Utilization of Iron Foam as Structured Catalyst for Fischer–Tropsch Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation and Characterization
2.1.1. Catalyst Preparation
2.1.2. Catalyst Characterization
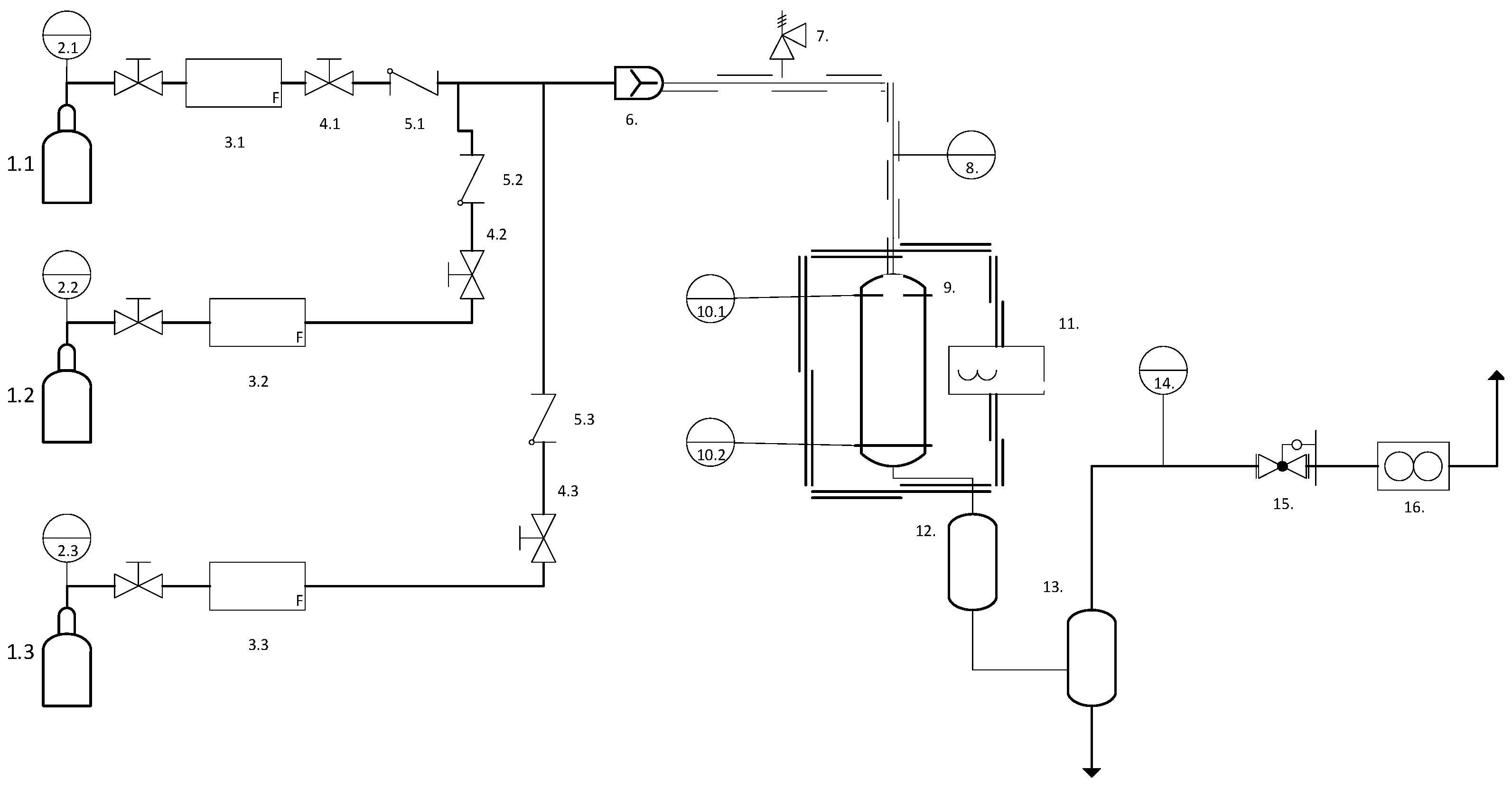
2.2. Catalyst Test
2.3. Product Characterization
3. Results
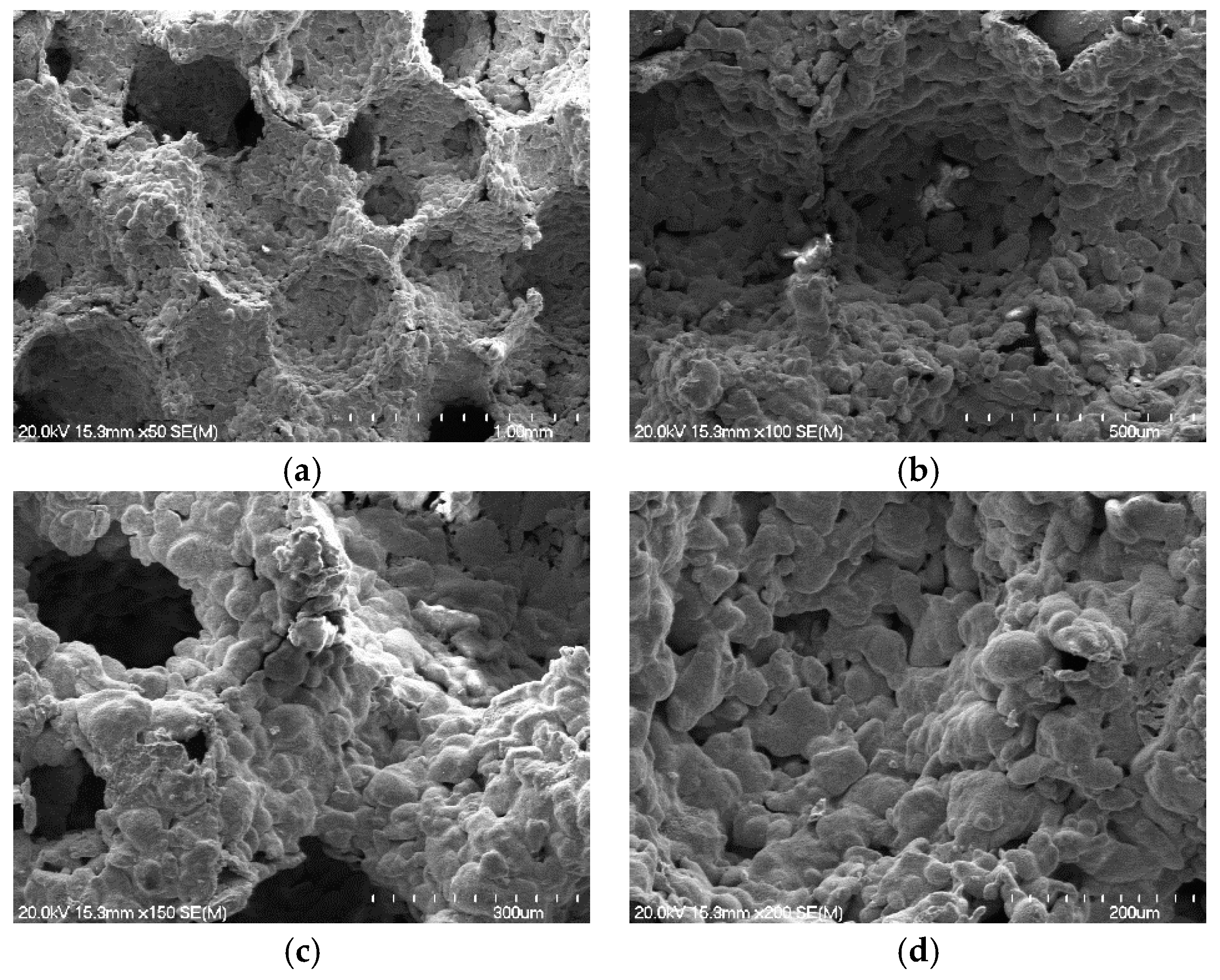
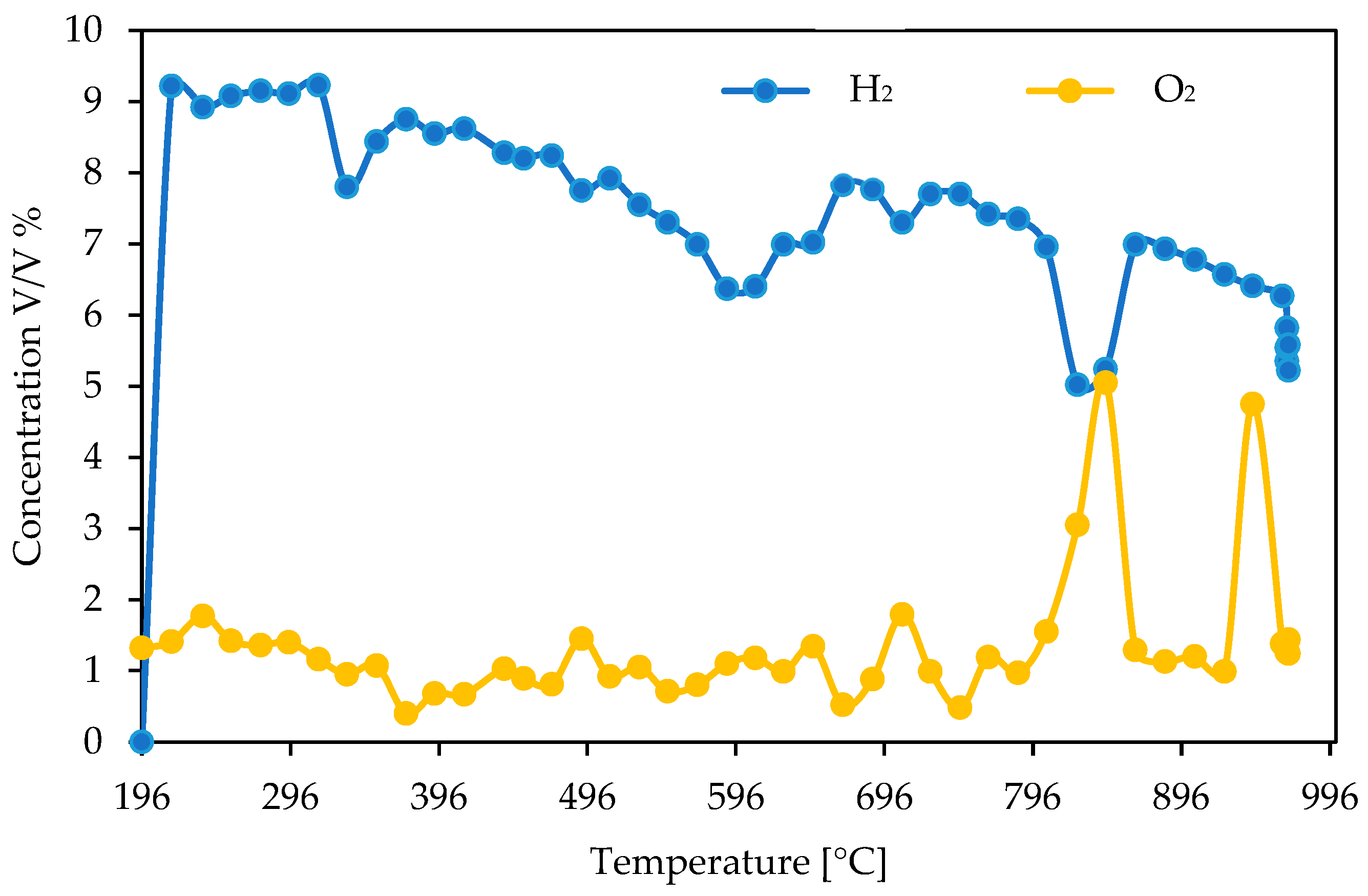
3.1. Catalyst Properties
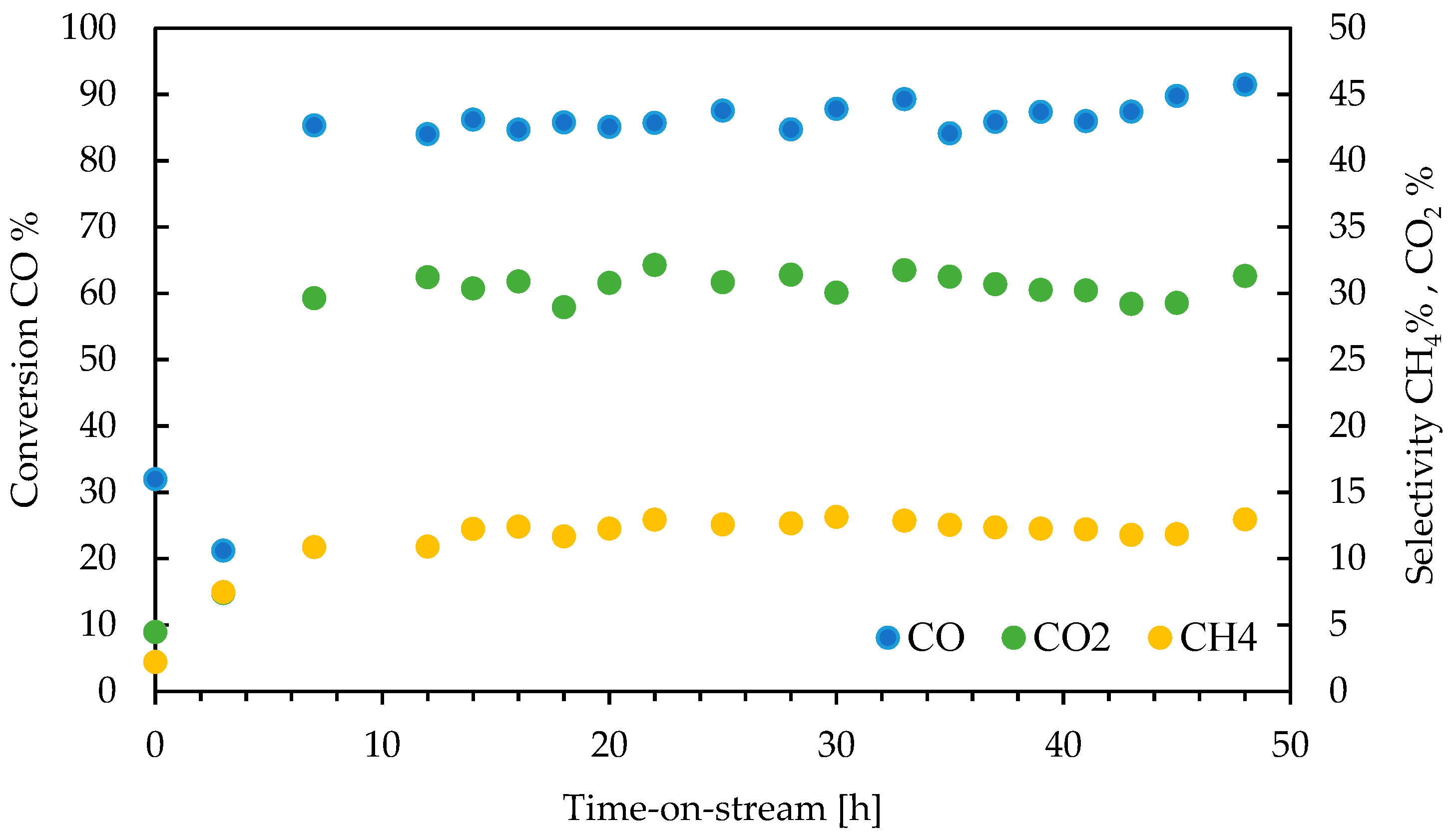
3.2. Catalytic Performance
3.3. Product Analysis
4. Discussion
5. Conclusions
6. Future Overview
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hurtado, Y.V.H. Development of a Structured Iron-Based Catalyst for Fischer–Tropsch Synthesis Using Bio-Syngas. Ph.D. Thesis, University of Sherbrooke, Sherbrooke, QC, Canada, 2023. Available online: http://hdl.handle.net/11143/21724 (accessed on 12 July 2021).
- BP. Energy Use by Sector. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/energy-outlook/bp-energy-outlook-2024.pdf (accessed on 12 July 2021).
- International Energy Agency. World Energy Balances 2019; OECD Publishing: Paris, France, 2019. [Google Scholar] [CrossRef]
- Our World in Data. Energy Production and Consumption. Available online: https://ourworldindata.org/energy-production-consumption (accessed on 12 July 2021).
- IEA. EIA Projects Nearly 50% Increase in World Energy Usage by 2050, Led by Growth in Asia. Available online: https://www.eia.gov/todayinenergy/detail.php?id=41433 (accessed on 6 March 2021).
- Canada Energy Regulator. Provincial and Territorial Energy Profiles–Canada. Available online: https://www.cer-rec.gc.ca/en/data-analysis/energy-markets/provincial-territorial-energy-profiles/provincial-territorial-energy-profiles-canada.html (accessed on 12 July 2021).
- Government of Canada. Greenhouse Gas Emissions: Drivers and Impacts. Available online: https://www.canada.ca/en/environment-climate-change/services/environmental-indicators/greenhouse-gas-emissions-drivers-impacts.html (accessed on 12 July 2021).
- IEA. Global Energy Review: CO2 Emissions in 2020. 2021. Available online: https://www.iea.org/articles/global-energy-review-co2-emissions-in-2020 (accessed on 2 March 2021).
- dos Santos, R.G.; Alencar, A.C. Biomass-derived syngas production via gasification process and its catalytic conversion into fuels by Fischer Tropsch synthesis: A review. Int. J. Hydrogen. Energy 2020, 45, 18114–18132. [Google Scholar] [CrossRef]
- Government of Canada. Renewable Fuels Regulations. 2020. Available online: https://www.canada.ca/en/environment-climate-change/services/managing-pollution/energy-production/fuel-regulations/renewable.html (accessed on 4 October 2021).
- Government of Canada. Energy and the Economy. 2021. Available online: https://natural-resources.canada.ca/science-data/data-analysis/sources-energy-information (accessed on 4 February 2025).
- Renewable Industries Canada. Fuelling a Way Forward. 2021. Available online: https://ricanada.org/ (accessed on 4 October 2021).
- Commit. European Union: Energy System Restructuring Towards a Long-Term Lowemission Pathway. 2021. Available online: https://unfccc.int/sites/default/files/resource/365_COMMIT%20Fact%20Sheet%20EU%20-%20a%20long-term%20low-emission%20pathway.pdf (accessed on 12 July 2021).
- Benedetti, V.; Ail, S.S.; Patuzzi, F.; Cristofori, D.; Rauch, R.; Baratieri, M. Investigating the feasibility of valorizing residual char from biomass gasification as catalyst support in Fischer-Tropsch synthesis. Renew. Energy 2020, 147, 884–894. [Google Scholar] [CrossRef]
- Lappas, A.; Heracleous, E. Production of biofuels via Fischer–Tropsch synthesis: Biomass-to-liquids. In Handbook of Biofuels Production; Elsevier: Amsterdam, The Netherlands, 2016; pp. 549–593. [Google Scholar]
- Ail, S.S.; Dasappa, S. Biomass to liquid transportation fuel via Fischer Tropsch synthesis–Technology review and current sce-nario. Renew. Sustain. Energy Rev. 2016, 58, 267–286. [Google Scholar] [CrossRef]
- Schulz, H. Short history and present trends of Fischer–Tropsch synthesis. Appl. Catal. A Gen. 1999, 186, 3–12. [Google Scholar] [CrossRef]
- Demirbas, A. Converting biomass derived synthetic gas to fuels via Fisher-Tropsch synthesis. Energy Sources Part A Recovery Util. Environ. Eff. 2007, 29, 1507–1512. [Google Scholar] [CrossRef]
- Davis, B.H. Fischer–Tropsch synthesis: Current mechanism and futuristic needs. Fuel Process. Technol. 2001, 71, 157–166. [Google Scholar] [CrossRef]
- Davis, B.H. Fischer–Tropsch synthesis: Overview of reactor development and future potentialities. Top. Catal. 2005, 32, 143–168. [Google Scholar] [CrossRef]
- Bezemer, G.L.; Bitter, J.H.; Kuipers, H.P.; Oosterbeek, H.; Holewijn, J.E.; Xu, X.; Kapteijn, F.; Van Dillen, A.J.; de Jong, K.P. Cobalt particle size effects in the Fischer−Tropsch reaction studied with carbon nanofiber supported catalysts. J. Am. Chem. Soc. 2006, 128, 3956–3964. [Google Scholar] [CrossRef]
- Bezemer, G.L.; Radstake, P.; Falke, U.; Oosterbeek, H.; Kuipers, H.; Van Dillen, A.; De Jong, K. Investigation of promoter effects of manganese oxide on carbon nanofiber-supported cobalt catalysts for Fischer–Tropsch synthesis. J. Catal. 2006, 237, 152–161. [Google Scholar] [CrossRef]
- Tu, J. Development of Catalysts for Biofuels Production from Biomass via Fischer-Tropsch Synthesis. Adv. New Renew. Energy 2014, 2, 94–103. [Google Scholar]
- Sharma, P.; Elder, T.; Groom, L.H.; Spivey, J.J. Effect of structural promoters on Fe-based Fischer–Tropsch synthesis of biomass derived syngas. Top. Catal. 2014, 57, 526–537. [Google Scholar] [CrossRef]
- Chun, D.H.; Park, J.C.; Hong, S.Y.; Lim, J.T.; Kim, C.S.; Lee, H.T.; Yang, J.-I.; Hong, S.J.; Jung, H. Highly selective iron-based Fischer–Tropsch catalysts activated by CO2-containing syngas. J. Catal. 2014, 317, 135–143. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, H.; Ning, W.; Wu, Y. Performance of fused-iron catalyst in Fischer-Tropsch synthesis in slurry reactor. Petrochem. Technol. 2009, 38, 593–597. [Google Scholar]
- Luque, R.; de la Osa, A.R.; Campelo, J.M.; Romero, A.A.; Valverde, J.L.; Sanchez, P. Design and development of catalysts for Biomass-To-Liquid-Fischer–Tropsch (BTL-FT) processes for biofuels production. Energy Environ. Sci. 2012, 5, 5186–5202. [Google Scholar] [CrossRef]
- Zhang, S. Production of Transportation Fuel Range Middle Distillates via Fischer-Tropsch Synthesis with Integrated Product Upgrading Under Supercritical Phase Conditions. Ph.D. Thesis, Auburn University, Auburn, AL, USA, 2013. [Google Scholar]
- Dry, M.E. High quality diesel via the Fischer–Tropsch process—A review. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2002, 77, 43–50. [Google Scholar] [CrossRef]
- Dry, M.E. The fischer–tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Chun, D.H.; Rhim, G.B.; Youn, M.H.; Deviana, D.; Lee, J.E.; Park, J.C.; Jeong, H. Brief review of precipitated iron-based catalysts for low-temperature fischer–tropsch synthesis. Top. Catal. 2020, 63, 793–809. [Google Scholar] [CrossRef]
- Shafer, W.D.; Gnanamani, M.K.; Graham, U.M.; Yang, J.; Masuku, C.M.; Jacobs, G.; Davis, B.H. Fischer–Tropsch: Product selectivity–the fingerprint of synthetic fuels. Catalysts 2019, 9, 259. [Google Scholar] [CrossRef]
- Rauch, R.; Kiennemann, A.; Sauciuc, A. Fischer-Tropsch synthesis to biofuels (BtL process). In The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 397–443. [Google Scholar]
- Guettel, R.; Kunz, U.; Turek, T. Reactors for Fischer-Tropsch Synthesis. Chem. Eng. Technol. Ind. Chem. Plant Equip. Process Eng. Biotechnol. 2008, 31, 746–754. [Google Scholar] [CrossRef]
- Parra-Cabrera, C.; Achille, C.; Kuhn, S.; Ameloot, R. 3D printing in chemical engineering and catalytic technology: Structured catalysts, mixers and reactors. Chem. Soc. Rev. 2018, 47, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Harmel, J.; Peres, L.; Estrader, M.; Berliet, A.; Maury, S.; Fécant, A.; Chaudret, B.; Serp, P.; Soulantica, K. hcp-Co Nanowires Grown on Metallic Foams as Catalysts for Fischer-Tropsch Synthesis. Angew. Chem. 2018, 130, 10739–10743. [Google Scholar] [CrossRef]
- Park, J.C.; Roh, N.S.; Chun, D.H.; Jung, H.; Yang, J.-I. Cobalt catalyst coated metallic foam and heat-exchanger type reactor for Fischer–Tropsch synthesis. Fuel Process. Technol. 2014, 119, 60–66. [Google Scholar] [CrossRef]
- Hilmen, A.-M.; Bergene, E.; Lindvåg, O.; Schanke, D.; Eri, S.; Holmen, A. Fischer–Tropsch synthesis on monolithic catalysts of different materials. Catal. Today 2001, 69, 227–232. [Google Scholar] [CrossRef]
- Tronconi, E.; Groppi, G.; Visconti, C.G. Structured catalysts for non-adiabatic applications. Curr. Opin. Chem. Eng. 2014, 5, 55–67. [Google Scholar] [CrossRef]
- Visconti, C.G.; Tronconi, E.; Lietti, L.; Groppi, G.; Forzatti, P.; Cristiani, C.; Zennaro, R.; Rossini, S. An experimental investiga-tion of Fischer–Tropsch synthesis over washcoated metallic structured supports. Appl. Catal. A Gen. 2009, 370, 93–101. [Google Scholar] [CrossRef]
- Visconti, C.G.; Tronconi, E.; Groppi, G.; Lietti, L.; Iovane, M.; Rossini, S.; Zennaro, R. Monolithic catalysts with high thermal conductivity for the Fischer–Tropsch synthesis in tubular reactors. Chem. Eng. J. 2011, 171, 1294–1307. [Google Scholar] [CrossRef]
- Visconti, C.G.; Groppi, G.; Tronconi, E. Highly conductive “packed foams”: A new concept for the intensification of strongly endo-and exo-thermic catalytic processes in compact tubular reactors. Catal. Today 2016, 273, 178–186. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Wilcox, W.; Li, S. A compact and high throughput reactor of monolithic-structured catalyst bed for con-version of syngas to liquid fuels. AIChE J. 2012, 58, 2820–2829. [Google Scholar] [CrossRef]
- Mehariya, S.; Iovine, A.; Casella, P.; Musmarra, D.; Figoli, A.; Marino, T.; Sharma, N.; Molino, A. Fischer–Tropsch synthesis of syngas to liquid hydrocarbons. In Lignocellulosic Biomass to Liquid Biofuels; Elsevier: Amsterdam, The Netherlands, 2020; pp. 217–248. [Google Scholar]
- Mai, K.; Elder, T.; Groom, L.H.; Spivey, J.J. Fe-based Fischer Tropsch synthesis of biomass-derived syngas: Effect of synthesis method. Catal. Commun. 2015, 65, 76–80. [Google Scholar] [CrossRef]
- Xue, Y.; Duan, S.; Chen, G.; Sun, J.; Abbas, M.; Chen, J. Effect of annealing atmosphere on Fischer-Tropsch synthesis perfor-mance of Fe/Fe foam structured catalyst. Fuel 2020, 262, 116570. [Google Scholar] [CrossRef]
- Feng, Y.; Fornell, J.; Zhang, H.; Solsona, P.; Barό, M.D.; Suriñach, S.; Pellicer, E.; Sort, J. Synthesis of α-Fe2O3 and Fe-Mn oxide foams with highly tunable magnetic properties by the replication method from polyurethane templates. Materials 2018, 11, 280. [Google Scholar] [CrossRef]
- Choi, J.H.; Yang, S.; Kim, Y.D.; Yun, J.Y. The effect of binder content for the pore properties of Fe foam fabricated by slurry coating process. J. Powder Mater. 2013, 20, 439–444. [Google Scholar] [CrossRef]
- Noor, F.M.; Bakar, M.I.A.; Mohamad, Z.; Ismail, A.E.; Ismon, M.; Ahmad, S.; Ismail, A.; Wahab, H.A.; Main, N.M.; Sanusi, S.H. Composition and type of a binder effects on the stainless steel foam microstructure prepared by sponge replication method. Int. J. Integr. Eng. 2019, 11, 90–94. [Google Scholar]
- Yeetsorn, R.; Tungkamani, S.; Maiket, Y. Fabrication of a ceramic Foam Catalyst using polymer foam scrap via the Replica technique for dry reforming. ACS Omega 2022, 7, 4202–4213. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.Y.; Park, D.H.; Wang, J.P. A Study on the Effect of Binder Content for Pore Property of Fe Foam Using Slurry Coating Method. In Advanced Materials Research; Trans Tech Publications, Ltd.: Bäch, Switzerland, 2015; Volume 1125, pp. 136–142. [Google Scholar] [CrossRef]
- ASTM D3663; Standard Test Method for Surface Area of Catalysts and Catalyst Carriers. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D3906; Standard Test Method for Determination of Relative X-ray Diffraction Intensities of Faujasite-Type Zeo-lite-Containing Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- Wan, H.; Wu, B.; An, X.; Tao, Z.; Li, T.; Xiang, H.; Li, Y. Structural properties, reduction and carburization behaviors of Fe/Cu/K/Al2O3 catalyst for Fischer-Tropsch synthesis. Acta Phys. Chim. Sin. 2007, 23, 1151–1156. [Google Scholar] [CrossRef]
- ASTM D1946; Standard Practice for Analysis of Reformed Gas by Gas Chromatography. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D2163; Standard Test Method for Determination of Hydrocarbons in Liquefied Petroleum (LP) Gases and Pro-pane/Propene Mixtures by Gas Chromatography. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D6730; Standard Test Method for Determination of Individual Components in Spark Ignition Engine Fuels by 100-Metre Capillary (with Precolumn) High-Resolution Gas Chromatography. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D5501; Standard Test Method for Determination of Ethanol and Methanol Content in Fuels Containing Greater than 20% Ethanol by Gas Chromatography. ASTM International: West Conshohocken, PA, USA, 2025.
- Cheng, X.; Yang, H.; Tatarchuk, B.J. Microfibrous entrapped hybrid iron-based catalysts for Fischer–Tropsch synthesis. Catal. Today 2016, 273, 62–71. [Google Scholar] [CrossRef]
- Quadbeck, P.; Kümmel, K.; Hauser, R.; Standke, G.; Adler, J.; Stephani, G. Open cell metal foams-application-oriented structure and material selection. In Proceedings of the CELLMAT 2010, Dresden, Germany, 27–29 October 2010. [Google Scholar]
- Sutygina, A.; Betke, U.; Hasemann, G.; Scheffler, M. Manufacturing of Open-Cell Metal Foams by the Sponge Replication Technique. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 882, p. 012022. [Google Scholar]
- Sutygina, A.; Betke, U.; Scheffler, M. Open-Cell Aluminum Foams by the Sponge Replication Technique: A Starting Powder Particle Study. Adv. Eng. Mater. 2020, 22, 1901194. [Google Scholar] [CrossRef]
- Sutygina, A.; Betke, U.; Scheffler, M. Hierarchical-Porous Copper Foams by a Combination of Sponge Replication and Freezing Techniques. Adv. Eng. Mater. 2022, 24, 2001516. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Pino, L.; Laganà, M.; Ferraro, M.; Antonucci, V. High-temperature CO2 methanation over structured Ni/GDC catalysts: Performance and scale-up for Power-to-Gas application. Fuel Process. Technol. 2020, 202, 106365. [Google Scholar] [CrossRef]
- Giani, L.; Groppi, G.; Tronconi, E. Mass-transfer characterization of metallic foams as supports for structured catalysts. Ind. Eng. Chem. Res. 2005, 44, 4993–5002. [Google Scholar] [CrossRef]
- Cruz, M.G.; Bastos-Neto, M.; Oliveira, A.C.; M Filho, J.; Soares, J.M.; Rodriguez-Castellon, E.; Fernandes, F.A. On the structural, textural and morphological features of Fe-based catalysts supported on polystyrene mesoporous carbon for Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2015, 495, 72–83. [Google Scholar] [CrossRef]
- Giani, L.; Groppi, G.; Tronconi, E. Heat transfer characterization of metallic foams. Ind. Eng. Chem. Res. 2005, 44, 9078–9085. [Google Scholar] [CrossRef]
- Giani, L.; Cristiani, C.; Groppi, G.; Tronconi, E. Washcoating method for Pd/γ-Al2O3 deposition on metallic foams. Appl. Catal. B Environ. 2006, 62, 121–131. [Google Scholar] [CrossRef]
- Wu, B.; Tian, L.; Xiang, H.; Zhang, Z.; Li, Y.W. Novel precipitated iron Fischer–Tropsch catalysts with Fe3O4 coexisting with α-Fe2O3. Catal. Lett. 2005, 102, 211–218. [Google Scholar] [CrossRef]
- Hu, J.; Yu, F.; Lu, Y. Application of Fischer–Tropsch synthesis in biomass to liquid conversion. Catalysts 2012, 2, 303–326. [Google Scholar] [CrossRef]
- Jin, Y.; Datye, A.K. Phase transformations in iron Fischer–Tropsch catalysts during temperature-programmed reduction. J. Catal. 2000, 196, 8–17. [Google Scholar] [CrossRef]
- Liu, J.-X.; Wang, P.; Xu, W.; Hensen, E.J. Particle size and crystal phase effects in Fischer-Tropsch catalysts. Engineering 2017, 3, 467–476. [Google Scholar] [CrossRef]
- Chun, D.H.; Park, J.C.; Rhim, G.B.; Lee, H.T.; Yang, J.I.; Hong, S.; Jung, H. Nanocrystalline ferrihydrite-based catalysts for Fischer-Tropsch synthesis: Part I. Reduction and carburization behavior. J. Nanosci. Nanotechnol. 2016, 16, 1660–1664. [Google Scholar] [CrossRef]
- Davis, B.H.; Occelli, M.L. Advances in Fischer-Tropsch Synthesis, Catalysts, and Catalysis; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Davis, B.H. Fischer–Tropsch synthesis: Reaction mechanisms for iron catalysts. Catal. Today 2009, 141, 25–33. [Google Scholar] [CrossRef]
- Banville, M.; Lee, R.; Labrecque, R.; Lavoie, J. Interaction of CO2/CH4 with steel WOOl in an electrocatalytic dry re-forming reactor. Energy Sust. IV 2013, 176, 17. [Google Scholar]
- Van Steen, E.; Prinsloo, F.F. Comparison of preparation methods for carbon nanotubes supported iron Fischer–Tropsch catalysts. Catal. Today 2002, 71, 327–334. [Google Scholar] [CrossRef]
- Li, S.; Li, A.; Krishnamoorthy, S.; Iglesia, E. Effects of Zn, Cu, and K promoters on the structure and on the reduction, carburization, and catalytic behavior of iron-based Fischer–Tropsch synthesis catalysts. Catal. Lett. 2001, 77, 197–205. [Google Scholar] [CrossRef]
- Espinoza, R.; Steynberg, A.; Jager, B.; Vosloo, A. Low temperature Fischer–Tropsch synthesis from a Sasol perspective. Appl. Catal. A Gen. 1999, 186, 13–26. [Google Scholar] [CrossRef]
- Jager, B.; Espinoza, R. Advances in low temperature Fischer-Tropsch synthesis. Catal. Today 1995, 23, 17–28. [Google Scholar] [CrossRef]
- Liu, K.; Suo, H.; Zhang, C.; Xu, J.; Yang, Y.; Xiang, H.; Li, Y. An active Fischer–Tropsch synthesis FeMo/SiO2 catalyst prepared by a modified sol–gel technique. Catal. Commun. 2010, 12, 137–141. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Gruber, H.; Weber, G.; Reichhold, A.; Pirone, R.; Bensaid, S. Aqueous phase reforming of pilot-scale Fischer-Tropsch water effluent for sustainable hydrogen production. Catal. Today 2021, 367, 239–247. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Li, A.; Iglesia, E. Pathways for CO2 formation and conversion during Fischer–Tropsch synthesis on iron-based catalysts. Catal. Lett. 2002, 80, 77–86. [Google Scholar] [CrossRef]
- Lim, D.-H.; Jo, J.H.; Shin, D.Y.; Wilcox, J.; Ham, H.C.; Nam, S.W. Carbon dioxide conversion into hydrocarbon fuels on defective graphene-supported Cu nanoparticles from first principles. Nanoscale 2014, 6, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- NIST/EPA/NIH Mass Spectral Library with Search Program (NIST 23); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2023.
- Dunn, B.C.; Cole, P.; Covington, D.J.; Heider, E.C.; Gasser, J.; Eyring, E.M.; Meuzelaar, H.L.; Pugmire, R.J. Fischer-Tropsch catalysts supported on sol-gel-derived silica. In Abstracts of Papers of the American Chemical Society; 1155 16TH ST, NW; American Chemical Society: Washington, DC, USA, 2003; Volume 225, p. U858. [Google Scholar]
- Karakaya, C.; White, E.; Jennings, D.; Kidder, M.; Deutschmann, O.; Kee, R.J. CO2 hydrogenation to hydrocarbons over Fe/BZY catalysts. ChemCatChem 2022, 14, e202200802. [Google Scholar] [CrossRef]
- Chernavskii, P.A.; Zaikovskii, V.I.; Pankina, G.V.; Khodakov, A.Y. Dimensional effects in the carbidization of supported iron nanoparticles. ChemCatChem 2013, 5, 1758–1761. [Google Scholar] [CrossRef]
- Oschatz, M.; van Deelen, T.W.; Weber, J.L.; Lamme, W.S.; Wang, G.; Goderis, B.; Verkinderen, O.; Dugulan, A.I.; De Jong, K.P. Effects of calcination and activation conditions on ordered mesoporous carbon supported iron catalysts for production of lower olefins from synthesis gas. Catal. Sci. Technol. 2016, 6, 8464–8473. [Google Scholar] [CrossRef]
- Durham, E.; Zhang, S.; Roberts, C. Diesel-length aldehydes and ketones via supercritical Fischer Tropsch Synthesis on an iron catalyst. Appl. Catal. A Gen. 2010, 386, 65–73. [Google Scholar] [CrossRef]


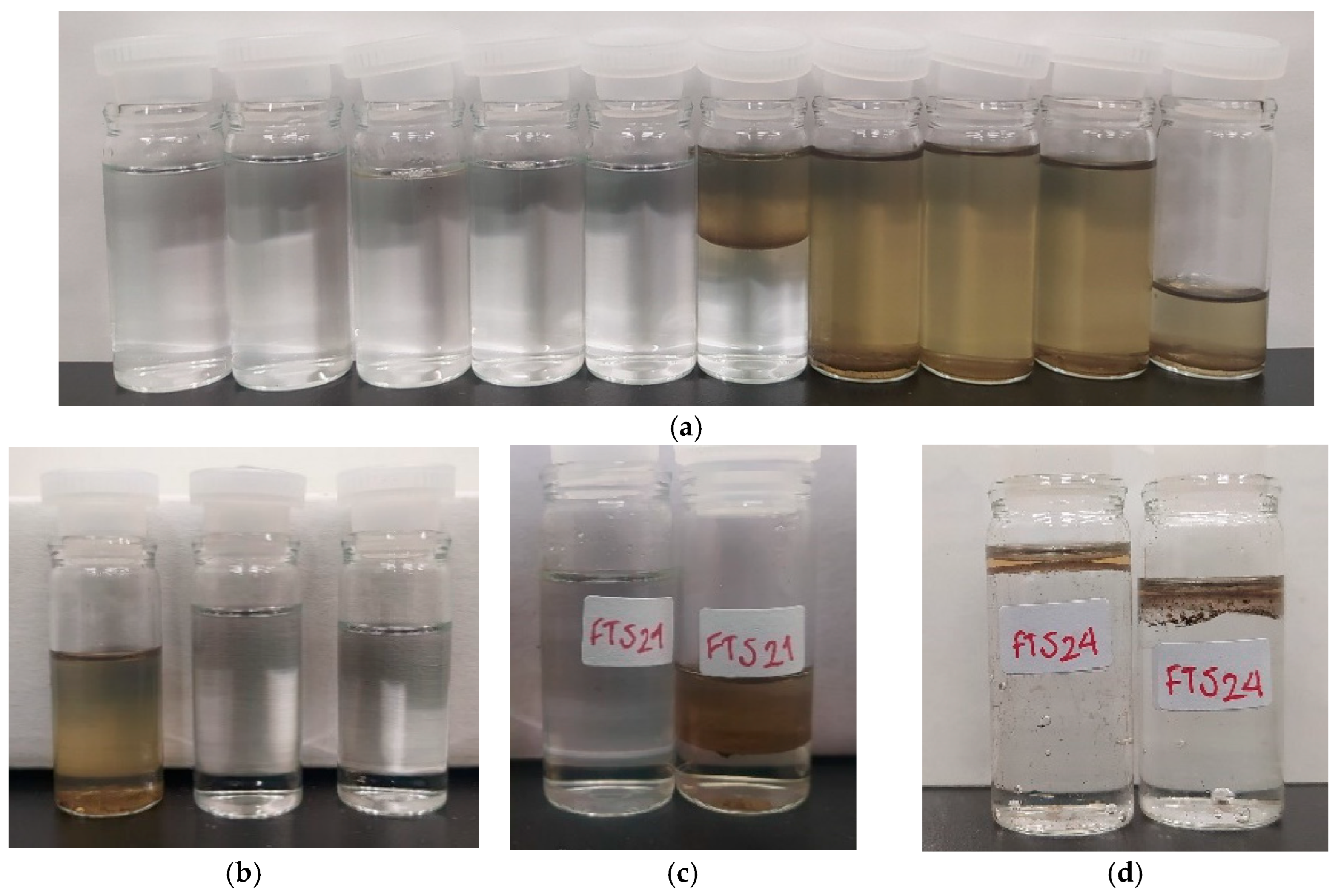
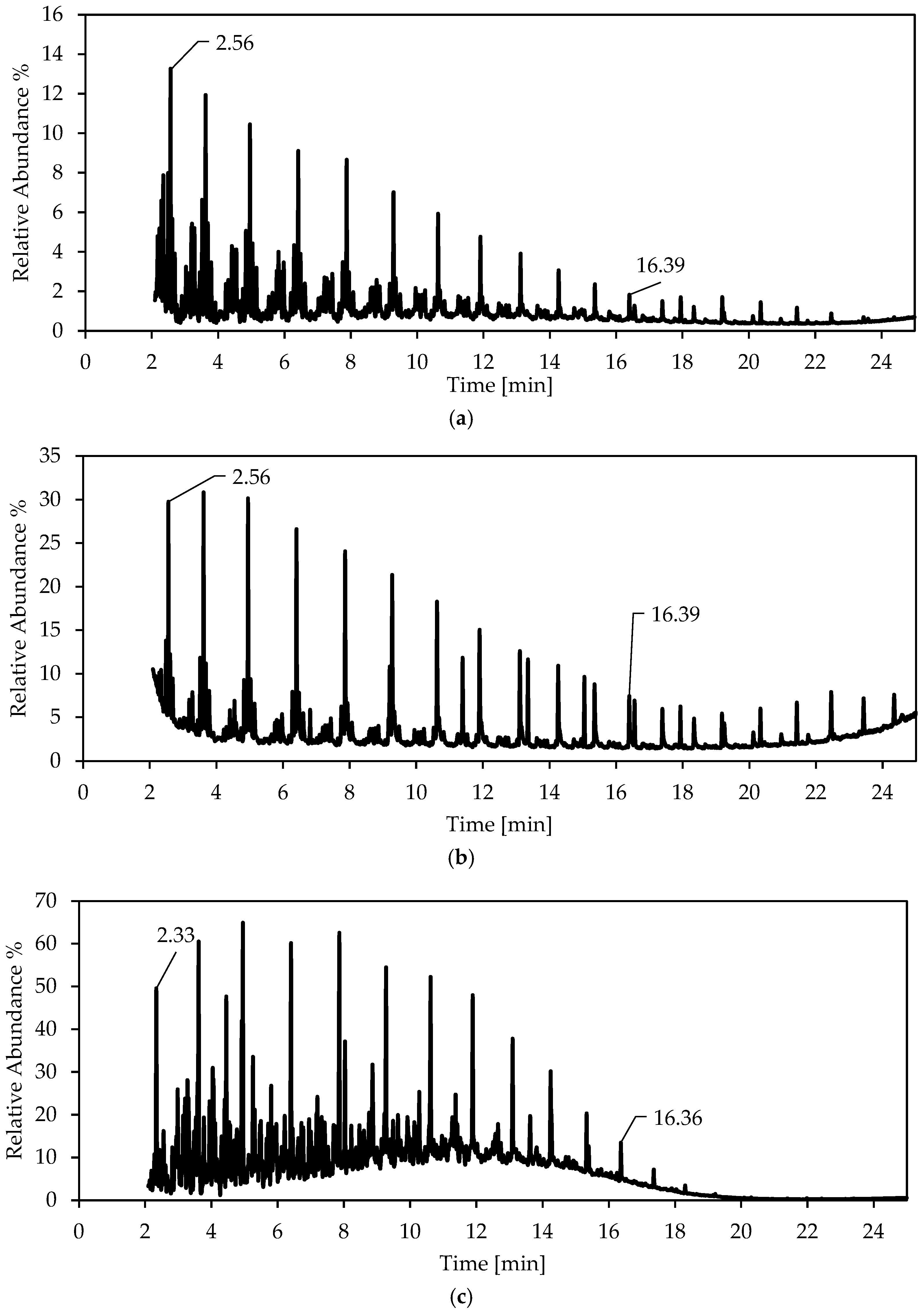
| Run | Catalyst | XCO | SCO2 | SCH4+ | SC3+ |
|---|---|---|---|---|---|
| 1 | Fe powder | 17.8 | 6.0 | 19.0 | 74.9 |
| Fe foam | 10.1 | 7.5 | 4.57 | 87.9 | |
| 2 | Fe powder | 16.3 | 6.5 | 14.0 | 78.5 |
| Fe foam | 9.6 | 10.8 | 10.8 | 89.1 |
| Temp. Red (°C) | XCO | SCO2 | SCH4 | SC+ |
|---|---|---|---|---|
| 400 | 15.2 | 4.2 | 10.1 | 85.7 |
| 325 | 36.2 | 18.6 | 14.7 | 66.6 |
| 250 | 16.1 | 3.5 | 9.7 | 86.8 |
| H2/CO | XCO | SCO2 | SCH4 | SC+ |
|---|---|---|---|---|
| 1.2 | 84.8 | 26.2 | 10.4 | 63.4 |
| 1.6 | 37.1 | 10.2 | 16.7 | 73.2 |
| 2 | 36.2 | 18.6 | 14.7 | 66.6 |
| 2.5 | 19.9 | 11.1 | 18.9 | 70.0 |
| H2/CO | 1.2 | 1.6 | ||
|---|---|---|---|---|
| Phase | Aqueous | Oil | Aqueous | Oil |
| Density [g·mL−1] | 0.992 | 0.750 | 0.988 | 0.772 |
| pH | 3.56 | -- | 3.49 | -- |
| Viscosity [cSt] | -- | 1.28 | -- | 1.37 |
| Amount [mg·kg−1] | ||
|---|---|---|
| Oxygenate | H2:CO = 1.2 | H2:CO = 1.6 |
| Ethanol | 19,796.6 | 10,480.9 |
| Acetic acid | 2418.1 | 1330.5 |
| H2/CO | 1.2 | 1.6 |
|---|---|---|
| C | 62.4 | 69.6 |
| H | 10.8 | 12.5 |
| O | 26.7 | 17.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurtado, Y.V.; Azadi, G.; Neto, E.L.d.B.; Lavoie, J.-M. Utilization of Iron Foam as Structured Catalyst for Fischer–Tropsch Synthesis. Fuels 2025, 6, 60. https://doi.org/10.3390/fuels6030060
Hurtado YV, Azadi G, Neto ELdB, Lavoie J-M. Utilization of Iron Foam as Structured Catalyst for Fischer–Tropsch Synthesis. Fuels. 2025; 6(3):60. https://doi.org/10.3390/fuels6030060
Chicago/Turabian StyleHurtado, Yira Victoria, Ghazal Azadi, Eduardo Lins de Barros Neto, and Jean-Michel Lavoie. 2025. "Utilization of Iron Foam as Structured Catalyst for Fischer–Tropsch Synthesis" Fuels 6, no. 3: 60. https://doi.org/10.3390/fuels6030060
APA StyleHurtado, Y. V., Azadi, G., Neto, E. L. d. B., & Lavoie, J.-M. (2025). Utilization of Iron Foam as Structured Catalyst for Fischer–Tropsch Synthesis. Fuels, 6(3), 60. https://doi.org/10.3390/fuels6030060







