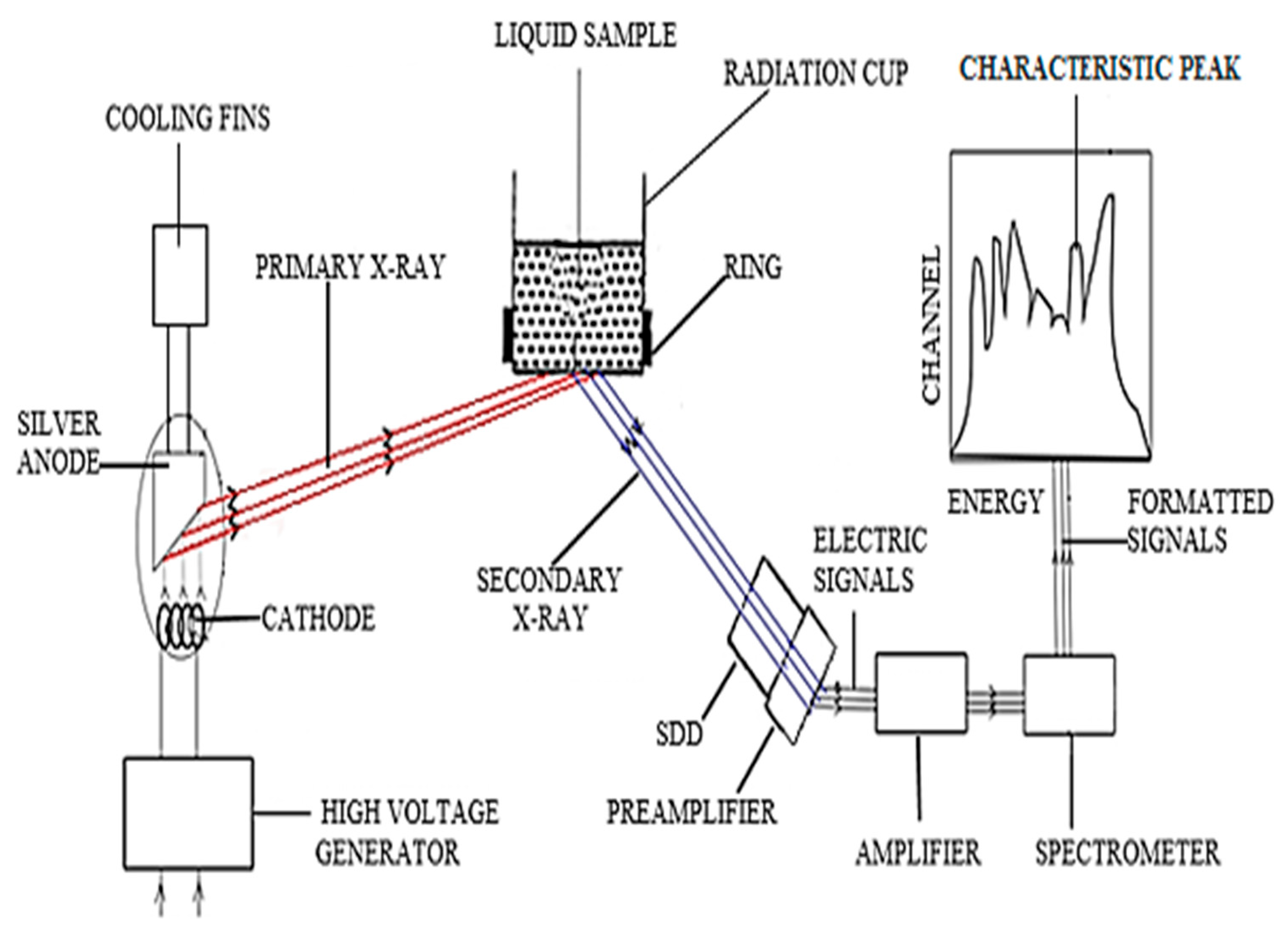
2.4. Results and Discussion
The elemental concentrations based on the analyses of fluorescence data acquired from every four samples of the seven refined fuels are shown in
Table 1,
Table 2,
Table 3,
Table 4,
Table 5,
Table 6 and
Table 7. Elements with concentrations below the detection limit of the fluorescence measurement technique are indicated as BL. From the seven tables, thirty-one elements were identified from each sample of the refined fuel. The elements are classified into non-metal, alkali metal, alkaline earth, transition metal, base metal and semimetal with an average concentration estimated in parts per million.
Table 1,
Table 2 and
Table 3 show the fluorescence results of fuel samples obtained from the service stations of Total Energies Ghana Limited, DE (diesel excellium), PE (petrol excellium) and KE (kerosene excellium), respectively.
Table 4 and
Table 5 present the fluorescence results of fuel samples acquired from the service stations of GOIL Company Limited, DXP (diesel xp) and PXP (petrol xp), respectively. Lastly,
Table 6 and
Table 7 present the fluorescence result of fuel samples acquired from the service stations of Shell Vivo Ghana Limited, VP (v-power petrol) and DFS (diesel fuelsave).
Among the thirty-one elements quantified in the twenty-eight fuel samples, In recorded relatively higher concentrations in each compared to the rest. From the seven tables, P, S, Cl, K, Cr, Fe and In recorded relatively higher concentrations in the samples from kerosene and diesel products, with S in higher concentrations in DE and DXP samples only. Similarly, in petrol products, the concentration of P, Cr and contaminants are relatively higher in fuel samples PE and PXP.
From the seven tables, the elemental concentrations of the non-metals identified were relatively lower compared to the metals. The concentrations of the element S in each fuel sample are between 20.0–50.0 ppm, which is slightly above the ultra-low level of 10.0 ppm per the standards of the Environment Protection Agency of the United States for fuels for on-road engines. On average, fuel sample KE recorded the highest sulphur concentration and sample VP the least. From
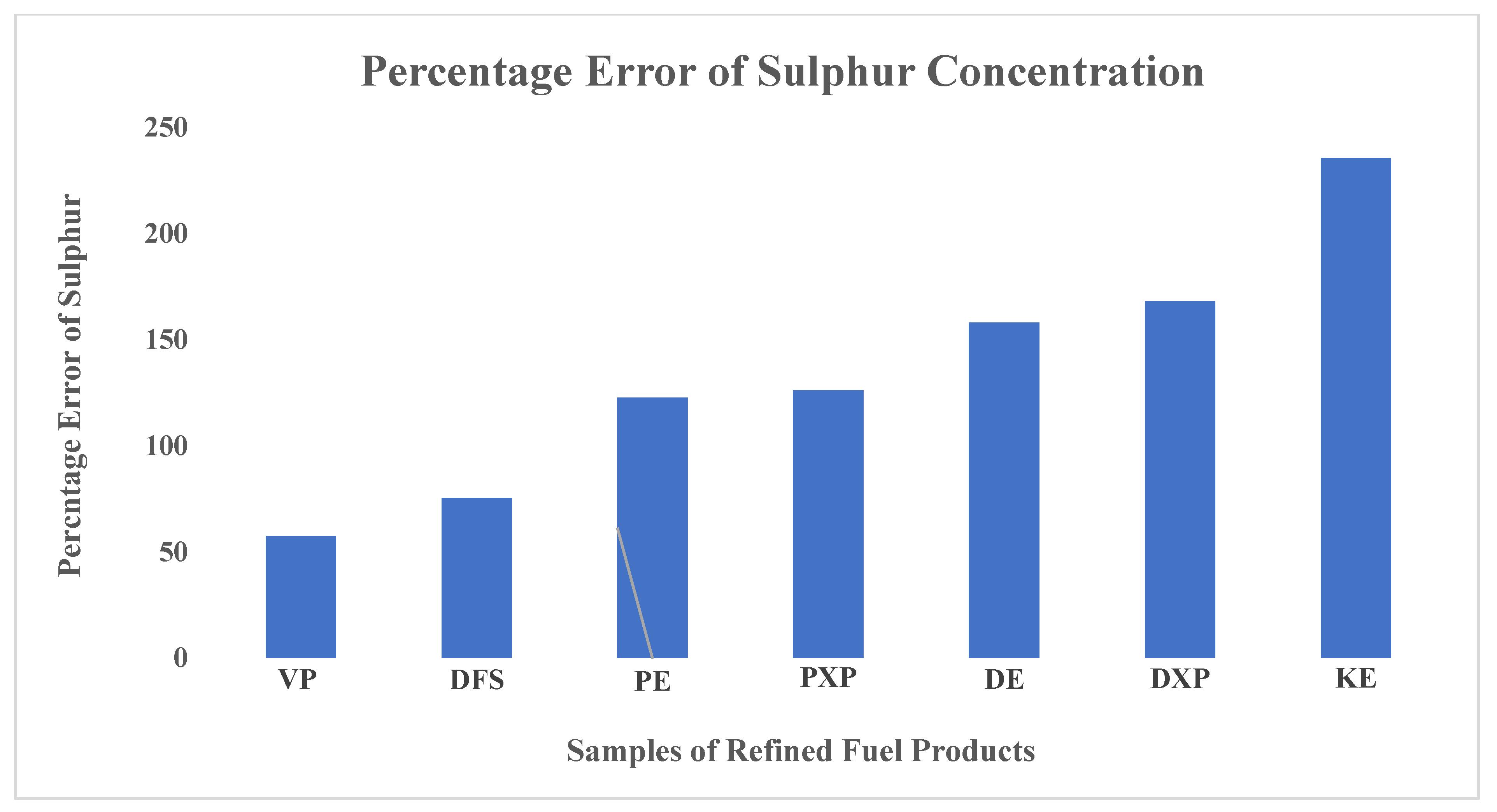
Table 3, the sulphur concentration in fuel sample KE is relatively higher compared to the rest. This is because kerosene fuel products usually have sulphur content much higher than petrol and diesel products. The study confirmed that sulphur concentration in on-road diesel fuels is usually more than in petrol fuels. From the results it is revealed that the concentration of sulphur in fuel sample DE (diesel) is approximately 1.16 times greater than sulphur in sample PE (petrol) even though both are imported by the same oil-marketing company, Total Energies Ghana Limited. Also, a similar effect happened between the fuel samples DXP (diesel) and PXP (petrol). However, there is a strong correlation between sulphur concentration in fuel sample PXP and PE, even though both are petrol products from different oil-marketing companies. Similarly, the sulphur concentration in sample DXP is approximately 1.02 times greater than that of sample DE. These slight anomalies may be because the major oil-marketing companies of Ghana import refined petroleum fuels from different Asian and European countries. Hence, on-road fuels for engines are acquired from any available source per the prevailing market conditions. Considering 10.0 ppm as the internationally accepted standard concentration of sulphur in on-road refined fuels, the estimated percentage error for the fuel samples—VP, DFS, PE, PXP, DE, DXP and KE—are 57.5, 75.4, 122.6, 126.2, 158.1, 168.1 and 235.5%, respectively, in ascending order.
Figure 2 is a bar graph showing the magnitude of the percentage error of sulphur concentration estimated for each sample.
Bromine is a naturally toxic halogen gas. The presence of mine elements in the crude sample may also be attributed to the chemical nature of the sedimentary rocks from which the crudes for the refined fuels were acquired. The comparison from the six tables reveals that, on average, the highest bromine concentration occurred in sample VP (v-power petrol), while sample PXP (petrol XP) recorded the least. The average concentrations of bromine in fuel samples PE (petrol excellium) and DE (diesel excellium) of Total Energies Ghana Limited are comparable. This suggests that the crude oils for fuel oil samples PE and DE were processed with similar refinery technology. Organic bromine contaminants in live tissues and cells cause malfunction of the nervous system and thyroid glands (WHO, 2009). Even though the concentration of bromine by the fluorescence measurement is in ultra-trace, however, when released into the atmosphere, gradual accumulation in the long term may produce dangerous effects.
The research also came up with four toxic metal elements namely mercury, lead, chromium and Manganese [
21]. The presence of these toxic metal elements may be attributed to the intrinsic chemical nature of the crude oils and the type of refinery technology during processing [
7,
22]. The presence of lead may also be from the introduction of tetraethyl lead, Pb(C
2H
5)
4 compound to the refined fuels by the manufacturers as an octane rating booster or antiknock agent [
7,
22,
23]. In the modern day, the octane rating of refined fuels is improved by the addition of methanol, ethanol, methyl tertiary butyl ether, ethyl tertiary butyl ether, etc. The results from the seven tables reveal that the average concentrations of the four identified toxic metals in each seven refined fuel products are non-uniform [
13,
17,
23]. This is because the three major oil-marketing companies in Ghana, import their fuel products from different sources and hence resulting in dissimilar concentrations. Mercury and lead are acute toxic metal elements and therefore their presence in the refined fuel samples will result in toxicity in the environment [
18]. The gradual long-period exposure of these toxic metals to animals and humans may cause the bio-amplification of toxic materials. Even though per the standards of the Environmental Protection Agency of the United States, the concentration levels of Hg, Pb and Mn are below ultra-low (10.0 ppm), however, the concentration of chromium is above the ultra-low. Therefore, among the heavy metal elements quantified, there is a relatively higher level of chromium emission from the exhausts of vehicles in Ghana. The sample DE recorded the highest chromium emission and DXP the least.
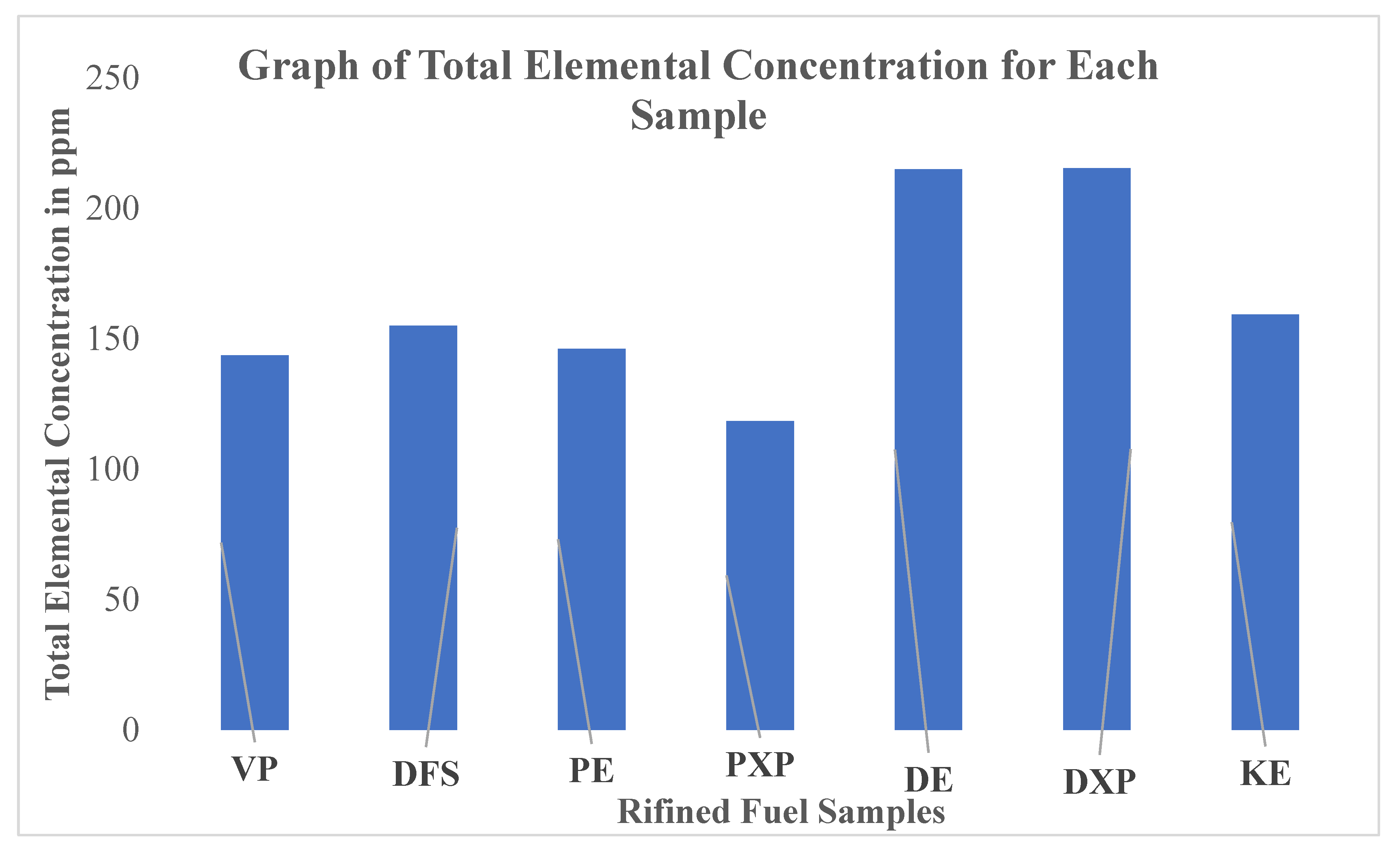
Studies in petrology have proven that metal compounds of alkali, alkaline earth, semimetal, transition and base metal in refined petroleum fuels are ash-producing agents [
19]. Salted compounds of these metals in refined fuels produce undesired results in the combustion chamber by causing a high-temperature corrosion effect on burner tips and refractories. The higher the ash-forming constituents, the greater the fouling deposits in the combustion equipment and vice versa. Based on the results for the diesel samples, DFS recorded an approximate total average elemental concentration of 155.0 ppm while DE and DXP recorded 215.0 and 215.3 ppm, respectively. It is seen that the total average concentrations for samples DXP and DE are most comparable, however, the individual elemental concentrations are not the same. Among the petrol products, samples PXP, VP and PE recorded approximate total average concentration values of 118.5, 143.6 and 146.1 ppm, respectively. Hence, the fuel sample PE recorded the highest elemental concentration among the petrol products consumed in Ghana. However, the fuel sample KE of kerosene product recorded an approximate significant value of 159.3 ppm, which represents lesser impurity content compared to diesel samples.
Figure 3 is the bar graph showing the impurity levels based on the total elemental concentrations present in the refined fuel samples.
The average specific gravity of each fuel sample was also determined at the Physics laboratory of NNRI. The specific gravity of the samples, DE, PE, KE, DXP, PXP, VP and DFS are 0.892, 0.704, 0.797, 0.881, 0.692, 0.697 and 0.894, respectively at 15.0 °C. Based on the values of specific gravities of the diesel samples, it indicates that DE, DXP and DFS are of grade 2D since diesel fuel of grade 2D has a specific gravity between 0.81–0.96. Grade 2D diesel fuels are recommended for relatively warmer weather conditions and tropical regions. This means the oil-marketing companies of Ghana import diesel fuels that are conducive to the environmental conditions of the country. There are variations among the values of specific gravity of diesel and petrol samples as shown. These anomalies may be due to the variations among both elemental concentrations and organic molecular weight in each sample.