Optimizing a Hydrogen and Methane Blending System Through Design and Simulation
Abstract
:1. Introduction
2. Materials and Methods
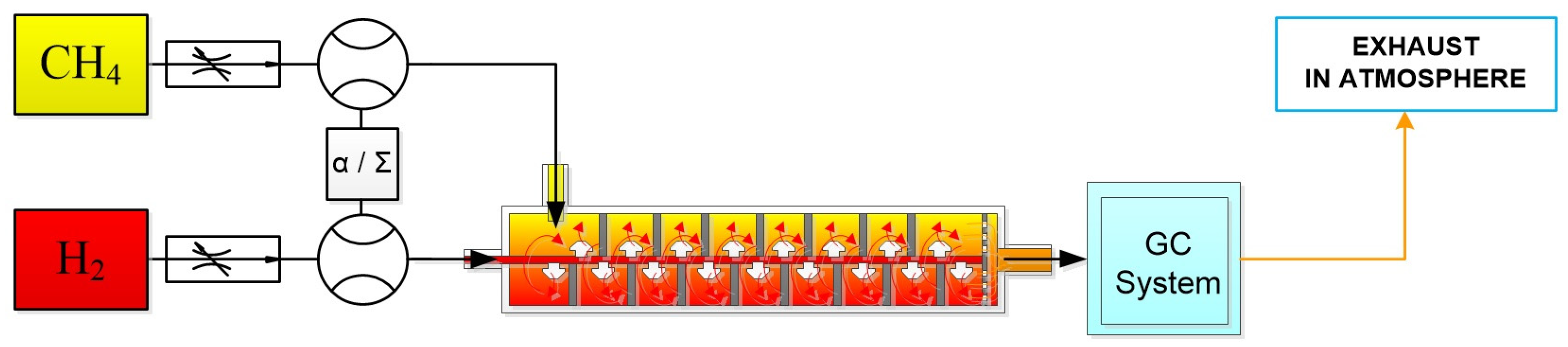
2.1. Experimental Setup
2.1.1. Mixing Flow and Design
2.1.2. Gas Chromatography and Measurement
2.1.3. Operational Conditions
2.1.4. Experimental Validation
2.2. Computational Model
2.2.1. Geometry
2.2.2. Simulation Parameters
- Solver type: pressure-based, steady-state solver;
- Turbulence model: SST k-ω, combining the strengths of k-ω (boundary layer flow description) for near-wall flow and k-ε (fluid free flow description) for free-stream regions [34]; This model was chosen because it effectively combines the advantages of the k-ε model, which performs well in free-stream regions, and the k-ω model, which provides higher accuracy near boundary layers close to the wall. Using only the k-ε model would have resulted in less accurate results.
- Species transport: modeled the convection–diffusion dynamics of CH4 and H2 using the species transport equation [35]
- Boundary conditions: For the boundary conditions, we applied (i) pressure conditions at the inlet and outlet, (ii) stationary walls with a no-slip condition, (iii) standard roughness, and (iv) zero heat flux (adiabatic conditions) in order to replicate realistic pipeline conditions and ensure the accuracy of the computational model. Inlet pressures for H2 ranged from 200 to 300 kPa, with CH4 pressures adjusted to achieve H2 mass fractions at the outlet between 5% and 18%. The set reference pressure and temperature were 101,325 Pa and 300 K, respectively. Mixture density: the compressible ideal gas, viscosity: 1.72 × 10−5 kg/m·s. Wall boundary conditions were stationary walls, no slip condition, standard roughness, and zero heat flux (adiabatic condition). The influence of gravitational forces was not considered.
2.2.3. Solver Configuration
3. Results and Discussions
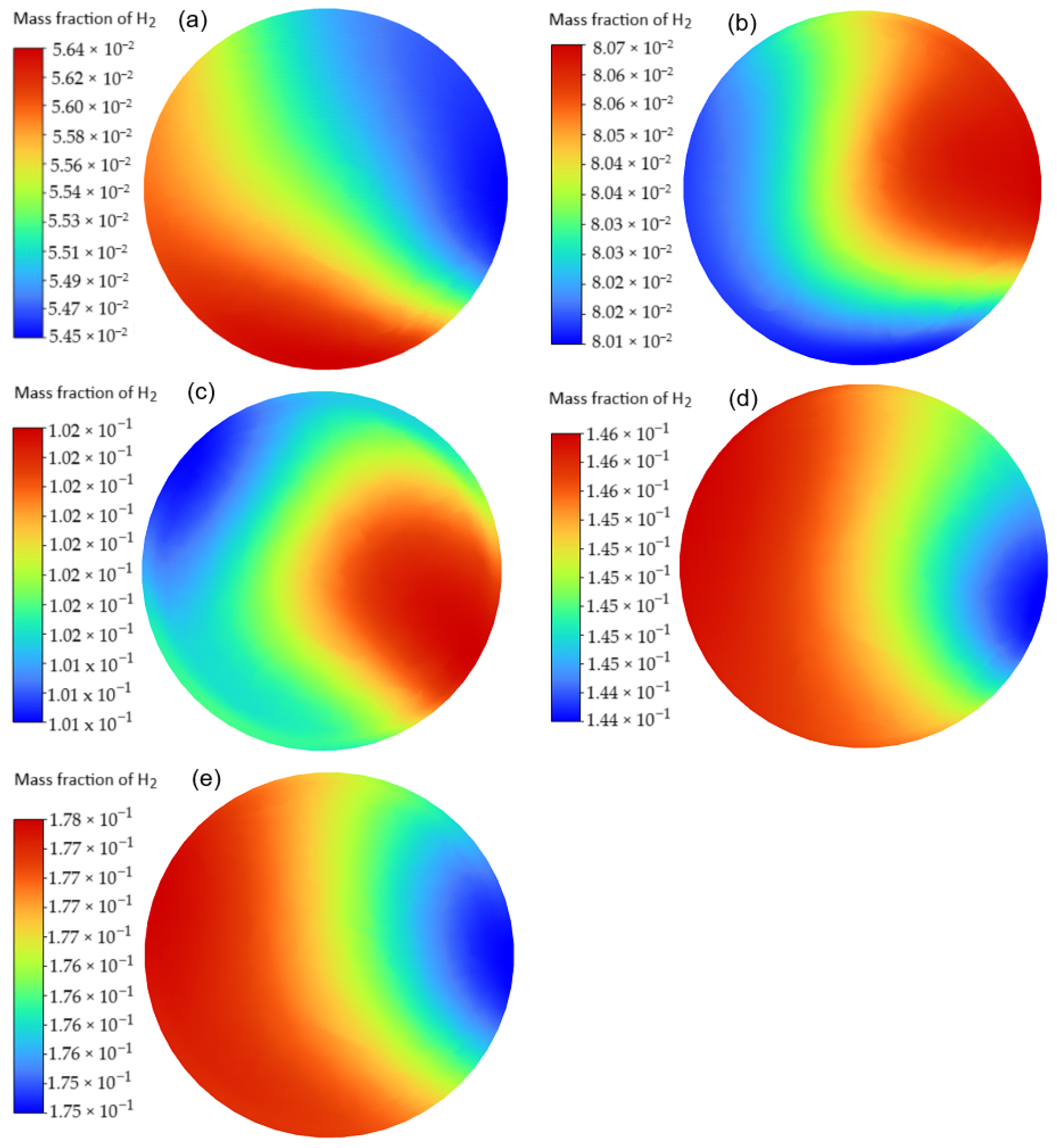
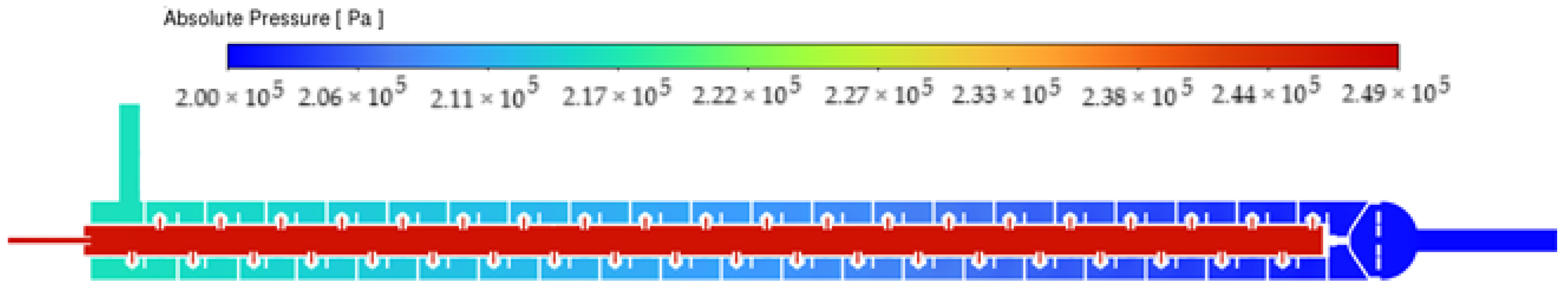
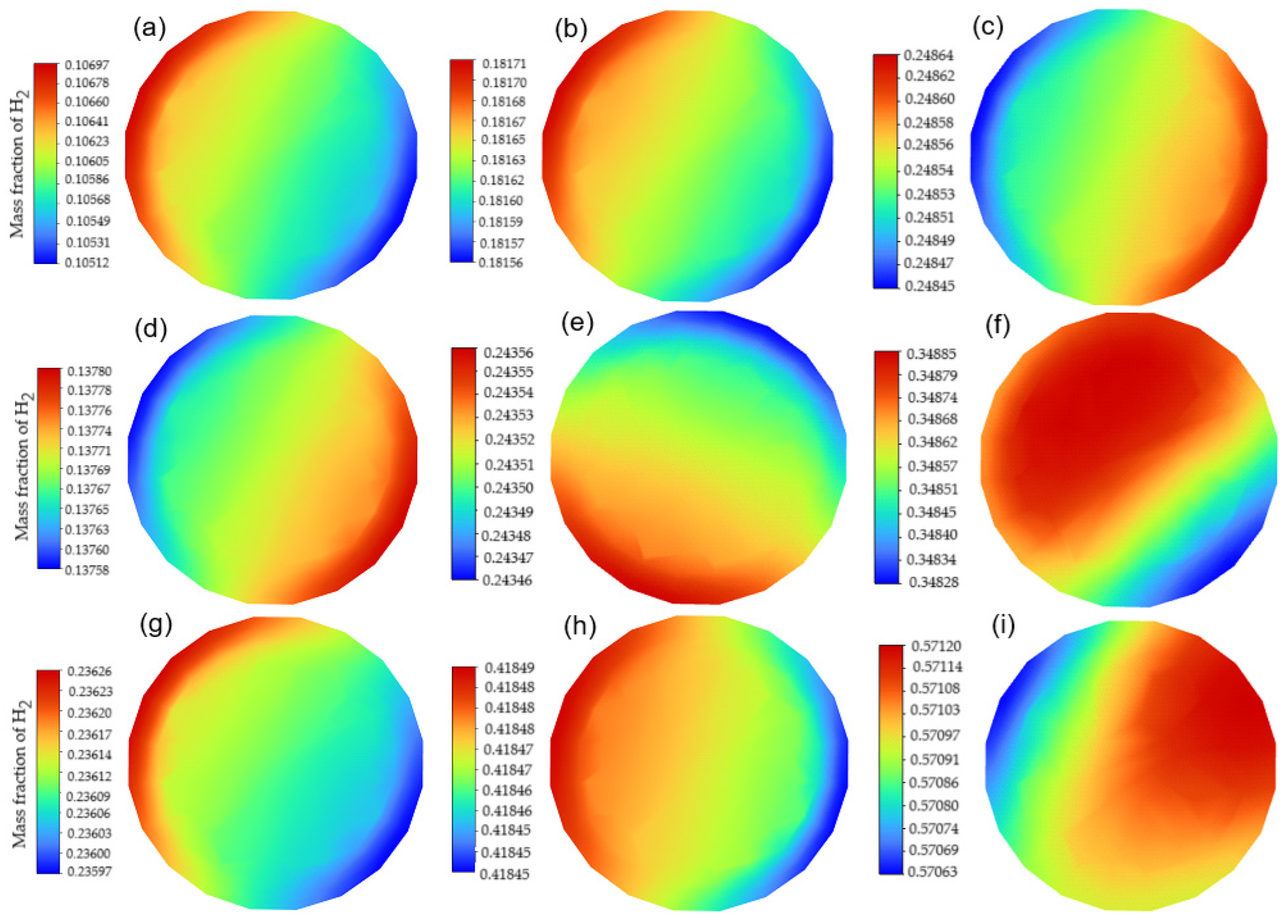
3.1. Mixing Performance
3.2. Impact of Nozzle Geometry
3.3. Comparison of Simulation and Experimental Data
3.4. System Optimization and Trade-Offs
- Smaller Nozzles (0.4 mm): Achieved highly uniform mixing with minimal pressure losses, making them ideal for applications requiring precise control of hydrogen concentration;
- Larger Nozzles (0.6 mm): Enabled higher hydrogen mass fractions but required careful management of pressure and flow conditions to maintain mixing efficiency.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bauer, C.G.; Forest, T.W. Effect of hydrogen addition on the performance of methane-fueled vehicles. Part I: Effect on S.I. engine performance. Int. J. Hydrogen Energy 2001, 26, 55–70. [Google Scholar] [CrossRef]
- Karim, G.A.; Wierzba, I.; Al-Alousi, Y. Methane-hydrogen mixtures as fuels. Int. J. Hydrogen Energy 1996, 21, 625–631. [Google Scholar] [CrossRef]
- Sierens, R.; Rosseel, E. Variable composition hydrogen/natural gas mixtures for increased engine efficiency and decreased emissions. J. Eng. Gas Turbines Power 2000, 122, 135–140. [Google Scholar] [CrossRef]
- Kong, M.; Feng, S.; Xia, Q.; Chen, C.; Pan, Z.; Gao, Z. Investigation of mixing behavior of hydrogen blended to natural gas in gas networks. Sustainability 2021, 13, 4255. [Google Scholar] [CrossRef]
- Sofian, M.; Haq, M.B.; Al Shehri, D.; Rahman, M.M.; Muhammed, N.S. A review on hydrogen blending in gas networks: Insight into safety, corrosion, embrittlement, and coatings. Int. J. Hydrogen Energy 2024, 60, 867–889. [Google Scholar] [CrossRef]
- Chugh, S.; Posina, V.A.; Sonkar, K.; Srivatsava, U.; Sharma, A.; Acharya, G.K. Modeling & simulation study to assess the effect of CO2 performance and emissions characteristics of 18% HCNG blend on a light duty SI engine. Int. J. Hydrogen Energy 2016, 41, 6155–6161. [Google Scholar] [CrossRef]
- Singh, A.P.; Pal, A.; Agarwal, A.K. Comparative particulate characteristics of hydrogen, CNG, HCNG, gasoline and diesel fueled engines. Fuel 2016, 185, 491–499. [Google Scholar] [CrossRef]
- Genovese, A.; Contrisciani, N.; Ortenzi, F.; Cazzola, V. On road experimental tests of hydrogen/natural gas blends on transit buses. Int. J. Hydrogen Energy 2011, 36, 1775–1783. [Google Scholar] [CrossRef]
- Luo, S.; Ma, F.; Mehra, R.K.; Huang, Z. Deep insights of HCNG engine research in China. Fuel 2020, 236, 116612. [Google Scholar] [CrossRef]
- Thakur, R.K.; Vial, C.; Nigam, K.D.P.; Nauman, E.B.; Djelveh, G. Static mixers in the process industries—A review. Chem. Eng. Res. Des. 2003, 81, 787–826. [Google Scholar] [CrossRef]
- Deng, J.; Ma, F.; Li, S.; He, Y.; Wang, M.; Jiang, L.; Zhao, S. Experimental study on combustion and emission characteristics of hydrogen-enriched compressed natural gas engine under idling condition. Int. J. Hydrogen Energy 2011, 36, 13150–13157. [Google Scholar] [CrossRef]
- Zidouni, F.; Krepper, E.; Rzehak, R.; Rabha, S.; Schubert, M.; Hampel, U. Simulation of gas–liquid flow in a helical static mixer. Chem. Eng. Sci. 2015, 137, 476–486. [Google Scholar] [CrossRef]
- Das, M.D.; Hrymak, A.N.; Baird, M.H. Laminar liquid–liquid dispersion in the SMX static mixer. Chem. Eng. Sci. 2013, 101, 329–344. [Google Scholar] [CrossRef]
- Melaina, M.W.; Antonia, O.; Penev, M. Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues; Report number: NREL/TP-5600-51995; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2013.
- Haddadi, M.M.; Hosseini, S.H.; Rashtchian, D.; Olazar, M. Comparative analysis of different static mixers performance by CFD technique. Chin. J. Chem. Eng. 2019, 28, 672–684. [Google Scholar] [CrossRef]
- Ohkawa, K.; Nakamoto, T.; Izuka, Y.; Hirata, Y.; Inoue, Y. Flow and mixing characteristics of σ-type plate static mixer with splitting and inverse recombination. Chem. Eng. Res. Des. 2008, 86, 1447–1453. [Google Scholar] [CrossRef]
- Static Mixers. Available online: https://www.staticmixers.com (accessed on 10 November 2024).
- Pang, Z.; Chen, R.; Cao, Y. Performance analysis and optimization for static mixer of SCR denitration system under different arrangements. Energies 2022, 15, 8977. [Google Scholar] [CrossRef]
- Static Pipeline Mixers. Available online: https://westfallstaticmixers.com/ (accessed on 12 November 2024).
- Ghanem, A.; Lemenand, T.; Della Valle, D.; Peerhossaini, H. Static mixers: Mechanisms, applications, and characterization methods–A review. Chem. Eng. Res. Des. 2014, 92, 205–228. [Google Scholar] [CrossRef]
- Helical Mixers. Available online: https://www.primix.com (accessed on 12 November 2024).
- Model HT. Available online: https://www.stamixco-usa.com/helical (accessed on 12 November 2024).
- Bakker, A.; LaRoche, R.D.; Marshall, E.M. Laminar flow in static mixers with helical elements. In The Online CFM Book; 2000; p. 546. Available online: https://www.bakker.org/cfm/publications/cfmbook/lamstat.pdf (accessed on 14 November 2024).
- Ujhidy, A.; Nemeth, J.; Szépvölgyi, J. Fluid flow in tubes with helical elements. Chem. Eng. Process. 2003, 42, 1–7. [Google Scholar] [CrossRef]
- John, T.P.; Poole, R.J.; Kowalski, A.; Fonte, C.P. Viscoelastic flow asymmetries in a helical static mixer and their impact on mixing performance. J. Nonnewton. Fluid Mech. 2024, 323, 105156. [Google Scholar] [CrossRef]
- SS Low Flow Binary Tee Static Bed Mixer. Available online: https://www.analytical-sales.com/product/ss-low-flow-binary-tee-static-bed-mixer-housing-only/ (accessed on 15 November 2024).
- Chen, Z.; Dewees, D.; Gustafsson, L.M. CFD modelling of mixing tees—Design of a thermal sleeve, Special Focus—Valves, Pumps and Turbomachinery. Hydrocarb. Process. 2020, 21–25. Available online: https://becht.com/wp-content/uploads/2020/08/mixing_tees_Hydroprocessing.pdf (accessed on 12 November 2024).
- High Pressure Static Mixing Tee. Available online: https://www.idex-hs.com (accessed on 16 November 2024).
- Kok, J.B.; Van der Wal, S. Mixing in T-junctions. Appl. Math. Model. 1996, 20, 232–243. [Google Scholar] [CrossRef]
- General Purpose Mixers. Available online: https://www.sulzer.com/en/products/agitators-mixers-and-heat-exchangers/static-mixers/general-purpose-mixers (accessed on 18 November 2024).
- Fradette, L.; Tanguy, P.; Li, H.Z.; Choplin, L. Liquid/liquid viscous dispersions with a SMX static mixer. Chem. Eng. Res. Des. 2007, 85, 395–405. [Google Scholar] [CrossRef]
- Lowry, E.; Yuan, Y.; Krishnamoorthy, G. A new correlation for single-phase pressure loss through SMV static mixers at high Reynolds numbers. Chem. Eng. Process. 2022, 171, 108716. [Google Scholar] [CrossRef]
- BOPI Nr. 12/2023. Available online: https://www.osim.ro/images/Publicatii/Inventii/2023/inv_12_2023.pdf (accessed on 12 November 2024).
- Bostan, V. Modele matematice în inginerie: Probleme de contact. In Modelări și Simulări Numerice în Aero-Hidrodinamică; BonsOffices: Chișinău, Moldova, 2014; 470p, ISBN 978-9975-80-831-6. [Google Scholar]
- Fluent. Ansys Fluent Theory Guide; ANSYS Inc.: Canonsburg, PA, USA, 2022. [Google Scholar]
- Monea, B.F.; Ionete, E.I.; Spiridon, S.I. Experimental investigation and CFD modeling of slush cryogen flow measurement using circular shape capacitors. Sensors 2020, 20, 2117. [Google Scholar] [CrossRef] [PubMed]

| Mixer Type | Primary Applications | Advantages | Disadvantages | Key References |
|---|---|---|---|---|
| Tab or Plate Mixers |
|
|
| [10,15,16,17,18,19,20] |
| Helical Mixers |
|
|
| [4,9,12,21,22,23,24,25] |
| Tee Mixers |
|
|
| [5,26,27,28,29] |
| Cartridge Mixers |
|
|
| [9,13,15,30,31,32] |
| Multi-Stage Mixers |
|
|
| [5,9,10] |
| Baffled Pipe Mixers |
|
|
| [4,12] |
| Case | H2 Mass Fraction (%) | H2 Inlet Pressure (kPa) | CH4 Inlet Pressure (kPa) |
|---|---|---|---|
| A1 | 5.45–5.64 | 150 | 130 |
| A2 | 8.01–8.07 | 150 | 115 |
| A3 | 10.1–10.2 | 150 | 110 |
| A4 | 14.4–14.6 | 180 | 110 |
| A5 | 17.5–17.8 | 200 | 110 |
| Case | H2 Mass Fraction (%) | H2 Inlet Pressure (kPa(r)) | CH4 Inlet Pressure (kPa(r)) |
|---|---|---|---|
| B1 | 10.6 | 150 | 130 |
| B2 | 18.2 | 200 | 130 |
| B3 | 24.8 | 250 | 130 |
| B4 | 13.8 | 150 | 120 |
| B5 | 24.3 | 200 | 120 |
| B6 | 34.9 | 250 | 120 |
| B7 | 23.6 | 150 | 110 |
| B8 | 41.8 | 200 | 110 |
| B9 | 57.1 | 250 | 110 |
| Case | H2 Mass Fraction (%) | Accuracy (%) | Accuracy Error (%) | |
|---|---|---|---|---|
| Simulated | Experimental | |||
| A1 | 5.45–5.64 | 5.54–5.78 | 97.97 | 2.02 |
| A2 | 8.01–8.07 | 8.18–8.23 | 97.98 | 2.01 |
| A3 | 10.10–10.20 | 10.25–10.44 | 98.11 | 1.88 |
| A4 | 14.40–14.60 | 14.46–14.60 | 99.79 | 0.21 |
| A5 | 17.5–17.8 | 17.6–18.25 | 98.48 | 1.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiridon, Ş.I.; Monea, B.F.; Ionete, E.I. Optimizing a Hydrogen and Methane Blending System Through Design and Simulation. Fuels 2025, 6, 28. https://doi.org/10.3390/fuels6020028
Spiridon ŞI, Monea BF, Ionete EI. Optimizing a Hydrogen and Methane Blending System Through Design and Simulation. Fuels. 2025; 6(2):28. https://doi.org/10.3390/fuels6020028
Chicago/Turabian StyleSpiridon, Ştefan Ionuţ, Bogdan Florian Monea, and Eusebiu Ilarian Ionete. 2025. "Optimizing a Hydrogen and Methane Blending System Through Design and Simulation" Fuels 6, no. 2: 28. https://doi.org/10.3390/fuels6020028
APA StyleSpiridon, Ş. I., Monea, B. F., & Ionete, E. I. (2025). Optimizing a Hydrogen and Methane Blending System Through Design and Simulation. Fuels, 6(2), 28. https://doi.org/10.3390/fuels6020028






