Forming Ni-Fe and Co-Fe Bimetallic Structures on SrTiO3-Based SOFC Anode Candidates
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Before Infiltration and Reduction (As-Prepared Samples)
3.2. After Infiltration and before Reduction in Dry H2
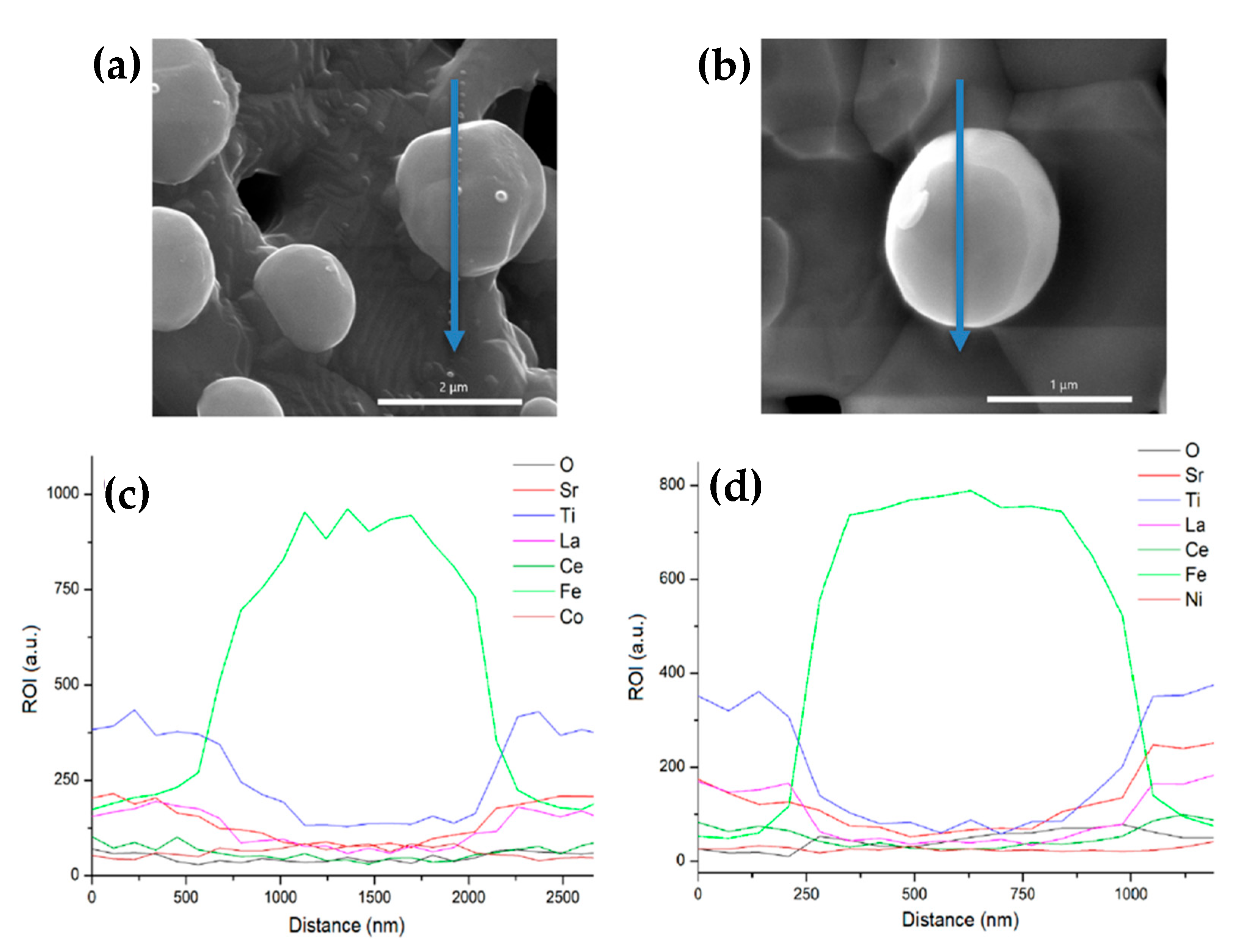
3.3. After Infiltration and Reduction in Dry H2–Topotactic Ion Exchange Exsolution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arshad, M.S.; Mbianda, X.Y.; Ali, I.; Wanbing, G.; Kamal, K.; Kauhaniemi, K.; Hassan, S.Z.; Yasin, G. Advances and Perspectives on Solid Oxide Fuel Cells: From Nanotechnology to Power Electronics Devices. Energy Technol. 2023, 11, 2300452. [Google Scholar] [CrossRef]
- Hanif, M.B.; Motola, M.; Gayyum, S.; Rauf, S.; Khalid, A.; Li, C.-J.; Li, C.-X. Recent advancements, doping strategies and the future perspective of perovskite-based solid oxide fuel cells for energy conversion. Chem. Eng. J. 2022, 428, 132603. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, B.; Su, P.-C.; He, C. Nanomaterials and technologies for low temperature solid oxide fuel cells: Recent advances, challenges and opportunities. Nano Energy 2018, 45, 148–176. [Google Scholar] [CrossRef]
- Bianchi, F.R.; Padinjarethil, A.K.; Hagen, A.; Bosio, B. Multiscale analysis of Ni-YSZ and Ni-CGO anode based SOFC degradation: From local microstructural variation to cell electrochemical performance. Electrochim. Acta 2023, 460, 142589. [Google Scholar] [CrossRef]
- Shu, L.; Sunarso, J.; Hashim, S.S.; Mao, J.; Zhou, W.; Liang, F. Advanced perovskite anodes for solid oxide fuel cells: A review. Int. J. Hydrogen Energy 2019, 44, 31275–31304. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, R.; Presto, S.; Carpanese, M.P.; Barbucci, A.; Viviani, M.; Singh, P. Suitability of Sm3+-Substituted SrTiO3 as Anode Materials for Solid Oxide Fuel Cells: A Correlation between Structural and Electrical Properties. Energies 2019, 12, 4042. [Google Scholar] [CrossRef]
- Vasyliv, B.D.; Podhurska, V.Y.; Ostash, O.P.; Vira, V.V. Effect of a Hydrogen Sulfide-Containing Atmosphere on the Physical and Mechanical Properties of Solid Oxide Fuel Cell Materials. In Nanochemistry, Biotechnology, Nanomaterials, and Their Applications, Proceedings of the 5th International Conference Nanotechnology and Nanomaterials (NANO2017), Chernivtsi, Ukraine, 23–26 August 2017; Fesenko, O., Yatsenko, L., Eds.; Springer Proceedings in Physics; Springer: Cham, Switzerland, 2018; pp. 475–485. [Google Scholar]
- Gao, Y.; Chen, D.; Saccoccio; Lu, Z.; Ciucci, F. From Material Design to Mechanism study: Nanoscale Ni Exsolution on a Highly Active A-site Deficient Anode Material for Solid Oxide Fuel Cells. Nano Energy 2016, 27, 499–508. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, D.; Xu, Y.; Wu, X.; Li, X. Comprehensive Analysis of Solid Oxide Fuel Cell Performance Degradation Mechanism, Prediction, and Optimization Studies. Energies 2023, 16, 788. [Google Scholar] [CrossRef]
- Mo, B.; Rix, J.; Pal, U.; Basu, S.; Gopalan, S. Improving SOFC Anode Electrocatalytic Activity Using Nanoparticle Infiltration into MIEC Compositions. J. Electrochem. Soc. 2020, 167, 134506. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.K.; Liu, J.; Curcio, A.; Jang, J.S.; Kim, I.D.; Ciucci, F.; Jung, W. Nanoparticle Ex-solution for Supported Catalysts: Materials Design, Mechanism and Future Perspectives. ACS Nano 2021, 15, 81–110. [Google Scholar] [CrossRef]
- Neagu, D.; Oh, T.-S.; Miller, D.N.; Menard, H.; Bukhari, S.M.; Gamble, S.R.; Gorte, R.J.; Vohs, J.M.; Irvine, J.T.S. Nano-socketed nickel particles with enhanced coking resistance grown in situ by redox exsolution. Nat. Commun. 2015, 6, 8120. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Azad, A.T.; Petra, P.M.I.; Begum, F.; Eriksson, S.G.; Azad, A.K. Nanomaterials for solid oxide fuel cells: A review. Renew. Sustain. Energy Rev. 2018, 82, 353–368. [Google Scholar] [CrossRef]
- Lin, Y.; Fang, S.; Su, D.; Brinkman, K.S.; Chen, F. Enhancing grain boundary ionic conductivity in mixed ionic-electronic conductors. Nat. Commun. 2015, 6, 6824. [Google Scholar] [CrossRef]
- Hussain, S.; Yangping, L. Review of solid oxide fuel cell materials: Cathode, anode, and electrolyte. Energy Transit. 2020, 4, 113–126. [Google Scholar] [CrossRef]
- Liu, M.; Lynch, M.E.; Blinn, K.; Alamgir, F.M.; Yong, M.C. Rational SOFC material design: New advances and tools. Mater. Today 2011, 14, 534–546. [Google Scholar] [CrossRef]
- Kan, W.H.; Samson, A.J.; Thangadurai, V. Trends in electrode development for next generation solid oxide fuel cells. J. Mater. Chem. A 2016, 4, 17913–17932. [Google Scholar] [CrossRef]
- Joo, S.; Kwon, O.; Kim, K.; Kim, S.; Kim, H.; Shin, J.; Jeong, H.Y.; Sengodan, S.; Han, J.W.; Kim, G. Cation-swapped homogeneous nanoparticles in perovskite oxides for high power density. Nat. Commun. 2019, 10, 697. [Google Scholar] [CrossRef]
- Kwon, O.; Joo, S.; Choi, S.; Sengodan, S.; Kim, G. Review on exsolution and its driving forces in perovskites. J. Phys. Energy 2020, 2, 032001. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jeong, H.; Won, B.R.; Jeon, H.; Park, C.H.; Park, D.; Kim, Y.; Lee, S.; Myung, J.H. Nanoparticle Exsolution on Perovskite Oxides: Insights into Mechanism, Characteristics and Novel Strategies. Nano-Micro Lett. 2024, 16, 33. [Google Scholar] [CrossRef]
- Joo, S.; Seong, A.; Kwon, O.; Kim, K.; Lee, J.H.; Gorte, R.J.; Vohs, J.M.; Han, J.W.; Kim, G. Highly active dry methane reforming catalysts with boosted in situ grown Ni-Fe nanoparticles on perovskite via atomic layer deposition. Sci. Adv. 2020, 6, eabb1573. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, X.; Zeng, Y.; Amirkhiz, B.S.; Wang, M.; Zhang, L.; Hua, B.; Li, J.; Li, J.; Luo, J. An ingenious Ni/Ce co-doped titanate based perovskite as a coking-tolerant anode material for direct hydrocarbon solid oxide fuel cells. J. Mater. Chem. A 2015, 3, 22830–22838. [Google Scholar] [CrossRef]
- Huízar-Félix, A.M.; Hernández, T.; de la Parra, S.; Ibarra, J.; Kharisov, B. Sol–gel based Pechini method synthesis and characterization of Sm1−x CaxFeO3 perovskite 0.1≤x≤0.5. Powder Technol. 2012, 229, 290–293. [Google Scholar] [CrossRef]
- Dimesso, L. Pechini Processes: An Alternate Approach of the Sol–Gel Method, Preparation, Properties, and Applications. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 1067–1088. [Google Scholar]
- Sunde, T.O.L.; Grande, T.; Einarsrud, M.-A. Modified Pechini Synthesis of Oxide Powders and Thin Films. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 1089–1118. [Google Scholar]
- Crystallography Open Database. Available online: https://www.crystallography.net/cod/1512124.html (accessed on 10 April 2024).
- Database of Ionic Radii. Available online: https://abulafia.mt.ic.ac.uk/shannon/ptable.php (accessed on 10 April 2024).
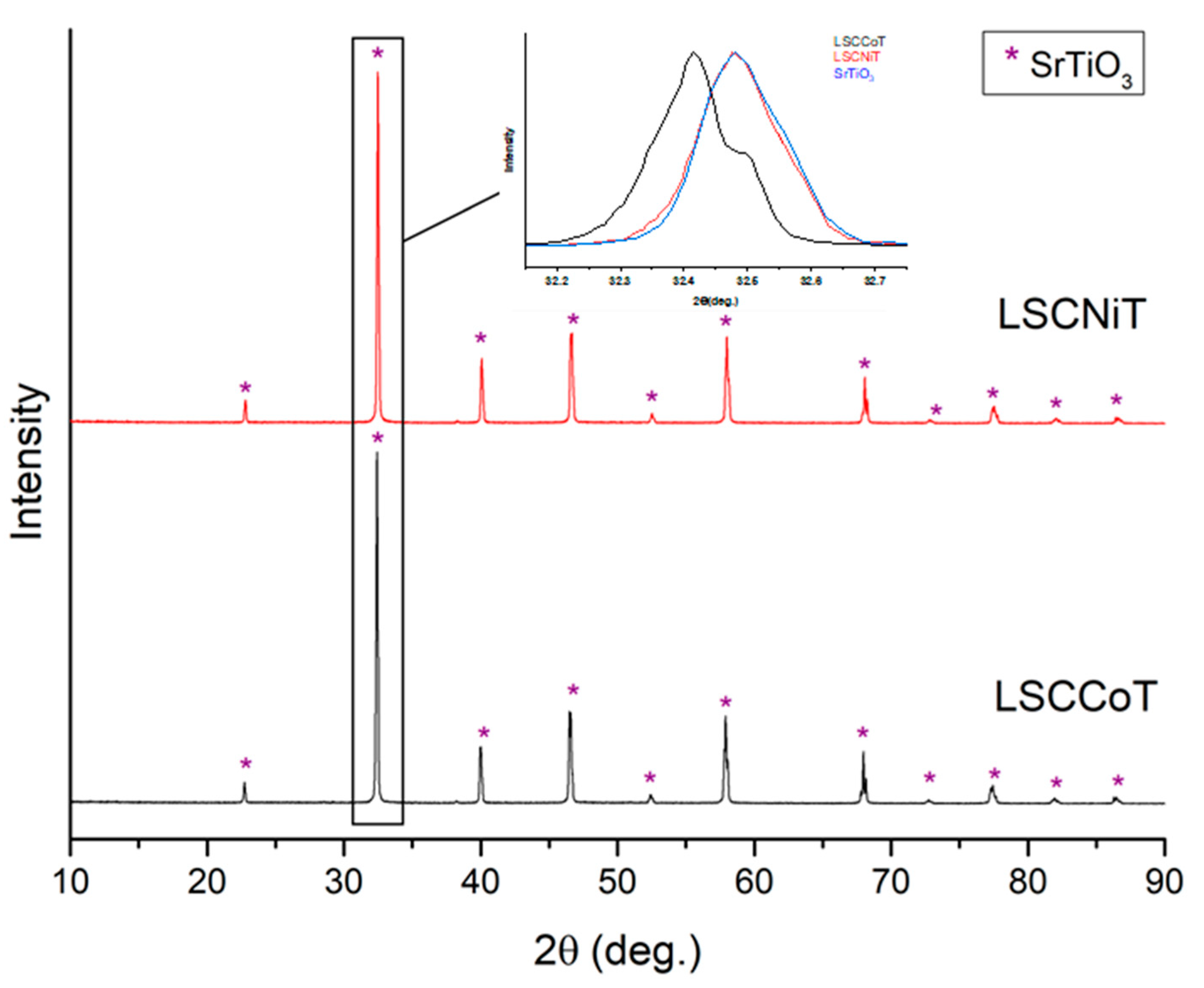
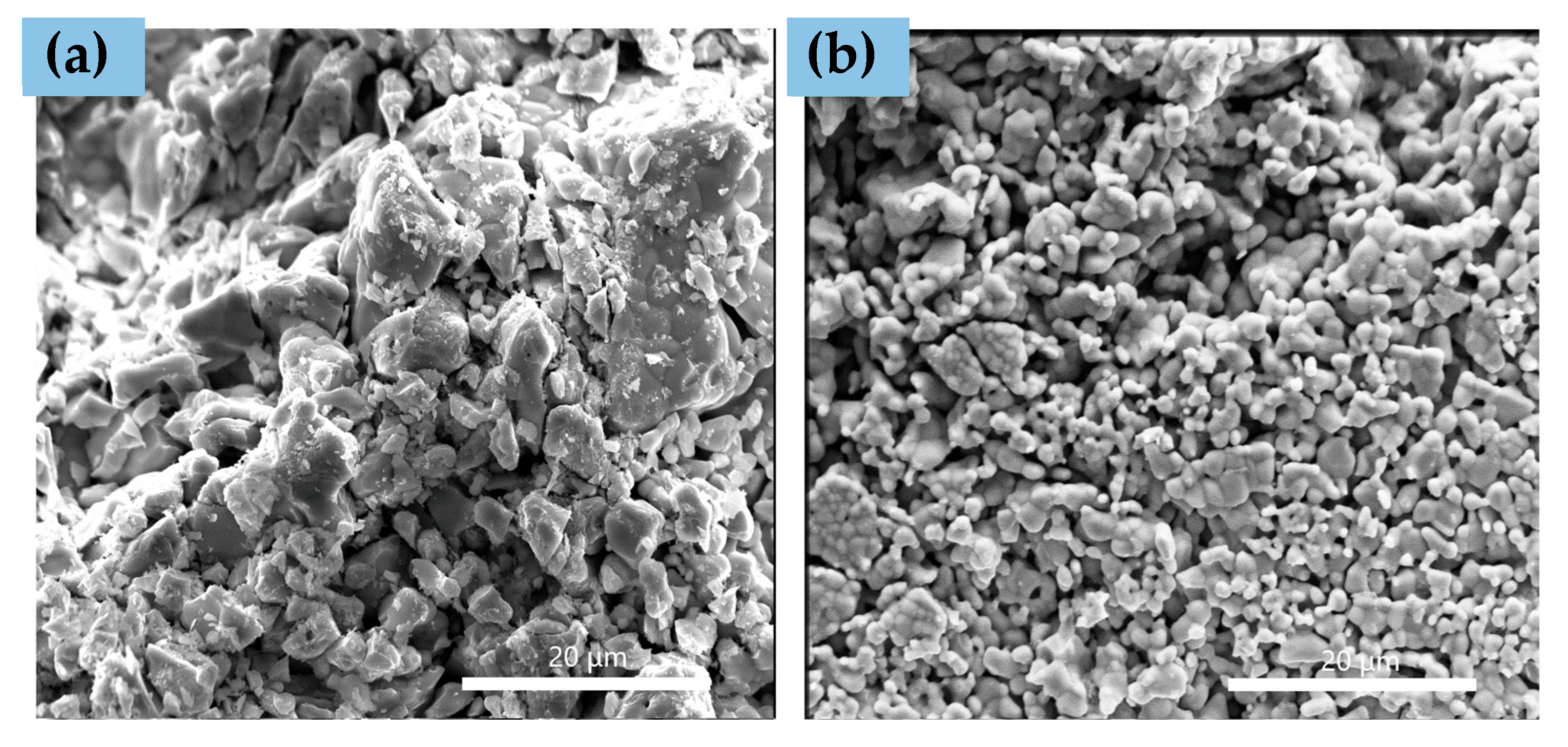
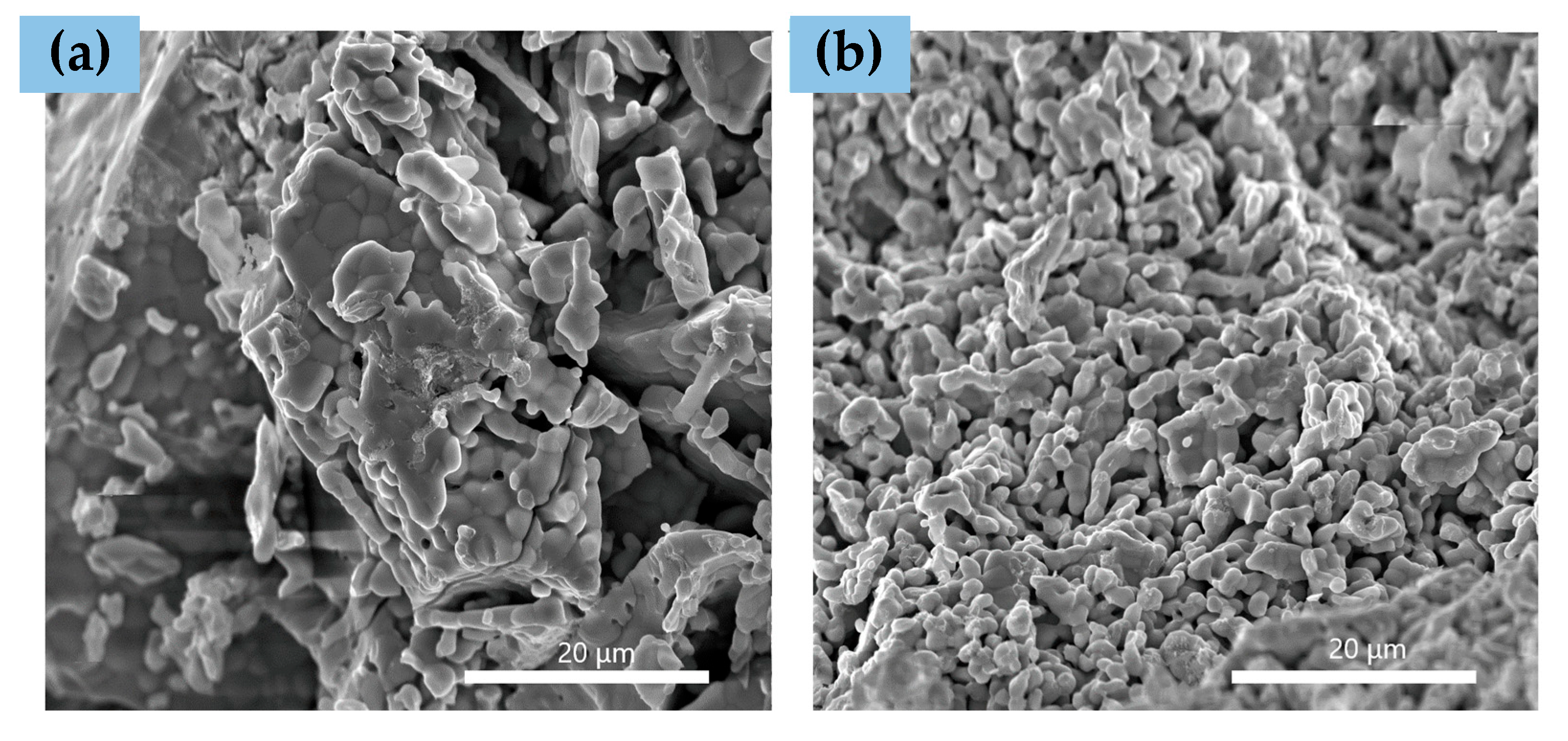
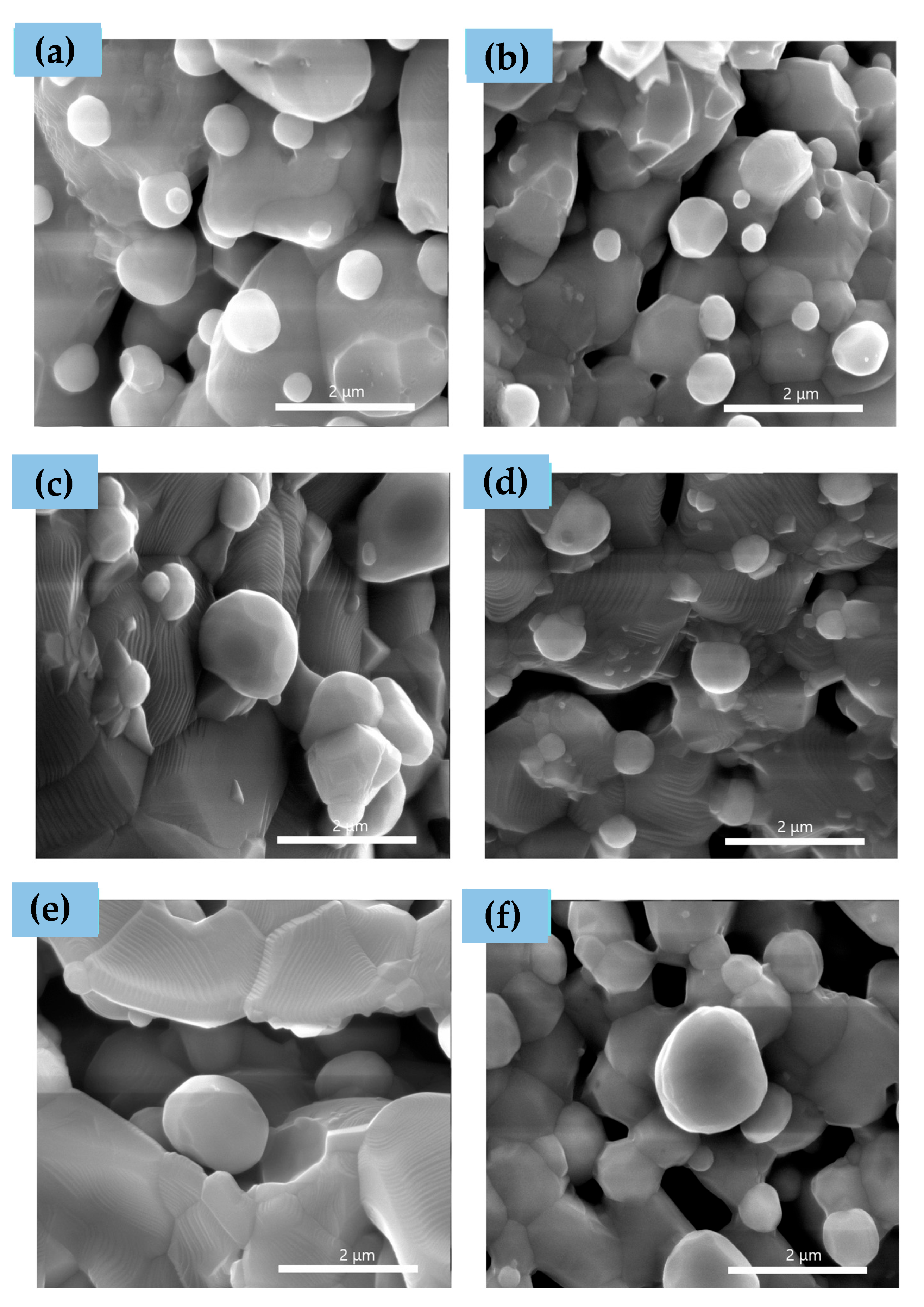

| Element | Ionic Radius [Å] |
|---|---|
| La3+ | 1.36 |
| Ce4+ | 1.14 |
| Ni4+ | 0.48 |
| Co4+ | 0.53 |
| Sr2+ | 1.44 |
| Ti4+ | 0.605 |
| Compound | Sample +5% | Sample +10% | Sample +15% |
|---|---|---|---|
| LSCCoT | 5.37% | 10.24% | 15.04% |
| LSCNiT | 5.60% | 10.56% | 15.08% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kujawska, K.; Koliński, W.; Bochentyn, B. Forming Ni-Fe and Co-Fe Bimetallic Structures on SrTiO3-Based SOFC Anode Candidates. Fuels 2024, 5, 564-573. https://doi.org/10.3390/fuels5030031
Kujawska K, Koliński W, Bochentyn B. Forming Ni-Fe and Co-Fe Bimetallic Structures on SrTiO3-Based SOFC Anode Candidates. Fuels. 2024; 5(3):564-573. https://doi.org/10.3390/fuels5030031
Chicago/Turabian StyleKujawska, Kinga, Wojciech Koliński, and Beata Bochentyn. 2024. "Forming Ni-Fe and Co-Fe Bimetallic Structures on SrTiO3-Based SOFC Anode Candidates" Fuels 5, no. 3: 564-573. https://doi.org/10.3390/fuels5030031
APA StyleKujawska, K., Koliński, W., & Bochentyn, B. (2024). Forming Ni-Fe and Co-Fe Bimetallic Structures on SrTiO3-Based SOFC Anode Candidates. Fuels, 5(3), 564-573. https://doi.org/10.3390/fuels5030031







