Opportunities for Emission Reduction in the Transformation of Petroleum Refining
Abstract
1. Introduction
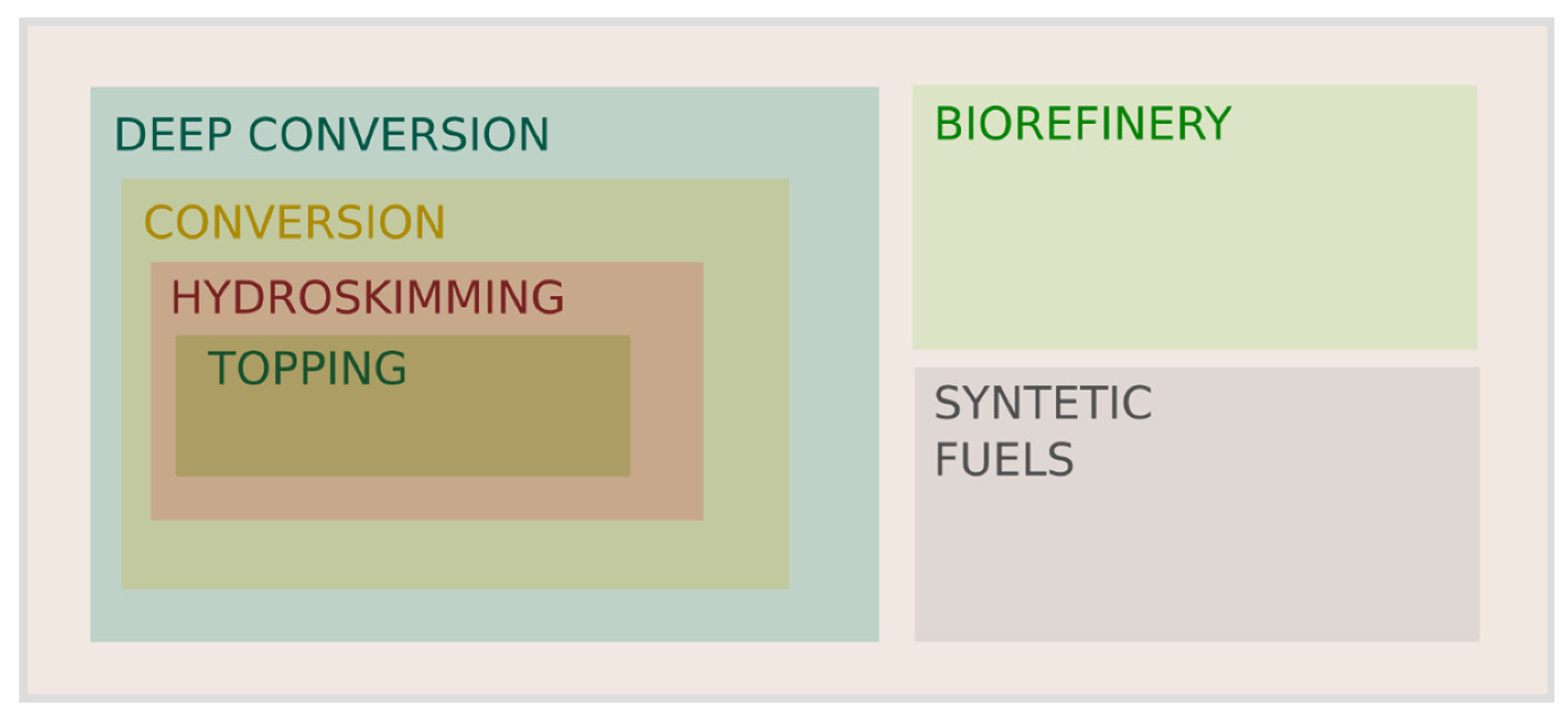
2. Typology of Refineries
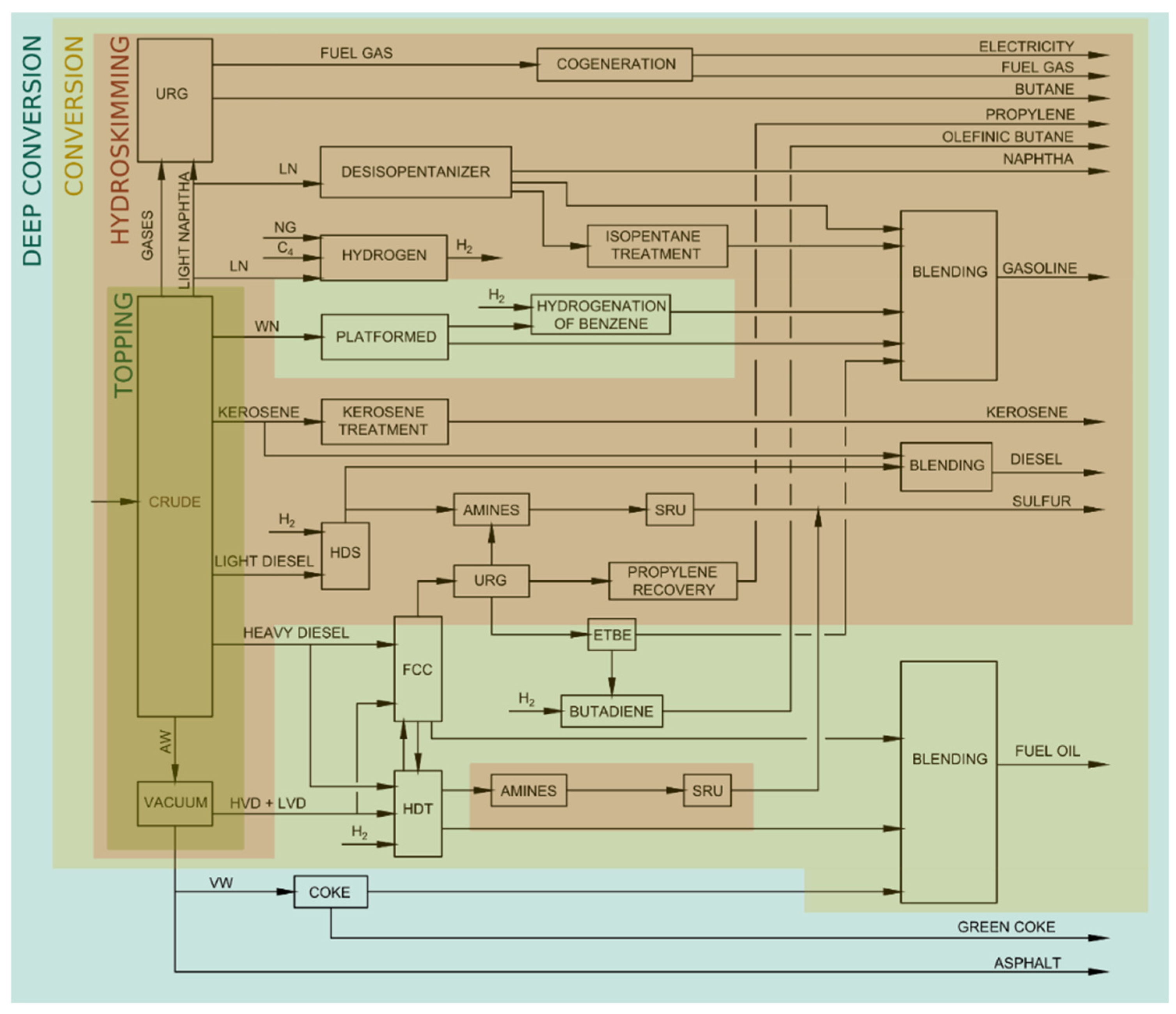
3. Identification of Units with Decarbonizing Potential
3.1. Units with Combustion
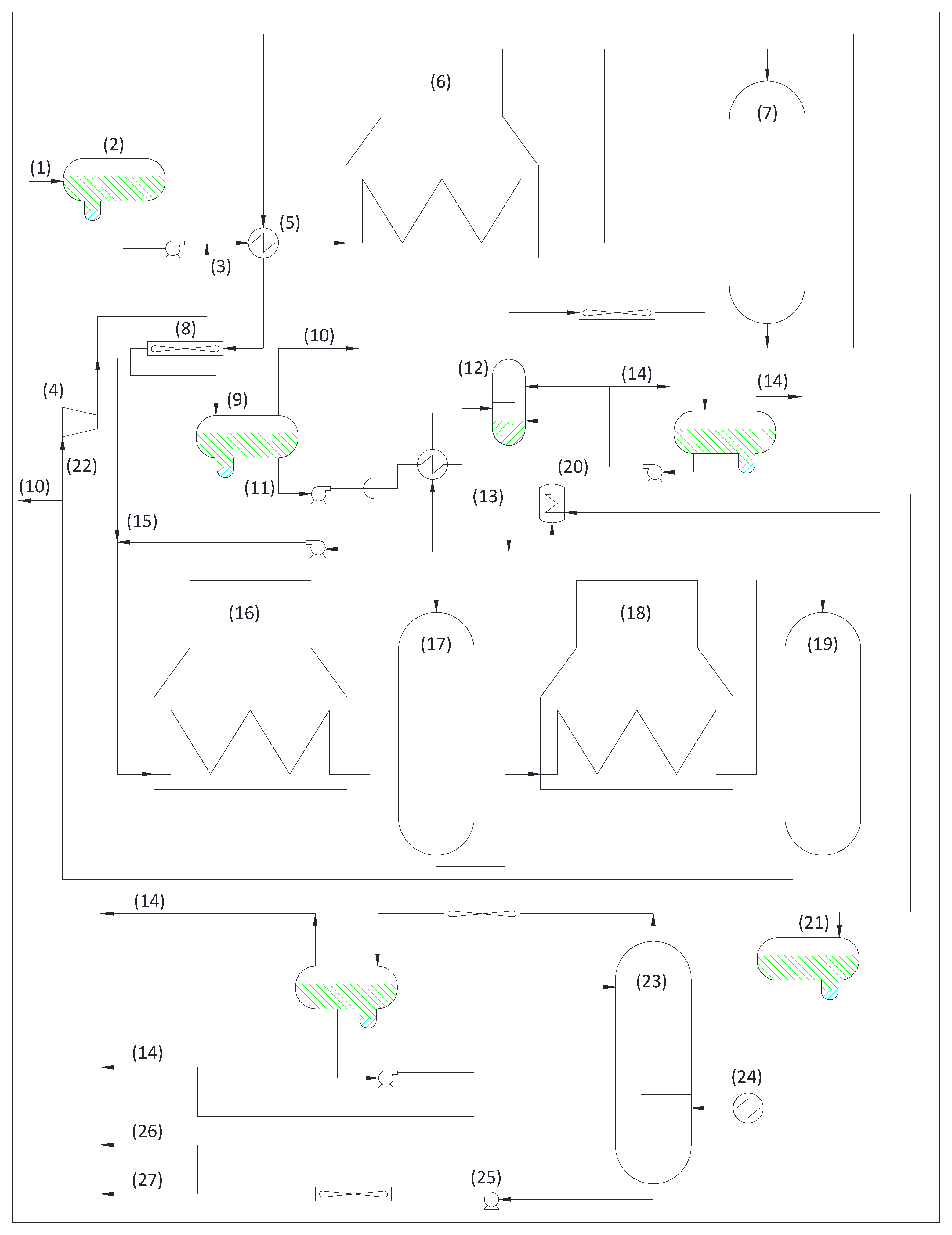
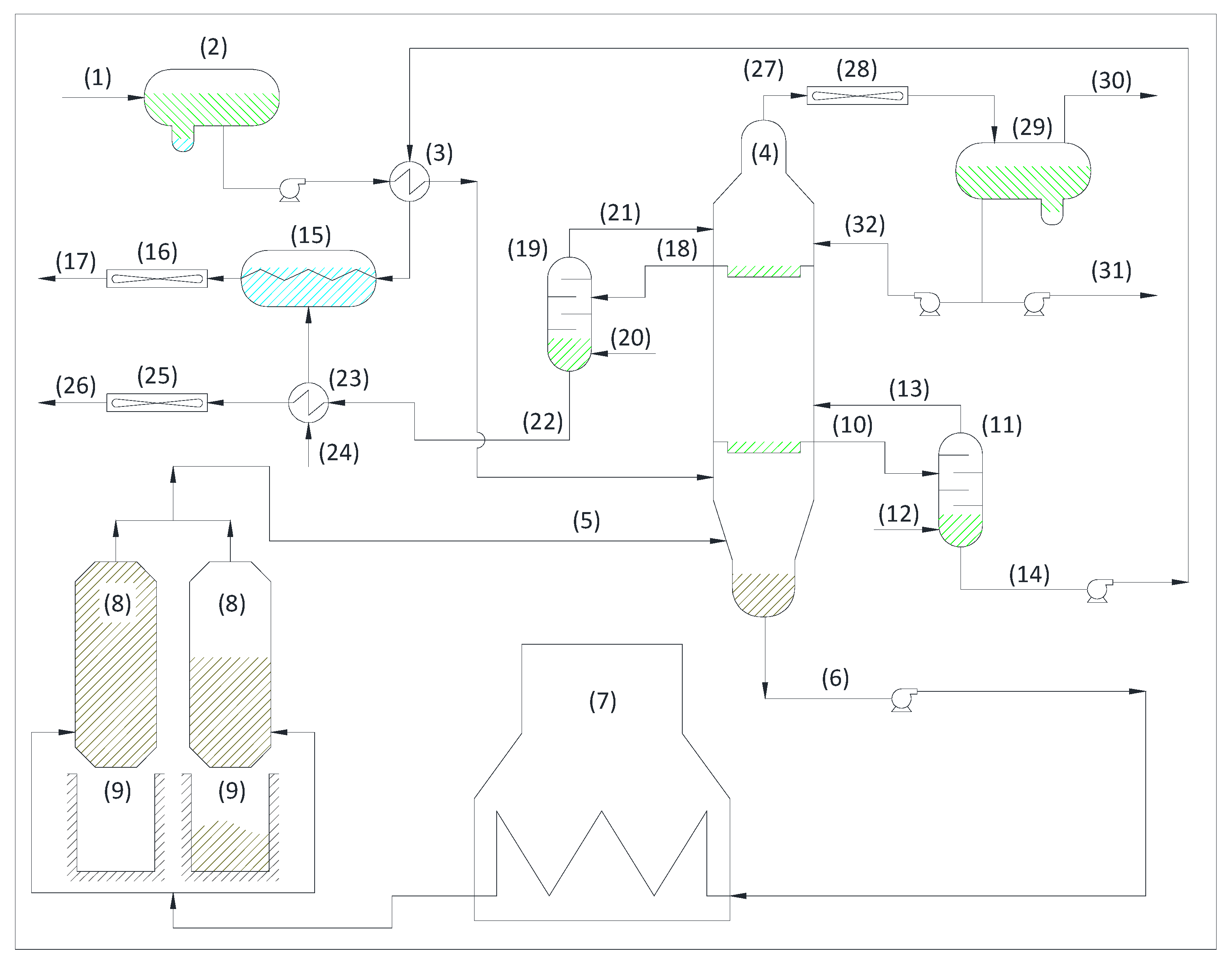
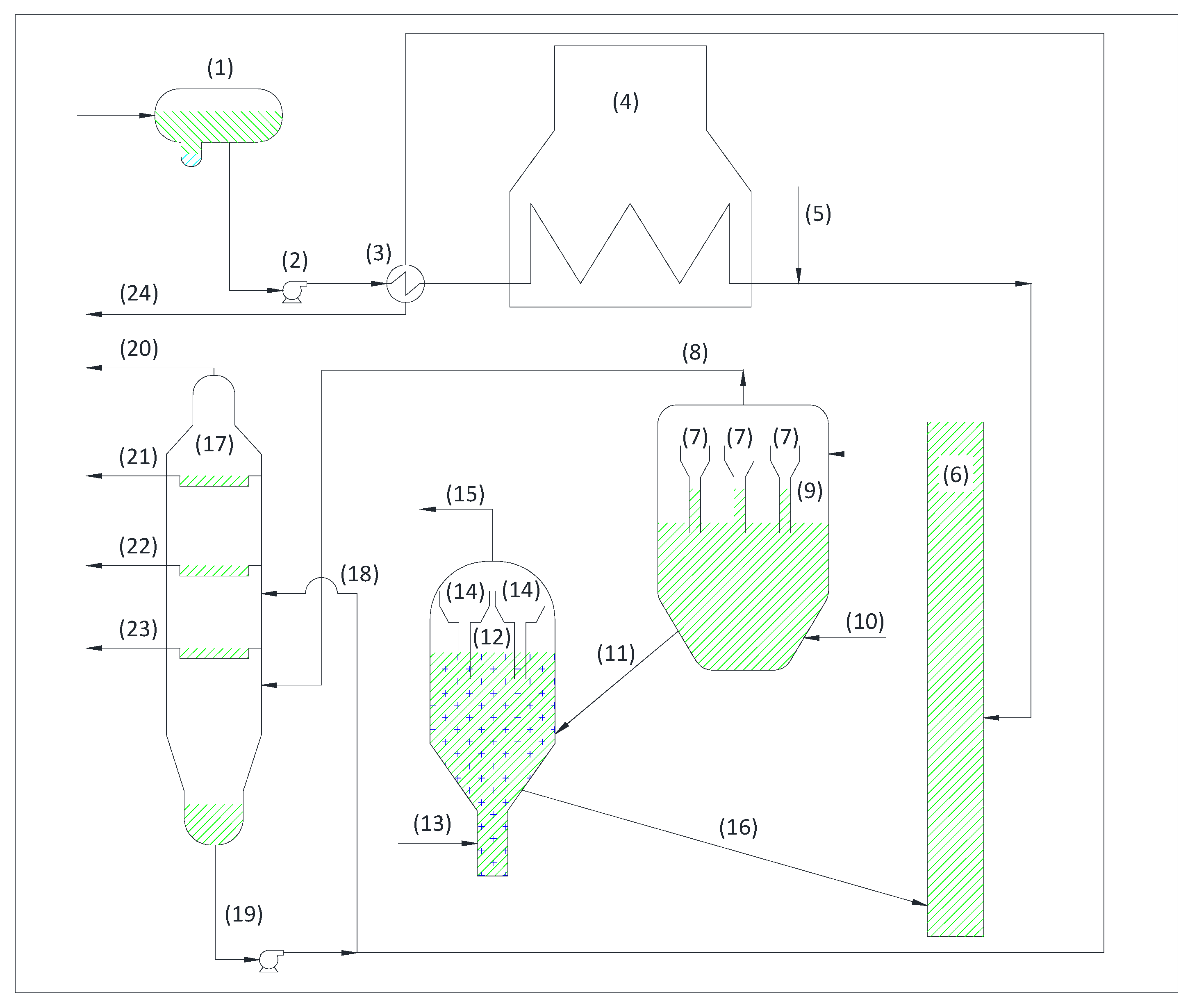
3.1.1. Crude Oil Unit and Vacuum Unit
- Establish an optimum tower cooling temperature at the reflux point.
- Eliminate with this reflux the heavy metals that may rise to the upper plates.
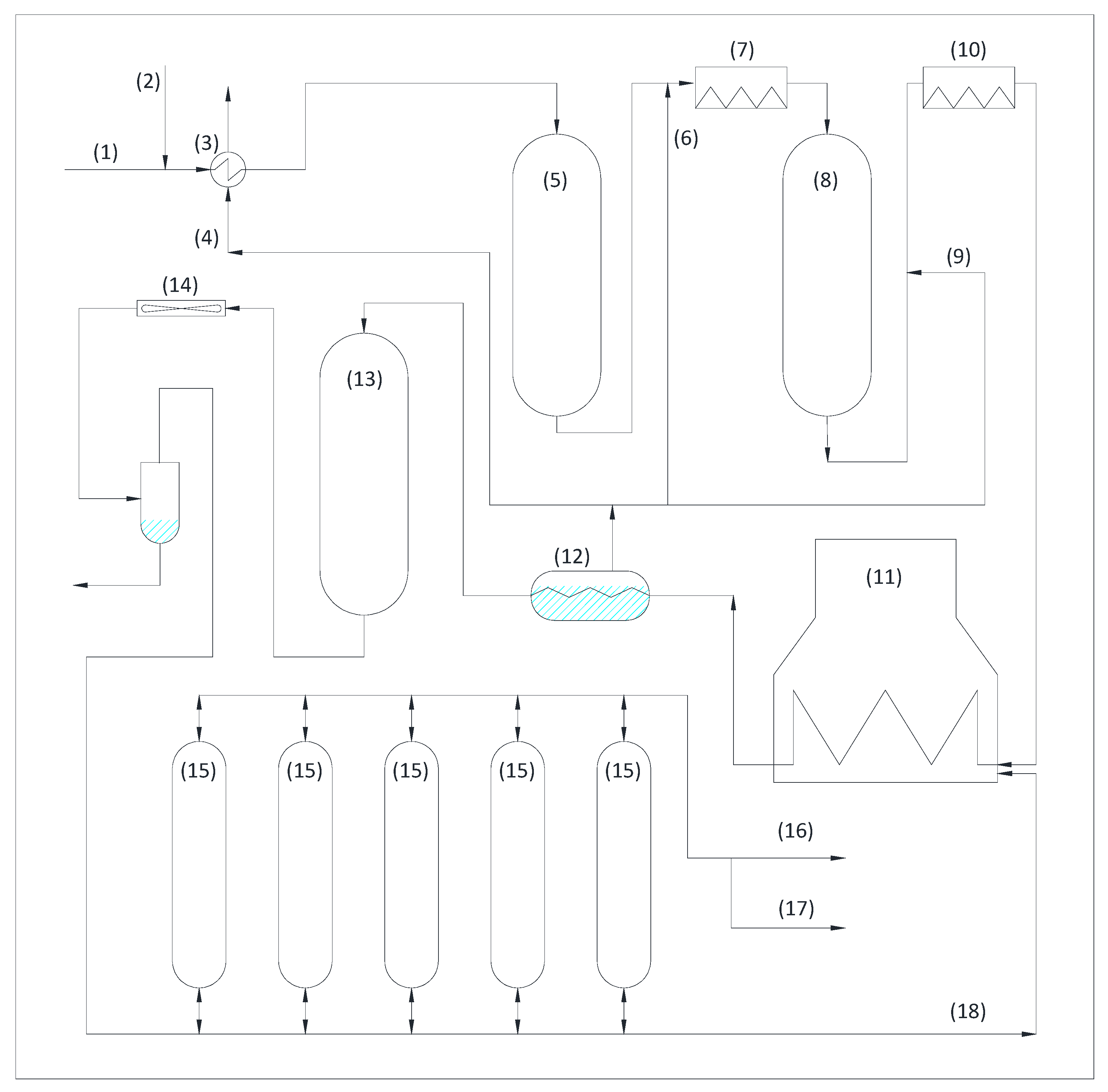
3.1.2. Platform
3.1.3. Hydrogen
3.1.4. Hydrodesulfurization
3.1.5. Coke
3.1.6. Fluid Catalytic Cracking
3.1.7. Hydrotreating
3.1.8. Identification of Decarbonization Potential in Combustion Units
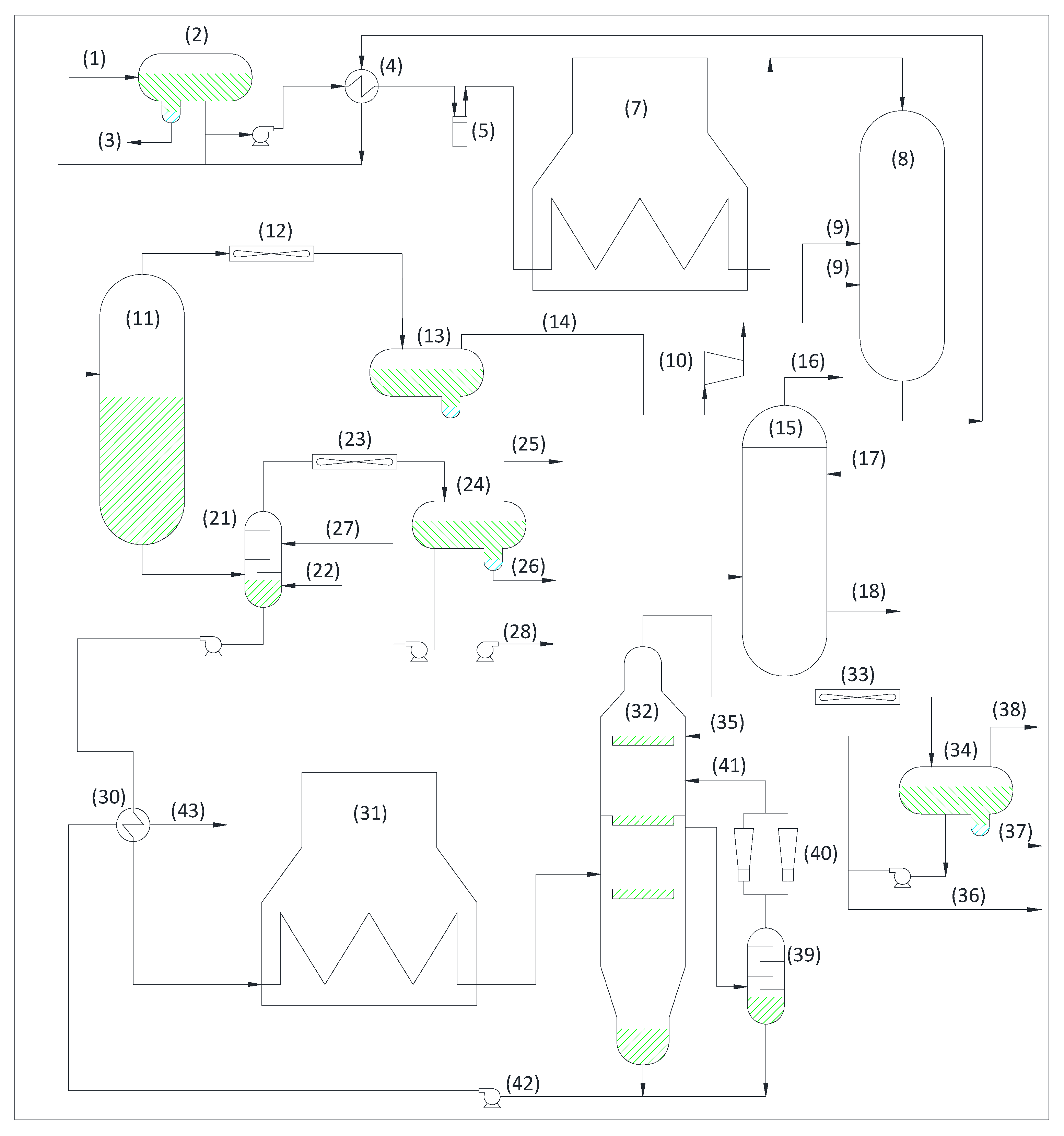
3.2. Non-Combustion Units:
3.2.1. Identification of Decarbonization
3.2.2. Identification of Decarbonization Potential in Non-Combustion Units
4. Analysis of Possible Actions for Decarbonization
4.1. Improvement Proposals for Decarbonization
4.2. Emission Reductions and Economic Implications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lo Piano, S.; Smith, S.T. Energy demand and its temporal flexibility: Approaches, criticalities and ways forward. Renew. Sustain. Energy Rev. 2022, 160, 112249. [Google Scholar] [CrossRef]
- Hourcade, J.-C.; Johnson, F.X.; Pachauri, S.; Simpson, N.P.; Singh, C.; Thomas, A.; Totin, E. Synthesis report of the IPCC Sixth Assessment Report (AR6). In Proceedings of the Fifty-Eight Session of the IPCC, Interlaken, Switzerland, 13–17 March 2023; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2023. [Google Scholar]
- IEA. The Oil and Gas Industry in Energy Transitions; OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Bill, C. 2017 Outlook for Energy: A View to 2040; Exxon Mobil: Irving, TX, USA, 2017. [Google Scholar]
- World Climate Action Summit and High-Level Segment at COP 28. Available online: https://unfccc.int/cop28/high-level (accessed on 25 February 2024).
- Total Energy Supply Outlook by Fuel and Scenario, 2000–2040. 2019. Available online: https://www.iea.org/data-and-statistics/charts/total-energy-supply-outlook-by-fuel-and-scenario-2000-2040 (accessed on 25 February 2024).
- Amado, M.; Poggi, F. Net-Zero Energy City Planning. In Sustainable Energy Transition for Cities; Elsevier: Amsterdam, The Netherlans, 2022; pp. 141–194. [Google Scholar] [CrossRef]
- Tribunal de Cuentas Europeo. Informe Especial Objetivosclimáticos y Energéticos de la UE; Tribunal de Cuentas Europeo: Luxembourg, 2023. [Google Scholar]
- Cordero-Lanzac, T.; Ramirez, A.; Navajas, A.; Gevers, L.; Brunialti, S.; Gandía, L.M.; Aguayo, A.T.; Sarathi, S.M.; Gascon, J. A techno-economic and life cycle assessment for the production of green methanol from CO2: Catalyst and process bottlenecks. J. Energy Chem. 2022, 68, 255–266. [Google Scholar] [CrossRef]
- Solakivi, T.; Paimander, A.; Ojala, L. Cost competitiveness of alternative maritime fuels in the new regulatory framework. Transp. Res. D Transp. Environ. 2022, 113, 103500. [Google Scholar] [CrossRef]
- Benajes, J.; García, A.; Monsalve-Serrano, J.; Martínez-Boggio, S. Potential of using OMEx as substitute of diesel in the dual-fuel combustion mode to reduce the global CO2 emissions. Transp. Eng. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Khalifa, R.; Alherbawi, M.; Elomri, A.; Al-Ansari, T. Optimization of Biofuel and Kerosene Fuel Blends to Support Sustainable Aviation. Comput. Aided Chem. Eng. 2022, 51, 85–90. [Google Scholar] [CrossRef]
- Colelli, L.; Segneri, V.; Bassano, C.; Vilardi, G. E-fuels, technical and economic analysis of the production of synthetic kerosene precursor as sustainable aviation fuel. Energy Convers. Manag. 2023, 288, 117165. [Google Scholar] [CrossRef]
- Elías, G.-H.; Xicoténcatl, L.-A.; Humberto, B.-Á.; Rodolfo, S.-E. Refinación de petróleo y suimpactoeconómico-tecnológico para la producción de gasolinas en México al 2030. Ing. Investig. Tecnol. 2013, 14, 475–487. [Google Scholar] [CrossRef]
- Elías, G.-H.; Humberto, B.-Á.; Rodolfo, S.-E.; Xicoténcatl, L.-A.; Claudia, G.-L.; Pablo, S.-Á. Consumo de energía y emisiones de bióxido de carbono del sector refinación de petróleo en México de 2015 a 2030. Ing. Investig. Tecnol. 2015, 16, 503–513. [Google Scholar] [CrossRef][Green Version]
- Szklo, A.; Schaeffer, R. Fuel specification, energy consumption and CO2 emission in oil refineries. Energy 2007, 32, 1075–1092. [Google Scholar] [CrossRef]
- Ma, S.; Lei, T.; Meng, J.; Liang, X.; Guan, D. Global oil refining’s contribution to greenhouse gas emissions from 2000 to 2021. Innovation 2023, 4, 100361. [Google Scholar] [CrossRef]
- Parkash, S. Refinery Distillation. In Refining Processes Handbook; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1–28. [Google Scholar] [CrossRef]
- Fraser, S. Distillation in Refining. In Distillation; Elsevier: Amsterdam, The Netherlands, 2014; pp. 155–190. [Google Scholar] [CrossRef]
- Lois, E.; Keating, E.L.; Gupta, A.K. Fuels. In Encyclopedia of Physical Science and Technology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 275–314. [Google Scholar] [CrossRef]
- Doble, M.; Kumar, A. Biodesulfurization. In Biotreatment of Industrial Effluents; Elsevier: Amsterdam, The Netherlands, 2005; pp. 255–265. [Google Scholar] [CrossRef]
- Sahl ABin Orosz, Á.; How, B.S.; Friedler, F.; Teng, S.Y. Electrification of oil refineries through multi-objective multi-period graph-theoretical planning: A crude distillation unit case study. J. Clean. Prod. 2024, 434, 140179. [Google Scholar] [CrossRef]
- van Straelen, J.; Geuzebroek, F.; Goodchild, N.; Protopapas, G.; Mahony, L. CO2 capture for refineries, a practical approach. Int. J. Greenh. Gas Control 2010, 4, 316–320. [Google Scholar] [CrossRef]
- Chan, W.N.; Walter, A.; Sugiyama, M.I.; Borges, G.C. Assessment of CO2 Emission Mitigation for a Brazilian Oil Refinery. Braz. J. Chem. Eng. 2016, 33, 835–850. [Google Scholar] [CrossRef]
- Cameron, D.B.; Waaler, A.; Komulainen, T.M. Oil and Gas digital twins after twenty years. How can they be made sustainable, maintainable and useful? In Proceedings of the 59th Conference on Simulation and Modelling (SIMS 59), Oslo, Norway, 26–28 September 2018; pp. 9–16. [Google Scholar] [CrossRef]
- Raj, P.; Surianarayanan, C. Digital twin: The industry use cases. In Advances in Computers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 285–320. [Google Scholar] [CrossRef]
- Giwa, T.; Akbari, M.; Kumar, A. Techno-economic assessment of an integrated biorefinery producing bio-oil, ethanol, and hydrogen. Fuel 2023, 332, 126022. [Google Scholar] [CrossRef]
- Hänggi, S.; Elbert, P.; Bütler, T.; Cabalzar, U.; Teske, S.; Bach, C.; Onder, C. A review of synthetic fuels for passenger vehicles. Energy Rep. 2019, 5, 555–569. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, X.; Zhang, Y.; Chen, G. Decarbonization pathways for the petroleum refining industry: A techno-economic assessment. J. Clean. Prod. 2023, 412, 137351. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Jin, Z.; Li, Z.; Liu, Q.; Zhang, K. Oil and gas pathway to net-zero: Review and outlook. Energy Strategy Rev. 2023, 45, 101048. [Google Scholar] [CrossRef]
- Pivac, I.; Šimunović, J.; Barbir, F.; Nižetić, S. Reduction of greenhouse gases emissions by use of hydrogen produced in a refinery by water electrolysis. Energy 2024, 296, 131157. [Google Scholar] [CrossRef]
- Boniek, D.; Figueiredo, D.; dos Santos, A.F.B.; de Resende Stoianoff, M.A. Biodesulfurization: A mini review about the immediate search for the future technology. Clean Technol. Environ. Policy 2015, 17, 29–37. [Google Scholar] [CrossRef]
- Available online: https://www.miteco.gob.es/es/costas/temas/proteccion-medio-marino/plan-ribera/contaminacion-marina-accidental/petroleo_y_comportamiento.aspx#:~:text=Al%20hablar%20de%20petr%C3%B3leo%20hay,que%20no%20se%20pueden%20destilar (accessed on 16 March 2024).
- Barker, C. Chapter 2 Origin, Composition and Properties of Petroleum. In Developments in Petroleum Science; Elsevier B.V.: Amsterdam, The Netherlands, 1985; pp. 11–45. [Google Scholar] [CrossRef]
- Hussain, N. Types and Configurations of Petroleum Oil Refineries. 2022. Available online: https://thepetrosolutions.com/types-configurations-petroleum-oil-refineries/ (accessed on 26 February 2024).
- Fahim, M.A.; Alsahhaf, T.A.; Elkilani, A. Hydroconversion. In Fundamentals of Petroleum Refining; Elsevier: Amsterdam, The Netherlands, 2010; pp. 153–198. [Google Scholar] [CrossRef]
- Farhan Hussain, R.; Mokhtari, A.; Ghalambor, A.; Amini Salehi, M. Smart downstream sector of O&G industry. In IoT for Smart Operations in the Oil and Gas Industry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 105–131. [Google Scholar] [CrossRef]
- Suganuma, S.; Katada, N. Innovation of catalytic technology for upgrading of crude oil in petroleum refinery. Fuel Process. Technol. 2020, 208, 106518. [Google Scholar] [CrossRef]
- Al-Samhan, M.; Al-Fadhli, J.; Al-Otaibi, A.M.; Al-Attar, F.; Bouresli, R.; Rana, M.S. Prospects of refinery switching from conventional to integrated: An opportunity for sustainable investment in the petrochemical industry. Fuel 2022, 310, 122161. [Google Scholar] [CrossRef]
- Rodionova, M.V.; Poudyal, R.S.; Tiwari, I.; Voloshin, R.A.; Zharmukhamedov, S.K.; Nam, H.G.; Zayadan, B.K.; Bruce, B.D.; Hou, H.J.; Allakhverdiev, S.I. Biofuel production: Challenges and opportunities. Int. J. Hydrogen Energy 2017, 42, 8450–8461. [Google Scholar] [CrossRef]
- Liang, Y.; Kashdan, T.; Sterner, C.; Dombrowski, L.; Petrick, I.; Kröger, M.; Hofer, R. Algal Biorefineries. In Industrial Biorefineries & White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 35–90. [Google Scholar] [CrossRef]
- Goria, K.; Singh, H.M.; Singh, A.; Kothari, R.; Tyagi, V.V. Insights into biohydrogen production from algal biomass: Challenges, recent advancements and future directions. Int. J. Hydrogen Energy 2024, 52, 127–151. [Google Scholar] [CrossRef]
- Berta, C.C. Ecocombustibles y Combustibles Sintéticos y Supapel Ante el Panorama Actual 2023; Ministerio de Defensa de España: Madrid, Spain, 2023. [Google Scholar]
- Willauer, H.D.; Hardy, D.R. Synthetic Fuel Development. In Future Energy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 561–580. [Google Scholar] [CrossRef]
- Ridjan, I.; Mathiesen, B.V.; Connolly, D.; Duić, N. The feasibility of synthetic fuels in renewable energy systems. Energy 2013, 57, 76–84. [Google Scholar] [CrossRef]
- Suppes, G.J.; Storvick, T.S. Energy and Sustainability. In Sustainable Power Technologies and Infrastructure; Academic Press: Cambridge, MA, USA, 2016; pp. 75–119. [Google Scholar] [CrossRef]
- Fahim, M.A.; Alsahhaf, T.A.; Elkilani, A. Crude Distillation. In Fundamentals of Petroleum Refining; Elsevier: Amsterdam, The Netherlands, 2010; pp. 69–93. [Google Scholar] [CrossRef]
- Fahim, M.A.; Alsahhaf, T.A.; Elkilani, A. Catalytic Reforming and Isomerization. In Fundamentals of Petroleum Refining; Elsevier: Amsterdam, The Netherlands, 2010; pp. 95–122. [Google Scholar] [CrossRef]
- IEA International Energy Agency. Net Zero by 2050 A Roadmap for the Global Energy Sector. 2021. Available online: https://iea.blob.core.windows.net/assets/deebef5d-0c34-4539-9d0c-10b13d840027/NetZeroby2050-ARoadmapfortheGlobalEnergySector_CORR.pdf (accessed on 16 March 2024).
- Herman, B. A case of multinational oligopoly in poor countries: Oil refinery investment in East Africa. J. Dev. Econ. 1975, 2, 121–143. [Google Scholar] [CrossRef]
- Manfroni, M.; Bukkens, S.G.F.; Giampietro, M. The declining performance of the oil sector: Implications for global climate change mitigation. Appl. Energy 2021, 298, 117210. [Google Scholar] [CrossRef]
- Pires, J.C.M.; Da Cunha Gonçalves, A.L. (Eds.) Bioenergy with Carbon Capture and Storage; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Boucheikhchoukh, A.; Berger, V.; Swartz, C.L.E.; Deza, A.; Nguyen, A.; Jaffer, S. Multiperiod refinery optimization for mitigating the impact of process unit shutdowns. Comput. Chem. Eng. 2022, 164, 107873. [Google Scholar] [CrossRef]
- McCay, M.H.; Shafiee, S. Hydrogen. In Future Energy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 475–493. [Google Scholar] [CrossRef]
- Gary, J.H.; Handwerk, G.E.; Kaiser, M.J.; Geddes, D. Petroleum Refining: Technology and Economics, 5th ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Eser, S. Atmospheric and Vacuum Distillation Units. Available online: https://courses.ems.psu.edu/fsc432/content/atmospheric-and-vacuum-distillation-units (accessed on 16 March 2024).
- Moran, S. Distillation Columns and Towers. In Process Plant Layout; Elsevier: Amsterdam, The Netherlands, 2017; pp. 325–338. [Google Scholar] [CrossRef]
- Luyben, W.L. Optimum vacuum distillation pressure. Chem. Eng. Process. -Process Intensif. 2022, 171, 108630. [Google Scholar] [CrossRef]
- Li, X.; Cui, C.; Sun, J. Enhanced product quality in lubricant type vacuum distillation unit by implementing dividing wall column. Chem. Eng. Process. -Process Intensif. 2018, 123, 1–11. [Google Scholar] [CrossRef]
- Amini, A.; Imaninasab, R. Investigating the effectiveness of Vacuum Tower Bottoms for Asphalt Rubber Binder based on performance properties and statistical analysis. J. Clean. Prod. 2018, 171, 1101–1110. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P.; Rosenfeld, P. The petroleum industry. In Handbook of Pollution Prevention and Cleaner Production—Best Practices in The Petroleum Industry; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1–97. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Jafari, M.; Iranshahi, D. Progress in catalytic naphtha reforming process: A review. Appl. Energy 2013, 109, 79–93. [Google Scholar] [CrossRef]
- Fink, J. Processes. In Guide to the Practical Use of Chemicals in Refineries and Pipelines; Elsevier: Amsterdam, The Netherlands, 2016; pp. 185–223. [Google Scholar] [CrossRef]
- Ancheyta-Juárez, J.; Villafuerte-Macías, E. Experimental validation of a kinetic model for naphtha reforming. Stud. Surf. Sci. Catal. 2001, 133, 615–618. [Google Scholar] [CrossRef]
- da Rosa, A.V.; Ordóñez, J.C. Hydrogen Production. In Fundamentals of Renewable Energy Processes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 419–470. [Google Scholar] [CrossRef]
- Iulianelli, A.; Liguori, S.; Wilcox, J.; Basile, A. Advances on methane steam reforming to produce hydrogen through membrane reactors technology: A review. Catal. Rev. 2016, 58, 1–35. [Google Scholar] [CrossRef]
- Lødeng, R.; Hannevold, L.; Bergem, H.; Stöcker, M. Catalytic Hydrotreatment of Bio-Oils for High-Quality Fuel Production. In The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 351–396. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P.; Rosenfeld, P.; Davletshin, A.R. Refineries. In Responsible Care; Gulf Publishing Company: Houston, TX, USA, 2008; pp. 236–316. [Google Scholar] [CrossRef]
- Parkash, S. Distillate Hydrotreating. In Refining Processes Handbook; Elsevier: Amsterdam, The Netherlands, 2003; pp. 29–61. [Google Scholar] [CrossRef]
- Miller, B. Introduction. In Fossil Fuel Emissions Control Technologies; Butterworth-Heinemann: Oxford, UK, 2015; pp. 1–45. [Google Scholar] [CrossRef]
- Errera, M.R.; Milanez, L.F. Thermodynamic analysis of a coke dry quenching unit. Energy Convers. Manag. 2000, 41, 109–127. [Google Scholar] [CrossRef]
- Alvarado, J.G.; Delgado Linares, J.G.; Medina, H.R. Rol de la Química Orgánica en losprocesos de conversión de hidrocarburos. Educ. Química 2015, 26, 288–298. [Google Scholar] [CrossRef]
- Boateng, A.A. Rotary Kiln Petroleum Coke Calcination Process: Some Design Considerations. In Rotary Kilns; Butterworth-Heinemann: Oxford, UK, 2016; pp. 265–290. [Google Scholar] [CrossRef]
- Tiwari, H.P.; Saxena, V.K. Industrial perspective of the cokemaking technologies. In New Trends in Coal Conversion: Combustion, Gasification, Emissions, and Coking; Woodhead Publishing: Cambridge, UK, 2019; pp. 203–246. [Google Scholar] [CrossRef]
- Speight, J.G. Refining Chemistry and Fouling Potential. In Fouling in Refineries; Elsevier: Amsterdam, The Netherlands, 2015; pp. 65–86. [Google Scholar] [CrossRef]
- Alnahdi, A.; Elkamel, A.; Shaik, M.A.; Al-Sobhi, S.A.; Erenay, F.S. Optimal Production Planning and Pollution Control in Petroleum Refineries Using Mathematical Programming and Dispersion Models. Sustainability 2019, 11, 3771. [Google Scholar] [CrossRef]
- Rodríguez, E.; Gutiérrez, A.; Palos, R.; Vela, F.J.; Azkoiti, M.J.; Arandes, J.M.; Bilbao, J. Co-cracking of high-density polyethylene (HDPE) and vacuum gasoil (VGO) under refinery conditions. Chem. Eng. J. 2020, 382, 122602. [Google Scholar] [CrossRef]
- Mujtaba, I.M.; John, Y.M.; Yusuf, A.Z.; Patel, R. Crude to chemicals: The conventional FCC unit still relevant. In Modelling of Chemical Process Systems; Elsevier: Amsterdam, The Netherlands, 2023; pp. 251–267. [Google Scholar] [CrossRef]
- Selalame, T.W.; Patel, R.; Mujtaba, I.M.; John, Y.M. A Review of Modelling of the FCC Unit—Part I: The Riser. Energies 2022, 15, 308. [Google Scholar] [CrossRef]
- Li, Z.; Lu, C.-X. FCC riser quick separation system: A review. Pet. Sci. 2016, 13, 776–781. [Google Scholar] [CrossRef]
- Rigutto, M.S.; van Veen, R.; Huve, L. Zeolites in Hydrocarbon Processing. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; pp. 855–913, XXIII–XXVI. [Google Scholar] [CrossRef]
- Selalame, T.W.; Patel, R.; Mujtaba, I.M.; John, Y.M. A Review of Modelling of the FCC Unit—Part II: The Regenerator. Energies 2022, 15, 388. [Google Scholar] [CrossRef]
- Ortega, E. An Overview of Hydrotreating. 2021. Available online: https://www.aiche.org/sites/default/files/cep/20211029.pdf (accessed on 16 March 2024).
- Rodríguez, E.; Félix, G.; Ancheyta, J.; Trejo, F. Modeling of hydrotreating catalyst deactivation for heavy oil hydrocarbons. Fuel 2018, 225, 118–133. [Google Scholar] [CrossRef]
- Speight, J.G. Hydrocarbons from crude oil. In Handbook of Industrial Hydrocarbon Processes; Gulf Professional Publishing: Houston, TX, USA, 2020; pp. 95–142. [Google Scholar] [CrossRef]
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture: Methods, Technologies and Applications; Woodhead Publishing: Cambridge, UK, 2020; pp. 3–28. [Google Scholar] [CrossRef]
- Delgado-Linares, J.G.; Delgado-Linares, G.A.; Mercado-Ojeda, R.A. Balances de masa y energía simplificados, aplicados a un proceso de craqueo catalítico de petróleo. Educ. Química 2009, 20, 456–460. [Google Scholar] [CrossRef]
- Nazerifard, R.; Mohammadpourfard, M.; Heris, S.Z. Optimization of the integrated ORC and carbon capture units coupled to the refinery furnace with the RSM-BBD method. J. CO2 Util. 2022, 66, 102289. [Google Scholar] [CrossRef]
- Sircar, A.; Nair, A.; Bist, N.; Yadav, K. Digital twin in hydrocarbon industry. Pet. Res. 2023, 8, 270–278. [Google Scholar] [CrossRef]
- Gary, J.H. Petroleum Refining. In Encyclopedia of Physical Science and Technology; Academic Press: Cambridge, MA, USA, 2003; pp. 741–761. [Google Scholar] [CrossRef]
- Yee, K.F.; Mohamed, A.R.; Tan, S.H. A review on the evolution of ethyl tert-butyl ether (ETBE) and its future prospects. Renew. Sustain. Energy Rev. 2013, 22, 604–620. [Google Scholar] [CrossRef]
- Ketabchi, E.; Mechleri, E.; Gu, S.; Arellano-Garcia, H. Modelling and Optimisation Approach of an Integrated Oil Refinery and a Petrochemical Plant. Comput. Aided Chem. Eng. 2018, 44, 1081–1086. [Google Scholar] [CrossRef]
- Treblig, B.; Garcia, G. Technical Description Unit of Isopentane; SSRN: Rochester, NY, USA, 2012. [Google Scholar]
- Speight, J.G. Gas cleaning processes. In Natural Gas; Elsevier: Amsterdam, The Netherlands, 2019; pp. 277–324. [Google Scholar] [CrossRef]
- Stewart, M.I. Gas Sweetening. In Surface Production Operations; Elsevier: Amsterdam, The Netherlands, 2014; pp. 433–539. [Google Scholar] [CrossRef]
- Bai, S.; Sridhar, S.; Khan, A.A. Recovery of propylene from refinery off-gas using metal incorporated ethylcellulose membranes. J. Membr. Sci. 2000, 174, 67–79. [Google Scholar] [CrossRef]
- Balogun, M.L.; Khan, W.U.; Shaikh, M.N.; Adamu, S.; Arjah, A.S.; Al-Bogami, S.A.; Al-Ghamdi, S.; Hossain, M.M. Redox MoO3/CaMnO3 catalysts for chemical-looping oxidative dehydrogenation of propane: A greener approach of propylene production. Appl. Catal. A Gen. 2023, 655, 119087. [Google Scholar] [CrossRef]
- Deraz, N.M.; Alarifi, A.; Shaban, S.A. Removal of sulfur from commercial kerosene using nanocrystalline NiFe2O4 based sorbents. J. Saudi Chem. Soc. 2010, 14, 357–362. [Google Scholar] [CrossRef]
- Rezvanpour, A.; Roostaazad, R.; Hesampour, M.; Nyström, M.; Ghotbi, C. Effective factors in the treatment of kerosene–water emulsion by using UF membranes. J. Hazard. Mater. 2009, 161, 1216–1224. [Google Scholar] [CrossRef]
- Bissett, J.R.; Rouanet, Y.; Thivnd, J.-M. Reduction of Benzene in Gasoline. U.S. Patent 5,099,543A, 5 March 1991. [Google Scholar]
- Process to Reduce the Benzene Content of Gasoline. Google Patents 1977.
- Mokhatab, S.; Poe, W.A. Sulfur Recovery and Handling. In Handbook of Natural Gas Transmission and Processing; Elsevier: Amsterdam, The Netherlands, 2012; pp. 291–316. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R.B. Sulfur Recovery Processes. In Gas Purification; Elsevier: Amsterdam, The Netherlands, 1997; pp. 670–730. [Google Scholar] [CrossRef]
- Reduce Benzene Content. WO Patent 2013028215A1, 19 August 2011.
- Low Benzene Content Gasoline Producing Process. U.S. Patent 4,209,383A, 3 November 1977.
- Zhao, S.; Song, Q.; Zhao, D.; Wang, Y. Identifying the spatiotemporal carbon footprint of the petroleum refining industry and its mitigation potential in China. Energy 2023, 284, 129240. [Google Scholar] [CrossRef]
- Madugula, A.C.S.; Sachde, D.; Hovorka, S.D.; Meckel, T.A.; Benson, T.J. Estimation of CO2 emissions from petroleum refineries based on the total operable capacity for carbon capture applications. Chem. Eng. J. Adv. 2021, 8, 100162. [Google Scholar] [CrossRef]
- Gailani, A.; Cooper, S.; Allen, S.; Pimm, A.; Taylor, P.; Gross, R. Assessing the potential of decarbonization options for industrial sectors. Joule 2024, 8, 576–603. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Karthikeyan, K.; Gandhi, R.; Grossmann, I.E.; Torres, A.I. Optimal Retrofit of Carbon Capture and Electrification Technologies in Oil Refineries for Reducing Direct CO2 Emissions. Ind. Eng. Chem. Res. 2025, 64, 10131–10147. [Google Scholar] [CrossRef]
- Popoola, L.T.; Nwogbu, C.C.; Taura, U.; Asmara, Y.P.; Nwobodo, L.O.; Agbo, A.O. Future prospects towards attaining zero-emission of greenhouse gases from crude oil refinery plants. In Cleaner Waste Systems; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar] [CrossRef]
- Feliu-Batlle, V.; Rivas-Perez, R. Control of the temperature in a petroleum refinery heating furnace based on a robust modified Smith predictor. ISA Trans. 2021, 112, 251–270. [Google Scholar] [CrossRef]
- Yu, H.; Feng, X.; Wang, Y.; Biegler, L.T.; Eason, J. A systematic method to customize an efficient organic Rankine cycle (ORC) to recover waste heat in refineries. Appl. Energy 2016, 179, 302–315. [Google Scholar] [CrossRef]
- de Maigret, J.; Viesi, D.; Mahbub, M.S.; Testi, M.; Cuonzo, M.; Thellufsen, J.Z. A Multi-Objective Optimization Approach in Defining the Decarbonization Strategy of a Refinery; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Bárkányi, Á.; Chován, T.; Németh, S.; Abonyi, J. Modelling for Digital Twins—Potential Role of Surrogate Models. Processes 2021, 9, 476. [Google Scholar] [CrossRef]
- Melesse, T.Y.; Pasquale, V.; Di Riemma, S. Digital Twin Models in Industrial Operations: A Systematic Literature Review. Procedia Manuf. 2020, 42, 267–272. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Wang, Q. Digital twin and AI applications for sustainable operation optimization in the refining industry. Chem. Eng. J. 2024, 478, 147819. [Google Scholar]
- Pour, N. Economics and policy of bioenergy with carbon capture and storage. In Bioenergy with Carbon Capture and Storage; Elsevier: Amsterdam, The Netherlands, 2019; pp. 257–271. [Google Scholar] [CrossRef]
- Revinova, S.; Lazanyuk, I.; Ratner, S.; Gomonov, K. Forecasting Development of Green Hydrogen Production Technologies Using Component-Based Learning Curves. Energies 2023, 16, 4338. [Google Scholar] [CrossRef]
- Song, Y.; Han, S.; Kim, J. Economic analysis of decarbonization investments in refinery systems focusing on carbon pricing and operational flexibility. Appl. Energy 2023, 345, 121345. [Google Scholar]


| Electrification of the Process | Gas Treatment of Exhaust | Complexity of the Process | Contribution H2 Renewable | Catalysts Improved | Heat Exchange System Optimized | Digital Twin | |
|---|---|---|---|---|---|---|---|
| Crude and vacuum | ✓✓✓ | ✓✓✓ | ✓ | ✓✓✓ | ✓✓✓ | ||
| Platforming | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | |
| Hydrogen | ✓✓ | ✓✓ | ✓✓✓ | ✓ | ✓✓ | ✓✓✓ | ✓ |
| HDS | ✓ | ✓ | ✓✓ | ✓✓ | ✓ | ✓ | |
| Coke | ✓✓ | ✓✓ | ✓✓ | ✓ | ✓ | ✓ | |
| FCC | ✓✓ | ✓✓ | ✓✓✓ | ✓ | ✓ | ✓ | |
| HDT | ✓ | ✓ | ✓✓ | ✓✓ | ✓ | ✓ | ✓ |
| Electrification of the Process | Treatment of Gases from Exhaust | Complexity of the Process | Contribution H2 Renewable | Catalysts Improved | Heat Exchange System Optimized | Digital Twin | |
|---|---|---|---|---|---|---|---|
| Gases | ✓✓ | ✓ | ✓ | ✓ | |||
| ETBE | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | ||
| Desisopentanizer | ✓ | ✓ | ✓ | ✓ | |||
| Isopentane | ✓ | ✓ | ✓ | ✓ | |||
| Amines | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓ | |||
| Propylene | ✓ | ✓✓ | ✓ | ✓ | |||
| Kerosene | ✓ | ✓✓ | ✓ | ✓ | ✓ | ||
| Benzene | ✓✓ | ✓✓✓ | ✓ | ✓ | ✓✓ | ✓✓ | |
| SRU | ✓✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓ |
| Method/Strategy | Description | Applicability/Units | Benefits | Challenges/Notes |
|---|---|---|---|---|
| Electrification | Replacement of fossil fuel combustion with electric-powered systems | Furnaces, reboilers, heating lines | Significant CO2 reduction | High investment, grid dependency, technical complexity |
| Treatment and Capture of Exhaust Gases | Technologies to capture or treat CO2, NOx, SOx emissions | FCC, SRU, HDT, HDS | Reduces emissions and pollutants | Requires process redesign, cost intensive |
| Catalysts Improvement | Use of advanced, high-performance catalysts | HDS, HDT, SRU | Improves conversion efficiency | Development time and cost |
| Heat Exchange Optimization | Integration of heat recovery systems and low-temp waste heat utilization | Most units, especially catalytic | Reduces fuel consumption and emissions | Design complexity |
| Renewable Hydrogen Use | Substitution of fossil H2 with renewable-produced H2 | HDS, HDT, hydrogen generation units | Lowers carbon footprint | Requires renewable H2 availability and infrastructure |
| Digital Twin and AI Tools | Process simulation and optimization through virtual replicas and AI | Entire refinery | Operational efficiency, energy savings | Algorithm refinement, data requirements |
| Advanced Combustion Technologies | Use of oxy-combustion, air preheating | Furnaces | Lower NOx emissions, better efficiency | Process modification needed |
| Biofuels and Synthetic Fuels | Integration or production of low-carbon fuels | Fuel blending, some refining processes | Potential for net-zero fuels | Market and supply chain integration |
| Carbon Capture and Storage (CCS) | Capture and geological storage of CO2 | Large emission sources | Essential for zero net emissions scenario | High CAPEX/OPEX, regulatory framework |
| Renewable Energy Integration | Use of on-site or purchased renewable electricity | Electrified units | Reduces indirect emissions | Requires energy management and investment |
| Investment Scenarios | ||||
|---|---|---|---|---|
| Base Case | Investment for 20% reduction CO2 | Investment for 40% reduction CO2 | Investment for 60% reduction CO2 | |
| Total emission (kt CO2/year) | 1342 | 1088 | 807 | 531.5 |
| Cost (M$/year) | 3.295 | 3.338 | 3.379 | 3.515 |
| Increase in annual cost (%) | 0.0 | 1.3 | 2.6 | 6.7 |
| Total emission/Cost | 0.41 | 0.32 | 0.24 | 0.15 |
| % Emissions Base Case | Emissions Base Case (kt/Year) | Simulation of Emission Reductions in Units (kt/Year) | |||
|---|---|---|---|---|---|
| 20% | 40% | 60% | |||
| Crude | 46.8 | 627 | 531 | 376 | 193 |
| Platformed | 12.3 | 165 | 130 | 107 | 50 |
| FCC | 1.5 | 20 | 17 | 13 | 8 |
| HDS | 15 | 201 | 159 | 124 | 62 |
| HDT | 18 | 242 | 184 | 137 | 162 |
| kerosene | 6.3 | 84 | 63 | 47.4 | 56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seijo-Bestilleiro, E.; Arias-Fernández, I.; Carro-López, D.; Naveiro, M. Opportunities for Emission Reduction in the Transformation of Petroleum Refining. Fuels 2025, 6, 66. https://doi.org/10.3390/fuels6030066
Seijo-Bestilleiro E, Arias-Fernández I, Carro-López D, Naveiro M. Opportunities for Emission Reduction in the Transformation of Petroleum Refining. Fuels. 2025; 6(3):66. https://doi.org/10.3390/fuels6030066
Chicago/Turabian StyleSeijo-Bestilleiro, Emilio, Ignacio Arias-Fernández, Diego Carro-López, and Manuel Naveiro. 2025. "Opportunities for Emission Reduction in the Transformation of Petroleum Refining" Fuels 6, no. 3: 66. https://doi.org/10.3390/fuels6030066
APA StyleSeijo-Bestilleiro, E., Arias-Fernández, I., Carro-López, D., & Naveiro, M. (2025). Opportunities for Emission Reduction in the Transformation of Petroleum Refining. Fuels, 6(3), 66. https://doi.org/10.3390/fuels6030066








