Gas Hydrate Plugging Mechanisms during Transient Shut–In/Restart Operation in Fully Dispersed Systems
Abstract
1. Introduction
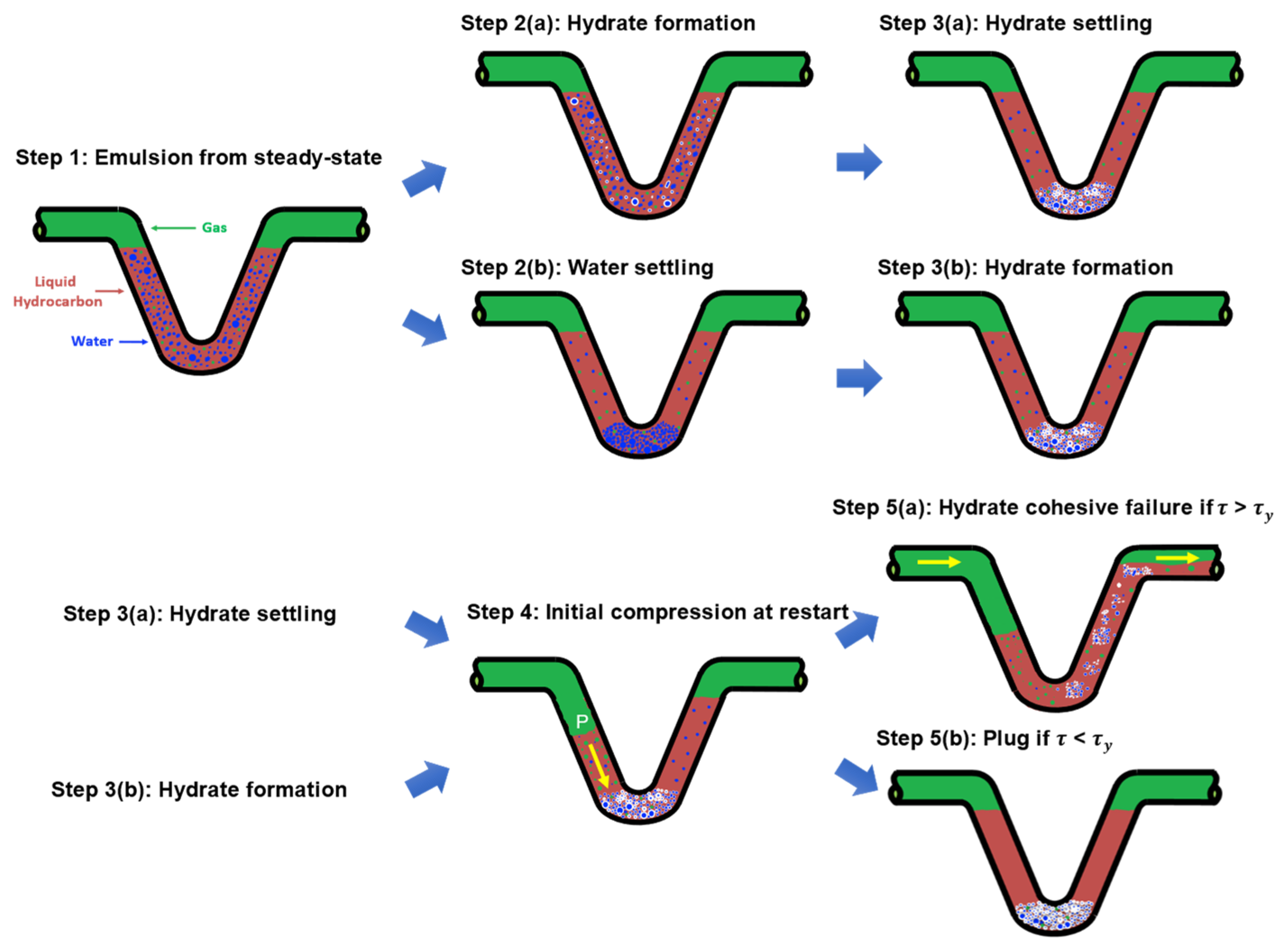
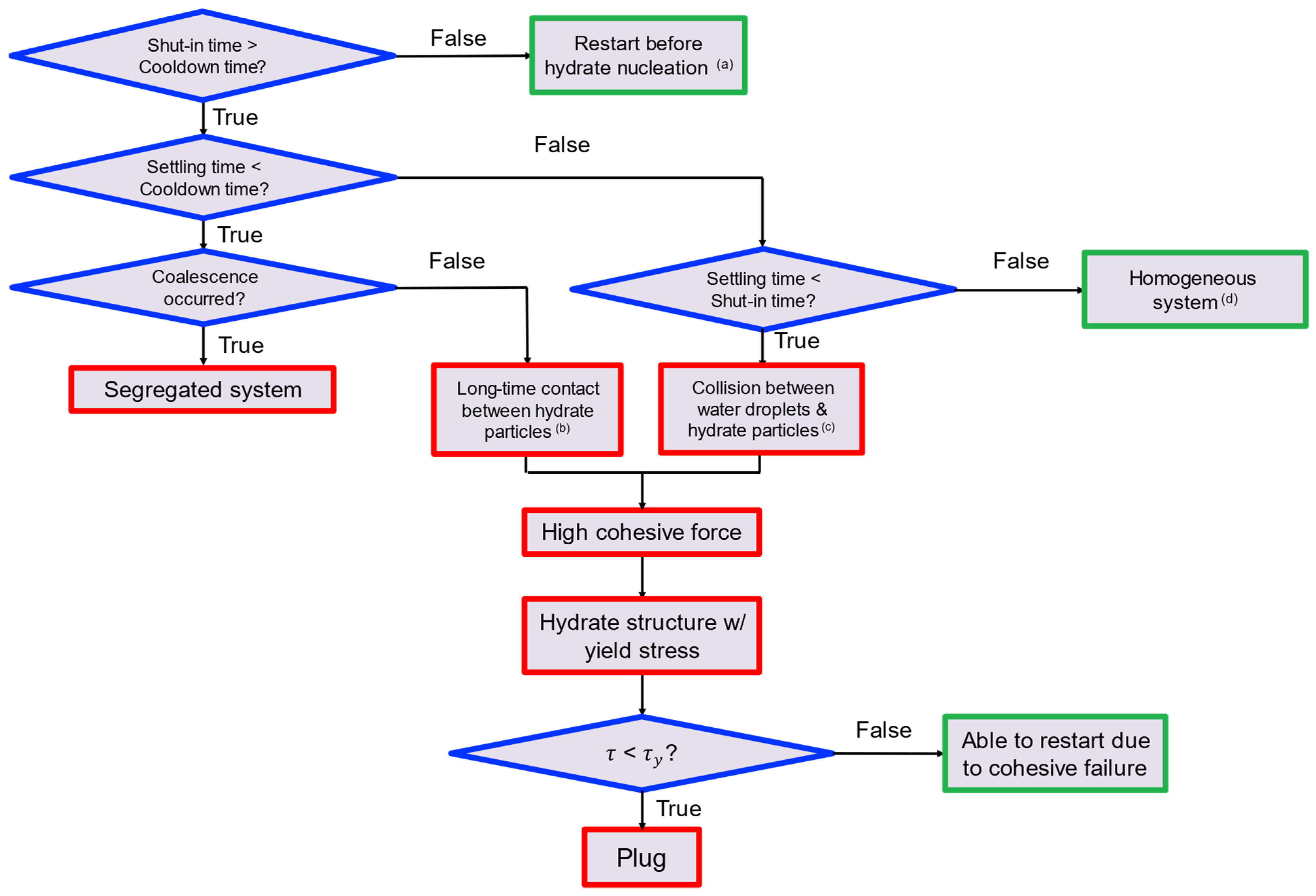
2. Conceptual Pictures for Transient Operations of Dispersed Systems
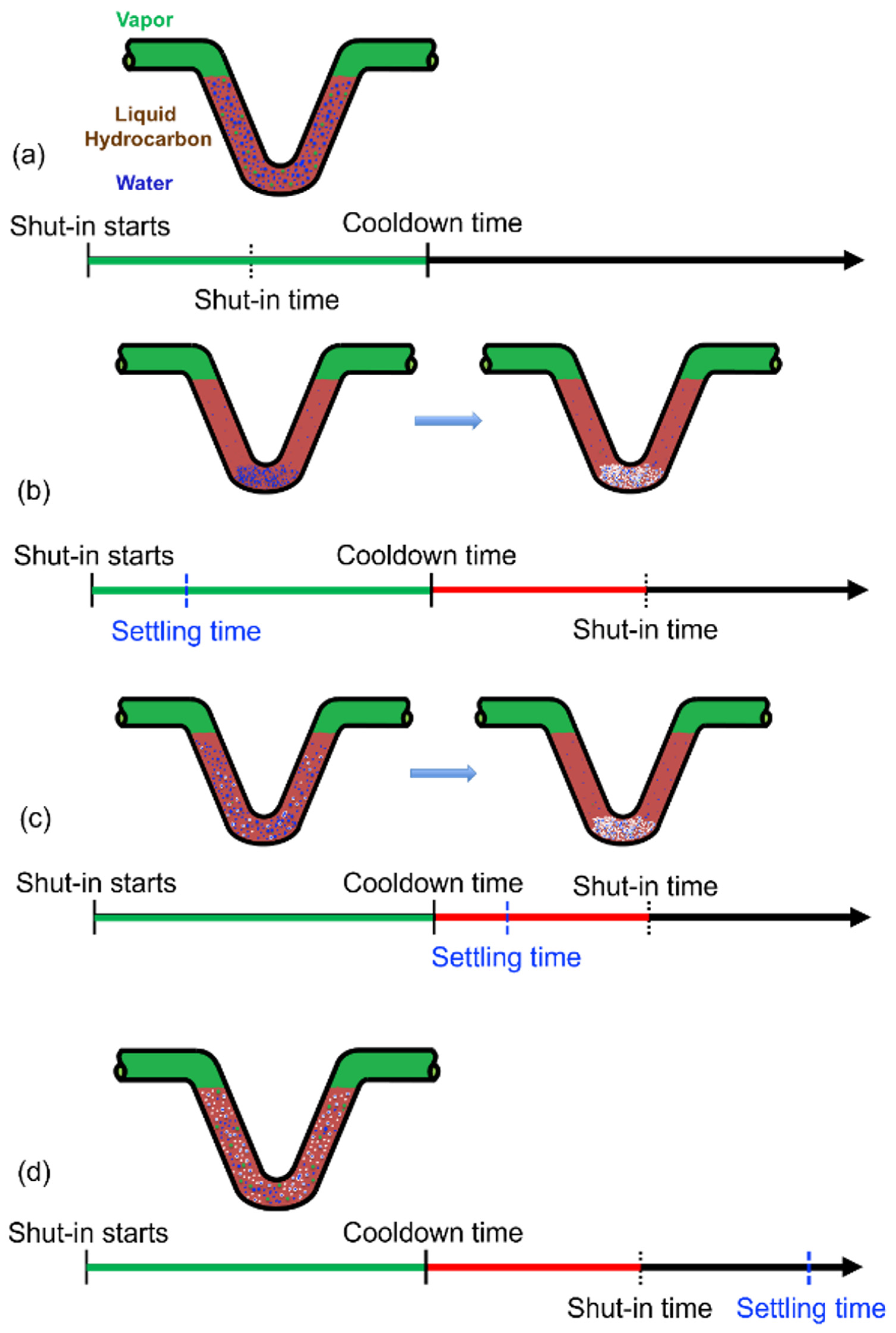
2.1. Step 1: Emulsifying and Water Droplet Settling during Shut–In
2.2. Steps 2 and 3: Hydrate Particle Formation
2.2.1. Steps 2(a) and 3(a): Water–Hydrate Interaction Dominates
2.2.2. Steps 2(b) and 3(b): Hydrate–Hydrate Interaction Dominates
2.3. Step 4: Hydrate Compression before Flowing
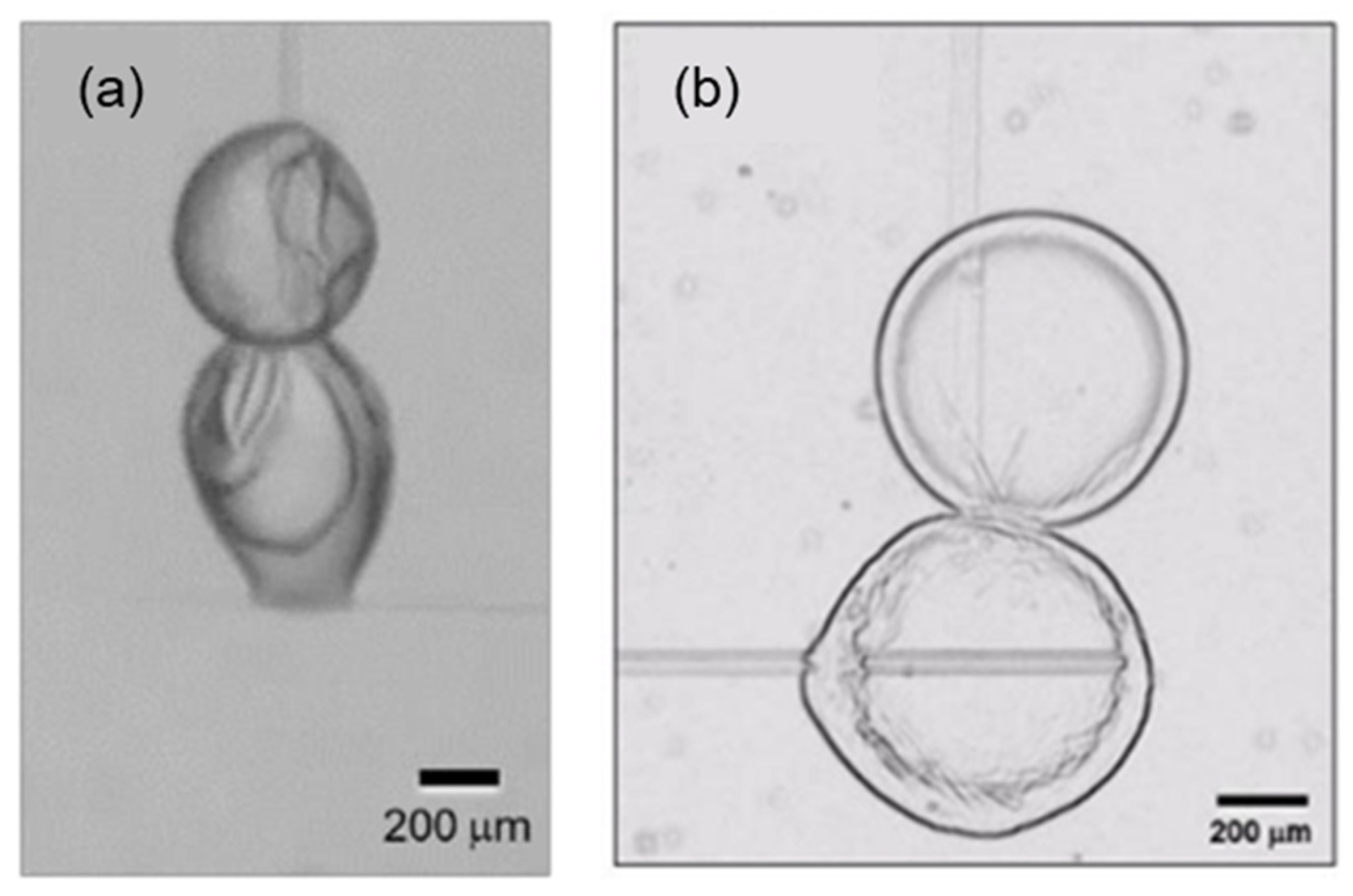
2.4. Step 5: Yield Stress of Hydrate Structures upon Restart
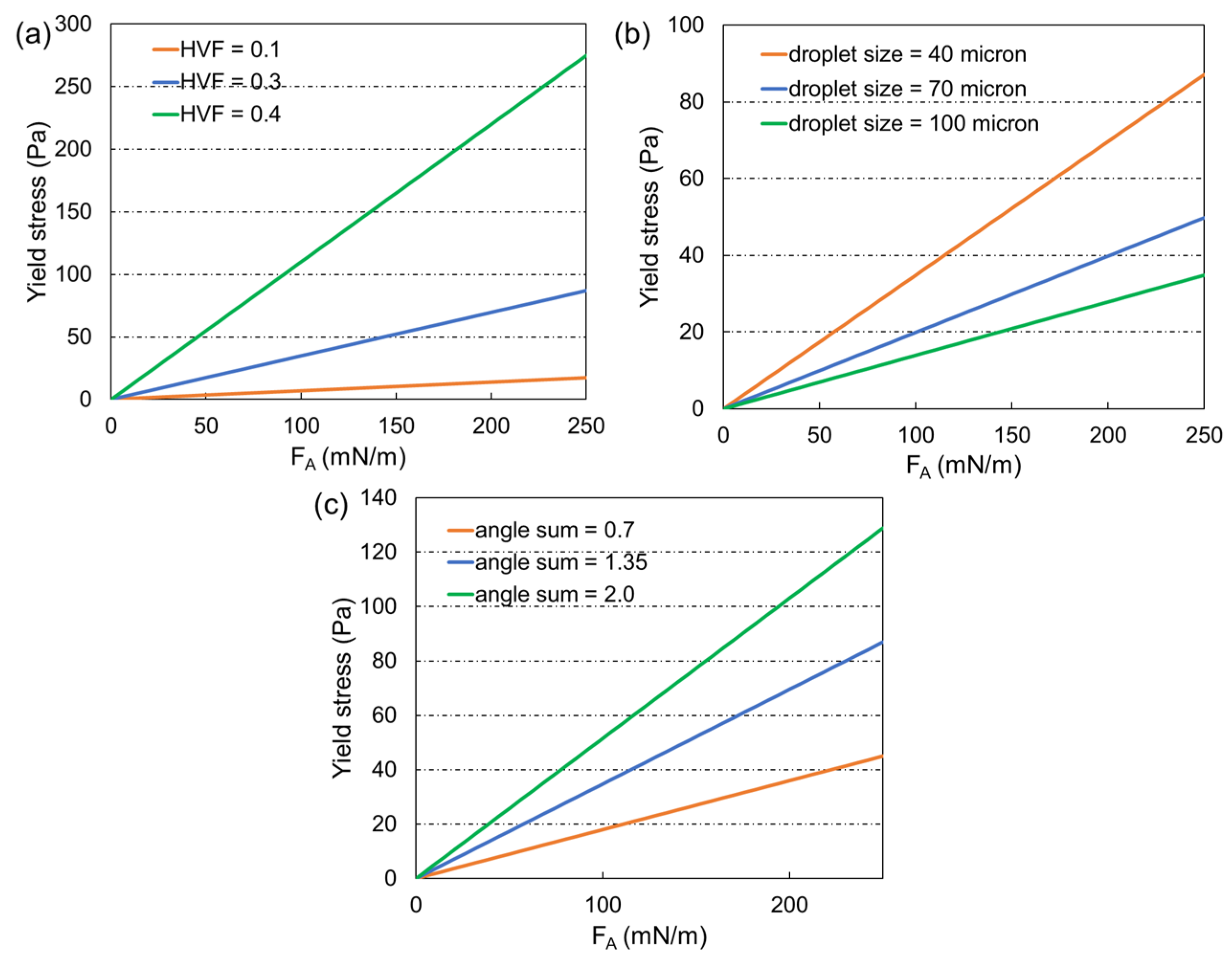
3. Yield Stress Model
4. Model Structure
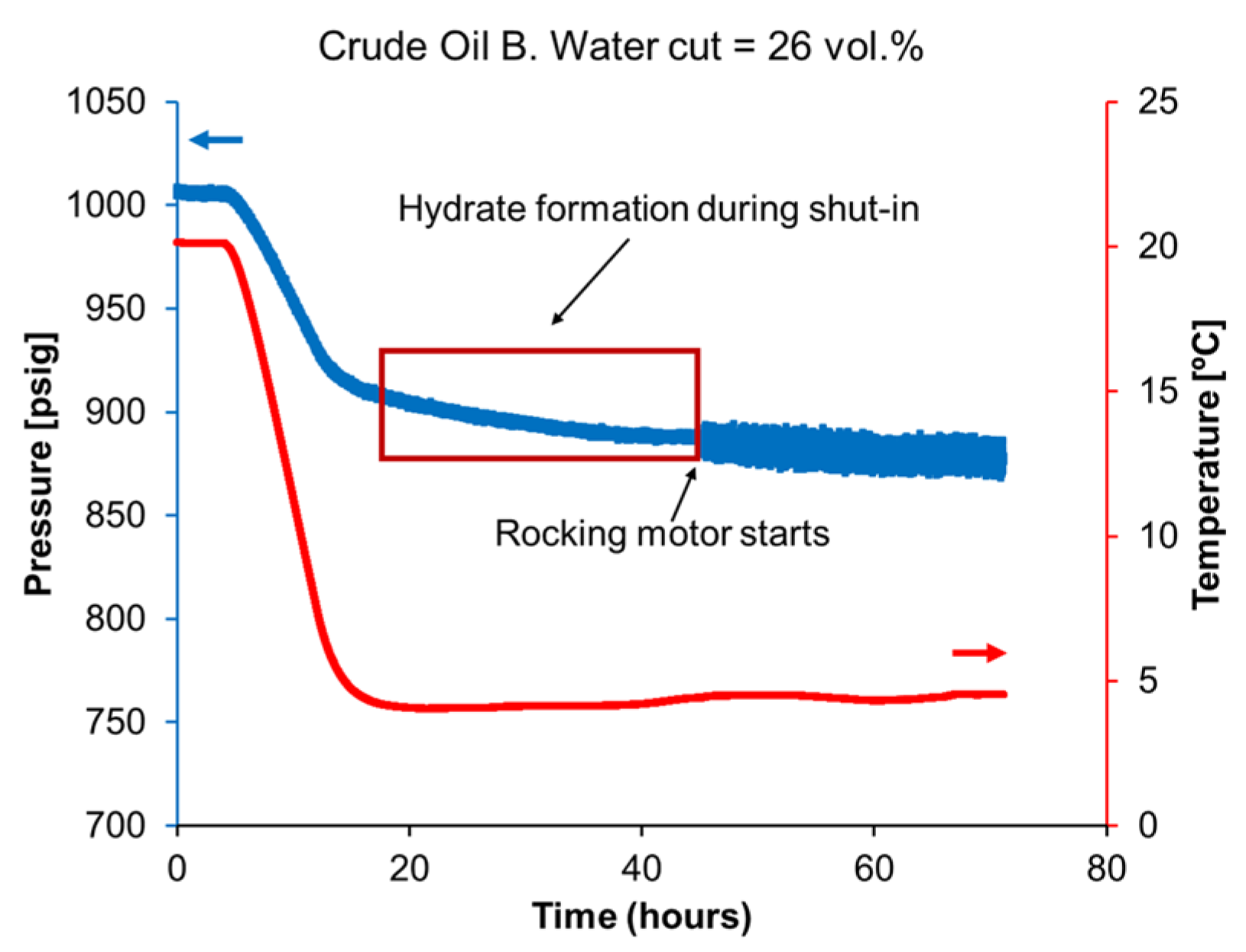
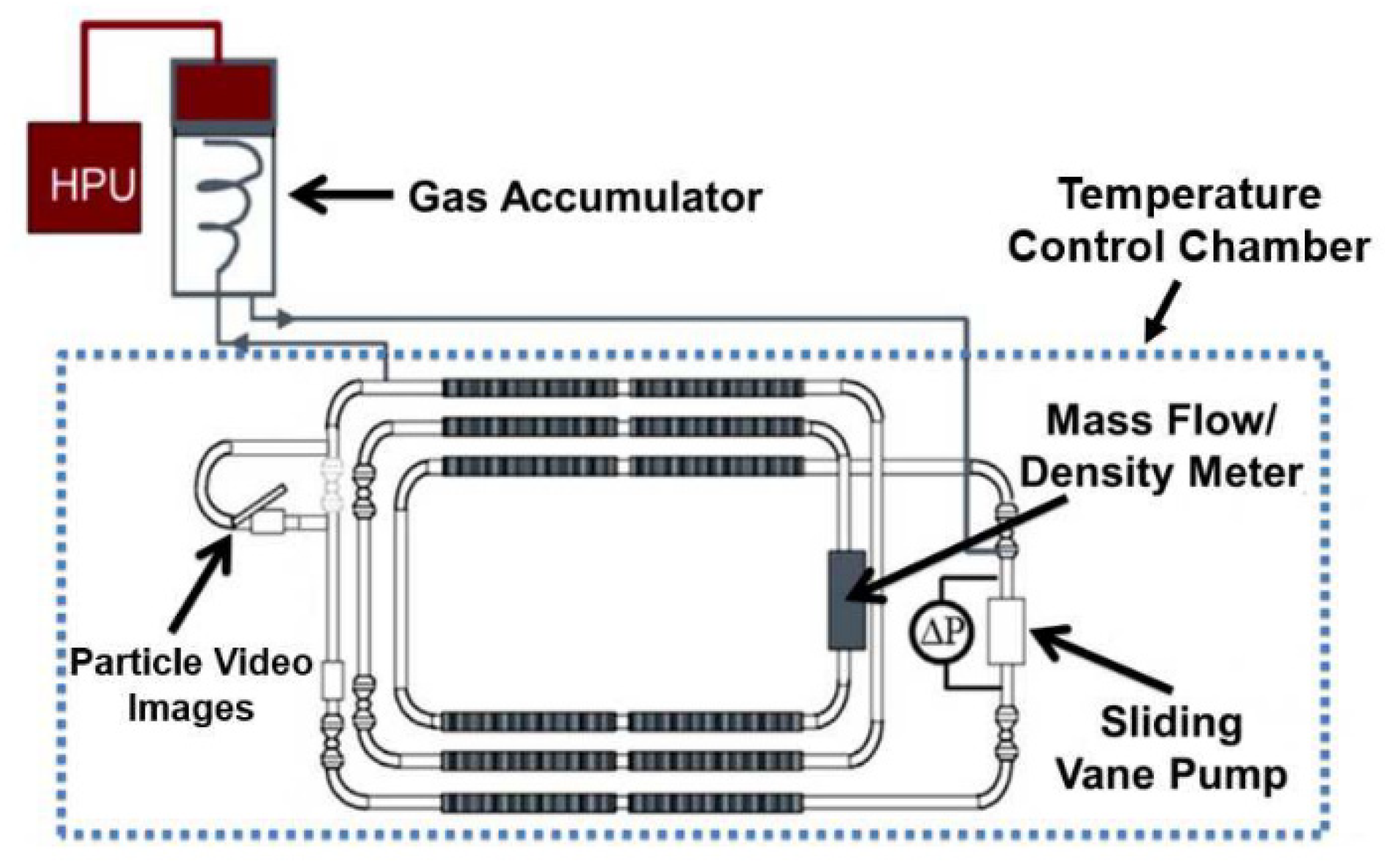
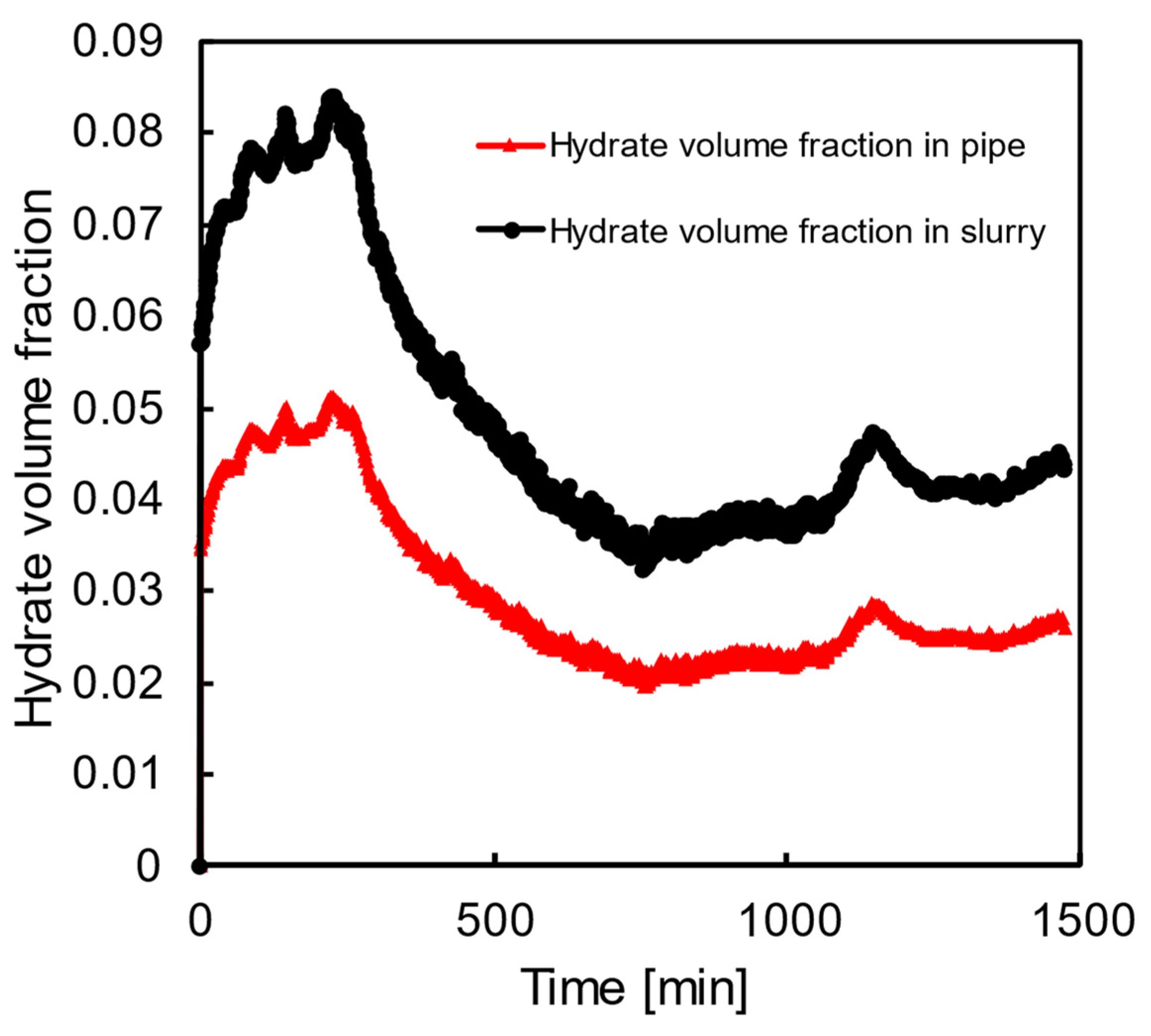
5. Model Validation with Large–Scale Flowloop Experiments
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| References | Methods | Yield Stress Measurement | Fluid | Hydrate Former | Hydrate Structure | Yield Stress Investigation |
|---|---|---|---|---|---|---|
| Webb (2012) [46] | Couette rheometer | Yield stress defined as the shear stress at which the shear rate increases rapidly. | Water and West African crude oil | Methane | Structure Ⅰ | Effect of WVF at 2 h annealing time: no yield stress measured below 30% WVF, small yield stress from 3.7 to 6.8 Pa at 30% to 45% WVF, high yield stress greater than 3000 Pa at 50% WVF. Effect of annealing time at 40% WVF: yield stress increased from 7 to 40.75 Pa from 2 to 48 h (increases with an annealing time up to 8 h and remains relatively unchanged. Effect of 3.5 wt% NaCl at 50% WVF: brine slurry has much lower yield stress 37 Pa compared with slurry formed using DI water. |
| Webb (2013) [47] | Couette rheometer | Yield stress defined as the shear stress at which the shear rate increases rapidly. | Water, dodecane, and aerosol dioctyl sodium sulfosuccinate (AOT) surfactant | Methane | Structure Ⅰ | Effect of WVF at 0 °C, 1500 psig P0 and 100 s−1 shear rate: yield stress of 5% WVF: 1 Pa; 10% WVF: 2 Pa; 25% WVF: 5 Pa; 30% WVF: 20 Pa. Effect of temperature at 30% WVF, 1500 psig P0 and 100 s−1 shear rate: yield stress of 2 °C: 4 Pa. Effect of WVF: yield stress at 30% WVF: 3 Pa; 20% WVF: 1 Pa. |
| Zylyftari, (2013) [49] | Strain–controlled Couette rheometer | Yield stress defined as the value of the stress plateau at a low shear rate. | Water, oil mixture of light mineral oil and Halocarbon 27, Span 80 surfactant and cyclopentane, and dissolved NaCl | Cyclopentane | Structure Ⅱ | Effect of salt concentration: yield stress of initial salt concentration 0.0 wt.%: 38 Pa; 3.4 wt.%: 145 Pa; 5.0 wt.%: 104 Pa; 7.5 wt.%: 90 Pa; 10.0 wt.%: 90 Pa; 12.5 wt.%: 0.3 Pa; 15.0 wt.%: 0.2 Pa. |
| Webb (2014) [50] | Couette rheometer | Yield stress defined as the shear stress at which the shear rate increases rapidly. | Water, mineral oil 70T, Span 80 surfactant, and dioctyl sodium sulfosuccinate (AOT) surfactant | Methane | Structure Ⅰ | Effect of WVF at 0 °C and 1500 psig P0: yield stress of 10% WVF: 3 Pa; 20% WVF: 11 Pa; 30% WVF: 18 Pa; 40% WVF: 21 Pa. Effect of temperature at 30% WVF and 1500 psig P0: yield stress of 2 °C: 43 Pa; 4 °C: 110 Pa; 6 °C: 30 Pa. Effect of P0 at 30% WVF and 0 °C: yield stress at 750 psig P0: 380 Pa; 1000 psig P0: 75 Pa; 1250 psig P0: 65 Pa. |
| Zylyftari, (2015) [51] | Four–bladed vane rheometer | Yield stress of the final structure measured with oscillatory stress ramp method without aging. Yield stress defined as the maximum value in elastic stress. | Water, oil mixture of light mineral oil and Halocarbon 27, Span 80 surfactant and cyclopentane, and dissolved NaCl | Cyclopentane | Structure Ⅱ | Effect of salt concentration: average yield stress of initial salt concentration 0.0 wt.%: 1250 Pa; 3.4 wt.%: 1960 Pa; 5.0 wt.%: 1620 Pa; 7.5 wt.%: 1700 Pa; 10.0 wt.%: 24 Pa; 12.5 wt.%: 0.14 Pa; 15.0 wt.%: 0.17 Pa. |
| Ahuja (2015) [52] | Stress–controlled Couette and four–bladed vane rheometer | Yield stress measured with 1. Oscillatory stress ramp method: Yield stress taken at shape decrease in storage and loss moduli over amplitude of oscillatory stress 2. Elastic stress maxima method: Yield stress defined as maximum elastic stress | Water, oil mixture of light mineral oil and Halocarbon 27, Span 80 surfactant, and cyclopentane | Cyclopentane | Structure Ⅱ | Effect of WVF: increasing yield stress with increasing water volume fraction above 15% WVF. Effect of shut–in time: increasing yield stress with increasing shut–in time for all water fractions. Effect of different rheological methods for yield stress measurement: in good agreement. |
| Alejandro (2019) [53] | Four–bladed vane rheometer | Yield stress measured with ramping shear stress from 0.01 to 2500 Pa. Yield stress defined as the shear stress at which the shear rate increases rapidly. | Water, oil mixture of mineral oil 70T, NaCl, and hydrate dispersants (HD) A–E | Methane | Structure Ⅰ | Effect of shut–in time and HD dosage: at 0.25 vol.% HD_A: yield stress of 0 h shut–in time: 55 Pa; 4 h: 2280 Pa; 8 h: 495 Pa; at 0.5 vol.% HD_A: 0 h: 45 Pa; 4 h: 60 Pa; 8 h: 75 Pa; at 1 vol.% HD_A: 0 h: 7 Pa; 4 h: 10 Pa; 8 h: 11 Pa; at 2 vol.% HD_A: 0 h: 9 Pa; 4 h:8 Pa; 8 h: 12 Pa. Effect of different HD type: at 2 vol.% HD_C: yield stress of 0 h shut–in time: 18 Pa; at 1 vol.% HD_D: 8 Pa; at 2 vol.% HD_E: 27 Pa; at 5 vol.% HD_E: 15 Pa. Effect of WVF: yield stress of 2 vol.% HD_A and 80% WC: at 0 h shut–in time: 27 Pa; 4 h: 22 Pa; 8 h: 26 Pa. |
| Qin (2020) [54] | Four–bladed vane rheometer | Yield stress measured with ramping shear stress from 0.1 to 300 Pa over 1080 s. Yield stress defined as the shear stress at which the shear rate increases rapidly. | Water, crude oil, and industrial AA | Methane | Structure Ⅰ | Effect of WVF at 5 °C and 1500 psig P0 without AA: yield stress of 5% WVF: 2.4 Pa; 10% WVF: 11 Pa; 20% WVF: 19 Pa; 30% WVF: 24.5 Pa. Effect of AA at 5 °C and 1500 psig P0 with AA: yield stress of 10% WVF: 3.7 Pa; 20% WVF: 4.0 Pa; 30% WVF: 4.5 Pa. |
| Liu (2020) [55] | Couette rheometer | Yield stress measured with ramping shear stress from 1 to 1000 Pa. Yield stress defined as the shear stress at which the shear rate increases rapidly. | Water, n–decane, and Span 80 and Tween 80 as surfactants (50 vol.% water cut) | Methane | Structure Ⅰ | Effect of annealing time: for 5 wt.% surfactant: yield stress of 5 min shut–in: 37 Pa; 10 min: 38 Pa; 20 min: 46 Pa; 40 min: 52 Pa; 80 min: 63 Pa; 160 min: 82 Pa; 320 min: 116 Pa; 640 min: 150 Pa; 1280 min: 217 Pa. For 10 wt.% surfactant: yield stress of 5 min shut–in: 25 Pa; 10 min: 29 Pa; 20 min: 33 Pa; 40 min: 40 Pa; 80 min: 45 Pa; 160 min: 59 Pa; 320 min: 76 Pa; 640 min: 88 Pa; 1280 min: 184 Pa. Effect of surfactant concentration: for 5 min shut–in: yield stress of no surfactant: exceed 1000 Pa; 3 wt.% surfactant: 615 Pa; 5 wt.% surfactant: 37 Pa; 10 wt.% surfactant: 25 Pa. |
| Sakurai (2021) [56] | Flowloop | Measure critical stress required for flow restart on a straight, horizontal 1.35 m pipe run with the dP transducer affixed to a 0.85 m length of pipe. | 100% water | Methane | Structure Ⅰ | Effect of HVF: yield stress of 0–20 vol.% HVF: 0–15 Pa. Effect of restart operation (slow linear or quick exponential): slow linear: hydrate blockage; quick exponential: no hydrate blockage. Effect of shut–in time (3 min or 8 h): 3 min shut–in: 0–10 Pa; 8 h shut–in: 0–14 Pa. |
| Liu (2022) [48] | Couette rheometer | Yield stress measured with ramping shear stress from 1 to 1000 Pa. Yield stress defined as the shear stress at which the shear rate increases rapidly. | Water, n–decane, Span 80 and Tween 80 as surfactants (50 vol.% water cut), and dissolved NaCl | Methane | Structure Ⅰ | Effect of annealing time and salinity: for 1 wt.% salinity and 53.2% water conversion fraction: yield stress of 5 min shut–in: 25 Pa; 10 min: 33 Pa; 20 min: 47 Pa; 40 min: 56 Pa; 80 min: 57 Pa; 160 min: 80 Pa; 320 min: 230 Pa; 640 min: 258 Pa; 1280 min: 270 Pa. For 5 wt.% salinity and 43.9% water conversion fraction: yield stress of 5 min shut–in: 12 Pa; 10 min: 15 Pa; 20 min: 19 Pa; 40 min: 20 Pa; 80 min: 21 Pa; 160 min: 16 Pa; 320 min: 19 Pa; 640 min: 27 Pa; 1280 min: 37 Pa. |
References
- Sloan, E.D., Jr.; Koh, C.A. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Davy, H. VIII. On a Combination of Oxymuriatic Gas and Oxygene Gas. R. Soc. 1811, 101, 155–162. [Google Scholar]
- Faraday, M. XIV. On Fluid Chlorine. R. Soc. 1823, 14, 160–165. [Google Scholar]
- Hammerschmidt, E.G. Formation of Gas Hydrates in Natural Gas Transmission Lines. Ind. Eng. Chem. 1934, 26, 851–855. [Google Scholar] [CrossRef]
- Sangwai, J.; Dandekar, A. Practical Aspects of Flow Assuracne in the Petroleum Industry; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2023. [Google Scholar]
- Chakrabarti, S.K. Handbook of Offshore Engineering; Elsevier: Amsterdam, The Netherlands, 2005; pp. 15–200. [Google Scholar] [CrossRef]
- Mokwenye, P.O. Evaluation of Gas Hydrate in Gas Pipeline Transportation. Master’s Thesis, Universit of North Dakota, Grand Forks, ND, USA, 2020. [Google Scholar]
- Bimuratkyzy, K.; Sagindykov, B. The Review of Flow Assurance Solutions with Respect to Wax and Asphaltene. Braz. J. Pet. Gas 2016, 10, 119–134. [Google Scholar] [CrossRef]
- Mullins, O.C. The Asphaltenes. Annu. Rev. Anal. Chem. 2011, 4, 393–418. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.M.; Mariow, D.; Johnson, T.L.; Johnston, C. The Evaluation of Enhanced (Carbonate/Sulfate) Scale-Dissolver Treatments for near-Wellbore Stimulation in Subsea Production Wells, Gulf of Mexico. In Proceedings of the SPE International Oilfield Scale Symposium, Aberdeen, UK, 31 May–1 June 2006; pp. 24–40. [Google Scholar] [CrossRef]
- Perumal, K.E. Corrosion Risk Analysis, Risk Based Inspection and a Case Study Concerning a Condensate Pipeline. Procedia Eng. 2014, 86, 597–605. [Google Scholar] [CrossRef]
- Kokal, S. Crude-Oil Emulsions: A State-of-the-Art Review. SPE Prod. Facil. 2005, 20, 5–12. [Google Scholar] [CrossRef]
- Gharaibah, E.; Zhang, Y. Flow Assurance Aspects and Optimization of Subsea Choke Valve Sand Management and Erosion. In Proceedings of the OTC Brasil, Rio de Janeiro, Brazil, 27–29 October 2015; pp. 1656–1665. [Google Scholar] [CrossRef]
- Vatamanu, J.; Kusalik, P.G. Molecular Insights into the Heterogeneous Crystal Growth of SImethane Hydrate. J. Phys. Chem. B 2006, 110, 15896–15904. [Google Scholar] [CrossRef]
- Cochran, S. Hydrate Control and Remediation Best Practices in Deepwater Oil Developments. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 5–8 May 2003; pp. 1696–1709. [Google Scholar] [CrossRef]
- Cardoso, C.A.B.R.; Gonçalves, M.A.L.; Camargo, R.M.T. Design Options for Avoiding Hydrates in Deep Offshore Production. J. Chem. Eng. Data 2015, 60, 330–335. [Google Scholar] [CrossRef]
- Volk, M.; Delle-Case, E.; Estanga, D. Risk-Based Restarts of Untreated Subsea Oil and Gas Flowlines in the GoM; The University of Tulsa: Tulsa, OK, USA, 2007. [Google Scholar]
- Turner, D.J. Clathrate Hydrate Formation in Water-in-Oil Dispersions. Ph.D. Thesis, Colorado School of Mines, Golden, CO, USA, 2005. [Google Scholar]
- Pickarts, M.A.; Ravichandran, S.; Ismail, N.A.; Stoner, H.M.; Delgado-Linares, J.; Sloan, E.D.; Koh, C.A. Perspective on the Oil-Dominated Gas Hydrate Plugging Conceptual Picture as Applied to Transient Shut-In/Restart. Fuel 2022, 324, 124606. [Google Scholar] [CrossRef]
- Zerpa, L.E.; Salager, J.L.; Koh, C.A.; Sloan, E.D.; Sum, A.K. Surface Chemistry and Gas Hydrates in Flow Assurance. Ind. Eng. Chem. Res. 2011, 50, 188–197. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Mironova, M.V.; Ilyin, S.O. Flow of Heavy Crude Oil-in-Water Emulsions in Long Capillaries Simulating Pipelines. J. Pet. Sci. Eng. 2017, 157, 117–123. [Google Scholar] [CrossRef]
- Wang, Y. The Development and Application of Hydrate Formation, Transportation and Bedding Models in Liquid-Dominated Systems. Ph.D. Thesis, Colorado School of Mines, Golden, CO, USA, 2019. [Google Scholar]
- Bhatnagar, G.; Gao, S. Gas Hydrate Management. In Flow Assurance; Gulf Professional Publishing: Cambridge, MA, USA, 2022; ISBN 9780128220108. [Google Scholar]
- Qian, Y.; Xu, J.; Yuan, H.; Peng, C.; Zhou, F. Study on the Hydrocyclonic Separation of Natural Gas Hydrate Slurries Combined with the Solution of Particles’ Settling Equation. Ind. Eng. Chem. Res. 2022, 61, 14637–14648. [Google Scholar] [CrossRef]
- Li, Q.; Shirazi, S.A.; McLaury, B.S.; Kouba, G.; Song, S. Measurements and Modeling of Particle Sedimentation Rate and Settling Velocity in a Vertical Pipe. In Proceedings of the ASME 2005 Fluids Engineering Division Summer Meeting, Houston, TX, USA, 19–23 June 2005. [Google Scholar]
- Navaneetha Kannan, S.; Daraboina, N.; Venkatesan, R.; Sarica, C. Settling and Re-Entrainment of Wax Particles in near-Gelling Systems. AIChE J. 2018, 64, 765–772. [Google Scholar] [CrossRef]
- Golchha, A.; Sarica, C.; Venkatesan, R. Settling of Wax Particles in Near-Gelling Systems under Quiescent Conditions. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 4–7 May 2015; pp. 83–99. [Google Scholar] [CrossRef]
- Petrosky, G.E.; Farshad, F.F. Viscosity Correlations for Gulf of Mexico Crude Oils. In Proceedings of the SPE Production Operations Symposium, Oklahoma City, OK, USA, 2–4 April 1995; pp. 249–258. [Google Scholar] [CrossRef]
- Qin, Y.; Aman, Z.M.; Pickering, P.F.; Johns, M.L.; May, E.F. High Pressure Rheological Measurements of Gas Hydrate-in-Oil Slurries. J. Nonnewton. Fluid Mech. 2017, 248, 40–49. [Google Scholar] [CrossRef]
- Guo, B.; Lyons, W.C.; Ghalambor, A. Petroleum Production Engineering a Computer Assisted Approach; Gulf Professional Publishing: Woburn, MA, USA, 2017; ISBN 9780128093740. [Google Scholar]
- Kurup, A.S.; Hernandez, O.; Idstein, T.; Zamora, C.A.; Greenly, L.; Anderson, J. Pushing Conventional Boundaries of Hydrate Management in a Dry Tree Facility. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 1–4 May 2017; pp. 1415–1427. [Google Scholar] [CrossRef]
- Salmin, D.C.; Delgado-Linares, J.G.; Wu, D.T.; Zerpa, L.E.; Koh, C.A. Hydrate Agglomeration in Crude Oil Systems in Which the Asphaltene Aggregation State Is Artificially Modified. SPE J. 2021, 26, 1189–1199. [Google Scholar] [CrossRef]
- Dykhno, L.A.; Jayawardena, S.S.; Schoppa, W. Blowdown Feasibility for Downhill Flowlines. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 5–8 May 2003; pp. 1710–1717. [Google Scholar] [CrossRef]
- Høiland, S.; Askvik, K.M.; Fotland, P.; Alagic, E.; Barth, T.; Fadnes, F. Wettability of Freon Hydrates in Crude Oil/Brine Emulsions. J. Colloid Interface Sci. 2005, 287, 217–225. [Google Scholar] [CrossRef]
- Fidel-Dufour, A.; Gruy, F.; Herri, J.M. Rheology of Methane Hydrate Slurries during Their Crystallization in a Water in Dodecane Emulsion under Flowing. Chem. Eng. Sci. 2006, 61, 505–515. [Google Scholar] [CrossRef]
- Colombel, E.; Gateau, P.; Barré, L.; Gruy, F.; Palermo, T. Discussion Sur Les Mécanismes d’agglomération Entre Particules d’hydrate Dans Les Émulsions Eau Dans Huile. Oil Gas Sci. Technol. 2009, 64, 629–636. [Google Scholar] [CrossRef]
- Ismail, N.A.; Delgado-Linares, J.G.; Koh, C.A. High Pressure Micromechanical Force Method to Assess the Non-Plugging Potential of Crude Oils and the Detection of Asphaltene-Hydrate Mixed Agglomerates. Fuel 2023, 335, 126871. [Google Scholar] [CrossRef]
- Song, J.H.; Couzis, A.; Lee, J.W. Direct Measurements of Contact Force between Clathrate Hydrates and Water. Langmuir 2010, 26, 9187–9190. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Couzis, A.; Lee, J.W. Investigation of Macroscopic Interfacial Dynamics between Clathrate Hydrates and Surfactant Solutions. Langmuir 2010, 26, 18119–18124. [Google Scholar] [CrossRef] [PubMed]
- Kokal, S.L.; Sayegh, S.G. Asphaltenes: The Cholesterol of Petroleum. Proc. Middle East Oil Show 1995, 1, 169–181. [Google Scholar] [CrossRef]
- Schramm, L.L. Emulsions, Foams, and Suspensions; Wiley-VCH: Weinheim, Germany, 2005; ISBN 9783527307432. [Google Scholar]
- Bui, T.; Phan, A.; Monteiro, D.; Lan, Q.; Ceglio, M.; Acosta, E.; Krishnamurthy, P.; Striolo, A. Evidence of Structure-Performance Relation for Surfactants Used as Antiagglomerants for Hydrate Management. Langmuir 2017, 33, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.; Zhang, J.S.; Couzis, A.; Somasundaran, P.; Lee, J.W. Adsorption of Cationic and Anionic Surfactants on Cyclopentane Hydrates. J. Phys. Chem. C 2010, 114, 13385–13389. [Google Scholar] [CrossRef]
- Phan, A.; Bui, T.; Acosta, E.; Krishnamurthy, P.; Striolo, A. Molecular Mechanisms Responsible for Hydrate Anti-Agglomerant Performance. Phys. Chem. Chem. Phys. 2016, 18, 24859–24871. [Google Scholar] [CrossRef] [PubMed]
- Aman, Z.M. Interfacial Phenomena of Cyclopentane Hydrate. Ph.D. Thesis, Colorado School of Mines, Golden, CO, USA, 2012. [Google Scholar]
- Webb, E.B.; Rensing, P.J.; Koh, C.A.; Sloan, E.D.; Sum, A.K.; Liberatore, M.W. High-Pressure Rheology of Hydrate Slurries Formed from Water-in-Oil Emulsions. Energy Fuels 2012, 26, 3504–3509. [Google Scholar] [CrossRef]
- Webb, E.B.; Koh, C.A.; Liberatore, M.W. Rheological Properties of Methane Hydrate Slurries Formed from AOT + Water + Oil Microemulsions. Langmuir 2013, 29, 10997–11004. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Liu, W.; Li, Y.; Lang, C.; Zhang, M.; Song, Y. Effect of Brine Salinity on the Rheological Properties of Hydrate-in-Oil Slurries. J. Pet. Sci. Eng. 2022, 208, 109756. [Google Scholar] [CrossRef]
- Zylyftari, G.; Lee, J.W.; Morris, J.F. Salt Effects on Thermodynamic and Rheological Properties of Hydrate Forming Emulsions. Chem. Eng. Sci. 2013, 95, 148–160. [Google Scholar] [CrossRef]
- Webb, E.B.; Koh, C.A.; Liberatore, M.W. High Pressure Rheology of Hydrate Slurries Formed from Water-in-Mineral Oil Emulsions. Ind. Eng. Chem. Res. 2014, 53, 6998–7007. [Google Scholar] [CrossRef]
- Zylyftari, G.; Ahuja, A.; Morris, J.F. Modeling Oilfield Emulsions: Comparison of Cyclopentane Hydrate and Ice. Energy Fuels 2015, 29, 6286–6295. [Google Scholar] [CrossRef]
- Ahuja, A.; Zylyftari, G.; Morris, J.F. Yield Stress Measurements of Cyclopentane Hydrate Slurry. J. Nonnewton. Fluid Mech. 2015, 220, 116–125. [Google Scholar] [CrossRef]
- Dapena, J.A. On the Kinetic Arrest of Hydrate Slurries. Ph.D. Thesis, Colorado School of Mines, Golden, CO, USA, 2019. [Google Scholar]
- Qin, Y.; Pickering, P.F.; Johns, M.L.; May, E.F.; Aman, Z.M. Rheological Method to Describe Metastable Hydrate-in-Oil Slurries. Energy Fuels 2020, 34, 7955–7964. [Google Scholar] [CrossRef]
- Liu, Z.; Song, Y.; Liu, W.; Liu, R.; Lang, C.; Li, Y. Rheology of Methane Hydrate Slurries Formed from Water-in-Oil Emulsion with Different Surfactants Concentrations. Fuel 2020, 275, 117961. [Google Scholar] [CrossRef]
- Sakurai, S.; Hoskin, B.; Choi, J.; Norris, B.W.E.; May, E.F.; Johns, M.L.; Aman, Z.M. Behavior of Methane Hydrate-in-Water Slurries from Shut-in to Flow Restart. Energy Fuels 2021, 35, 13086–13097. [Google Scholar] [CrossRef]
- Studart, A.R.; Amstad, E.; Gauckler, L.J. Yielding of Weakly Attractive Nanoparticle Networks. Soft Matter 2011, 7, 6408–6412. [Google Scholar] [CrossRef]
- Hu, S. Interfacial Properties of CH4/C2H6 Gas Hydrate Particles with Chemical Additives. Ph.D. Thesis, Colorado School of Mines, Golden, CO, USA, 2019. [Google Scholar]
- Lee, H.S.; Singh, P.; Thomason, W.H.; Fogler, H.S. Waxy Oil Gel Breaking Mechanisms: Adhesive versus Cohesive Failure. Energy Fuels 2008, 22, 480–487. [Google Scholar] [CrossRef]
- Ekweribe, C.; Civan, F.; Lee, H.S.; Singh, P. Effect of System Pressure on Restart Conditions of Subsea Pipelines. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008; pp. 1754–1775. [Google Scholar] [CrossRef]
- Srivastava, V. Quantitative Risk Modeling of Hydrate Bedding Using Mechanistic, Statistical, and Artifical Neural Network Frameworks. Ph.D. thesis, Colorado School of Mines, Golden, CO, USA, 2018. [Google Scholar]




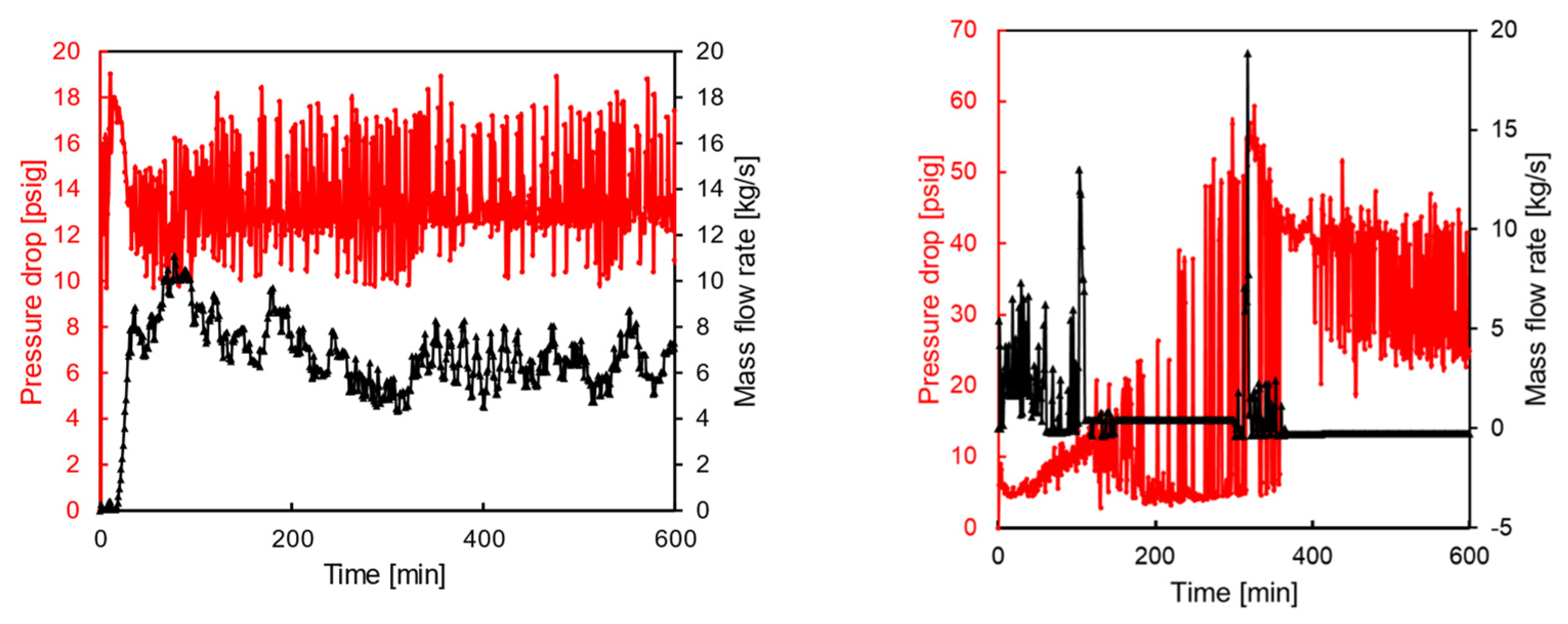
| Experiment No. | Water Cut [vol.%] | Pump Speed [rpm] | Mixture Velocity [m/s] | Plug Observed |
|---|---|---|---|---|
| 1 | 30 | 500 | 1.13 | No |
| 2 | 30 | 750 | 1.74 | No |
| 3 | 30 | 1200 | 2.87 | No |
| 4 | 50 | 350 | 0.73 | Yes |
| 5 | 50 | 750 | 1.74 | Yes |
| 6 | 50 | 1200 | 2.87 | No |
| Experiment No. | Loop Pressure Drop at Restart [psig] | Shear Stress [Pa] | Yield Stress [Pa] | Plug Based on Comparison |
|---|---|---|---|---|
| 1 | 10.1 | 43.3 | 8.4 | No |
| 2 | 11.1 | 45.1 | 16.8 | No |
| 3 | 19 | 58.9 | 6.9 | No |
| 4 | 8.2 | 40.0 | 185.8 | Yes |
| 5 | 11.3 | 45.4 | 140.2 | Yes |
| 6 | 27.1 | 73.0 | 60.0 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, A.; Ismail, N.A.; Delgado-Linares, J.G.; Majid, A.A.A.; Zerpa, L.E.; Koh, C.A. Gas Hydrate Plugging Mechanisms during Transient Shut–In/Restart Operation in Fully Dispersed Systems. Fuels 2024, 5, 297-316. https://doi.org/10.3390/fuels5030017
Qu A, Ismail NA, Delgado-Linares JG, Majid AAA, Zerpa LE, Koh CA. Gas Hydrate Plugging Mechanisms during Transient Shut–In/Restart Operation in Fully Dispersed Systems. Fuels. 2024; 5(3):297-316. https://doi.org/10.3390/fuels5030017
Chicago/Turabian StyleQu, Anqi, Nur Aminatulmimi Ismail, Jose G. Delgado-Linares, Ahmad A. A. Majid, Luis E. Zerpa, and Carolyn A. Koh. 2024. "Gas Hydrate Plugging Mechanisms during Transient Shut–In/Restart Operation in Fully Dispersed Systems" Fuels 5, no. 3: 297-316. https://doi.org/10.3390/fuels5030017
APA StyleQu, A., Ismail, N. A., Delgado-Linares, J. G., Majid, A. A. A., Zerpa, L. E., & Koh, C. A. (2024). Gas Hydrate Plugging Mechanisms during Transient Shut–In/Restart Operation in Fully Dispersed Systems. Fuels, 5(3), 297-316. https://doi.org/10.3390/fuels5030017








