Sub-Supercritical Hydrothermal Liquefaction of Lignocellulose and Protein-Containing Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Feedstocks and Their Analysis
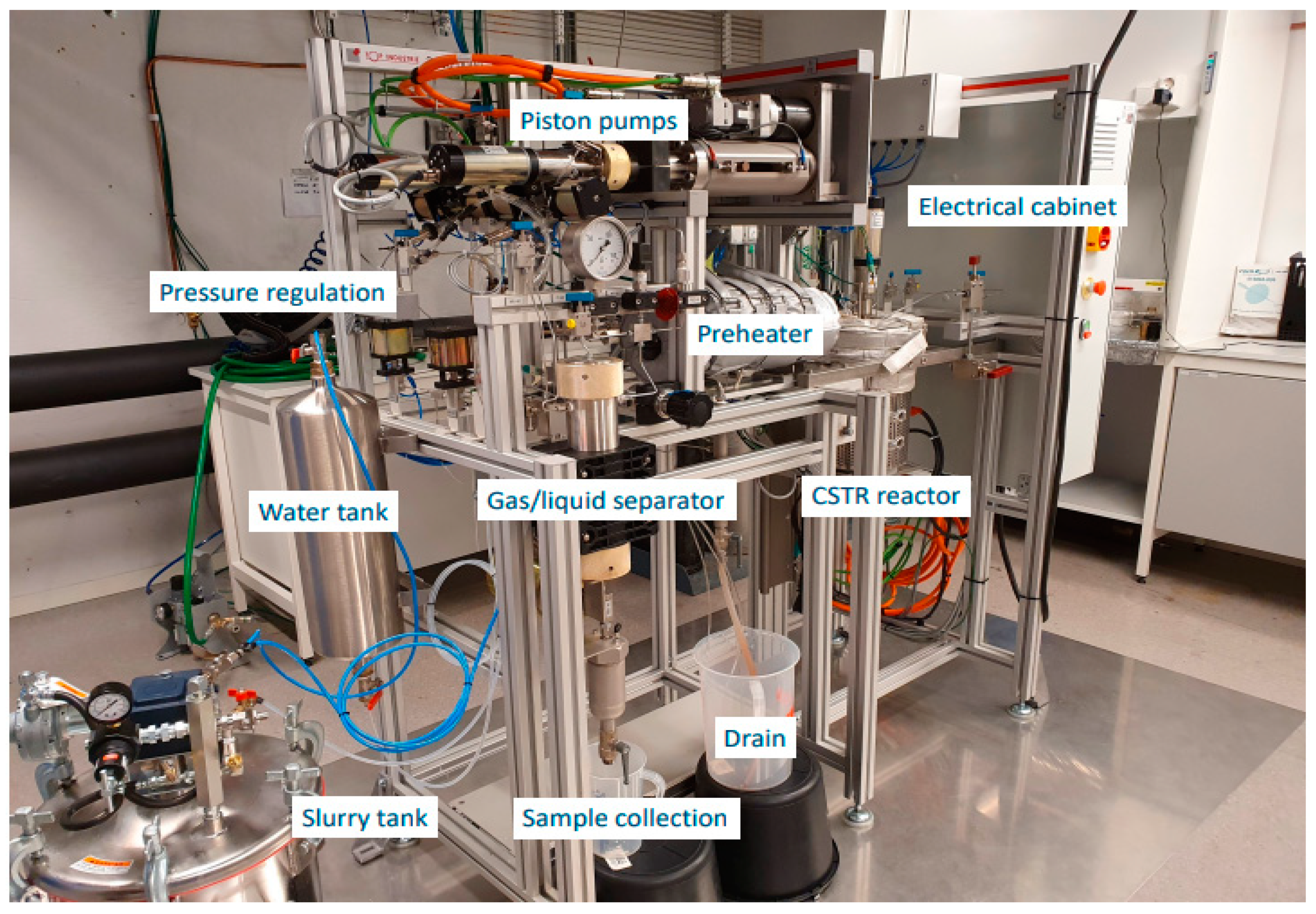
2.2. HTL Experiments
2.3. Analysis of HTL Products
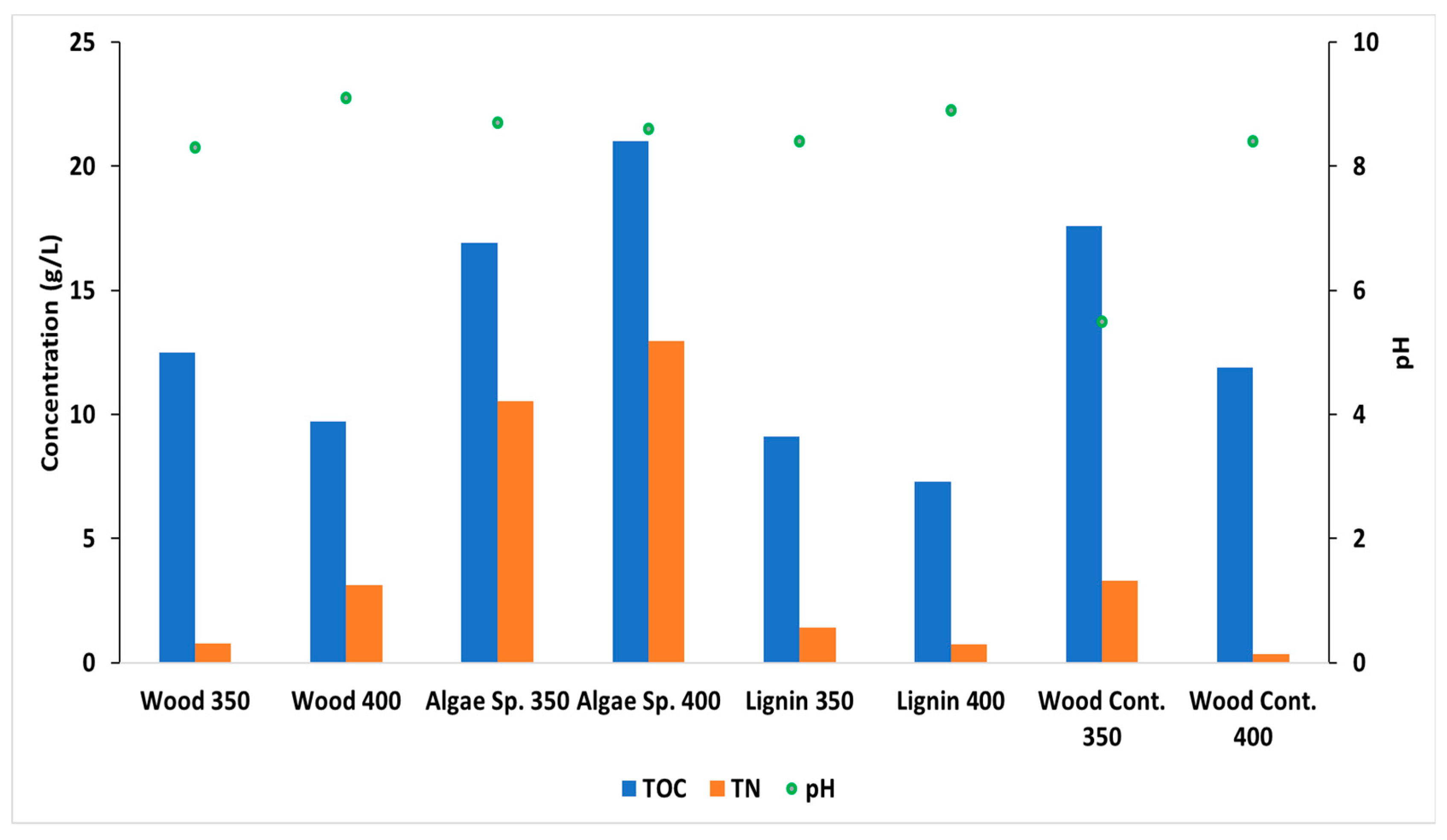
3. Results and Discussion
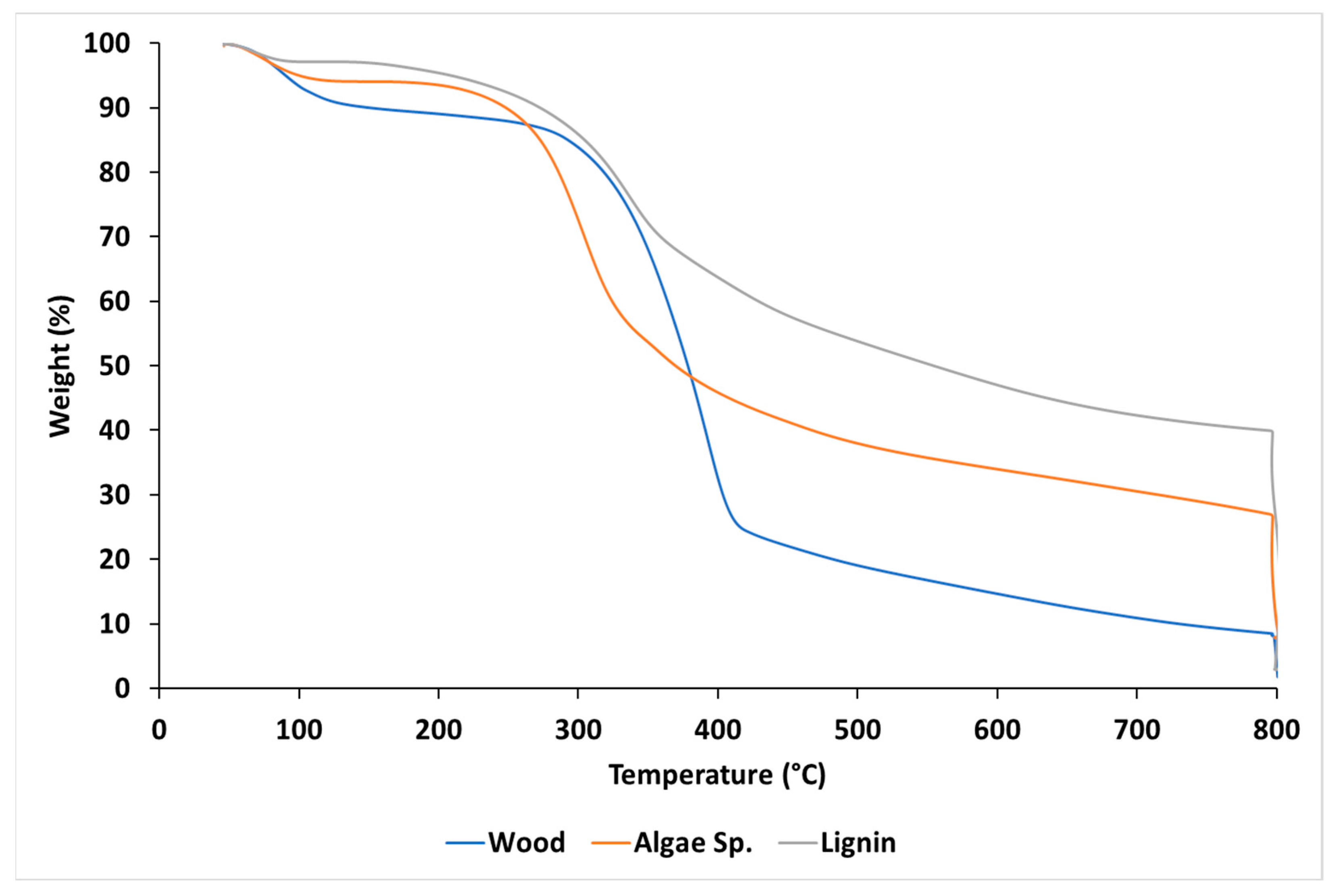
3.1. Feedstock Characterization
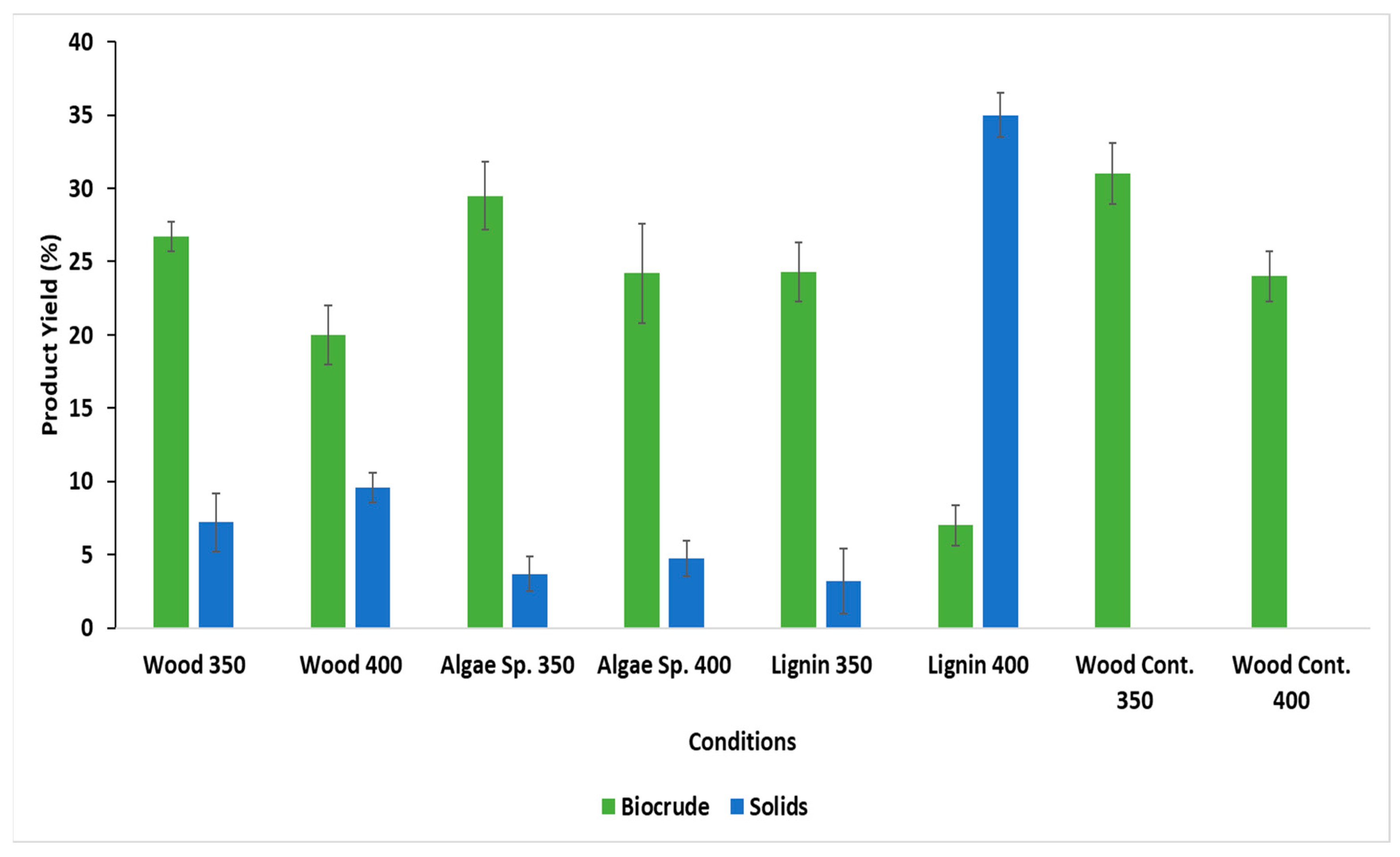
3.2. Yield of HTL Products
3.3. Quality of Bio-Crude
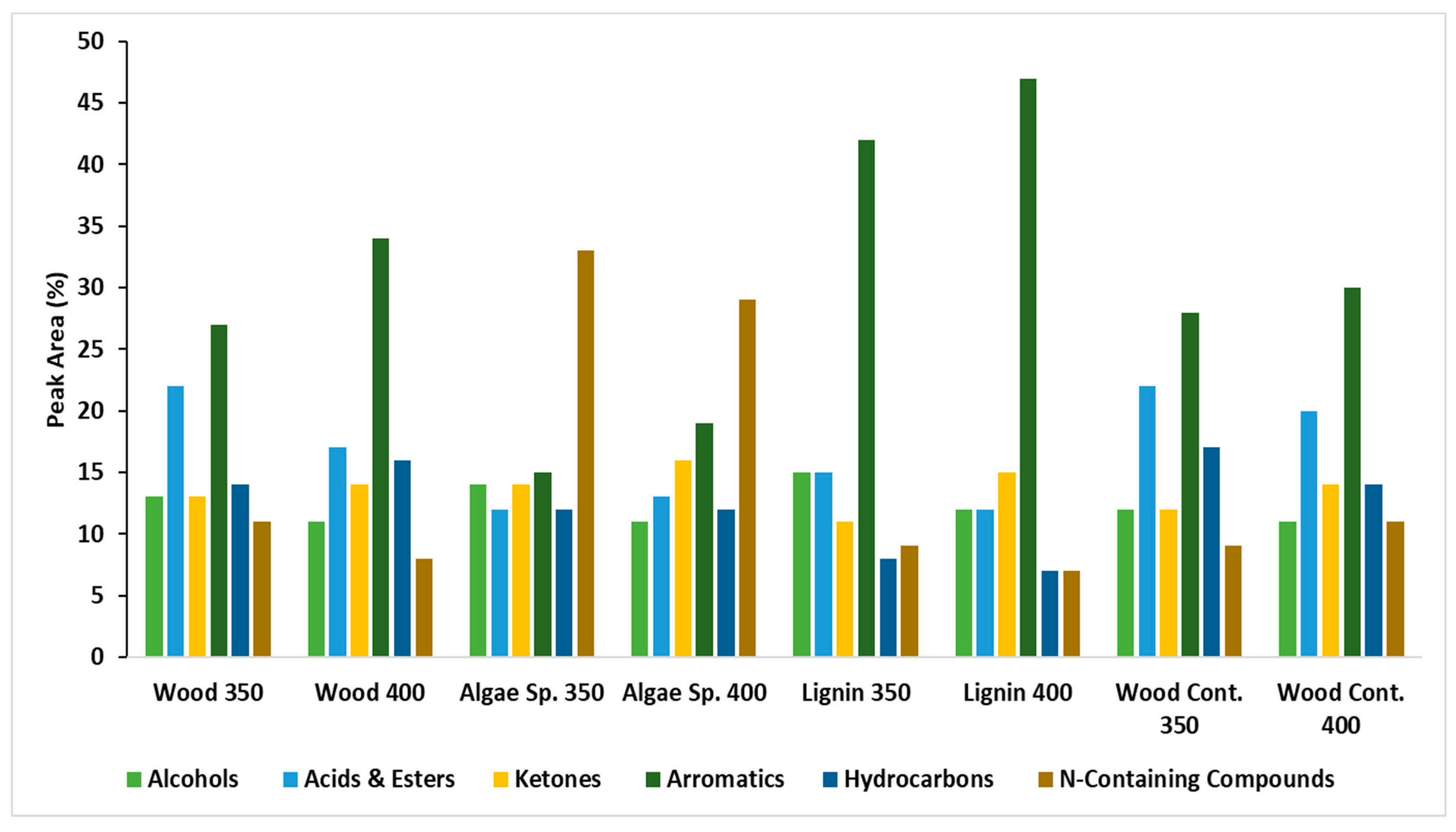
3.4. Compounds Composition of Bio-Crude
3.5. Analysis of Solid Residue
3.6. Analysis of Aqueous Phase
4. Conclusions
5. Challenges and Recommendations for Future Prospective of HTL
5.1. Temperature
5.2. Feedstock
5.3. Process Mode
5.4. HTL with Hydrotreatment (Taking as a Whole-Process Chain)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ocampo, E.; Beltrán, V.V.; Gómez, E.A.; Ríos, L.A.; Ocampo, D. Hydrothermal liquefaction process: Review and trends. Curr. Res. Green Sustain. Chem. 2023, 7, 100382. [Google Scholar] [CrossRef]
- Castello, D.; Pedersen, T.H.; Rosendahl, L.A. Continuous hydrothermal liquefaction of biomass: A critical review. Energies 2018, 11, 3165. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumar, V.; Kumar, P.; Mohanty, K. Hydrothermal liquefaction of biomass for bio-crude production: A review on feedstocks, chemical compositions, operating parameters, reaction kinetics, techno-economic study, and life cycle assessment. Fuel 2022, 316, 123377. [Google Scholar] [CrossRef]
- Shahbeik, H.; Panahi, H.K.S.; Dehhaghi, M.; Guillemin, G.J.; Fallahi, A.; Hosseinzadeh-Bandbafha, H.; Amiri, H.; Rehan, M.; Raikwar, D.; Latine, H.; et al. Biomass to biofuels using hydrothermal liquefaction: A comprehensive review. Renew. Sustain. Energy Rev. 2024, 189, 113976. [Google Scholar] [CrossRef]
- Kruse, A.; Dinjus, E. Hot compressed water as reaction medium and reactant: Properties and synthesis reactions. J. Supercrit. Fluids 2007, 39, 362–380. [Google Scholar] [CrossRef]
- de Caprariis, B.; Bracciale, M.; Bavasso, I.; Chen, G.; Damizia, M.; Genova, V.; Marra, F.; Paglia, L.; Pulci, G.; Scarsella, M.; et al. Unsupported Ni metal catalyst in hydrothermal liquefaction of oak wood: Effect of catalyst surface modification. Sci. Total Environ. 2020, 709, 136215. [Google Scholar] [CrossRef]
- de Caprariis, B.; De Filippis, P.; Petrullo, A.; Scarsella, M. Hydrothermal liquefaction of biomass: Influence of temperature and biomass composition on the bio-oil production. Fuel 2017, 208, 618–625. [Google Scholar] [CrossRef]
- Shi, W.; Li, S.; Jin, H.; Zhao, Y.; Yu, W. The hydrothermal liquefaction of rice husk to bio-crude using metallic oxide catalysts. Energy Sources Part A Recovery Util. Environ. Eff. 2013, 35, 2149–2155. [Google Scholar] [CrossRef]
- Patil, P.T.; Armbruster, U.; Martin, A. Hydrothermal liquefaction of wheat straw in hot compressed water and subcritical water–alcohol mixtures. J. Supercrit. Fluids 2014, 93, 121–129. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Y.; Park, E.; Han, X.; Zeng, Y.; Xu, C. Hydrothermal Liquefaction of Pinewood Sawdust: Influence of Reaction Atmosphere. Sustainability 2023, 15, 6698. [Google Scholar] [CrossRef]
- Bassoli, S.C.; da Fonseca, Y.A.; Wandurraga, H.J.L.; Baeta, B.E.L.; Amaral, M.D.S. Research progress, trends, and future prospects on hydrothermal liquefaction of algae for bio-crude production: A bibliometric analysis. Biomass Convers. Biorefin. 2023, 10, 1–16. [Google Scholar] [CrossRef]
- Costa, P.A.; Mata, R.M.; Pinto, M.F.; Paradela, F.; Dutra, F. Hydrothermal Liquefaction of Microalgae for the Production of Bio-crude and Value-added Chemicals. Chem. Eng. Trans. 2022, 94, 865–870. [Google Scholar] [CrossRef]
- Jena, U.; Eboibi, B.E.; Das, K.C. Co-Solvent Assisted Hydrothermal Liquefaction of Algal Biomass and Bio-crude Upgrading. Fuels 2022, 3, 326–341. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour. Technol. 2011, 102, 215–225. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Feng, L.; Li, X.; Wang, Z.; Liu, B. Catalytic hydrothermal liquefaction of lignin for production of aromatic hydrocarbon over metal supported mesoporous catalyst. Bioresour. Technol. 2021, 323, 124569. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Fu, P.; Song, C.; Fan, Y. Valorization of waste biomass through hydrothermal liquefaction: A review with focus on linking hydrothermal factors to products characteristics. Ind. Crops Prod. 2023, 191, 116017. [Google Scholar] [CrossRef]
- Belkheiri, T.; Andersson, S.-I.; Mattsson, C.; Olausson, L.; Theliander, H.; Vamling, L. Hydrothermal Liquefaction of Kraft Lignin in Subcritical Water: Influence of Phenol as Capping Agent. Energy Fuels 2018, 32, 5923–5932. [Google Scholar] [CrossRef]
- Arturi, K.R.; Strandgaard, M.; Nielsen, R.P.; Søgaard, E.G.; Maschietti, M. Hydrothermal liquefaction of lignin in near-critical water in a new batch reactor: Influence of phenol and temperature. J. Supercrit. Fluids 2017, 123, 28–39. [Google Scholar] [CrossRef]
- Yang, J.; He, Q.; Niu, H.; Corscadden, K.; Astatkie, T. Hydrothermal liquefaction of biomass model components for product yield prediction and reaction pathways exploration. Appl. Energy 2018, 228, 1618–1628. [Google Scholar] [CrossRef]
- Mathanker, A.; Pudasainee, D.; Kumar, A.; Gupta, R. Hydrothermal liquefaction of lignocellulosic biomass feedstock to produce biofuels: Parametric study and products characterization. Fuel 2020, 271, 117534. [Google Scholar] [CrossRef]
- Pińkowska, H.; Wolak, P.; Złocińska, A. Hydrothermal decomposition of alkali lignin in sub- and supercritical water. Chem. Eng. J. 2012, 187, 410–414. [Google Scholar] [CrossRef]
- Belkheiri, T.; Andersson, S.-I.; Mattsson, C.; Olausson, L.; Theliander, H.; Vamling, L. Hydrothermal liquefaction of kraft lignin in sub-critical water: The influence of the sodium and potassium fraction. Biomass Convers. Biorefin. 2018, 8, 585–595. [Google Scholar] [CrossRef]
- Madsen, R.B.; Biller, P.; Jensen, M.M.; Becker, J.; Iversen, B.B.; Glasius, M. Predicting the Chemical Composition of Aqueous Phase from Hydrothermal Liquefaction of Model Compounds and Biomasses. Energy Fuels 2016, 30, 10470–10483. [Google Scholar] [CrossRef]
- Ross, A.; Biller, P.; Kubacki, M.; Li, H.; Lea-Langton, A.; Jones, J. Hydrothermal processing of microalgae using alkali and organic acids. Fuel 2010, 89, 2234–2243. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, W.-T.; Zhang, P.; Luo, Z.; Zhang, Y. Hydrothermal liquefaction of Chlorella pyrenoidosa in sub- and supercritical ethanol with heterogeneous catalysts. Bioresour. Technol. 2013, 133, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, Z.; Feng, L. Effects of reaction parameter on catalytic hydrothermal liquefaction of microalgae into hydrocarbon rich bio-oil. J. Energy Inst. 2021, 94, 22–28. [Google Scholar] [CrossRef]
- Vo, T.K.; Lee, O.K.; Lee, E.Y.; Kim, C.H.; Seo, J.-W.; Kim, J.; Kim, S.-S. Kinetics study of the hydrothermal liquefaction of the microalga Aurantiochytrium sp. KRS101. Chem. Eng. J. 2016, 306, 763–771. [Google Scholar] [CrossRef]
- Conti, F.; Toor, S.S.; Pedersen, T.H.; Nielsen, A.H.; Rosendahl, L.A. Bio-crude production and nutrients recovery through hydrothermal liquefaction of wastewater irrigated willow. Biomass Bioenergy 2018, 118, 24–31. [Google Scholar] [CrossRef]
- Zhu, Z.; Toor, S.S.; Rosendahl, L.; Yu, D.; Chen, G. Influence of alkali catalyst on product yield and properties via hydrothermal liquefaction of barley straw. Energy 2015, 80, 284–292. [Google Scholar] [CrossRef]
- Parikh, J.; Channiwala, S.; Ghosal, G. A correlation for calculating HHV from proximate analysis of solid fuels. Fuel 2005, 84, 487–494. [Google Scholar] [CrossRef]
- Yong, T.L.-K.; Matsumura, Y. Reaction kinetics of the lignin conversion in supercritical water. Ind. Eng. Chem. Res. 2012, 51, 11975–11988. [Google Scholar] [CrossRef]
- Leng, L.; Zhou, J.; Li, T.; Vlaskin, M.; Zhan, H.; Peng, H.; Huang, H.; Li, H. Nitrogen heterocycles in bio-oil produced from hydrothermal liquefaction of biomass: A review. Fuel 2023, 335, 126995. [Google Scholar] [CrossRef]
- Toor, S.S.; Reddy, H.; Deng, S.; Hoffmann, J.; Spangsmark, D.; Madsen, L.B.; Holm-Nielsen, J.B.; Rosendahl, L.A. Hydrothermal liquefaction of Spirulina and Nannochloropsis salina under subcritical and supercritical water conditions. Bioresour. Technol. 2013, 131, 413–419. [Google Scholar] [CrossRef]
- Jensen, C.U.; Guerrero, J.K.R.; Karatzos, S.; Olofsson, G.; Iversen, S.B. Fundamentals of Hydrofaction™: Renewable crude oil from woody biomass. Biomass Convers. Biorefin. 2017, 7, 495–509. [Google Scholar] [CrossRef]
- Kruse, A.; Maniam, P.; Spieler, F. Influence of proteins on the hydrothermal gasification and liquefaction of biomass. 2. Model compounds. Ind. Eng. Chem. Res. 2007, 46, 87–96. [Google Scholar] [CrossRef]
- Singh, R.; Prakash, A.; Balagurumurthy, B. Hydrothermal liquefaction of agricultural and forest biomass residue: Comparative study. J. Mater. Cycles Waste Manag. 2015, 17, 442–452. [Google Scholar] [CrossRef]
- Zhu, Z.; Rosendahl, L.; Toor, S.S.; Yu, D.; Chen, G. Hydrothermal liquefaction of barley straw to bio-crude oil: Effects of reaction temperature and aqueous phase recirculation. Appl. Energy 2015, 137, 183–192. [Google Scholar] [CrossRef]
- Biller, P.; Sharma, B.K.; Kunwar, B.; Ross, A.B. Hydroprocessing of bio-crude from continuous hydrothermal liquefaction of microalgae. Fuel 2015, 159, 197–205. [Google Scholar] [CrossRef]
- Rizwan, M.; Murtaza, G.; Zulfiqar, F.; Moosa, A.; Iqbal, R.; Ahmed, Z.; Irshad, S.; Khan, I.; Li, T.; Chen, J.; et al. Sustainable manufacture and application of biochar to improve soil properties and remediate soil contaminated with organic impurities: A systematic review. Front. Environ. Sci. 2023, 11, 1277240. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nagappan, S.; Bhosale, R.R.; Lay, C.-H.; Nguyen, D.D.; Pugazhendhi, A.; Chang, S.W.; Kumar, G. Review on sustainable production of biochar through hydrothermal liquefaction: Physico-chemical properties and applications. Bioresour. Technol. 2020, 310, 123414. [Google Scholar] [CrossRef]
- Zheng, M.; Schideman, L.C.; Tommaso, G.; Chen, W.-T.; Zhou, Y.; Nair, K.; Qian, W.; Zhang, Y.; Wang, K. Anaerobic digestion of wastewater generated from the hydrothermal liquefaction of Spirulina: Toxicity assessment and minimization. Energy Convers. Manag. 2017, 141, 420–428. [Google Scholar] [CrossRef]
- Watson, J.; Si, B.; Li, H.; Liu, Z.; Zhang, Y. Influence of catalysts on hydrogen production from wastewater generated from the HTL of human feces via catalytic hydrothermal gasification. Int. J. Hydrogen Energy 2017, 42, 20503–20511. [Google Scholar] [CrossRef]
- Watson, J.; Wang, T.; Si, B.; Chen, W.-T.; Aierzhati, A.; Zhang, Y. Valorization of hydrothermal liquefaction aqueous phase: Pathways towards commercial viability. Prog. Energy Combust. Sci. 2020, 77, 100819. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, S.; Yuan, Z.; Xu, C.C.; Bassi, A. Investigation of aqueous phase recycling for improving bio-crude oil yield in hydrothermal liquefaction of algae. Bioresour. Technol. 2017, 239, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Biller, P.; Madsen, R.B.; Klemmer, M.; Becker, J.; Iversen, B.B.; Glasius, M. Effect of hydrothermal liquefaction aqueous phase recycling on bio-crude yields and composition. Bioresour. Technol. 2016, 220, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Toor, S.S.; Seehar, T.H.; Nielsen, R.S.; Nielsen, A.H.; Pedersen, T.H.; Rosendahl, L.A. Bio-Crude Production through Aqueous Phase Recycling of Hydrothermal Liquefaction of Sewage Sludge. Energies 2020, 13, 493. [Google Scholar] [CrossRef]
- SundarRajan, P.; Gopinath, K.; Arun, J.; GracePavithra, K.; Joseph, A.A.; Manasa, S. Insights into valuing the aqueous phase derived from hydrothermal liquefaction. Renew. Sustain. Energy Rev. 2021, 144, 111019. [Google Scholar] [CrossRef]
- Leng, L.; Li, J.; Wen, Z.; Zhou, W. Use of microalgae to recycle nutrients in aqueous phase derived from hydrothermal liquefaction process. Bioresour. Technol. 2018, 256, 529–542. [Google Scholar] [CrossRef]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Ocfemia, K.S.; Zhang, Y.; Funk, T. Hydrothermal Processing of Swine Manure Into Oil using a Contineous Reactor System: Development and Testing. Trans. ASABE 2006, 49, 533–541. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Shah, A.A.; Ahmed, J.; Rehman, S.; Sultan, S.H.; Shah, A.K.; Raza, A.; Mubarak, N.M.; Hashmi, Z.; Usto, M.A.; et al. Hydrothermal Liquefaction of Lignocellulosic and Protein-Containing Biomass: A Comprehensive Review. Catalysts 2022, 12, 1621. [Google Scholar] [CrossRef]
- Anastasakis, K.; Biller, P.; Madsen, R.B.; Glasius, M.; Johannsen, I. Continuous Hydrothermal Liquefaction of Biomass in a Novel Pilot Plant with Heat Recovery and Hydraulic Oscillation. Energies 2018, 11, 2695. [Google Scholar] [CrossRef]
- Ghavami, N.; Özdenkçi, K.; Salierno, G.; Björklund-Sänkiaho, M.; De Blasio, C. Analysis of operational issues in hydrothermal liquefaction and supercritical water gasification processes: A review. Biomass Convers. Biorefin. 2023, 13, 12367–12394. [Google Scholar] [CrossRef]
- Elhassan, M.; Abdullah, R.; Kooh, M.R.R.; Chau, Y.-F.C. Hydrothermal liquefaction: A technological review on reactor design and operating parameters. Bioresour. Technol. Rep. 2023, 21, 101314. [Google Scholar] [CrossRef]

| Feedstocks | Moisture (%) | Ash a | VM a | FC a | C (%) b | H (%) b | N (%) b | O (%) b,d | H/C | HHV (MJ/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Wood Powder | 9.88 | 0.40 | 91.11 | 8.60 | 46.09 | 6.59 | 0.09 | 47.24 | 1.71 | 18.36 |
| Algae Sp. | 5.87 | 8.92 | 72.23 | 19.08 | 45.80 | 6.83 | 10.66 | 36.72 | 1.79 | 18.50 |
| Lignin | 4.41 | 0.20 | 60.45 | 39.80 | 61.85 | 6.08 | 0.63 | 31.45 | 1.18 | 24.72 |
| Samples | C (%) a | H (%) a | N (%) a | O (%) a,b | H/C | HHV (MJ/kg) c | Ash (%) | Water Content (%) |
|---|---|---|---|---|---|---|---|---|
| Wood 350 | 77.76 | 7.45 | 0.72 | 14.07 | 1.15 | 33.43 | 2.22 | 5.93 |
| Wood 400 | 82.50 | 7.97 | 0.75 | 8.79 | 1.16 | 36.15 | 1.21 | 4.21 |
| Algae Sp. 350 | 77.16 | 10.01 | 6.06 | 6.78 | 1.56 | 36.26 | 1.15 | 4.59 |
| Algae Sp. 400 | 77.77 | 9.76 | 6.45 | 6.03 | 1.51 | 36.22 | 0.13 | 6.11 |
| Lignin 350 | 72.03 | 7.23 | 1.15 | 19.60 | 1.20 | 30.62 | 15.30 | 5.54 |
| Lignin 400 | 79.96 | 7.91 | 1.07 | 11.07 | 1.19 | 34.95 | 12.65 | 6.54 |
| Wood Cont. 350 | 73.23 | 7.26 | 0.44 | 19.08 | 1.19 | 31.19 | 0.75 | 9.44 |
| Wood Cont. 400 | 77.69 | 7.81 | 0.65 | 13.87 | 1.21 | 33.83 | 1.16 | 8.51 |
| Samples | C (%) a | H (%) a | N (%) a | O (%) a,b | H/C | O/C | HHV (MJ/kg) c | Ash (%) |
|---|---|---|---|---|---|---|---|---|
| Wood 350 | 77.15 | 4.11 | 0.18 | 18.58 | 0.64 | 0.18 | 29.08 | 20.12 |
| Wood 400 | 83.95 | 4.01 | 0.14 | 11.90 | 0.57 | 0.11 | 31.97 | 11.27 |
| Algae Sp 350 | 32.08 | 2.58 | 1.94 | 63.41 | 0.97 | 1.48 | 7.28 | 26.15 |
| Algae Sp. 400 | 28.00 | 1.01 | 1.34 | 69.66 | 0.43 | 1.87 | 3.56 | 29.37 |
| Lignin 350 | 71.76 | 2.92 | 1.34 | 23.99 | 0.49 | 0.25 | 25.24 | 22.76 |
| Lignin 400 | 79.63 | 4.48 | 0.63 | 15.27 | 0.67 | 0.14 | 30.63 | 17.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, A.A.; Sharma, K.; Seehar, T.H.; Toor, S.S.; Sandquist, J.; Saanum, I.; Pedersen, T.H. Sub-Supercritical Hydrothermal Liquefaction of Lignocellulose and Protein-Containing Biomass. Fuels 2024, 5, 75-89. https://doi.org/10.3390/fuels5010005
Shah AA, Sharma K, Seehar TH, Toor SS, Sandquist J, Saanum I, Pedersen TH. Sub-Supercritical Hydrothermal Liquefaction of Lignocellulose and Protein-Containing Biomass. Fuels. 2024; 5(1):75-89. https://doi.org/10.3390/fuels5010005
Chicago/Turabian StyleShah, Ayaz Ali, Kamaldeep Sharma, Tahir Hussain Seehar, Saqib Sohail Toor, Judit Sandquist, Inge Saanum, and Thomas Helmer Pedersen. 2024. "Sub-Supercritical Hydrothermal Liquefaction of Lignocellulose and Protein-Containing Biomass" Fuels 5, no. 1: 75-89. https://doi.org/10.3390/fuels5010005
APA StyleShah, A. A., Sharma, K., Seehar, T. H., Toor, S. S., Sandquist, J., Saanum, I., & Pedersen, T. H. (2024). Sub-Supercritical Hydrothermal Liquefaction of Lignocellulose and Protein-Containing Biomass. Fuels, 5(1), 75-89. https://doi.org/10.3390/fuels5010005





