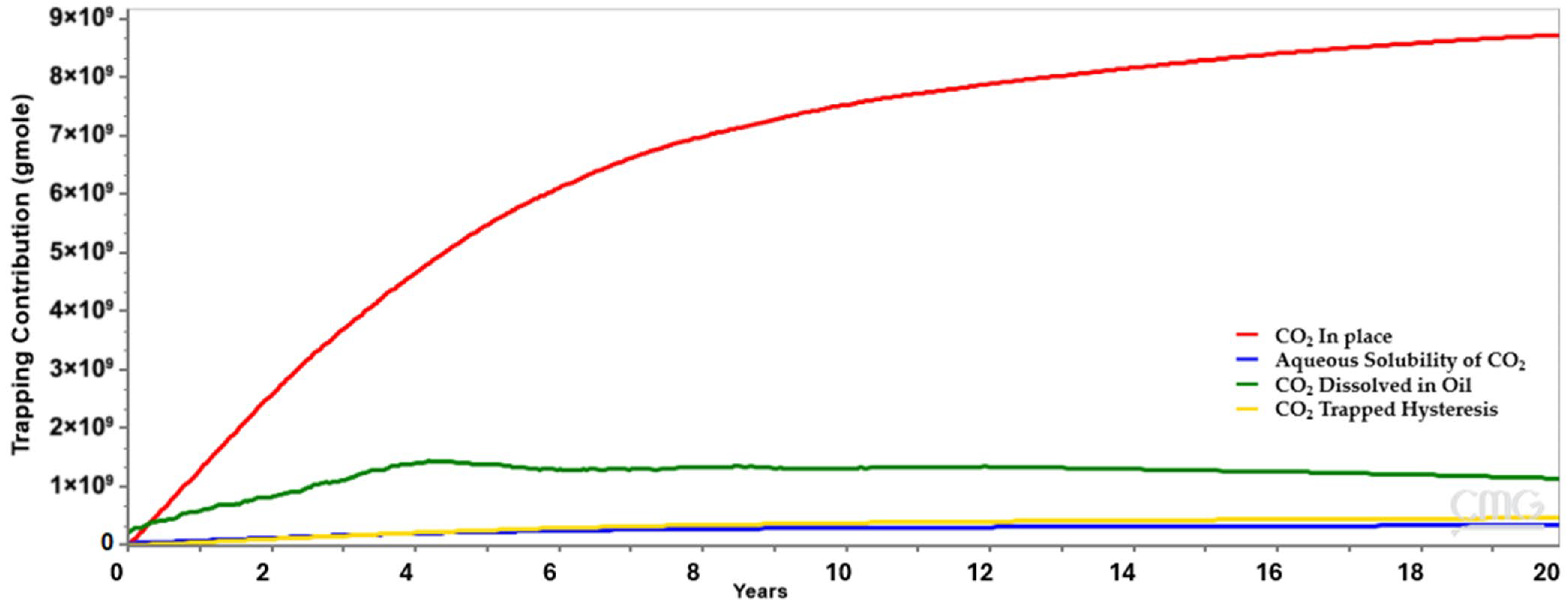
This study focuses on a carbonate limestone reservoir over a 20-year EOR-forecast-timeframe. Mineral interaction and trapping play important roles in the long term; however, this numerical study is not long enough to account for mineralization but will account for solubility trapping and residual trapping.
4. Discussion
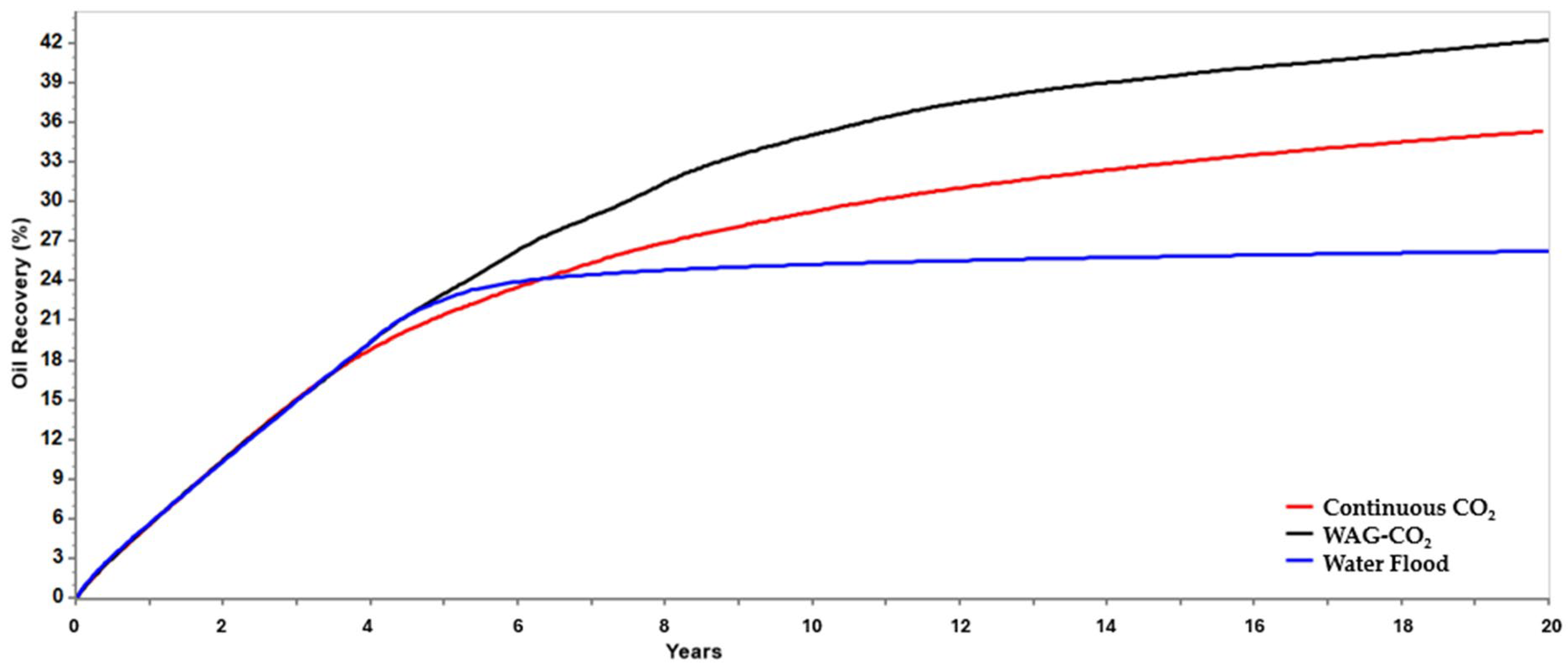
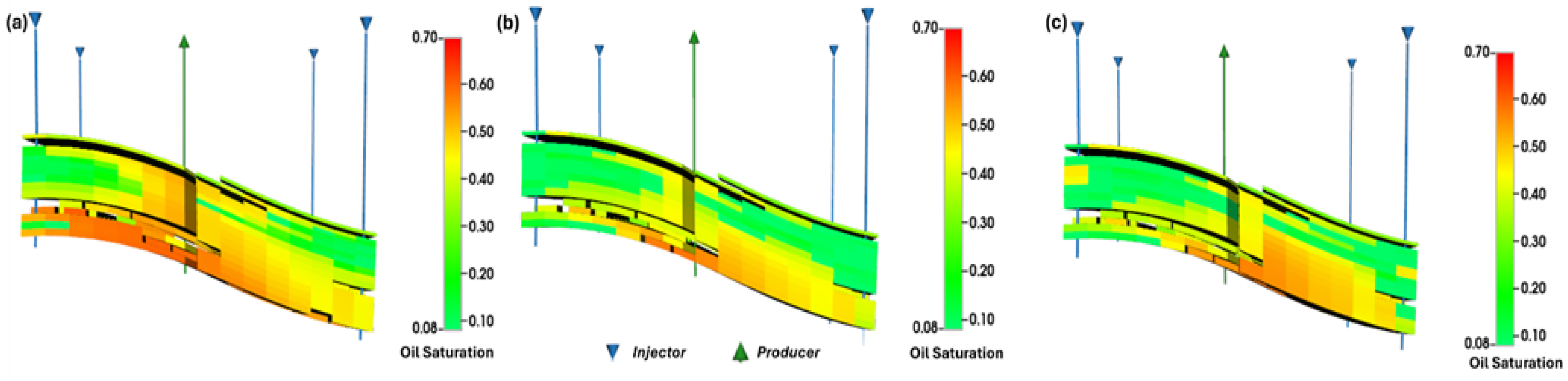
The simulation results reveal that the WAG-CO
2 injection scenario in a small sector of a target limestone reservoir achieved a recovery factor (RF) of 42%, outperforming the continuous CO
2 injection scenario which attained 35% (
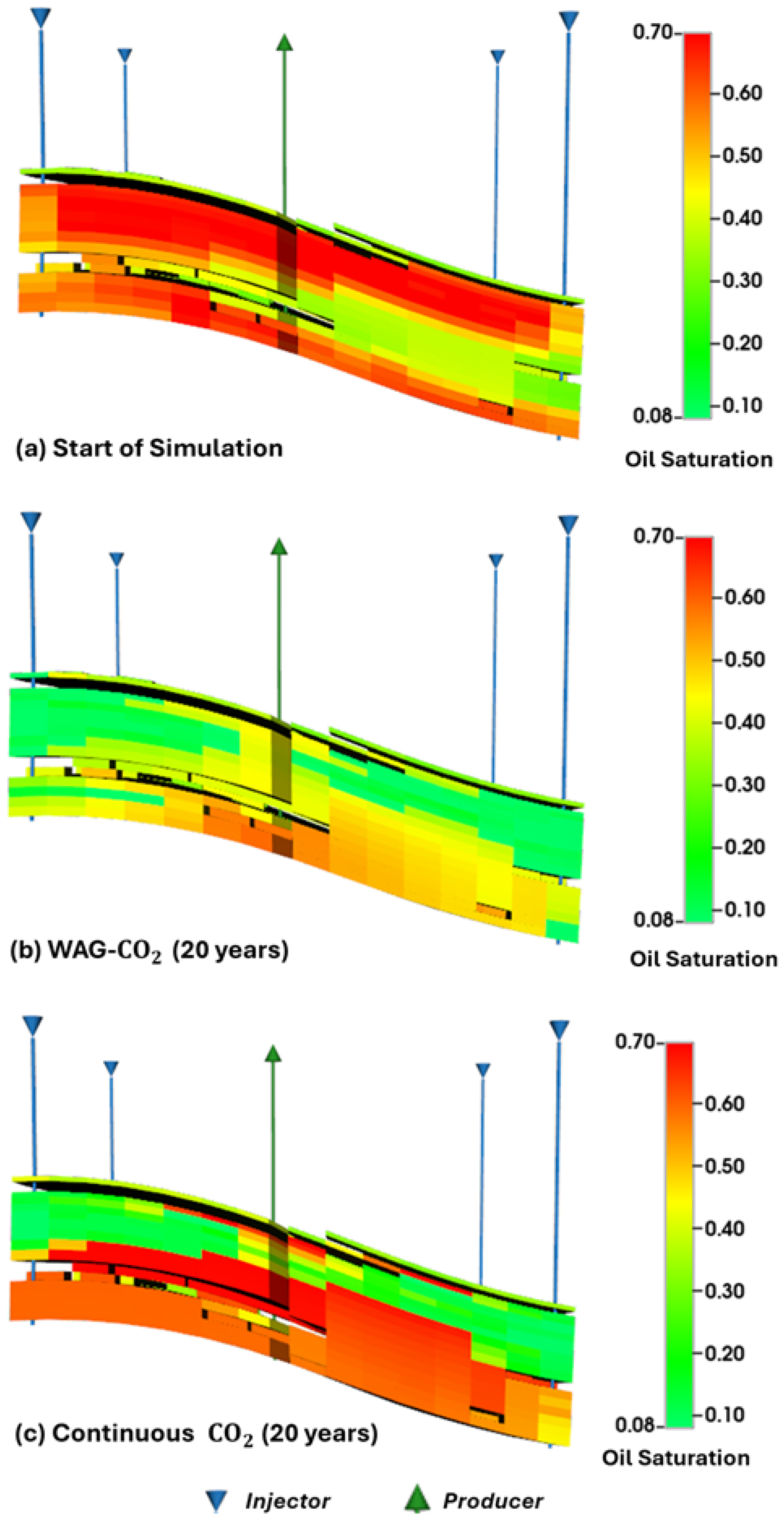
Figure 6). This improvement is attributed to enhanced sweep efficiency and reduced gravity override in the WAG-CO
2 case, as evidenced by saturation profiles (
Figure 7) showing more uniform displacement and better oil bank formation. Further alignment with our results is provided by the numerical simulation study conducted by Jia et al. [
46], who evaluated a CO
2-carbonated water–alternating–gas (WAG-CO
2) injection process using data from the Weyburn field, a well-characterized carbonate reservoir. Their simulation revealed a 6.7% improvement in oil recovery compared to continuous CO
2 injection and a 6.7% increase in CO
2 storage capacity when the carbonated water concentration was increased from 0 to 1.2 mol/L. Additionally, it was stated that sweep efficiency was improved especially in the lower layers of the reservoir, due to reduced gravity override and better mobility control mechanisms that directly align with our simulation findings in the WAG-CO
2 scenario. The use of Weyburn field parameters adds credibility and real-field relevance to their observations.
Experimental evidence [
47] strongly supports our simulation findings regarding the benefits of WAG-CO
2 injection in carbonate reservoirs. Core-flood experiments demonstrated that WAG-CO
2 achieves the highest oil recovery compared to continuous gas or water flooding, with approximately 30% improvement over waterfloods and 15% over continuous gas injection which is consistent with our simulation.
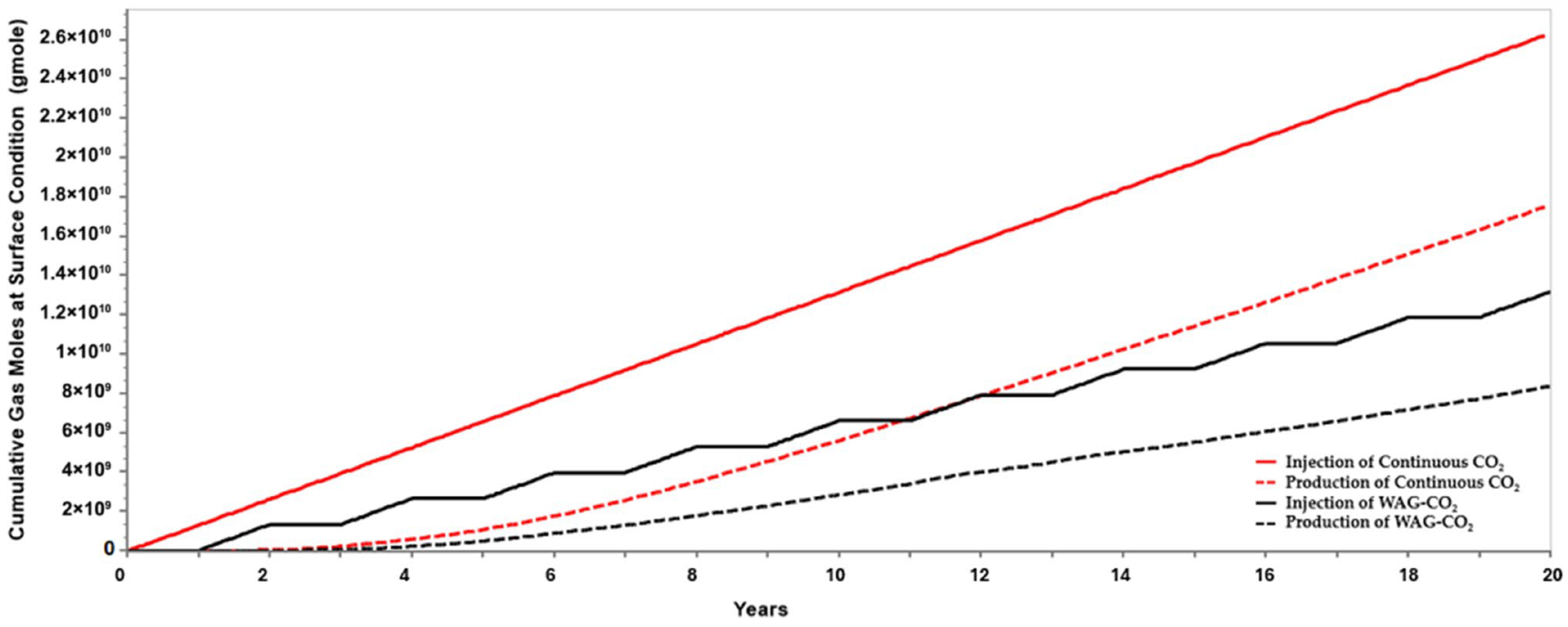
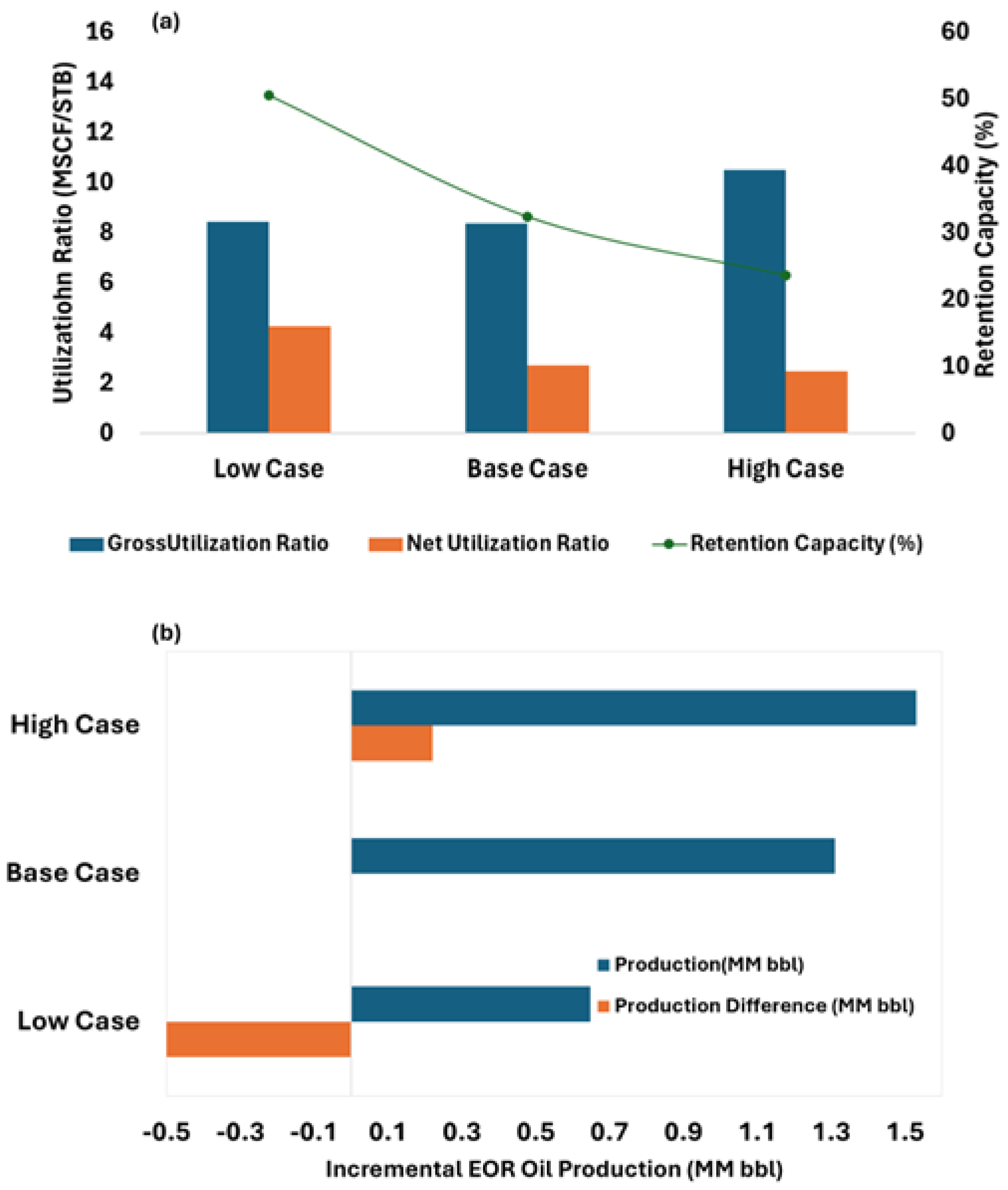
The simulation results demonstrated a net CO
2 utilization ratio (NUR) of 3.2 MSCF/STB and a gross utilization ratio (GUR) of 8.3 MSCF/STB for the WAG-CO
2 scenario within a small 5-spot sector model. These values are encouraging when benchmarked against real field data from the Sacroc Unit and Salt Creek Field, both of which are limestone and part of the Canyon Reef Formation in Texas. These mature CO
2-EOR projects have reported net utilization ratio (NUR) values ranging from 5 to 10 MSCF/STB and gross utilization ratio (GUR) values between 10 and 15 MSCF/STB [
18]. Some publications [
48] reported gross utilization ratios of 7.4–27.0 M SCF/STB and net utilization ratios of 2.4–12.6 M SCF/STB for conventional reservoirs which align with our results.
Further supporting the superior performance of the WAG strategy in CO
2 sequestration were a numerical study conducted in the Cuu Long Basin of Vietnam [
49]. Their findings confirmed that WAG-CO
2 significantly improved both residual and solubility trapping compared to continuous CO
2 injection.
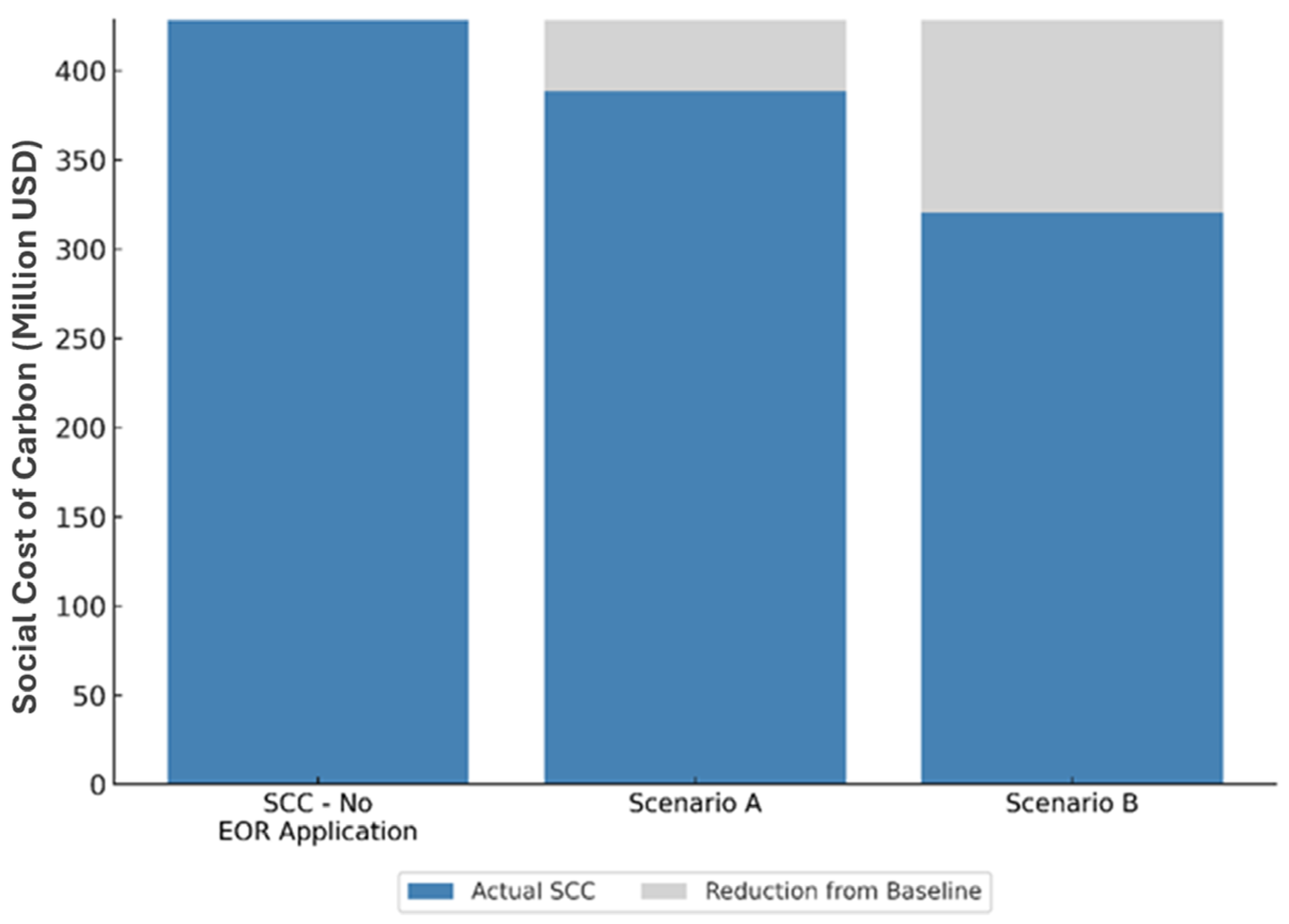
As per the SCC estimation, two scenarios were evaluated to assess the potential cost savings associated with the integration of EOR and sequestration. Scenario A demonstrated a reduction of approximately USD 40 million (10%) in the Social Cost of Carbon (SCC), while Scenario B indicated a more substantial reduction of USD 108 million (24%), both relative to the total volume of captured CO2 emissions. These estimations are based on a single five-spot sector model utilizing only 8–23% of the total captured CO2. Expanding the implementation to multiple injection patterns across the reservoir could potentially amplify these benefits by increasing storage capacity, lowering the SCC, and enhancing the economic feasibility through additional oil production revenues. The CO2 emissions from the source amount to 119,000 metric tons per year. This would amount to 135,000, 165,000, and 213,000 metric tons of sequestered CO2 in five, ten, and twenty years, respectively, in a single five-spot pattern. For a full-field application, the sequestered CO2 would increase by a factor of 4 to 11 based on the CO2 recycling mechanism and the number of patterns.
From the economic viability standpoint, the CCUS project proposed by our study is similar to the Weyburn Field project in terms of the basic properties with even closer proximity to the emissions source which reduces the transport cost. The cost of the Weyburn field project to store CO
2 is USD 20.04 per ton of CO
2 [
50] as compared to USD 125 to USD 335 per ton from the direct air capture (DAC) process [
51]. The target limestone reservoir sector that was simulated in this study uses 119,000 tons of CO
2/year, leading to an annual cost of USD 2,383,000 with an assumed crude oil price of USD 63.32/barrel [
52] (37,650 barrels of oil production is required annually to cover the cost of the project). Thus, this cost is minimal when compared to the total income from the sale of the incremental oil from the proposed EOR project. According to Kwak and Kim [
53], the CO
2 purchase is the largest cost in the early phase of the CO
2 EOR operation (they considered a range of CO
2 price between USD 12/t and USD 32/t). These observations signify the importance of using captured CO
2 for the economic viability of the CO
2-EOR project.
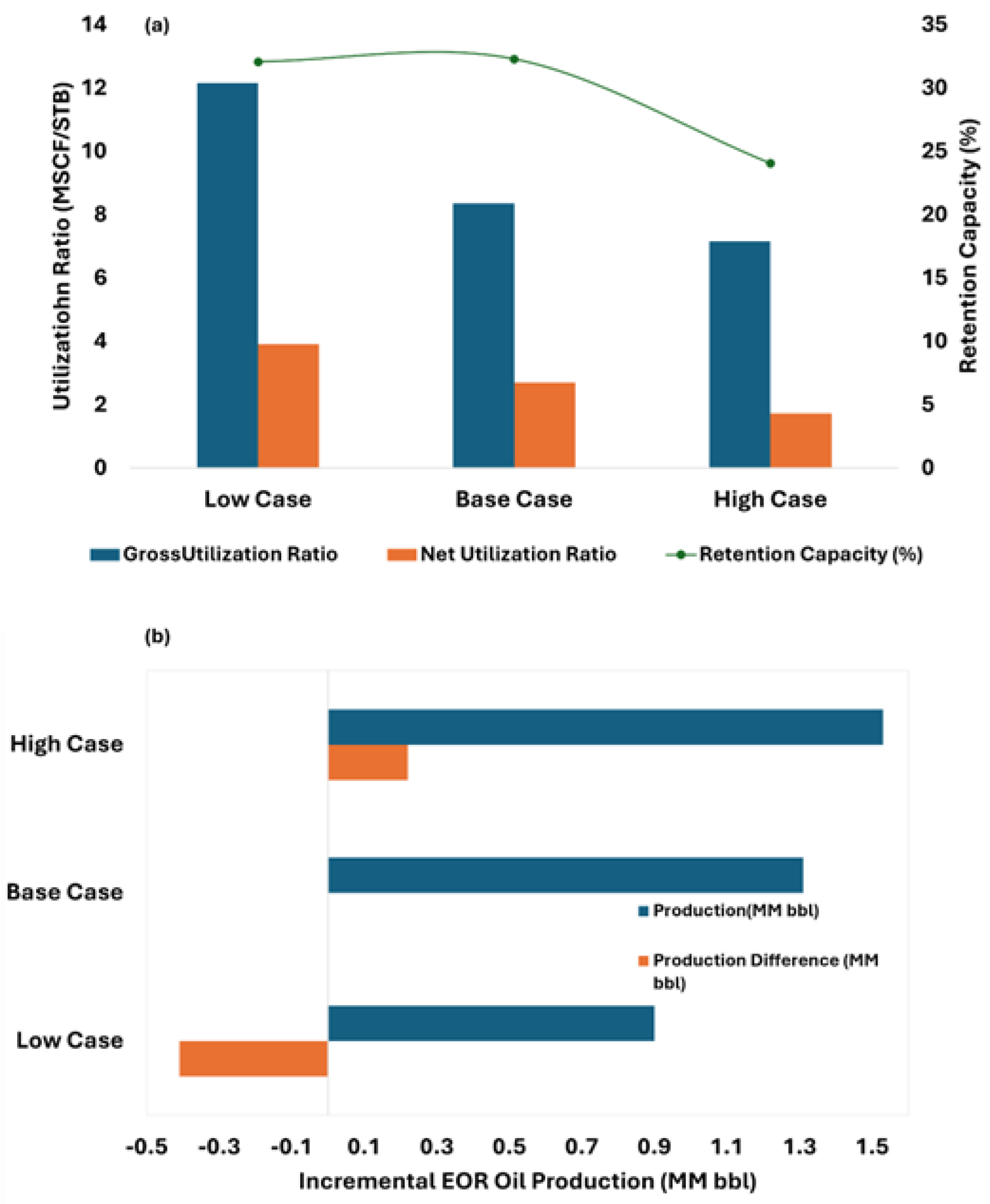
The sensitivity analysis conducted on residual oil saturation (Sor) in this study aligns with established trends reported in the literature. As illustrated in
Figure 13a, scenarios with lower Sor resulted in significantly higher gross and net CO
2 utilization ratios (GUR and NUR), indicating that more CO
2 was required per barrel of oil produced due to the limited remaining mobile oil. These outcomes are consistent with the findings of Ren and Duncan [
54], who demonstrated through numerical simulation that increasing initial Sor leads to reduced CO
2 utilization ratios and improved oil production efficiency in a five-spot WAG-CO
2 pattern. Regarding the HCPV injection sensitivity analysis, results indicate that reducing the CO
2 injection below the base HCPV value leads to a significant decline in oil recovery—up to 50% less production—rendering it suboptimal from an EOR standpoint. Conversely, increasing the injection to 2.0 HCPV enhances cumulative oil recovery by approximately 220,000 barrels, representing a 16% improvement over the base case. However, such a high injection volume may present economic and operational challenges, particularly due to increased CO
2 recycling demands. Based on the observed trends, an optimal injection range appears to lie between 1.0 and 1.5 HCPV, balancing enhanced oil recovery and long-term sequestration objectives.
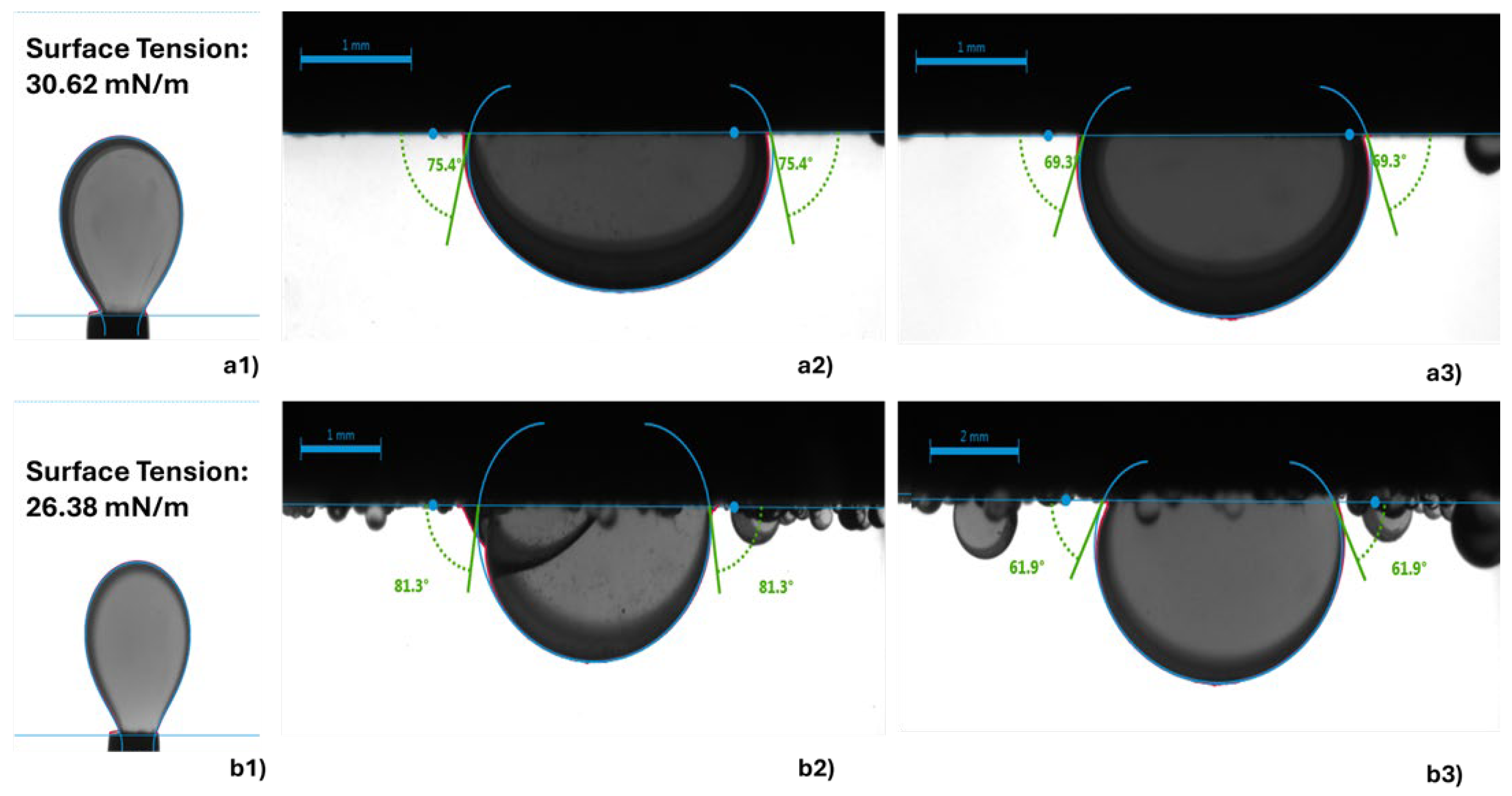
The wettability alteration induced by WAG-CO
2 injection plays a pivotal role in enhancing oil recovery and CO
2 trapping efficiency in carbonate formations. Under WAG-CO
2, reservoir rocks tend to shift toward a more water-wet state, which promotes increased capillary trapping of CO
2 and amplifies relative permeability hysteresis. Morrow [
55] emphasized that alterations in wettability, as evidenced by variations in interfacial tension (IFT) and contact angle, significantly affect capillary pressure, film stability, and, ultimately, oil recovery. Increased water-wetness, shown by reduced contact angles, typically improves displacement efficiency, especially in carbonate systems.