Consequence Analysis of LPG-Related Hazards: Ensuring Safe Transitions to Cleaner Energy
Abstract
1. Introduction
2. Characteristics and Properties of Liquified Petroleum Gas
3. Applications
3.1. Transportation
3.2. Heating and Cooking
3.3. Fuel Cells
3.4. Refrigeration
4. Biopropane Perspectives
5. Highlighted Accidents Involving LPG Storage and Transportation
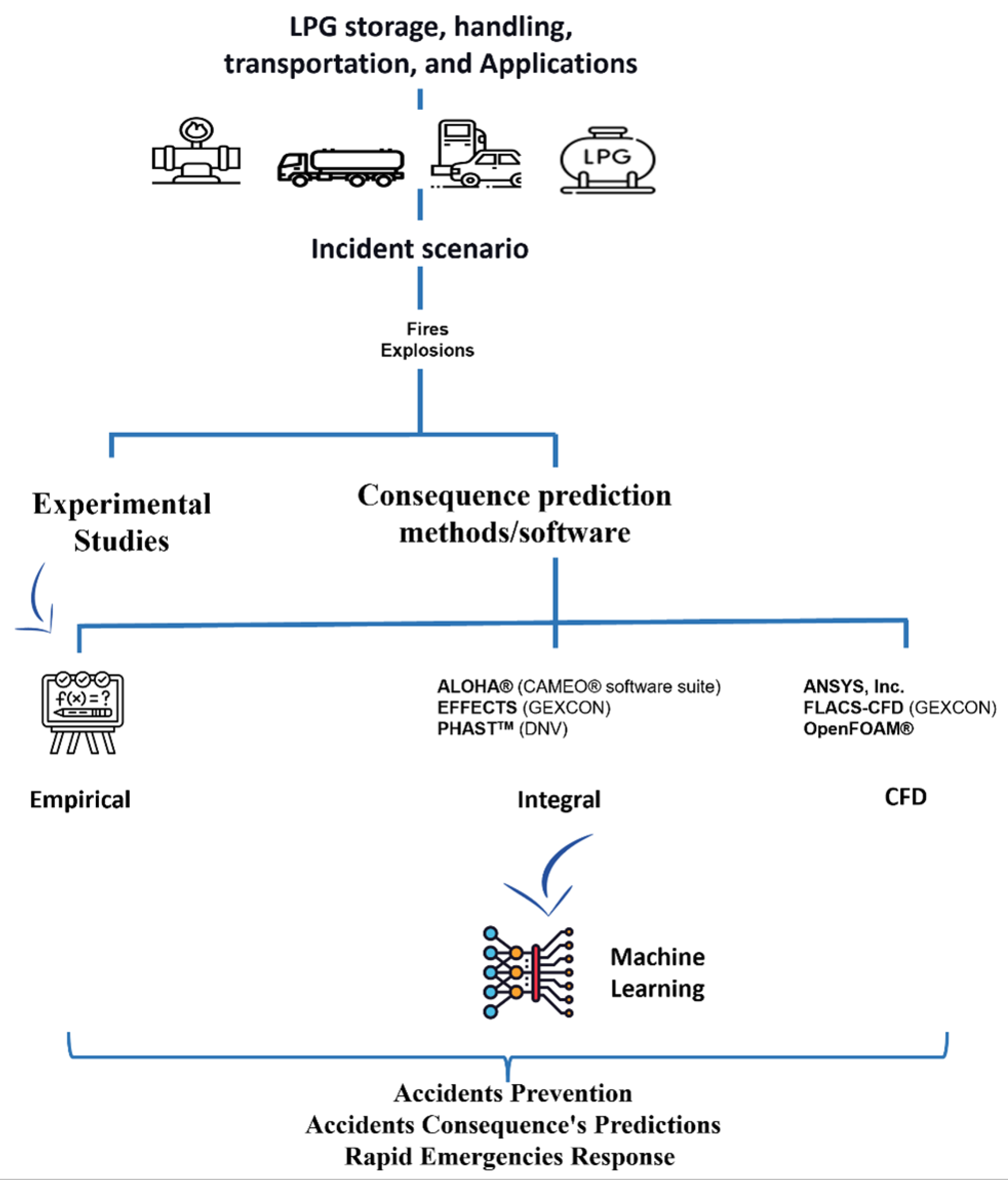
6. Risk Analysis
6.1. Experimental Research and Consequence Prediction Methods
6.1.1. Experimental Assessment
| Place | Year | Cause | Fatalities | Reference |
|---|---|---|---|---|
| Feyzin, France | 1966 | The operational failure of the plant operator caused an LPG (propane) leak. | 18 | [127] |
| Mexico City, Mexico | 1984 | LPG (propane/butane mixture) leakage followed by ignition caused several explosions—domino effect. | >500 | [128] |
| Rio de Janeiro, Brazil | 1972 | The operator lost control in a draining operation on an LPG sphere, leading to a BLEVE. | 38 | [129] |
| Sainte-Élizabeth-de-Warwick, QC, Canada | 1993 | An LPG (propane) tank near a barn was involved in a violent fire and ruptured by BLEVE. | 4 | [130] |
| Visakhapatnam, India | 1997 | LPG leakage in a storage vessel caused a flammable vapor cloud followed by ignition and explosion. | >60 | [131] |
| Bucheon, Korea | 1998 | LPG (propane/butane mixture) leakage during the discharge process from the tank into subterranean storage. | 1 | [132] |
| Toronto, Canada | 2008 | Propane release at a transfer facility. An unknown ignition source led to a VCE and BLEVEs. | 2 | [133] |
| Viareggio, Italy | 2009 | The derailment of a train carrying LPG (propane) caused a release. The gas cloud formed led to a flash fire. | 31 | [134] |
| Chiba, Japan | 2011 | Earthquakes led to LPG (propane) vessels collapsing, resulting in a BLEVE. | [135] | |
| Kannur, India | 2012 | A truck tanker overturned, producing an LPG leak and a large vapor cloud that ignited, leading to a BLEVE. | 20 | [136] |
| Linyi, China | 2017 | An LPG tanker leaked during unloading, leading to a significant explosion and fire. | 10 | [137] |
| Wenling, China | 2020 | An LPG (propane/butane mixture) tank truck overturned while transiting at high speed on an expressway ramp. The tank collided with a concrete guardrail and exploded. | 20 | [138] |
| Fires/Explosions | Definition | Graphical Description | References |
|---|---|---|---|
| Pool fire | Combustion of a substance that evaporates from a layer formed by a liquid fuel pool. A pool fire exhibits high flame temperature and heat flux to its surroundings. |  | [139] |
| Jet fire | Results from a liquid, vapor, or gas discharge into a free space from an orifice. The momentum of the discharge induces the mixture of the discharged material with the atmosphere. |  | [140] |
| Fireball | It is a fire that burns sufficiently rapidly for the burning mass to rise into the air as a cloud or a ball. It occurs if a flammable liquid, gas, or dust cloud abruptly releases and has limited mixing with air before ignition. |  | [141] |
| Flash fire | The combustion of the flammable vapor or gas is mixed with air, and the flame propagates through that mixture with no overpressure generation. |  | [142] |
| Vapor cloud explosion (VCE) | Results from igniting a cloud of flammable vapor or gas in which flame velocities are sufficiently high to produce a pressure wave. |  | [143] |
| Boiling liquid expanding vapor explosion (BLEVE) | A BLEVE could be described as the explosive release of expanding vapor and boiling liquid when a catastrophic failure occurs in a pressure vessel holding a pressure-liquified gas. |  | [144] |
6.1.2. Empirical Modeling
| Incident | Objective | Experiment Details | Studied Variables | Main Conclusions | Reference |
|---|---|---|---|---|---|
| Jet fire | Study on the horizontal jet flame that impinges a vertical plate. | Plate (Q235 low-carbon steel) dimensions: 1 m × 1 m × 5 mm, thermal conductivity: 53.6 W/(mK). Nozzle (stainless steel): inner diameters of 2.0 mm, 3.0 mm, and 4.2 mm. Spacings between nozzle exit and plate: 0.20, 0.25, 0.30, 0.35 and 0.40 m. | Effect of nozzle exit velocity, exit diameter, and exit-plate spacing on the horizontally impinging jet fire. | A new correlation coupling the turbulent Karlovitz stretch factor and the ratio of nozzle exit diameter to exit-plate spacing was developed for the flame extension area of both horizontally and vertically impinging jet fire. It is noted that the temperature profile holds a big difference in the upward and downward directions along the vertical plate. | [152] |
| LPG tank under fire | Study the consequences of an LPG vehicle tank failure under fire conditions. | Ten fire tests on toroidal LPG vehicle tanks with no safety devices were conducted. | Tank filling level, fragmentation distance, and the radius of the danger zone. | All the tested tanks failed with a BLEVE within a t < 5 min after ignition, accompanied by a fireball, a near-field blast wave, and enormous fragmentation, leading to a high risk to rescue services when an LPG tank is affected by a fire. | [153] |
| BLEVE | Study the first moments (early milliseconds) of small-scale BLEVE in propane vessels | A small-scale apparatus was constructed to record detailed images of the failure process and measure overpressures near the vessel. The apparatus is an aluminum tube with D = 50 mm and L = 300 mm. | Failure pressures: from 10 to 33 bar. Measurement of (i) properties: temperature and pressure and (ii) consequences: blast overpressure, loud imaging, and shock around the vessel, among others. | The observation revealed the presence of a Mach shock at the vessel at the early stage of the opening. The results also demonstrated that the lead shock is generated before and gone before the liquid starts boiling, which indicates that the vapor expansion is primarily responsible for the first shock overpressure. | [144] |
| BLEVE | Analysis of the peak overpressure from the lead shock produced by a BLEVE using a new method: spherical shock theory. | A small-scale apparatus was constructed to record detailed images of the failure process and measure overpressures near the vessel (R/Dtube = 0.175/0.050 = 3.5): Aluminum tube with D = 50 mm and L = 300 mm. | Overpressure in the near-field (distance from the BLEVE center to the target, measured in the range within ten times the diameter of the BLEVE vessel); high-speed images. | Using shock tube overpressure prediction and spherical shock propagation model, a model based on the vapor phase properties at failure and a spherical shock propagation model was developed to predict BLEVEs overpressures in the near-field. | [154] |
| BLEVE | Study the ground force effect of BLEVEs: their impact on bridges and other infrastructure. | A small-scale apparatus was constructed to record detailed images of the failure process and measure overpressures near the vessel (R/Dtube = 0.175/0.050 = 3.5): Aluminum tube with D = 50.8 mm and L = 300 mm. | The failure pressure Pfail (from 11.7 bar to 32.7 bar); liquid fill level φliq (from 0 to 87%); the weakened length through machining at the top of the tube, Lc (from 50 mm to 150 mm). | The liquid fill ratio and the length of the debilitated vessel govern the magnitude of the ground force, which, jointly with the impulse, were linearly related to the rupture pressure and liquid fill ratio. | [155] |
| Deflagration-to-detonation transitions (DDTs) | Prediction of DDTs at large scales in congested areas. | Different tests on DDTs using propane in a test rig of 50,000 ft3 (1500 m3) gross volume were performed by SRI International and Gexcon. | Variables: levels of congestion, confinement, and gas concentrations. The flame speed and overpressure measurements were used for validation. | The congested area plays a significant role in the occurrence probability of DDTs. Also, the effects of detonating clouds are more critical than previously thought, which should be considered in future plant layout assessments. | [156] |
6.1.3. Integral Methods
6.1.4. Computational Fluid Dynamics (CFD)
| Incident | Objective | CFD Code | Models | Scenario | Main Conclusions | Reference |
|---|---|---|---|---|---|---|
| Pool fire | Heat radiation from large LPG pool fires | ANSYS Fluent | k-ɛ model; radiation: P-1 model; non-premixed combustion model; surface emissive power (SEP) model; | Three different pool diameters of 12.9, 14.9, and 16.9 m with atmosphere temperatures of 309, 306, and 312 K, mass burning rates of 29.087, 47.328, 44.426 kg/s, and wind velocity of 3, 2.5, 0 m/s, respectively. | The employed CFD model (compared to experimental data) accurately predicted the radiation of large LPG pool fires. Safe separation distances between LPG facilities and surroundings were estimated. | [174] |
| Pool fire | Flame height and flame tilt as functions of pool diameter and wind velocity. | ANSYS Fluent | k-ɛ model; radiation: P-1 model; non-premixed combustion model; | Air velocities of 0, 0.5, 2.5, and 3 m/s were selected for each of the following pool fires with diameter, ambient temperature, and average mass burning rate of (i): 10.4 m, 306 K, and 8.406 kg/s (ii): 12.9 m, 309 K, 12.932 kg/s, (iii): 14.9 m, 306 K, and 17.254 kg/s, and (iv) 16.9 m, 312 K, and 22.196 kg/s. | Higher horizontal wind velocity → stronger convection effect in the horizontal direction, reducing the flame height and increasing the flame tilt angle. Larger diameters are less sensitive to the wind velocity. | [139] |
| Jet fire | Compare different turbulence models for vertical propane jet fire simulation. | Home code coupled with ANSYS CFX | Turbulence models: k-ε, SST, BSL, BSL RS, and RNG k-ε; EDC combustion model; Monte Carlo radiation model. | Computational domain: Cylinder with L = 10 m and D = 2 m. Fuel inlet to simulate the vertical jet fire: Nozzle with D = 12.75 mm. Temperature range: 1500–1700 K. Fuel rates: 148.41 m/s (0.03 kg/s) and 252.75 m/s (0.19 kg/s). | The SST turbulence model is the most suitable, with an average error of 4.7% for a jet fire simulation | [176] |
| Jet fire | Flame geometry of horizontal turbulent jet fires in reduced pressures | FDS | LES turbulence model; EDC combustion model. | The domain size is 8 m × 8 m × 7 m. The release square nozzle (20 mm × 20 mm) is provided in the Y-Z plane, 1.3 m above the ground. Five ambient pressure conditions, ranging between 0.6 and 1 atm, and eight jet fuel exit velocities, between 27.5 and 125 m/s, were studied. | Low-pressure conditions could lead to higher jet flame areas, and the flame length mainly depends on the exit momentum | [177] |
| Vapor Cloud Esplosion | Study the hazard evolution of considerable LPG leakage and vapor cloud explosion | FLACS | RANS turbulence model | The model size of the LPG plant was 450 × 300 m, including six oil tanks, six refrigerated tanks, and four pressure tanks. The LPG vapor cloud with stoichiometric concentration was ignited at the top of the refrigerated tank; the initial temperature was 301 K. | LPG expansion about the ground occurs along the gaps between structural congestion. Continuous large-scale congestions, such as walls, significantly enhance the LPG expansion and concentration accumulation. | [178] |
| Vapor cloud Explosion | Evaluate the PDR approach (PDRFoam) to model the effect of small-scale obstacles: pipes on flame propagation and explosion overpressure. | OpenFOAM | κ-ϵ—PDR-based modifications for turbulence; laminar flamelet combustion model | Pipe geometry: 3 m in length, 1 m in breadth, and 1 m in height. The pitch is 0.33 m vertically and horizontally, and the obstacle diameter (D) is 0.01 m. The fuel-air mixture was filled in the domain with an equivalence ratio of unity. | The flame propagation is well modeled considering the database of vapor cloud explosion experiments. However, over-predictions for peak pressure were found. | [181] |
| BLEVE fireball | Analyze the consequences of the fireball from an LPG tanker BLEVE accident. | FDS | LES | A 200 m3 cube was selected as the domain. The sides and top of the domain behaved as an open atmosphere. Initial pressure: atmospheric pressure; initial oxygen mass fraction: 0.232; initial temperature: 20 °C. | The bigger the fuel mass, the larger the longitudinal diameter of the fireball, as well as the fireball’s diameter and aspect ratio. Fireball gradually changes from momentum-driven to buoyancy-driven as the mass of fuel increases. | [182] |
| BLEVE fireball | Evaluate the BLEVE thermal effects on a gas processing plant. | FDS | LES turbulence model; EDC combustion model; thermal radiation model | Plant: open calculation domain of 300 m × 300 m × 360 m. Relative humidity: 40%, ambient temperature: 20 °C. Accumulator: Temperature 40 °C; Pressure: 14.5 barg; Volume: 50 m3. | The procedure showed good agreement with experimental data. However, the authors noted the importance of the liquid-gas transition and the container disintegration in modeling a BLEVE process. | [183] |
| LPG BLEVE in a tunnel | Investigate the rock vibrations induced by an LPG BLEVE inside a tunnel. | FLACS and ANSYS LS-DYNA | κ-ϵ model; ALE algorithm | Model domain: 30 m length, 30 m width, 60 m height. LPG tank: volume 20 m3; diameter 2.4 m. The tank center is located at the center of the upper arc of the curved-wall-arched tunnel. | The cover depth of the tunnel, the rock type, and the porosity are significantly more influential than the tunnel lining concrete grade in the LPG BLEVE-induced ground vibrations. | [186] |
| BLEVE | Blast wave prediction of large-scale BLEVE in the open space. | FLACS | RANS turbulence model | A 2000 L propane tank is modeled as a 2.6 m long cuboid. Stretched grids start at 4 m from the edge of the core grid domain. | The liquid flashing from the LPG tanks is slower than vapor expansion when generating shock waves. The maximum peak pressures from models were mainly attributed to vapor expansion. | [195] |
| BLEVE | Blast wave prediction of medium to large-scale BLEVE in an obstructed environment. | FLACS | RANS turbulence model | Two domains were simulated with dimensions of 65 × 40 × 30 m3 and 230 × 60 × 40 m3. The obstacles in the following parametric study are located on the ground and at least 5 m away from the BLEVE source. | Only the vapor expansion from BLEVE was considered to simulate the shock waves in an obstructed environment. Good agreement was obtained between the CFD results and medium-large-scale experimental data. Simulation-based correlations for pressure predictions that could predict explosion loads were proposed. | [196] |
| BLEVE-VCE Wenling accident | Simulation of gas dispersion resulting from the accidental instantaneous release of LPG and reconstructing actual accident processes. | Ansys Fluent | RANS turbulence model; SIMPLE algorithm | CFD simulation domain: A high-resolution terrain geometric model of the region around the accident site with an area of 3.83 km2 and height of 300 m (ICEM software), containing buildings, viaducts, ramps, and trees. GIS data from Google Maps at a horizontal resolution of 10 m. | The LPG instantaneous-release model was constructed considering the multicomponent liquid flash and liquid pool evaporation and spreading. The droplets in the release source, terrains, and obstacles significantly affected the vapor cloud’s dispersion behavior and extension distance. | [201] |
6.1.5. Application of Machine Learning
7. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Natural Resources Canada—NRCAN. Hydrogen Strategy for Canada—Seizing the Opportunities for Hydrogen; Natural Resources Canada: Ottawa, ON, Canada, 2020. [Google Scholar]
- Gouvernement du Québec. Stratégie Québécoise sur l’Hydrogène Vert et les Bioénergies 2030; Goverment of Québec: Quebec City, QC, Canada, 2022. [Google Scholar]
- Rej, S.; Bandyopadhyay, A.; Mahmood, H.; Murshed, M.; Mahmud, S. The role of liquefied petroleum gas in decarbonizing India: Fresh evidence from wavelet–partial wavelet coherence approach. Environ. Sci. Pollut. Res. 2022, 29, 35862–35883. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, G.H.; Allen, C.I.; Wiedmann, T.; Metternicht, G.I. Accelerating electric vehicle uptake: Modelling public policy options on prices and infrastructure. Transp. Res. Part A Policy Pract. 2022, 162, 155–174. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.W.; Kim, M.K. Economics of charging infrastructure for electric vehicles in Korea. Energy Policy 2022, 164, 112875. [Google Scholar] [CrossRef]
- Talib Hashem, G.; Al-Dawody, M.F.; Sarris, I.E. The characteristics of gasoline engines with the use of LPG: An experimental and numerical study. Int. J. Thermofluids 2023, 18, 100316. [Google Scholar] [CrossRef]
- Usman, M.; Ijaz Malik, M.A.; Ranjha, Q.A.; Arif, W.; Jamil, M.K.; Miran, S.; Siddiqui, S. Experimental assessment of performance, emission and lube oil deterioration using gasoline and LPG for a sustainable environment. Case Stud. Therm. Eng. 2023, 49, 103300. [Google Scholar] [CrossRef]
- Chun, K.W.; Kim, M.; Hur, J.-J. Development of a Marine LPG-Fueled High-Speed Engine for Electric Propulsion Systems. J. Mar. Sci. Eng. 2022, 10, 1498. [Google Scholar] [CrossRef]
- Baek, S.; Lee, S.; Shin, M.; Lee, J.; Lee, K. Analysis of combustion and exhaust characteristics according to changes in the propane content of LPG. Energy 2022, 239, 122297. [Google Scholar] [CrossRef]
- Haselip, J.; Chen, K.; Marwah, H.; Puzzolo, E. Cooking in the margins: Exploring the role of liquefied petroleum gas for refugees in low-income countries. Energy Res. Soc. Sci. 2022, 83, 102346. [Google Scholar] [CrossRef]
- Kashyap, S.R.; Pramanik, S.; Ravikrishna, R.V. A review of energy-efficient domestic cookstoves. Appl. Therm. Eng. 2024, 236, 121510. [Google Scholar] [CrossRef]
- Kulkarni, S.; Chavali, S.; Dikshit, S. A review on analysis of Vapour Compression Refrigeration System (VCRS) for its performance using different ecofriendly refrigerants and nanofluids. Mater. Today Proc. 2023, 72, 878–883. [Google Scholar] [CrossRef]
- Warguła, Ł.; Kukla, M.; Lijewski, P.; Dobrzyński, M.; Markiewicz, F. Influence of the Use of Liquefied Petroleum Gas (LPG) Systems in Woodchippers Powered by Small Engines on Exhaust Emissions and Operating Costs. Energies 2020, 13, 5773. [Google Scholar] [CrossRef]
- Bariha, N.; Srivastava, V.C.; Mishra, I.M. Theoretical and experimental studies on hazard analysis of LPG/LNG release: A review. Rev. Chem. Eng. 2017, 33, 387–432. [Google Scholar] [CrossRef]
- Sarvestani, K.; Ahmadi, O.; Mortazavi, S.B.; Mahabadi, H.A. Development of a predictive accident model for dynamic risk assessment of propane storage tanks. Process Saf. Environ. Prot. 2021, 148, 1217–1232. [Google Scholar] [CrossRef]
- Yang, D.; Peng, K.; Zheng, J.; Xie, B.; Wang, J.; Xu, B.; Li, F. Consequences analysis of the LPG tank truck traffic accident: A case study of the Wenling explosion accident. J. Loss Prev. Process Ind. 2023, 87, 105228. [Google Scholar] [CrossRef]
- Huffman, M.; Hutchison, V.; Ranganathan, S.; Noll, G.; Baxter, C.; Hildebrand, M.; Wang, Q. A comparative bibliometric study of the transport risk considerations of liquefied natural gas and liquefied petroleum gas. Can. J. Chem. Eng. 2024, 102, 2019–2038. [Google Scholar] [CrossRef]
- Kim, C.; Cho, S.H.; Cho, S.M.; Na, Y.; Kim, S.; Kim, D.K. Review of hydrogen infrastructure: The current status and roll-out strategy. Int. J. Hydrogen Energy 2023, 48, 1701–1716. [Google Scholar] [CrossRef]
- Schühle, P.; Stöber, R.; Semmel, M.; Schaadt, A.; Szolak, R.; Thill, S.; Alders, M.; Hebling, C.; Wasserscheid, P.; Salem, O. Dimethyl ether/CO2—A hitherto underestimated H2 storage cycle. Energy Env. Sci. 2023, 16, 3002–3013. [Google Scholar] [CrossRef]
- Schöne, N.; Heinz, B. Semi-Systematic Literature Review on the Contribution of Hydrogen to Universal Access to Energy in the Rationale of Sustainable Development Goal Target 7.1. Energies 2023, 16, 1658. [Google Scholar] [CrossRef]
- Chakraborty, A.; Biswas, S.; Meitei, S.; Sengupta, A.; Kakati, D.; Banerjee, R. Examining the significance of the ignition characteristics of hydrogen and liquefied-petroleum-gas on the reactivity controlled compression ignition and its interspersed profiles induced in an existing diesel engine: A comparative perspective. Energy Convers. Manag. 2022, 268, 115976. [Google Scholar] [CrossRef]
- Payne, A.; Garcia-Garcia, G.; Styring, P. Production of propane and propene via carbon capture utilisation: Comparison of its environmental and economic performance against conventional production methods. Green Chem. 2023, 25, 4029–4057. [Google Scholar] [CrossRef]
- Canada Energy Regulator. Propane Market Review—Final Report. Calgary, AB, Canada, 2014. Available online: https://www.cer-rec.gc.ca/en/data-analysis/energy-commodities/natural-gas-liquids/report/2014/propane-market-review.html (accessed on 2 May 2025).
- Walls, M.; Joo, M.; Ross, M. Impact of the Direct Injection of Liquid Propane on the Efficiency of a Light-Duty, Spark-Ignited Engine; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2017. [Google Scholar]
- CAN/CGSB-3.14-2018; Propane for Fuel Purposes. Canadian General Standards Board Issuing Body: Ottawa, ON, Canada, 2018.
- Wallner, T.; Miers, S.A. Internal Combustion Engines, Alternative Fuels for. In Electric, Hybrid, and Fuel Cell Vehicles; Elgowainy, A., Ed.; Springer: New York, NY, USA, 2021; pp. 27–66. [Google Scholar] [CrossRef]
- CAN/CGSB-3.14-2023; Propane for Fuel Purposes. Canadian General Standards Board Issuing Body: Ottawa, ON, Canada, 2023.
- Bulut, B.; İşbilen, E.; Atakül, H.; Tantekin-Ersolmaz, B. Adsorptive removal of dimethyl disulfide and thiophene from liquefied petroleum gas by zeolite-based adsorbents. Microporous Mesoporous Mater. 2022, 337, 111924. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, B.J.; Chen, Q.L. A New Adsorption Process to Intensify Liquefied Petroleum Gas Recovery from Raw Natural Gas. Energy Procedia 2015, 75, 853–859. [Google Scholar] [CrossRef]
- Selvan, K.K.; Mundra, M.; Panda, R.C. Steady-state and transient dynamics for sweetening of LPG process. Digit. Chem. Eng. 2022, 4, 100035. [Google Scholar] [CrossRef]
- Available online: https://www.iso.org/standard/15315.html (accessed on 3 May 2025).
- Thompson, S.M.; Robertson, G.; Myers, R.; Schütze, A. Liquefied Petroleum Gas. In Handbook of Fuels; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 101–117. [Google Scholar] [CrossRef]
- Available online: https://www.dinmedia.de/en/standard/din-51622/322786220 (accessed on 3 May 2025).
- Van Orsdol, F. ASTM D1835; Discussion on Uses of the Specification for Liquefied Petroleum Gas. ASTM International: West Conshohocken, PA, USA, 2016.
- Kumar Ramalingam, A.; Krieck, M.; Pischinger, S.; Alexander Heufer, K. Understanding the Oxidation Behavior of Automotive Liquefied Petroleum Gas Fuels: Experimental and Kinetic Analyses. Energy Fuels 2020, 34, 2323–2333. [Google Scholar] [CrossRef]
- Demirbas, A. Fuel Properties of Hydrogen, Liquefied Petroleum Gas (LPG), and Compressed Natural Gas (CNG) for Transportation. Energy Sources 2002, 24, 601–610. [Google Scholar] [CrossRef]
- Cipollone, R.; Villante, C. A Dynamical Model for the Design of LPG Liquid-Phase Injection Systems. In Proceedings of the ASME 2000 ICE Fall Engine Technology Conference, Paper No. 2000-ICE-319, Peoria, IL, USA, 23–26 September 2000. [Google Scholar]
- Yamamoto, H.; Pruess, K. Numerical Simulations of Leakage from Underground LPG Storage Caverns; Lawrence Berkeley National Lab (LBNL): Berkeley, CA, USA, 2004. [Google Scholar]
- Abd, A.A.; Naji, S.Z.; Tye, C.T.; Othman, M.R. Evaluation the effect of the ambient temperature on the liquid petroleum gas transportation pipeline. Chem. Prod. Process Model. 2022, 17, 479–488. [Google Scholar] [CrossRef]
- Paltrinieri, N.; Bonvicini, S.; Spadoni, G.; Cozzani, V. Cost-Benefit Analysis of Passive Fire Protections in Road LPG Transportation. Risk Anal. 2012, 32, 200–219. [Google Scholar] [CrossRef]
- Kang, Y.; Wu, Z.; Ma, S.; Zhao, M.; Li, W. CFD-based assessment and visualization of the failure consequences of LPG tankers. J. Loss Prev. Process Ind. 2023, 82, 105008. [Google Scholar] [CrossRef]
- Mądziel, M. Liquified Petroleum Gas-Fuelled Vehicle CO2 Emission Modelling Based on Portable Emission Measurement System, On-Board Diagnostics Data, and Gradient-Boosting Machine Learning. Energies 2023, 16, 2754. [Google Scholar] [CrossRef]
- Elvers, B.; Schütze, A. Handbook of Fuels; Wiley Online Library: Hoboken, NJ, USA, 2022. [Google Scholar]
- Sarvestani, K.; Ahmadi, O.; Alenjareghi, M.J. LPG Storage Tank Accidents: Initiating Events, Causes, Scenarios, and Consequences. J. Fail. Anal. Prev. 2021, 21, 1305–1314. [Google Scholar] [CrossRef]
- Pio, G.; Salzano, E. Evaluation of safety parameters of light alkenes by means of detailed kinetic models. Process Saf. Environ. Prot. 2018, 119, 131–137. [Google Scholar] [CrossRef]
- Canada Measurement. Bulletin. Selection of Volume Correction Factor Tables and Standard Density Values for Some Common Products; Canada Measurement: Ottawa, ON, Canada, 2013. [Google Scholar]
- Yeo, S.J.; Kim, J.; Lee, W.J. Potential economic and environmental advantages of liquid petroleum gas as a marine fuel through analysis of registered ships in South Korea. J. Clean. Prod. 2022, 330, 129955. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I. Life cycle environmental impact assessments and comparisons of alternative fuels for clean vehicles. Resour. Conserv. Recycl. 2018, 132, 141–157. [Google Scholar] [CrossRef]
- Woo Jeong, J.; Baek, S.; Kim, Y.; Woo, S.; Lim, Y.; Lee, K. Investigation of CO2 and PN emission characteristics according to the propane content for a LPG engine. Fuel 2024, 357, 129877. [Google Scholar] [CrossRef]
- World LPG Association (WLPGA); Liquid Gas Europe. Autogas Incentive Policies—A Country-by-Country Analysis of Why and How Governments Encourage Autogas and What Works; World LPG Association: Neuilly-sur-Seine, France; Liquid Gas Europe: Bruxelles, Belgium, 2019. [Google Scholar]
- Kivevele, T.; Raja, T.; Pirouzfar, V.; Waluyo, B.; Setiyo, M. LPG-fueled vehicles: An overview of technology and market trend. Automot. Exp. 2020, 3, 6–19. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration. Global Transportation Energy Consumption: Examination of Scenarios to 2040 Using ITEDD; U.S. Energy Information Administration: Washington, DC, USA, 2017. [Google Scholar]
- Setiyo, M.; Condro Purnomo, B.; Waluyo, B.; Munahar, S.; Latifur Rochman, M.; Rahman Saleh, A.; Desy Fatmaryanti, S.; David Samuel, O. Cooling power characteristics of half-cycle refrigeration system in LPG fuelled vehicles by auxiliary chiller as heat exchanger. Therm. Sci. Eng. Prog. 2022, 27, 101145. [Google Scholar] [CrossRef]
- Chu, W.; Li, H.; Liu, Y.; Zhou, B.; Luo, H.; Kim, W. Three-dimensional simulation analysis of in-cylinder combustion in space in-orbit hydrogen–oxygen internal combustion engine. Appl. Therm. Eng. 2025, 263, 125391. [Google Scholar] [CrossRef]
- Chang, F.; Luo, H.; Zhai, C.; Jin, Y.; Xiong, P.; Wang, J.; Song, B.; Zhang, J.; Nishida, K. Experimental investigation of fuel adhesion from wall-impinging spray with various injection mass ratios. Exp. Therm. Fluid Sci. 2025, 163, 111403. [Google Scholar] [CrossRef]
- Waluyo, B.; Setiyo, M.; Purnomo, B.C.; Rochman, M.L.; Habibi, I.; Saleh, A.R.; Fatmaryanti, S.D.; Kolakoti, A. Cooling effect characteristic of the novel half-cycle refrigeration system on a liquefied petroleum gas (LPG) fueled vehicle. Therm. Sci. Eng. Prog. 2022, 34, 101405. [Google Scholar] [CrossRef]
- Simsek, S.; Uslu, S.; Simsek, H.; Uslu, G. Improving the combustion process by determining the optimum percentage of liquefied petroleum gas (LPG) via response surface methodology (RSM) in a spark ignition (SI) engine running on gasoline-LPG blends. Fuel Process. Technol. 2021, 221, 106947. [Google Scholar] [CrossRef]
- Cardone, M.; Marialto, R.; Ianniello, R.; Lazzaro, M.; Di Blasio, G. Spray Analysis and Combustion Assessment of Diesel-LPG Fuel Blends in Compression Ignition Engine. Fuels 2021, 2, 1–15. [Google Scholar] [CrossRef]
- Oliva, F.; Fernández-Rodríguez, D. Autoignition study of LPG blends with diesel and HVO in a constant-volume combustion chamber. Fuel 2020, 267, 117173. [Google Scholar] [CrossRef]
- Fabiś, P.; Flekiewicz, M. The Influence of LPG and DME Mixtures on Passenger Car Performance. Energies 2022, 15, 7144. [Google Scholar] [CrossRef]
- Dinesh, M.H.; Pandey, J.K.; Kumar, G.N. Effect of parallel LPG fuelling in a methanol fuelled SI engine under variable compression ratio. Energy 2022, 239, 122134. [Google Scholar] [CrossRef]
- Jemni, M.A.; Kassem, S.H.; Driss, Z.; Abid, M.S. Effects of hydrogen enrichment and injection location on in-cylinder flow characteristics, performance and emissions of gaseous LPG engine. Energy 2018, 150, 92–108. [Google Scholar] [CrossRef]
- Aravindan, M.; Praveen Kumar, G.; Arulanandam, M.K.; Murali, S.; Sheoran, N.; Waykole, N.; Muthaiah, R.; Sharma, P. Multi-objective optimization and analysis of chemical kinetics properties: Exploring the impact of different hydrogen blending ratios on LPG and methane-air mixtures. Energy Convers. Manag. X 2024, 22, 100532. [Google Scholar] [CrossRef]
- Kim, J.K.; Yeo, S.; Choi, J.-H.; Lee, W.-J. LPG, Gasoline, and Diesel Engines for Small Marine Vessels: A Comparative Analysis of Eco-Friendliness and Economic Feasibility. Energies 2024, 17, 450. [Google Scholar] [CrossRef]
- Hakam, D.F.; Nugraha, H.; Wicaksono, A.; Rahadi, R.A.; Kanugrahan, S.P. Mega conversion from LPG to induction stove to achieve Indonesia’s clean energy transition. Energy Strategy Rev. 2022, 41, 100856. [Google Scholar] [CrossRef]
- Wichangarm, M.; Matthujak, A.; Sriveerakul, T.; Sucharitpwatskul, S.; Phongthanapanich, S. Investigation on thermal efficiency of LPG cooking burner using computational fluid dynamics. Energy 2020, 203, 117849. [Google Scholar] [CrossRef]
- Shupler, M.; O’Keefe, M.; Puzzolo, E.; Nix, E.; Anderson de Cuevas, R.; Mwitari, J.; Gohole, A.; Sang, E.; Čukić, I.; Menya, D.; et al. Pay-as-you-go liquefied petroleum gas supports sustainable clean cooking in Kenyan informal urban settlement during COVID-19 lockdown. Appl. Energy 2021, 292, 116769. [Google Scholar] [CrossRef]
- Mhd Safri, N.A.; Zainuddin, Z.; Mohd Azmi, M.S.; Zulkifle, I.; Fudholi, A.; Ruslan, M.H.; Sopian, K. Current status of solar-assisted greenhouse drying systems for drying industry (food materials and agricultural crops). Trends Food Sci. Technol. 2021, 114, 633–657. [Google Scholar] [CrossRef]
- Gunasegaran, M.K.; Hasanuzzaman, M.; Tan, C.; Bakar, A.H.A.; Ponniah, V. Energy Consumption, Energy Analysis, and Solar Energy Integration for Commercial Building Restaurants. Energies 2023, 16, 7145. [Google Scholar] [CrossRef]
- Deb, S.; Muthukumar, P. Development and performance assessment of LPG operated cluster Porous Radiant Burner for commercial cooking and industrial applications. Energy 2021, 219, 119581. [Google Scholar] [CrossRef]
- Herrera, B.; Cacua, K.; Olmos-Villalba, L. Combustion stability and thermal efficiency in a porous media burner for LPG cooking in the food industry using Al2O3 particles coming from grinding wastes. Appl. Therm. Eng. 2015, 91, 1127–1133. [Google Scholar] [CrossRef]
- Su, X.; Zhang, F.; Yin, Y.; Tu, B.; Cheng, M. Thermodynamic analysis and fuel processing strategies for propane-fueled solid oxide fuel cell. Energy Convers. Manag. 2020, 204, 112279. [Google Scholar] [CrossRef]
- Corigliano, O.; Pagnotta, L.; Fragiacomo, P. On the Technology of Solid Oxide Fuel Cell (SOFC) Energy Systems for Stationary Power Generation: A Review. Sustainability 2022, 14, 15276. [Google Scholar] [CrossRef]
- Antolini, E. Direct propane fuel cells. Fuel 2022, 315, 123152. [Google Scholar] [CrossRef]
- Jena, S.K.; Bose, S.; Patle, S.D. Comparison of the performance of propane (R290) and propene (R1270) as alternative refrigerants for cooling during expansion in a helical capillary tube: A CFD-based insight investigation. Int. J. Refrig. 2023, 146, 300–313. [Google Scholar] [CrossRef]
- Muzaffar, A.; Tariq, M.H.; Abbas, A.; Tayyab, M.; Cheema, T.A. Refrigeration Potential Investigation of Liquefied Petroleum Gas under Atmospheric Conditions. Eng. Proc. 2022, 23, 32. [Google Scholar] [CrossRef]
- Babarinde, T.O.; Madyira, D.M.; Mashinini, P.M. Performance evaluation of graphene-enhanced LPG in a vapour compression refrigeration system: An experimental approach. Energy Rep. 2022, 8, 1226–1235. [Google Scholar] [CrossRef]
- Sobieraj, M.; Ksionek, D.; Pavković, B. Investigation on solid-liquid equilibrium for binary mixtures of carbon dioxide (R744) and alkanes: Propane (R290) and isobutane (R600a). Int. J. Refrig. 2023, 154, 205–214. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.; Fan, S.; Peng, X.; Tsubaki, N.; Zhao, T.-S. Transformation of LPG to light olefins on composite HZSM-5/SAPO-5. New J. Chem. 2021, 45, 4860–4866. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, S.; Zhang, J.; Ma, Q.; Wang, K.; Zhao, T.S. Transformation of LPG on HZSM-5 catalyst: Effects of tuned pores and acidity on product distribution. Fuel 2019, 254, 115615. [Google Scholar] [CrossRef]
- Hajimirzaee, S.; Soleimani Mehr, A.; Kianfar, E. Modified ZSM-5 Zeolite for Conversion of LPG to Aromatics. Polycycl. Aromat. Compd. 2022, 42, 2334–2347. [Google Scholar] [CrossRef]
- Ramantani, T.; Evangeliou, V.; Kormentzas, G.; Kondarides, D.I. Hydrogen production by steam reforming of propane and LPG over supported metal catalysts. Appl. Catal. B 2022, 306, 121129. [Google Scholar] [CrossRef]
- Johnson, E. New biofuel debut: Biopropane. Biofuels Bioprod. Biorefin. 2015, 9, 627–629. [Google Scholar] [CrossRef]
- Ajuka, L.O.; Kazeem, R.A.; Kuti, O.A.; Jen, T.C.; Afolalu, A.S.; Akinlabi, E.T. Decarbonized automotive fuel: Liquefied petroleum gas biosynthesis, benefits and drawbacks. Results Eng. 2024, 21, 101889. [Google Scholar] [CrossRef]
- Johnson, E. A carbon footprint of HVO biopropane. Biofuels Bioprod. Biorefin. 2017, 11, 887–896. [Google Scholar] [CrossRef]
- Johnson, E. Process Technologies and Projects for BioLPG. Energies 2019, 12, 250. [Google Scholar] [CrossRef]
- Tirumareddy, P.; Esmi, F.; Masoumi, S.; Borugadda, V.B.; Dalai, A.K. Introduction to Green Diesel. In Green Diesel: An Alternative to Biodiesel and Petrodiesel; Aslam, M., Shivaji Maktedar, S., Sarma, A.K., Eds.; Springer Nature: Singapore, 2022; pp. 1–40. [Google Scholar] [CrossRef]
- Hulteberg, C.; Leveau, A. Scaling up a Gas-Phase Process for Converting Glycerol to Propane. Catalysts 2020, 10, 1007. [Google Scholar] [CrossRef]
- Abafe Diejomaoh, O.T.; Onwudili, J.A.; Simons, K.E.; Maziero, P. On-purpose production of propane fuel gas from the hydrothermal reactions of n-butanol over Pt/Al2O3 catalyst: A parametric and mechanistic study. Fuel 2024, 365, 131140. [Google Scholar] [CrossRef]
- Chen, K.C.; Leach, M.; Black, M.J.; Tesfamichael, M.; Kemausuor, F.; Littlewood, P.; Marker, T.; Mwabonje, O.; Mulugetta, Y.; Murphy, R.J.; et al. BioLPG for Clean Cooking in Sub-Saharan Africa: Present and Future Feasibility of Technologies, Feedstocks, Enabling Conditions and Financing. Energies 2021, 14, 3916. [Google Scholar] [CrossRef]
- Singh, H.; Padhi, T.; Kashyap, A.; Taneja, S. Recent advances in biogas production using various bio-waste’s and its potential application: An overview. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Amer, M.; Hoeven, R.; Kelly, P.; Faulkner, M.; Smith, M.H.; Toogood, H.S.; Scrutton, N.S. Renewable and tuneable bio-LPG blends derived from amino acids. Biotechnol. Biofuels 2020, 13, 125. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Ramirez, A.; Cruz-Fernandez, M.; Zander, H.J.; Joensen, F.; Woolass, S.; Meiswinkel, A.; Styring, P.; Gascon, J.; Olsbye, U. A CO2 valorization plant to produce light hydrocarbons: Kinetic model, process design and life cycle assessment. J. CO2 Util. 2023, 67, 102337. [Google Scholar] [CrossRef]
- Moura, R.; Beer, M.; Patelli, E.; Lewis, J. Learning from major accidents: Graphical representation and analysis of multi-attribute events to enhance risk communication. Saf. Sci. 2017, 99, 58–70. [Google Scholar] [CrossRef]
- Lindberg, A.K.; Hansson, S.O.; Rollenhagen, C. Learning from accidents—What more do we need to know? Saf. Sci. 2010, 48, 714–721. [Google Scholar] [CrossRef]
- Khan, A.; Sattari, F.; Lefsrud, L.; Tufail, M. Enhancing regional process safety management. J. Loss Prev. Process Ind. 2021, 71, 104444. [Google Scholar] [CrossRef]
- Abedsoltan, H.; Abedsoltan, A. Future of process safety: Insights, approaches, and potential developments. Process Saf. Environ. Prot. 2024, 185, 684–707. [Google Scholar] [CrossRef]
- Lacoursiere, J.P. A risk management initiative implemented in Canada. J. Hazard. Mater. 2006, 130, 311–320. [Google Scholar] [CrossRef]
- API Standard 2510; Design and Construction of LPG Installations. API—American Petroleum Institute: Washington, DC, USA, 2020.
- EPA—U.S. Environmental Protection Agency. Risk Management Program Guidance for Propane Storage Facilities (40 CFR Part 68); EPA 550-B-00-001; Office of Solid Waste and Emergency Response: Washington, DC, USA, 2009. Available online: https://www.epa.gov/sites/default/files/2013-11/documents/storage.pdf (accessed on 30 January 2025).
- OSHA—Occupational Safety and Health Administration. Process Safety Management for Storage Facilities; The Occupational Safety and Health Administration: Washington, DC, USA, 2017. [Google Scholar]
- CSA B149.2:20; Propane Storage and Handling Code. CSA Group: Toronto, ON, Canada, 2020.
- CSA Z767:24; Process Safety Management—National Standard of Canada. CSA Group: Toronto, ON, Canada, 2024.
- Ni, Y.; Sattari, F.; Lefsrud, L.; Tufail, M. A rising tide raises all boats: Regional promotion of process safety through joint government/industry management. J. Loss Prev. Process Ind. 2020, 68, 104331. [Google Scholar] [CrossRef]
- European Major Accident Hazards Bureau The EMARS (Major Accident Reporting System) Database. Available online: https://emars.jrc.ec.europa.eu/en/emars/content (accessed on 4 March 2024).
- Akel, A.J.N.; Patriarca, R.; Di Gravio, G.; Antonioni, G.; Paltrinieri, N. Business intelligence for the analysis of industrial accidents based on MHIDAS database. Chem. Eng. Trans. 2021, 86, 229–234. [Google Scholar]
- Dakkoune, A.; Vernières-Hassimi, L.; Leveneur, S.; Lefebvre, D.; Estel, L. Risk analysis of French chemical industry. Saf. Sci. 2018, 105, 77–85. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Jang, D.; Lee, M.C. Property-based quantitative risk assessment of hydrogen, ammonia, methane, and propane considering explosion, combustion, toxicity, and environmental impacts. J. Energy Storage 2022, 54, 105344. [Google Scholar] [CrossRef]
- Det Norske Veritas (DNV). Closing the Safety Gap in an Era of Transformation—Maritime; DNV: Høvik, Norway, 2021; Available online: https://www.dnv.com/maritime/publications/closing-the-safety-gap-in-an-era-of-transformation-download/ (accessed on 20 May 2025).
- Bilgili, L. A systematic review on the acceptance of alternative marine fuels. Renew. Sustain. Energy Rev. 2023, 182, 113367. [Google Scholar] [CrossRef]
- Yoo, B.H.; Wilailak, S.; Bae, S.H.; Gye, H.R.; Lee, C.J. Comparative risk assessment of liquefied and gaseous hydrogen refueling stations. Int. J. Hydrogen Energy 2021, 46, 35511–35524. [Google Scholar] [CrossRef]
- MacNguyen, R. Comparison of hydrogen and hydrocarbon fuels hazards and practical risk management strategies. Process Saf. Prog. 2023, 42, 225–241. [Google Scholar] [CrossRef]
- Pasman, H.; Sripaul, E.; Khan, F.; Fabiano, B. Energy transition technology comes with new process safety challenges and risks. Process Saf. Environ. Prot. 2023, 177, 765–794. [Google Scholar] [CrossRef]
- Roy, N.; Laboureur, D.M.; Yu, M.; Pasman, H.J.; O’Connor, M.; Mannan, M.S. Patterns and Trends in Injuries Due to Consumer Propane Incidents. ACS Chem. Health Saf. 2020, 27, 251–258. [Google Scholar] [CrossRef]
- Birk, A.M. Hazards from propane BLEVEs: An update and proposal for emergency responders. J. Loss Prev. Process Ind. 1996, 9, 173–181. [Google Scholar] [CrossRef]
- Transport Canada. BLEVE—Response and Prevention. 2024. Available online: https://tc.canada.ca/en/dangerous-goods/bleve-response-prevention (accessed on 30 January 2025).
- Transport Canada; U.S. Department of Transportation; Secretariat of Transport and Communications of Mexico. Emergency Response Guidebook; United States Pipeline and Hazardous Materials Safety Administration: Washington, DC, USA, 2024. [Google Scholar]
- Witlox, H.W.M.; Fernandez, M.; Harper, M.; Oke, A.; Stene, J.; Xu, Y. Verification and validation of Phast consequence models for accidental releases of toxic or flammable chemicals to the atmosphere. J. Loss Prev. Process Ind. 2018, 55, 457–470. [Google Scholar] [CrossRef]
- Juwari, J.; Anugraha, R.P.; Zanata, M.; Leksono, N.A.; Hamid, M.D.; Cahyono, T. Quantitative risk assessment and risk reduction of worst-case accident scenario at fuel storage terminal. J. Loss Prev. Process Ind. 2024, 88, 105272. [Google Scholar] [CrossRef]
- Abdulkareem Abdulraheem, K.; Adeniran, J.A.; Aremu, A.S.; Yusuf, M.; Oyeneye, A.K.; Atanda, S.A.; Abubakar, I. Consequence modeling of liquefied petroleum gas accidental release from retail outlets in an African city residential environment. Process Saf. Prog. 2023, 42, 299–309. [Google Scholar] [CrossRef]
- Pouyakian, M.; Ashouri, M.; Eidani, S.; Madvari, R.F.; Laal, F. A systematic review of consequence modeling studies of the process accidents in Iran from 2006 to 2022. Heliyon 2023, 9, e13550. [Google Scholar] [CrossRef]
- Kwak, H.; Kim, M.; Min, M.; Park, B.; Jung, S. Assessing the Quantitative Risk of Urban Hydrogen Refueling Station in Seoul, South Korea, Using SAFETI Model. Energies 2024, 17, 867. [Google Scholar] [CrossRef]
- Park, B.; Kim, Y.; Paik, S.; Kang, C. Numerical and experimental analysis of jet release and jet flame length for qualitative risk analysis at hydrogen refueling station. Process Saf. Environ. Prot. 2021, 155, 145–154. [Google Scholar] [CrossRef]
- Shen, R.; Jiao, Z.; Parker, T.; Sun, Y.; Wang, Q. Recent application of Computational Fluid Dynamics (CFD) in process safety and loss prevention: A review. J. Loss Prev. Process Ind. 2020, 67, 104252. [Google Scholar] [CrossRef]
- Paltrinieri, N.; Comfort, L.; Reniers, G. Learning about risk: Machine learning for risk assessment. Saf. Sci. 2019, 118, 475–486. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, Q. Consequence Prediction Using Quantitative Property–Consequence Relationship Models. In Machine Learning in Chemical Safety and Health; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 81–92. [Google Scholar] [CrossRef]
- Bunn, A.; Hailwood, M. Fire and Explosion of LPG Tanks at Feyzin, France, No1300 T. IChemE Saf. Train. 2016, 251, 11. [Google Scholar]
- Pietersen, C.M. Analysis of the LPG-disaster in Mexico city. J. Hazard. Mater. 1988, 20, 85–107. [Google Scholar] [CrossRef]
- de Souza, A.B., Jr. Emergency planning for hazardous industrial areas: A Brazilian case study. Risk Anal. 2000, 20, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.M.; Xu, J.; Venart, J.E.S. Fire-induced failure of a propane tank: Some lessons to be learnt. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2003, 217, 79–91. [Google Scholar] [CrossRef]
- Khan, F.I.; Abbasi, S.A. The world’s worst industrial accident of the 1990s what happened and what might have been: A quantitative study. Process Saf. Prog. 1999, 18, 135–145. [Google Scholar] [CrossRef]
- Park, K.; Sam Mannan, M.; Jo, Y.D.; Kim, J.Y.; Keren, N.; Wang, Y. Incident analysis of Bucheon LPG filling station pool fire and BLEVE. J. Hazard. Mater. 2006, 137, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Birk, A.M. Shock waves and condensation clouds from industrial BLEVEs and VCEs. Process Saf. Environ. Prot. 2017, 110, 15–20. [Google Scholar] [CrossRef]
- Landucci, G.; Tugnoli, A.; Busini, V.; Derudi, M.; Rota, R.; Cozzani, V. The Viareggio LPG accident: Lessons learnt. J. Loss Prev. Process Ind. 2011, 24, 466–476. [Google Scholar] [CrossRef]
- Li, X.; Koseki, H.; Sam Mannan, M. Case study: Assessment on large scale LPG BLEVEs in the 2011 Tohoku earthquakes. J. Loss Prev. Process Ind. 2015, 35, 257–266. [Google Scholar] [CrossRef]
- Bariha, N.; Mishra, I.M.; Srivastava, V.C. Fire and explosion hazard analysis during surface transport of liquefied petroleum gas (LPG): A case study of LPG truck tanker accident in Kannur, Kerala, India. J. Loss Prev. Process Ind. 2016, 40, 449–460. [Google Scholar] [CrossRef]
- Jia, Q.; Fu, G.; Xie, X.; Hu, S.; Wu, Y.; Li, J. LPG leakage and explosion accident analysis based on a new SAA method. J. Loss Prev. Process Ind. 2021, 71, 104467. [Google Scholar] [CrossRef]
- Lyu, S.; Zhang, S.; Huang, X.; Peng, S.; Li, J. Investigation and modeling of the LPG tank truck accident in Wenling, China. Process Saf. Environ. Prot. 2022, 157, 493–508. [Google Scholar] [CrossRef]
- Yi, H.; Feng, Y.; Park, H.; Wang, Q. Configuration predictions of large liquefied petroleum gas (LPG) pool fires using CFD method. J. Loss Prev. Process Ind. 2020, 65, 104099. [Google Scholar] [CrossRef]
- Laboureur, D.M.; Gopalaswami, N.; Zhang, B.; Liu, Y.; Mannan, M.S. Experimental study on propane jet fire hazards: Assessment of the main geometrical features of horizontal jet flames. J. Loss Prev. Process Ind. 2016, 41, 355–364. [Google Scholar] [CrossRef]
- Hurley, M.J.; Gottuk, D.T.; Hall, J.R., Jr.; Harada, K.; Kuligowski, E.D.; Puchovsky, M.; Watts, J.M., Jr.; Wieczorek, C.J. (Eds.) SFPE Handbook of Fire Protection Engineering; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Center for Chemical Process Safety. Flash Fires—Sample Problems. In Guidelines for Evaluating the Characteristics of Vapor Cloud Explosions, Flash Fires, and BLEVEs; Wiley Online Library: Hoboken, NJ, USA, 1994; pp. 277–284. [Google Scholar] [CrossRef]
- Center for Chemical Process Safety. Basic Principles of Vapor Cloud Explosions. In Guidelines for Evaluating the Characteristics of Vapor Cloud Explosions, Flash Fires, and BLEVEs; Wiley Online Library: Hoboken, NJ, USA, 1994; pp. 69–145. [Google Scholar] [CrossRef]
- Birk, A.M.; Eyssette, R.; Heymes, F. Early moments of BLEVE: From vessel opening to liquid flashing release. Process Saf. Environ. Prot. 2019, 132, 35–46. [Google Scholar] [CrossRef]
- Le, S.T.; Nguyen, T.N.; Linforth, S.; Ngo, T.D. Safety investigation of hydrogen energy storage systems using quantitative risk assessment. Int. J. Hydrogen Energy 2023, 48, 2861–2875. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Hao, H. A state-of-the-art review of experimental and numerical studies on BLEVE overpressure prediction. J. Loss Prev. Process Ind. 2022, 80, 104920. [Google Scholar] [CrossRef]
- Lv, J.; Wang, Q.; Tang, F.; Sun, X. The evolution of flame geometrical characteristics and air entrainment of inclined jet flames. Process Saf. Environ. Prot. 2023, 178, 414–422. [Google Scholar] [CrossRef]
- Kraft, R.A.; Orellano, S.; Mores, P.L.; Scenna, N.J. BLEVE: Safety distances estimation by simple models based on the Jakob number. J. Loss Prev. Process Ind. 2023, 83, 105069. [Google Scholar] [CrossRef]
- Elizaryev, A.; Nasyrova, E.; Cattani, C.; Tarakanov, D.; Tarakanov, D.; Khasanov, I. Mathematical Models for Assessment the Thermal Radiation of a Fireball During Bleve. In Safety in Aviation and Space Technologies; Bieliatynskyi, A., Breskich, V., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 323–333. [Google Scholar]
- Roberts, M.W. Analysis of Boiling Liquid Expanding Vapor Explosion (BLEVE) Events at DOE Sites; Safety Analysis Workshop; EQE International, Inc.: Oakland, CA, USA, 2000; pp. 1–20. [Google Scholar]
- Dhurandher, B.K.; Kumar, R.; Dhiman, A. Impact Assessment of Thermal Radiation Hazard from LPG Fireball. Procedia Earth Planet. Sci. 2015, 11, 499–506. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, K.; Zhang, L.; Nie, X.; Wu, Y.; Jiang, J.; Dederichs, A.S.; He, L. Flame extension area and temperature profile of horizontal jet fire impinging on a vertical plate. Process Saf. Environ. Prot. 2021, 147, 547–558. [Google Scholar] [CrossRef]
- Tschirschwitz, R.; Krentel, D.; Kluge, M.; Askar, E.; Habib, K.; Kohlhoff, H.; Krüger, S.; Neumann, P.P.; Storm, S.U.; Rudolph, M.; et al. Experimental investigation of consequences of LPG vehicle tank failure under fire conditions. J. Loss Prev. Process Ind. 2018, 56, 278–288. [Google Scholar] [CrossRef]
- Birk, A.M.; Eyssette, R.; Heymes, F. Analysis of BLEVE overpressure using spherical shock theory. Process Saf. Environ. Prot. 2020, 134, 108–120. [Google Scholar] [CrossRef]
- Eyssette, R.; Heymes, F.; Birk, A.M. Ground loading from BLEVE through small scale experiments: Experiments and results. Process Saf. Environ. Prot. 2021, 148, 1098–1109. [Google Scholar] [CrossRef]
- Davis, S.; Merilo, E.; Engel, D.; Ziemba, A.; Pinto, M.; van Wingerden, K. Large scale detonation testing: New findings in the prediction of DDTs at large scales. J. Loss Prev. Process Ind. 2017, 48, 345–357. [Google Scholar] [CrossRef]
- Reinders, J.E.A.; Velthuis, J.F.M.; Spruijt, M.P.N. Pressure and temperature increase of LPG in a thermally coated pressure vessel exposed to fire: Experimental and model results. J. Loss Prev. Process Ind. 2019, 57, 55–60. [Google Scholar] [CrossRef]
- Hemmatian, B.; Casal, J.; Planas, E. A new procedure to estimate BLEVE overpressure. Process Saf. Environ. Prot. 2017, 111, 320–325. [Google Scholar] [CrossRef]
- Hemmatian, B.; Planas, E.; Casal, J. Comparative analysis of BLEVE mechanical energy and overpressure modelling. Process Saf. Environ. Prot. 2017, 106, 138–149. [Google Scholar] [CrossRef]
- TNOYB. Methods for the Calculation of Physical Effects; The Committee for the Prevention of Disasters by Hazardous Materials: The Hague, The Netherlands, 2005. [Google Scholar]
- Casal, J.; Salla, J.M. Using liquid superheating energy for a quick estimation of overpressure in BLEVEs and similar explosions. J. Hazard. Mater. 2006, 137, 1321–1327. [Google Scholar] [CrossRef]
- Planas-Cuchi, E.; Salla, J.M.; Casal, J. Calculating overpressure from BLEVE explosions. J. Loss Prev. Process Ind. 2004, 17, 431–436. [Google Scholar] [CrossRef]
- Laamarti, E.M.; Birk, A.M.; Chanut, C.; Heymes, F. Correlations to estimate the ground loading from small scale propane BLEVE experiments. Process Saf. Environ. Prot. 2024, 185, 876–889. [Google Scholar] [CrossRef]
- Sellami, I.; Nait-Said, R.; de Izarra, C.; Chetehouna, K.; Zidani, F. Quantitative consequence analysis using Sedov-Taylor blast wave model. Part I: Model description and validation. Process Saf. Environ. Prot. 2018, 116, 763–770. [Google Scholar] [CrossRef]
- Sellami, I.; Nait-Said, R.; Chetehouna, K.; de Izarra, C.; Zidani, F. Quantitative consequence analysis using Sedov-Taylor blast wave model. Part II: Case study in an Algerian gas industry. Process Saf. Environ. Prot. 2018, 116, 771–779. [Google Scholar] [CrossRef]
- Jiao, Z.; Hu, P.; Xu, H.; Wang, Q. Machine Learning and Deep Learning in Chemical Health and Safety: A Systematic Review of Techniques and Applications. ACS Chem. Health Saf. 2020, 27, 316–334. [Google Scholar] [CrossRef]
- Bariha, N.; Ojha, D.K.; Srivastava, V.C.; Mishra, I.M. Fire and risk analysis during loading and unloading operation in liquefied petroleum gas (LPG) bottling plant. J. Loss Prev. Process Ind. 2023, 81, 104928. [Google Scholar] [CrossRef]
- Beheshti, M.H.; Dehghan, S.F.; Hajizadeh, R.; Jafari, S.M.; Koohpaei, A. Modelling the consequences of explosion, fire and gas leakage in domestic cylinders containing LPG. Ann. Med. Health Sci. Res. 2018, 8, 83–88. [Google Scholar]
- Terzioglu, L.; Iskender, H. Modeling the consequences of gas leakage and explosion fire in liquefied petroleum gas storage tank in Istanbul technical university, Maslak campus. Process Saf. Prog. 2021, 40, 319–326. [Google Scholar] [CrossRef]
- Kukfisz, B.; Kuczyńska, A.; Piec, R.; Szykuła-Piec, B. Research on the Safety and Security Distance of Above-Ground Liquefied Gas Storage Tanks and Dispensers. Int. J. Environ. Res. Public Health 2022, 19, 839. [Google Scholar] [CrossRef]
- Kumar Malviya, R.; Rushaid, M. Consequence Analysis of LPG Storage Tank. Mater. Today Proc. 2018, 5, 4359–4367. [Google Scholar] [CrossRef]
- Alfatesha, M.T.; Biaka, D.R.A. Analysis of Boiling Liquid Expanding Vapour Explosion (BLEVE) Due To Loss of Containment for Liquefied Petroleum Gas (LPG) Road Tankers. J. Occup. Saf. Health 2019, 16, 11–19. [Google Scholar]
- Gargiulo, A.; Duetsch-Patel, J.E.; Borgoltz, A.; Devenport, W.J.; Roy, C.J.; Lowe, K.T. Strategies for Computational Fluid Dynamics Validation Experiments. J. Verif. Valid. Uncertain. Quantif. 2023, 8, 031004. [Google Scholar] [CrossRef]
- Yi, H.; Feng, Y.; Wang, Q. Computational fluid dynamics (CFD) study of heat radiation from large liquefied petroleum gas (LPG) pool fires. J. Loss Prev. Process Ind. 2019, 61, 262–274. [Google Scholar] [CrossRef]
- Scarponi, G.E.; Landucci, G.; Birk, A.M.; Cozzani, V. Three dimensional CFD simulation of LPG tanks exposed to partially engulfing pool fires. Process Saf. Environ. Prot. 2021, 150, 385–399. [Google Scholar] [CrossRef]
- Mashhadimoslem, H.; Ghaemi, A.; Behroozi, A.H.; Palacios, A. A New simplified calculation model of geometric thermal features of a vertical propane jet fire based on experimental and computational studies. Process Saf. Environ. Prot. 2020, 135, 301–314. [Google Scholar] [CrossRef]
- Vijayan, P.; Thampi, G.K.; Vishwakarma, P.K.; Palacios, A. Evaluation of flame geometry of horizontal turbulent jet fires in reduced pressures: A numerical approach. J. Loss Prev. Process Ind. 2022, 80, 104931. [Google Scholar] [CrossRef]
- Hu, Q.; Qian, X.; Shen, X.; Zhang, Q.; Ma, C.; Pang, L.; Liang, Y.; Feng, H.; Yuan, M. Investigations on vapor cloud explosion hazards and critical safe reserves of LPG tanks. J. Loss Prev. Process Ind. 2022, 80, 104904. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Wang, H.; Liu, X.; Jiao, Y.; Wu, Y. Dynamic Process and Damage Evaluation Subject to Explosion Consequences Resulting from a LPG Tank Trailer Accident. Processes 2023, 11, 1514. [Google Scholar] [CrossRef]
- Wang, K.; Hu, Q.; Qian, X.; Li, M.; Shi, T. Cause analysis and damage mechanism of explosive destruction with case investigation involving LPG tank trailer. Eng. Fail. Anal. 2022, 133, 106002. [Google Scholar] [CrossRef]
- Dhiman, M.; Zambare, A.; Sathiah, P.; Narasimhamurthy, V.D. CFD simulations of vapour cloud explosions using PDRFoam. J. Loss Prev. Process Ind. 2023, 85, 105164. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, X.; Xia, L.; Pan, Y.; Ni, Y.; Wang, S.; Zhou, W. Hazard analysis on LPG fireball of road tanker BLEVE based on CFD simulation. J. Loss Prev. Process Ind. 2020, 68, 104319. [Google Scholar] [CrossRef]
- Sellami, I.; Manescau, B.; Chetehouna, K.; de Izarra, C.; Nait-Said, R.; Zidani, F. BLEVE fireball modeling using Fire Dynamics Simulator (FDS) in an Algerian gas industry. J. Loss Prev. Process Ind. 2018, 54, 69–84. [Google Scholar] [CrossRef]
- Weerheijm, J.; Verreault, J.; van der Voort, M.M. Quantitative risk analysis of gas explosions in tunnels. Fire Saf. J. 2018, 97, 146–158. [Google Scholar] [CrossRef]
- Li, Y.Z. Study of fire and explosion hazards of alternative fuel vehicles in tunnels. Fire Saf. J. 2019, 110, 102871. [Google Scholar] [CrossRef]
- Cheng, R.; Chen, W.; Hao, H.; Li, J. Numerical prediction of ground vibrations induced by LPG boiling liquid expansion vapour explosion (BLEVE) inside a road tunnel. Undergr. Space 2023, 12, 44–64. [Google Scholar] [CrossRef]
- Shelke, A.V.; Maheshwari, N.K.; Gera, B.; Singh, R.K. CFD Analysis of Hydrocarbon Fireballs. Combust. Sci. Technol. 2017, 189, 1440–1466. [Google Scholar] [CrossRef]
- Abdel-Jawad, M. Validation of BLEVE events using the hybrid code exploCFD. Process Saf. Prog. 2021, 40, e12208. [Google Scholar] [CrossRef]
- Laboureur, D.; Birk, A.M.; Buchlin, J.M.; Rambaud, P.; Aprin, L.; Heymes, F.; Osmont, A. A closer look at BLEVE overpressure. Process Saf. Environ. Prot. 2015, 95, 159–171. [Google Scholar] [CrossRef]
- Abdel-Jawad, M. CSC coupled Graham Kenney source term for hybrid models of BLEVE events: Formulation, implementation and validation. Process Saf. Prog. 2021, 40, 281–288. [Google Scholar] [CrossRef]
- Davidy, A. CFD Simulation and Mitigation with Boiling Liquid Expanding Vapor Explosion (BLEVE) Caused by Jet Fire. ChemEngineering 2019, 3, 1. [Google Scholar] [CrossRef]
- Scarponi, G.E.; Landucci, G.; Birk, A.M.; Cozzani, V. LPG vessels exposed to fire: Scale effects on pressure build-up. J. Loss Prev. Process Ind. 2018, 56, 342–358. [Google Scholar] [CrossRef]
- Scarponi, G.E.; Landucci, G.; Birk, A.M.; Cozzani, V. An innovative three-dimensional approach for the simulation of pressure vessels exposed to fire. J. Loss Prev. Process Ind. 2019, 61, 160–173. [Google Scholar] [CrossRef]
- Scarponi, G.E.; Cozzani, V.; Antonioni, G.; Doghieri, F. Modeling the behavior of LPG tanks exposed to partially engulfing pool fires. Process Saf. Environ. Prot. 2024, 182, 1072–1085. [Google Scholar] [CrossRef]
- Li, J.; Hao, H. Numerical study of medium to large scale BLEVE for blast wave prediction. J. Loss Prev. Process Ind. 2020, 65, 104107. [Google Scholar] [CrossRef]
- Li, J.; Hao, H. Numerical simulation of medium to large scale BLEVE and the prediction of BLEVE’s blast wave in obstructed environment. Process Saf. Environ. Prot. 2021, 145, 94–109. [Google Scholar] [CrossRef]
- Ma, Q.; Zhong, M.; Guo, Y.; You, J.; He, Y.; Chen, J.; Zhang, Z. Study on the characteristic of boiling expansion process of superheated LPG and its vapor cloud explosion. J. Loss Prev. Process Ind. 2022, 78, 104831. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, H.; Chen, W.; Li, J.; Wang, Z. Prediction of BLEVE loading on a rigid structure. Process Saf. Environ. Prot. 2023, 175, 1–16. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Hao, H. Prediction of BLEVE loading on structures. J. Loss Prev. Process Ind. 2024, 90, 105325. [Google Scholar] [CrossRef]
- Yuan, M.; Hu, Q.; Huang, Z.; Shen, X.; Qian, X.; Yang, H.; Qi, S.; Jiang, J.; Xia, D. Gas explosion impact behavior and disaster analysis based on structural failure: Numerical modeling. J. Loss Prev. Process Ind. 2024, 87, 105234. [Google Scholar] [CrossRef]
- Lyu, S.; Zhang, S.; Huang, X.; Peng, S.; Yang, D.; Sun, M.; Qi, Q. CFD simulations of instantaneously released liquefied gas in urban areas: A case study of LPG tank truck accident in Wenling, China. Sustain. Cities Soc. 2023, 94, 104550. [Google Scholar] [CrossRef]
- Harhara, A.; Arora, A.; Faruque Hasan, M.M. Process safety consequence modeling using artificial neural networks for approximating heat exchanger overpressure severity. Comput. Chem. Eng. 2023, 170, 108098. [Google Scholar] [CrossRef]
- Tamascelli, N.; Solini, R.; Paltrinieri, N.; Cozzani, V. Learning from major accidents: A machine learning approach. Comput. Chem. Eng. 2022, 162, 107786. [Google Scholar] [CrossRef]
- Tamascelli, N.; Campari, A.; Parhizkar, T.; Paltrinieri, N. Artificial Intelligence for safety and reliability: A descriptive, bibliometric and interpretative review on machine learning. J. Loss Prev. Process Ind. 2024, 90, 105343. [Google Scholar] [CrossRef]
- Hemmatian, B.; Casal, J.; Planas, E.; Hemmatian, B.; Rashtchian, D. Prediction of BLEVE mechanical energy by implementation of artificial neural network. J. Loss Prev. Process Ind. 2020, 63, 104021. [Google Scholar] [CrossRef]
- Gabhane, L.R.; Kanidarapu, N. Environmental Risk Assessment Using Neural Network in Liquefied Petroleum Gas Terminal. Toxics 2023, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Q.; Hao, H.; Li, L. Prediction of BLEVE blast loading using CFD and artificial neural network. Process Saf. Environ. Prot. 2021, 149, 711–723. [Google Scholar] [CrossRef]
- Li, J.; Hao, H.; Chen, W.; Cheng, R. Calculation of BLEVE energy and overpressures inside a tunnel using analytical and CFD methods. Tunn. Undergr. Space Technol. 2022, 120, 104263. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Hao, H. Development of efficient methods for prediction of medium to large scale BLEVE pressure in open space. Process Saf. Environ. Prot. 2022, 161, 421–435. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Shao, Y.; Li, L.; Hao, H. A comparative study on the most effective machine learning model for blast loading prediction: From GBDT to Transformer. Eng. Struct. 2023, 276, 115310. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, L.; Hao, H.; Wang, R.; Li, J. Prediction of BLEVE loads on structures using machine learning and CFD. Process Saf. Environ. Prot. 2023, 171, 914–925. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Chen, W.; Li, L.; Hao, H. Machine learning prediction of BLEVE loading with graph neural networks. Reliab. Eng. Syst. Saf. 2024, 241, 109639. [Google Scholar] [CrossRef]


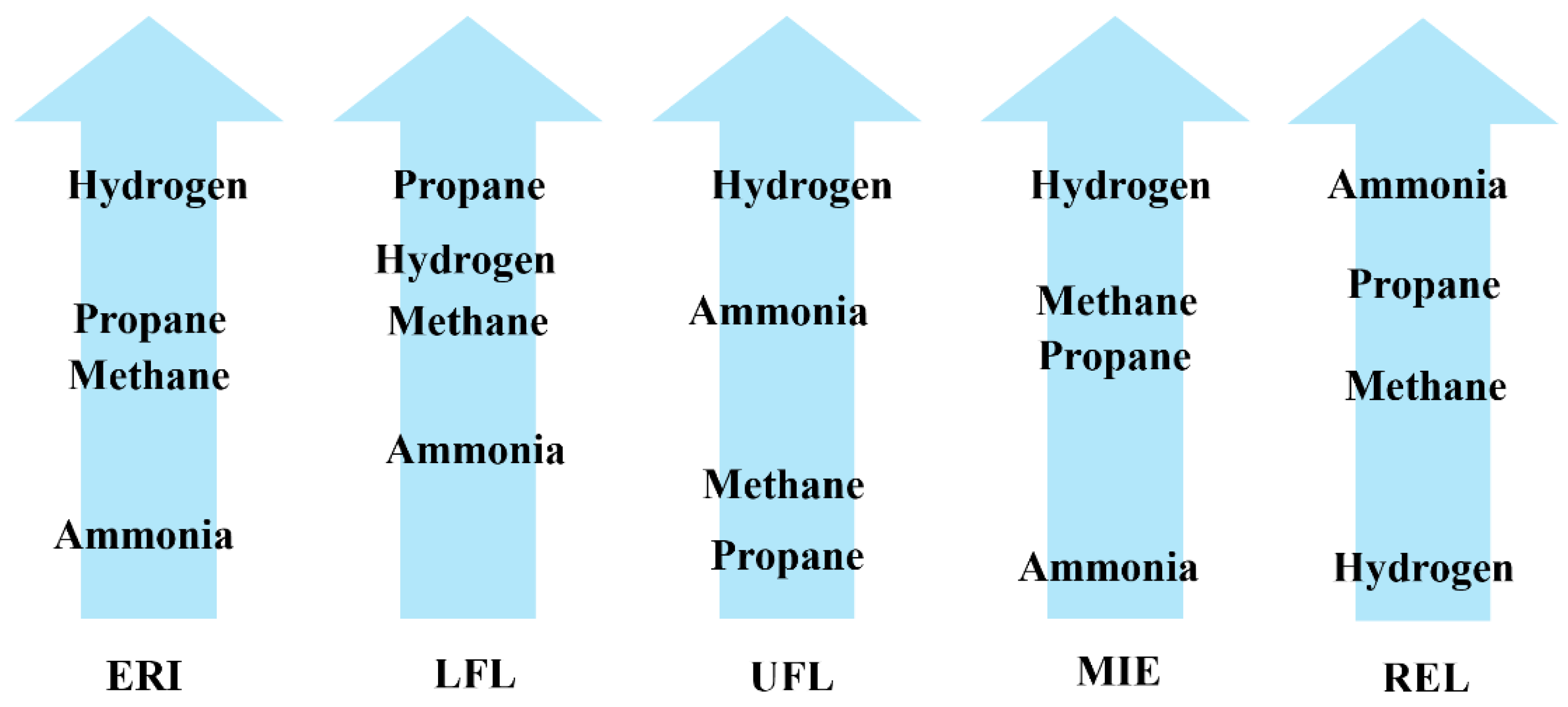
| Component | ISO 9162:2013 Commercial Propane ISO-F-LP | ASTM D 1835-Special-Duty Propane (USA) | CAN/CGSB-3.14-2023 Grade 1 (Canada) |
|---|---|---|---|
| Propane | - | - | 90% min. by volume |
| Butane C4 hydrocarbons | 7.5% max. %(molar) | 2.5% max. by volume | 2.5% max. by volume |
| Sulfur | 50 mg/kg max | 123 mg/kg max | 123 mg/kg max |
| Evaporation residue | 60 mg/kg max | 0.05 mL max per 100 ml | 0.05 mL max per 100 mL |
| Vapor pressure | 1550 kPa max at 40 °C | 1435 kPa max at 37.8 °C | 1435 kPa max at 37.8 °C |
| Component | Propane | Propene | n-Butane | Gasoline | Diesel |
|---|---|---|---|---|---|
| Boiling point @ 101.3 kPa (°C) | −42.1 | −47.7 | −0.5 | 30–220 | 160–380 |
| Vapor pressure @ 37.8 °C (kPa) | 1310 | 1561 | 356 | ~64 | ~2 |
| Density @ 15 °C (kg/m3) | 506.0 * | 520.4 * | 583.0 * | ~730 | ~840 |
| Gross calorific value @ 25 °C (kJ/kg) | 50,014 | 48,954 | 49,155 | ~44,300 | ~45,500 |
| Lower Flammability Limit, LFL (% vol. in air) | 2.3 | 2.2 | 1.9 | 1.4 | 0.7 |
| Upper Flammability Limit, UFL (% vol. in air) | 9.5 | 9.6 | 8.5 | 7.6 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardila-Suarez, C.; Lacoursière, J.-P.; Soucy, G.; Rego de Vasconcelos, B. Consequence Analysis of LPG-Related Hazards: Ensuring Safe Transitions to Cleaner Energy. Fuels 2025, 6, 45. https://doi.org/10.3390/fuels6020045
Ardila-Suarez C, Lacoursière J-P, Soucy G, Rego de Vasconcelos B. Consequence Analysis of LPG-Related Hazards: Ensuring Safe Transitions to Cleaner Energy. Fuels. 2025; 6(2):45. https://doi.org/10.3390/fuels6020045
Chicago/Turabian StyleArdila-Suarez, Carolina, Jean-Paul Lacoursière, Gervais Soucy, and Bruna Rego de Vasconcelos. 2025. "Consequence Analysis of LPG-Related Hazards: Ensuring Safe Transitions to Cleaner Energy" Fuels 6, no. 2: 45. https://doi.org/10.3390/fuels6020045
APA StyleArdila-Suarez, C., Lacoursière, J.-P., Soucy, G., & Rego de Vasconcelos, B. (2025). Consequence Analysis of LPG-Related Hazards: Ensuring Safe Transitions to Cleaner Energy. Fuels, 6(2), 45. https://doi.org/10.3390/fuels6020045







