Aspen Plus® Modeling and Simulation of an Industrial Biomass Direct Liquefaction Process
Abstract
1. Introduction
2. Liquefaction Process and Model Implementation
2.1. The Energreen Liquefaction Process
2.1.1. Biomass and Catalyst Feeding
2.1.2. Pretreatment
2.1.3. Liquefaction
2.1.4. Filtration
2.2. Aspen Plus® Model Implementation
2.2.1. Chemical Compound Setup
2.2.2. Missing Physical and Thermodynamic Properties
2.2.3. Feedstock Characterization
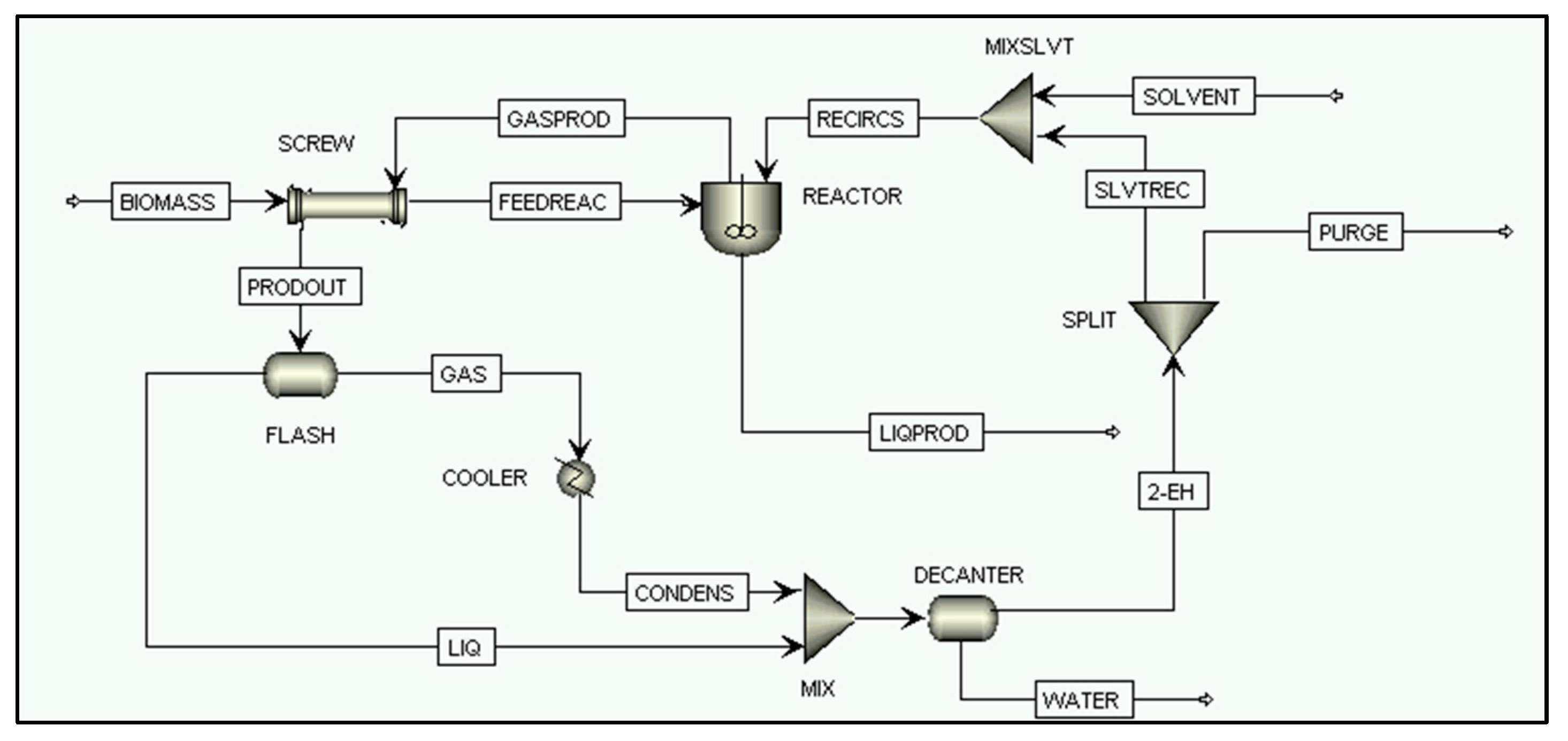
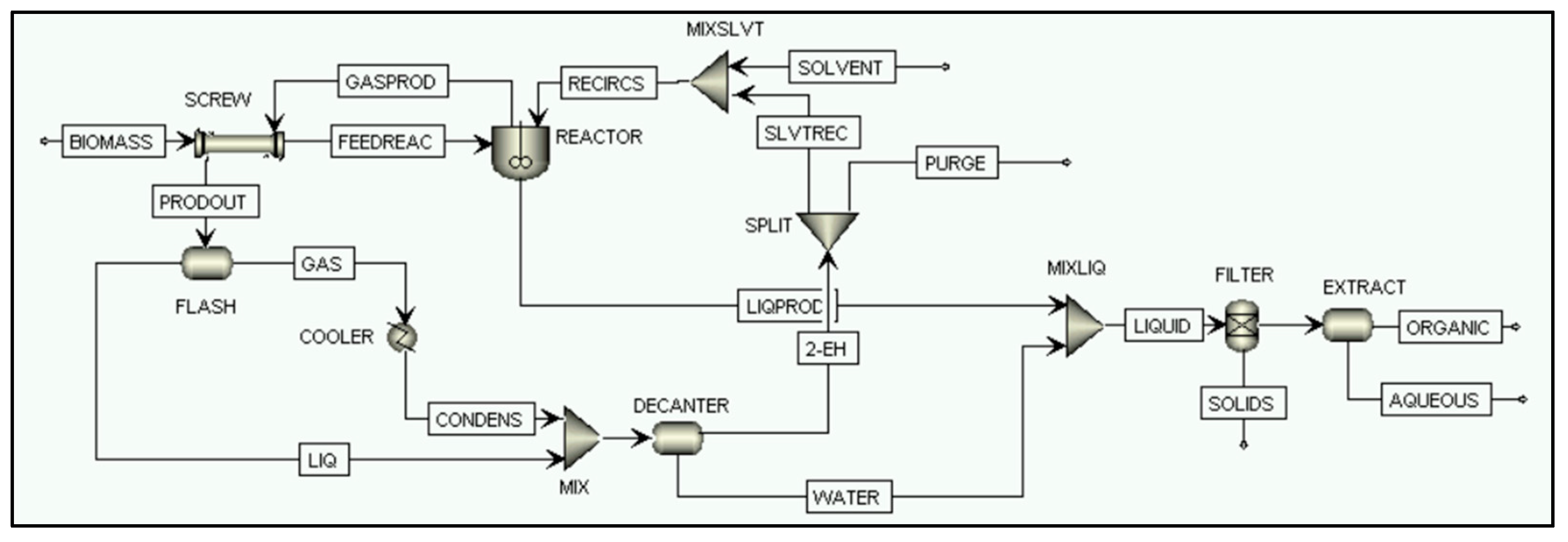
2.2.4. Direct Biomass Liquefaction Process Simulation
SCREW
REACTOR
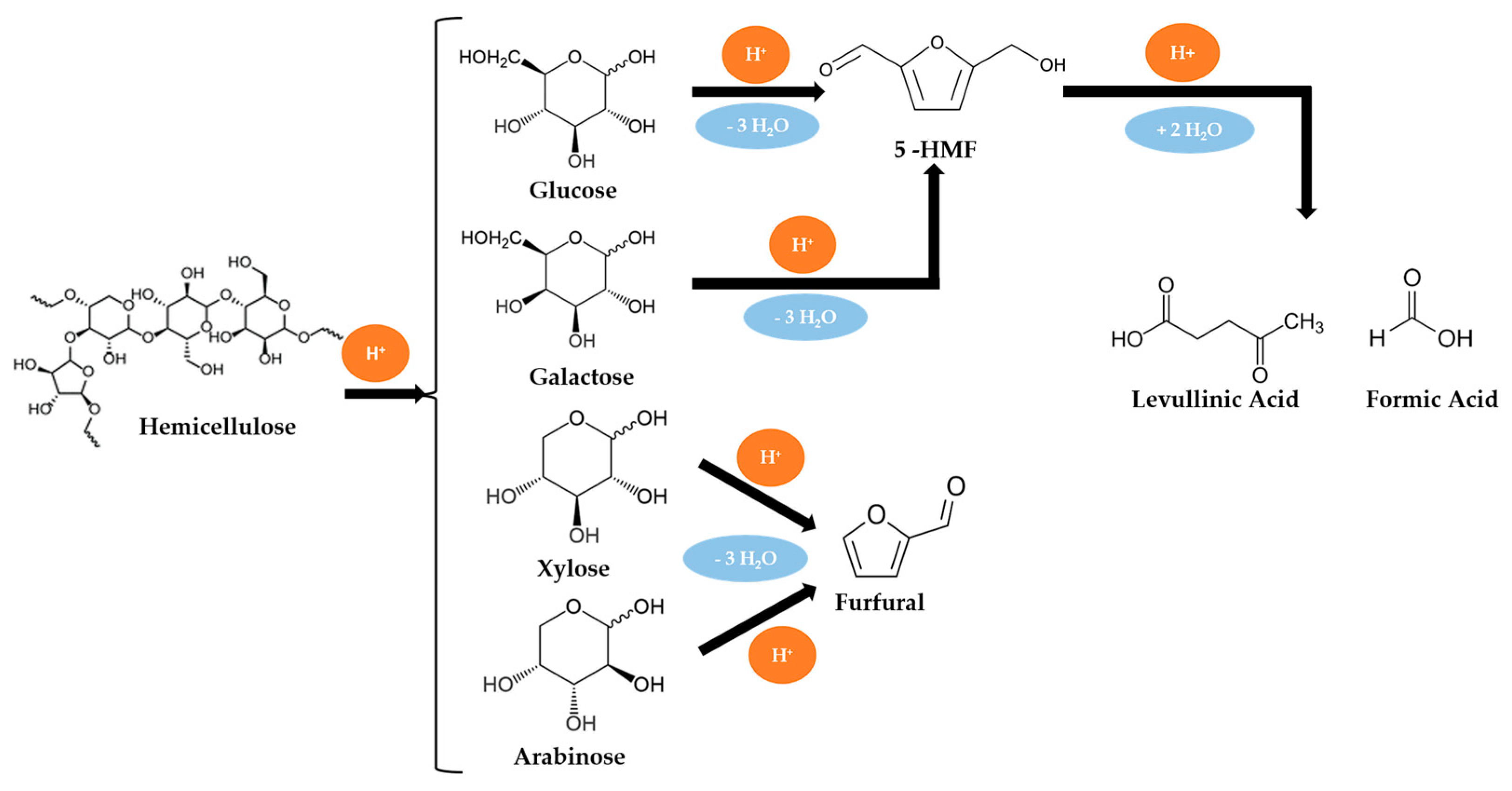
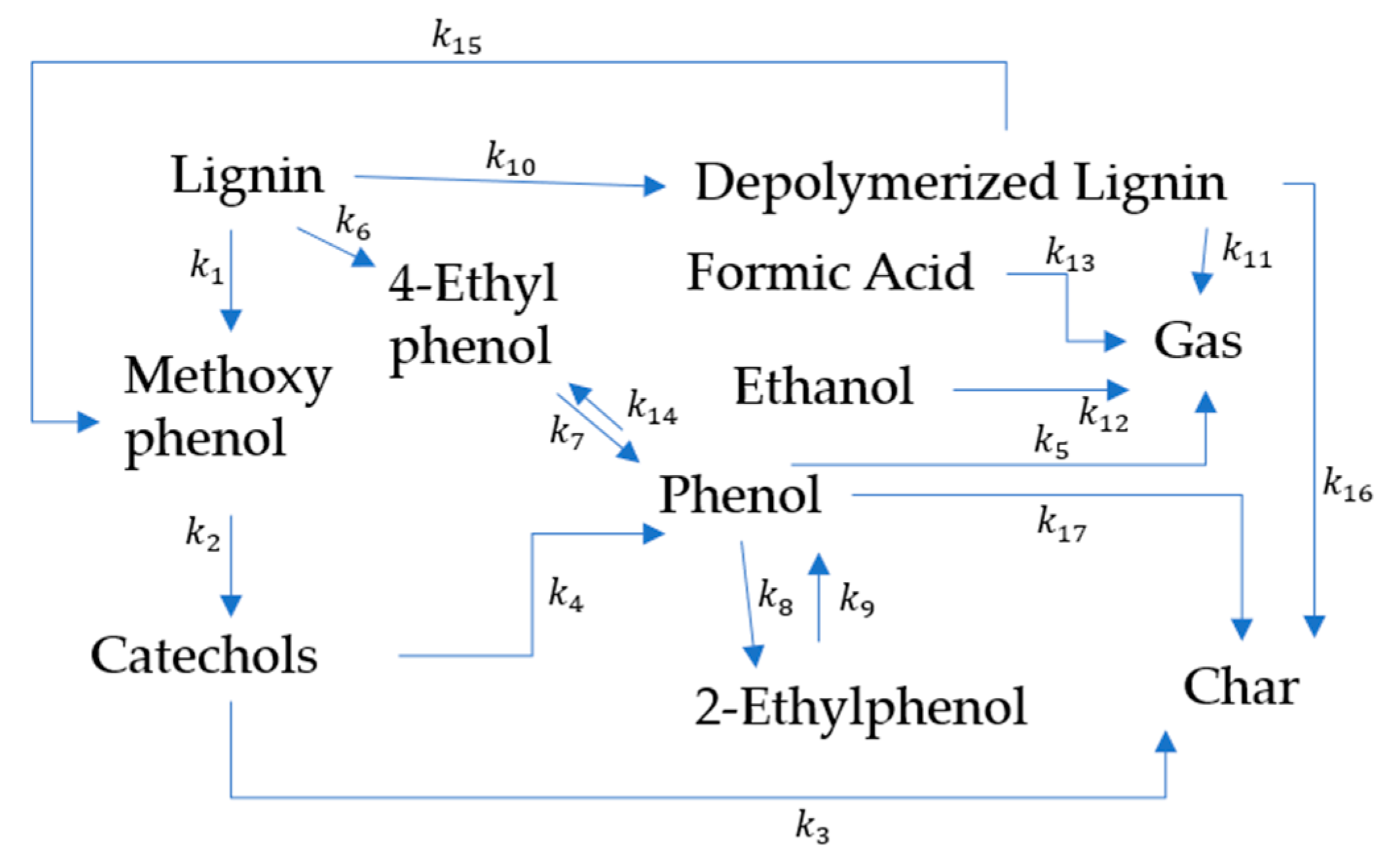
Biomass Liquefaction Reactions
FLASH
COOLER
MIX, MIXSLVT, MIXLIQ
DECANTER
SPLIT
FILTER
EXTRACT
3. Simulation Results
3.1. Results
3.1.1. Model Validation
3.1.2. Fossil Fuel Substitution Simulation
3.2. Sensitivity Analysis
3.2.1. Temperature
3.2.2. Purge Fraction
3.2.3. Biomass Moisture Content
3.2.4. Biomass/Solvent Fraction
3.2.5. Activation Energy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Braz, A.; Mateus, M.M.; dos Santos, R.G.; Machado, R.; Bordado, J.M.; Correia, M.J.N. Modelling of Pine Wood Sawdust Thermochemical Liquefaction. Biomass Bioenergy 2019, 120, 200–210. [Google Scholar] [CrossRef]
- Pan, H. Synthesis of Polymers from Organic Solvent Liquefied Biomass: A Review. Renew. Sustain. Energy Rev. 2011, 15, 3454–3463. [Google Scholar] [CrossRef]
- Moser, L.; Penke, C.; Batteiger, V. An In-Depth Process Model for Fuel Production via Hydrothermal Liquefaction and Catalytic Hydrotreating. Processes 2021, 9, 1172. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.C.; Champagne, P. Overview of Recent Advances in Thermo-Chemical Conversion of Biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Hao, B.; Xu, D.; Wei, Y.; Diao, Y.; Yang, L.; Fan, L.; Guo, Y. Mathematical Models Application in Optimization of Hydrothermal Liquefaction of Biomass. Fuel Process. Technol. 2023, 243, 107673. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Liu, T.; Leng, S.; Yang, L.; Peng, H.; Jiang, S.; Zhou, W.; Leng, L.; Li, H. Machine Learning Prediction and Optimization of Bio-Oil Production from Hydrothermal Liquefaction of Algae. Bioresour. Technol. 2021, 342, 126011. [Google Scholar] [CrossRef]
- Katongtung, T.; Onsree, T.; Tippayawong, N. Machine Learning Prediction of Biocrude Yields and Higher Heating Values from Hydrothermal Liquefaction of Wet Biomass and Wastes. Bioresour. Technol. 2022, 344, 126278. [Google Scholar] [CrossRef]
- Ranganathan, P.; Savithri, S. Computational Fluid Dynamics Simulation of Hydrothermal Liquefaction of Microalgae in a Continuous Plug-Flow Reactor. Bioresour. Technol. 2018, 258, 151–157. [Google Scholar] [CrossRef]
- Ou, L.; Thilakaratne, R.; Brown, R.C.; Wright, M.M. Techno-Economic Analysis of Transportation Fuels from Defatted Microalgae via Hydrothermal Liquefaction and Hydroprocessing. Biomass Bioenergy 2015, 72, 45–54. [Google Scholar] [CrossRef]
- Hoffmann, J.; Rudra, S.; Toor, S.S.; Holm-Nielsen, J.B.; Rosendahl, L.A. Conceptual Design of an Integrated Hydrothermal Liquefaction and Biogas Plant for Sustainable Bioenergy Production. Bioresour. Technol. 2013, 129, 402–410. [Google Scholar] [CrossRef]
- Pedersen, T.H.; Hansen, N.H.; Pérez, O.M.; Cabezas, D.E.V.; Rosendahl, L.A. Renewable Hydrocarbon Fuels from Hydrothermal Liquefaction: A Techno-Economic Analysis. Biofuels, Bioprod. Biorefining 2018, 12, 213–223. [Google Scholar] [CrossRef]
- Ong, B.H.Y.; Walmsley, T.G.; Atkins, M.J.; Walmsley, M.R.W. Hydrothermal Liquefaction of Radiata Pine with Kraft Black Liquor for Integrated Biofuel Production. J. Clean. Prod. 2018, 199, 737–750. [Google Scholar] [CrossRef]
- Mishra, V.K.; Goswami, R. A Review of Production, Properties and Advantages of Biodiesel. Biofuels 2018, 9, 273–289. [Google Scholar] [CrossRef]
- Jiang, W.; Kumar, A.; Adamopoulos, S. Liquefaction of Lignocellulosic Materials and Its Applications in Wood Adhesives—A Review. Ind. Crops Prod. 2018, 124, 325–342. [Google Scholar] [CrossRef]
- Zhong, C.; Wei, X. A Comparative Experimental Study on the Liquefaction of Wood. Energy 2004, 29, 1731–1741. [Google Scholar] [CrossRef]
- Zhang, H.; Pang, H.; Shi, J.; Fu, T.; Liao, B. Investigation of Liquefied Wood Residues Based on Cellulose, Hemicellulose, and Lignin. J. Appl. Polym. Sci. 2012, 123, 850–856. [Google Scholar] [CrossRef]
- Isa, K.M.; Abdullah, T.A.T.; Ali, U.F.M. Hydrogen Donor Solvents in Liquefaction of Biomass: A Review. Renew. Sustain. Energy Rev. 2018, 81, 1259–1268. [Google Scholar] [CrossRef]
- Bordado, J.; Mateus, M.; Lopes, R.; Salva, J.; Nunes, Â.; Cachaço, A.; Mina, B.; Correia, J. Instalação para a Realização de um Processo de Conversão de Materiais Lignocelulósicos em Biocombustível Líquido. PT Patent 108816, 2017. [Google Scholar]
- Nunes, Â.; Bordado, J.; Correia, J.; Mateus, M.; Lopes, R. Catalytic and Continuous Thermochemical Process of Production of Valuable Derivatives from Organic Materials and Waste. EP Patent 18398010, 4 May 2023. [Google Scholar]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin Structure and Its Engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Ruddy, D.A.; Nash, C.P.; Harris, K.; Christensen, E.D.; Dupuis, D.P.; Tan, E.C.D. A Separations and Purification Process for Improving Yields and Meeting Fuel Contaminant Specifications for High-Octane Gasoline Produced from Dimethyl-Ether over a Cu/BEA Catalyst. Biofuels, Bioprod. Biorefining 2022, 16, 1469–1477. [Google Scholar] [CrossRef]
- Ur’yash, V.F.; Larina, V.N.; Kokurina, N.Y.; Novoselova, N.V. The Thermochemical Characteristics of Cellulose and Its Mixtures with Water. Russ. J. Phys. Chem. A 2010, 84, 915–921. [Google Scholar] [CrossRef]
- Gorensek, M.B.; Shukre, R.; Chen, C.-C. Development of a Thermophysical Properties Model for Flowsheet Simulation of Biomass Pyrolysis Processes. ACS Sustain. Chem. Eng. 2019, 7, 9017–9027. [Google Scholar] [CrossRef]
- Yan, L.; Greenwood, A.A.; Hossain, A.; Yang, B. A Comprehensive Mechanistic Kinetic Model for Dilute Acid Hydrolysis of Switchgrass Cellulose to Glucose, 5-HMF and Levulinic Acid. RSC Adv. 2014, 4, 23492–23504. [Google Scholar] [CrossRef]
- Girisuta, B.; Danon, B.; Manurung, R.; Janssen, L.P.B.M.; Heeres, H.J. Experimental and Kinetic Modelling Studies on the Acid-Catalysed Hydrolysis of the Water Hyacinth Plant to Levulinic Acid. Bioresour. Technol. 2008, 99, 8367–8375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, H.; Guo, H.; Huang, C.; Xiong, L.; Chen, X. Kinetic Study on the Liquefaction of Wood and Its Three Cell Wall Component in Polyhydric Alcohols. Appl. Energy 2014, 113, 1596–1600. [Google Scholar] [CrossRef]
- Forchheim, D.; Gasson, J.R.; Hornung, U.; Kruse, A.; Barth, T. Modeling the Lignin Degradation Kinetics in a Ethanol/Formic Acid Solvolysis Approach. Part 2. Validation and Transfer to Variable Conditions. Ind. Eng. Chem. Res. 2012, 51, 15053–15063. [Google Scholar] [CrossRef]
- Poulikidou, S.; Heyne, S.; Grahn, M.; Harvey, S. Lifecycle Energy and Greenhouse Gas Emissions Analysis of Biomass-Based 2-Ethylhexanol as an Alternative Transportation Fuel. Energy Sci. Eng. 2019, 7, 851–867. [Google Scholar] [CrossRef]
- Enes, T.; Aranha, J.; Fonseca, T.; Lopes, D.; Alves, A.; Lousada, J. Thermal Properties of Residual Agroforestry Biomass of Northern Portugal. Energies 2019, 12, 1418. [Google Scholar] [CrossRef]
- Fuels—Higher and Lower Calorific Values. Available online: https://www.engineeringtoolbox.com/fuels-higher-calorific-values-d_169.html (accessed on 6 January 2023).
- Jethmalani, H. Easing Petroleum Coke Prices a Respite for Cement Investors | Mint. Available online: https://www.livemint.com/market/mark-to-market/easing-petroleum-coke-prices-a-respite-for-cement-investors-11656954592385.html (accessed on 6 January 2023).



| Component ID | Component Name |
|---|---|
| 2EH | 2-ETHYLHEXANOL |
| H2O | WATER |
| CELL | CELLULOSE |
| GLUCOSE | DEXTROSE |
| GALACT | D-GALACTOSE |
| COUMARIL | TRANS-P-COUMARYL-ALCOHOL |
| XYLOSE | D-XYLOSE |
| ARABIN | D-(-)-ARABINOSE |
| HMF | 5-HYDROXYMETHYLFURFURAL |
| LA | LEVULINIC-ACID |
| FA | FORMIC-ACID |
| FURFURAL | FURFURAL |
| PHENOL | PHENOL |
| Operating Condition | Value |
|---|---|
| Temperature (°C) | 25 |
| Pressure (bar) | 1 |
| Mass Flow (kg/h) | 74.27 |
| Component | Value |
|---|---|
| H2O | 12.38 |
| CELL | 30.95 |
| GLUCOSE | 4.64 |
| GALACT | 4.64 |
| COUMARYL | 12.38 |
| XYLOSE | 4.64 |
| ARABIN | 4.64 |
| k0 (s−1) | Ea (kJ/mol) | |
|---|---|---|
| k1 | 3.66 × 109 | 109.35 |
| k2 | 5.31 × 105 | 74.37 |
| k3 | 9.27 × 107 | 87.13 |
| k0 (s−1) | Ea (kJ/mol) | |
|---|---|---|
| Hemicellulose decomposition (galactose, arabinose, xylose) | 284.29 | 40.49 |
| Lignin decomposition (p-coumaryl alcohol) | 528.48 | 44.31 |
| Biomass Property | Value |
|---|---|
| Moisture (%) | 15.0 |
| Ash content (%) | 10.0 |
| Dry ash-free biomass (%) | 75.0 |
| Run Parameter | Value |
|---|---|
| Solvent feed (kg) | 1960 |
| Biomass feed (kg) | 2600 |
| Catalyst feed (kg) | 180 |
| Reactor temperature (°C) | 160 |
| Reactor pressure (atm) | 1 |
| Water recovered (kg) | 990 |
| Solvent recovered (kg) | 1270 |
| Liquefied product (kg) | 3720 |
| Run Parameter | Experimental Run | Model Run | Deviation (%) |
|---|---|---|---|
| Temperature (°C) | 160 | 160 | 0 |
| Liquefied bio-oil (kg/h) | 42.6 | 39.9 | 6.4 |
| Liquefaction yield (m/m %) | 68.9 | 64.5 | 6.4 |
| Biomass Component | Sample 1 | Sample 2 | Sample 3 |
|---|---|---|---|
| Cellulose (%) | 50 | 60 | 45 |
| Hemicellulose (%) | 30 | 30 | 40 |
| Lignin (%) | 20 | 10 | 15 |
| Biomass Sample | CBIOMASS | CCELLULOSE | CARABINOSE | CGALACTOSE | CXYLOSE | Cp-COUMARYL |
|---|---|---|---|---|---|---|
| Sample 1 | 72 | 63 | 96 | 96 | 96 | 97 |
| Sample 2 | 67 | 63 | 96 | 96 | 96 | 97 |
| Sample 3 | 74 | 63 | 96 | 96 | 96 | 97 |
| Biomass Sample | Sample 1 |
|---|---|
| Sample 1 | 61 |
| Sample 2 | 58 |
| Sample 3 | 61 |
| Biomass Sample | Yield Deviation (%) | CBIOMASS Deviation (%) |
|---|---|---|
| Sample 2 | −4.7 | −6.6 |
| Sample 3 | −0.7 | 2.7 |
| Biomass Sample | QFEEDREAC (kg/h) | QRECIRCS (kg/h) | QGASPROD (kg/h) | QLIQROD (kg/h) |
|---|---|---|---|---|
| Sample 1 | 74 | 71 | 81 | 65 |
| Sample 2 | 74 | 63 | 76 | 61 |
| Sample 3 | 74 | 73 | 88 | 60 |
| Biomass Sample | QDry Biomass (kg/h) | Q2-EH (kg/h) | Total (kg/h) | Biomass Fraction | Solvent Fraction | LHV (GJ/t) |
|---|---|---|---|---|---|---|
| Sample 1 | 39.9 | 17.71 | 57.7 | 0.69 | 0.31 | 24.4 |
| Sample 2 | 37.4 | 15.0 | 52.4 | 0.71 | 0.29 | 23.9 |
| Sample 3 | 37.4 | 15.0 | 52.4 | 0.71 | 0.29 | 23.9 |
| Biomass Sample | Released Energy (GJ/h) | Qpetcoke equivalent (kg/h) | USD/Year |
|---|---|---|---|
| Sample 1 | 1.41 | 47.6 | 102,000 |
| Sample 2 | 1.25 | 42.5 | 91,000 |
| Sample 3 | 1.25 | 42.5 | 91,000 |
| ΔT (%) | ΔQFEEDREAC (%) | ΔQRECIRCS (%) | ΔQGASPROD (%) | ΔQLIQPROD (%) | ΔQAQUEOUS (%) | ΔQORGANIC (%) |
|---|---|---|---|---|---|---|
| −20 | 0 | −30 | −66 | 49 | −29 | 21 |
| −10 | 0 | −22 | −43 | 29 | −19 | 14 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 67 | 84 | −30 | 5 | −15 |
| 20 | 0 | 278 | 297 | −63 | 244 | −100 |
| ΔT (%) | ΔYield (%) | ΔCBIOMASS (%) | ΔCCELOBIOSE (%) | ΔCARABINOSE (%) | ΔCGALACTOSE (%) | ΔCXYLOSE (%) | ΔCp-COUMARYL (%) |
|---|---|---|---|---|---|---|---|
| −20 | −42 | −37 | −79 | −5 | −5 | −5 | −5 |
| −10 | −25 | −22 | −45 | −2 | −2 | −2 | −2 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 20 | 19 | 32 | 1 | 1 | 1 | 1 |
| 20 | 32 | 29 | 48 | 1 | 2 | 2 | 1 |
| ΔQH2O (%) | ΔQcooler (%) |
|---|---|
| −20 | −54.4 |
| −10 | −35.9 |
| 0 | 0 |
| 10 | 78.5 |
| 20 | 309 |
| ΔPurge Fraction (%) | ΔQFEEDREAC (%) | ΔQRECIRCS (%) | ΔQGASPROD (%) | ΔQLIQPROD (%) | ΔQAQUEOUS (%) | ΔQORGANIC (%) |
|---|---|---|---|---|---|---|
| −50 | 0 | 4 | 3 | 1 | 2 | 2 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 50 | 0 | −4 | −3 | −1 | −2 | −1 |
| 100 | 0 | −7 | −5 | −2 | −4 | −3 |
| 200 | 0 | −13 | −9 | −3 | −9 | −4 |
| ΔQH2O (%) | ΔQcooler (%) |
|---|---|
| −50 | 3 |
| 0 | 0 |
| 50 | −2 |
| 100 | −4 |
| 200 | −8 |
| ΔQH2O (%) | ΔQFEEDREAC (%) | ΔQRECIRCS (%) | ΔQGASPROD (%) | ΔQLIQPROD (%) | ΔQAQUEOUS (%) | ΔQORGANIC (%) |
|---|---|---|---|---|---|---|
| −20 | −3.4 | −3.4 | −8.5 | 2.9 | −32.8 | 6.3 |
| −10 | −1.7 | −1.7 | −4.2 | 1.4 | −16.1 | 3.0 |
| 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 10 | 1.6 | 1.7 | 4.1 | −1.3 | 15.5 | −2.8 |
| 20 | 3.3 | 3.3 | 8.1 | −2.6 | 30.4 | −5.4 |
| ΔQH2O (%) | ΔQcooler (%) |
|---|---|
| −20 | −11.2 |
| −10 | −5.6 |
| 0 | 0.0 |
| 10 | 5.5 |
| 20 | 10.9 |
| ΔQH2O (%) | ΔQFEEDREAC (%) | ΔQRECIRCS (%) | ΔQGASPROD (%) | ΔQLIQPROD (%) | ΔQAQUEOUS (%) | ΔQORGANIC (%) |
|---|---|---|---|---|---|---|
| −20 | 0.0 | 17.2 | 8.9 | 7.9 | −9.3 | 8.6 |
| −10 | 0.0 | 7.3 | 3.7 | 3.4 | −3.9 | 3.6 |
| 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 10 | 0.0 | −6.0 | −3.1 | −2.8 | 3.2 | −3.0 |
| 20 | 0.0 | −11.3 | −5.7 | −5.3 | 6.0 | −5.5 |
| ΔQH2O (%) | ΔQcooler (%) |
|---|---|
| −20 | 6.6 |
| −10 | 2.8 |
| 0 | 0.0 |
| 10 | −2.2 |
| 20 | −4.2 |
| ΔEa (%) | ΔQFEEDREAC (%) | ΔQRECIRCS (%) | ΔQGASPROD (%) | ΔQLIQPROD (%) | ΔQAQUEOUS (%) | ΔQORGANIC (%) |
|---|---|---|---|---|---|---|
| −20 | 0.0 | 13.2 | 23.8 | −15.0 | −16.5 | 25.4 |
| −10 | 0.0 | 10.8 | 20.3 | −13.2 | −12.6 | 22.1 |
| 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 10 | 0.0 | −9.3 | −22.2 | 17.3 | −5.21 | −20.4 |
| 20 | 0.0 | −16.1 | −32.6 | 22.7 | −7.94 | −26.5 |
| ΔEa (%) | ΔYield (%) | ΔCBIOMASS (%) | ΔCCELOBIOSE (%) | ΔCARABINOSE (%) | ΔCGALACTOSE (%) | ΔCXYLOSE (%) | ΔCp-COUMARYL (%) |
|---|---|---|---|---|---|---|---|
| −20 | 41.9 | 38.5 | 58.1 | 3.4 | 3.3 | 3.3 | 2.7 |
| −10 | 37.2 | 34.0 | 54.0 | 2.5 | 2.5 | 2.5 | 2.0 |
| 0 | 0 | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 10 | −46.3 | −41.6 | −88.0 | −7.0 | −7.0 | −7.0 | −6.4 |
| 20 | −60.47 | −55.5 | −99.4 | −23.5 | −23.4 | −23.4 | −23.2 |
| ΔQH2O (%) | ΔQcooler (%) |
|---|---|
| −20 | 19.6 |
| −10 | 16.5 |
| 0 | 0.0 |
| 10 | −17.2 |
| 20 | −26.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecílio, D.M.; Gonçalves, J.R.M.; Correia, M.J.N.; Mateus, M.M. Aspen Plus® Modeling and Simulation of an Industrial Biomass Direct Liquefaction Process. Fuels 2023, 4, 221-242. https://doi.org/10.3390/fuels4020014
Cecílio DM, Gonçalves JRM, Correia MJN, Mateus MM. Aspen Plus® Modeling and Simulation of an Industrial Biomass Direct Liquefaction Process. Fuels. 2023; 4(2):221-242. https://doi.org/10.3390/fuels4020014
Chicago/Turabian StyleCecílio, Duarte M., J. Ricardo M. Gonçalves, Maria Joana Neiva Correia, and Maria Margarida Mateus. 2023. "Aspen Plus® Modeling and Simulation of an Industrial Biomass Direct Liquefaction Process" Fuels 4, no. 2: 221-242. https://doi.org/10.3390/fuels4020014
APA StyleCecílio, D. M., Gonçalves, J. R. M., Correia, M. J. N., & Mateus, M. M. (2023). Aspen Plus® Modeling and Simulation of an Industrial Biomass Direct Liquefaction Process. Fuels, 4(2), 221-242. https://doi.org/10.3390/fuels4020014









