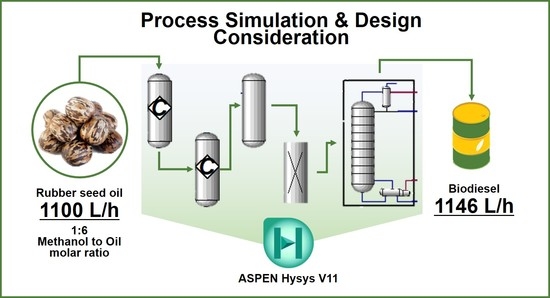
Process Simulation and Design Considerations for Biodiesel Production from Rubber Seed Oil
Abstract
1. Introduction
2. Design Considerations
2.1. Biodiesel Feedstock
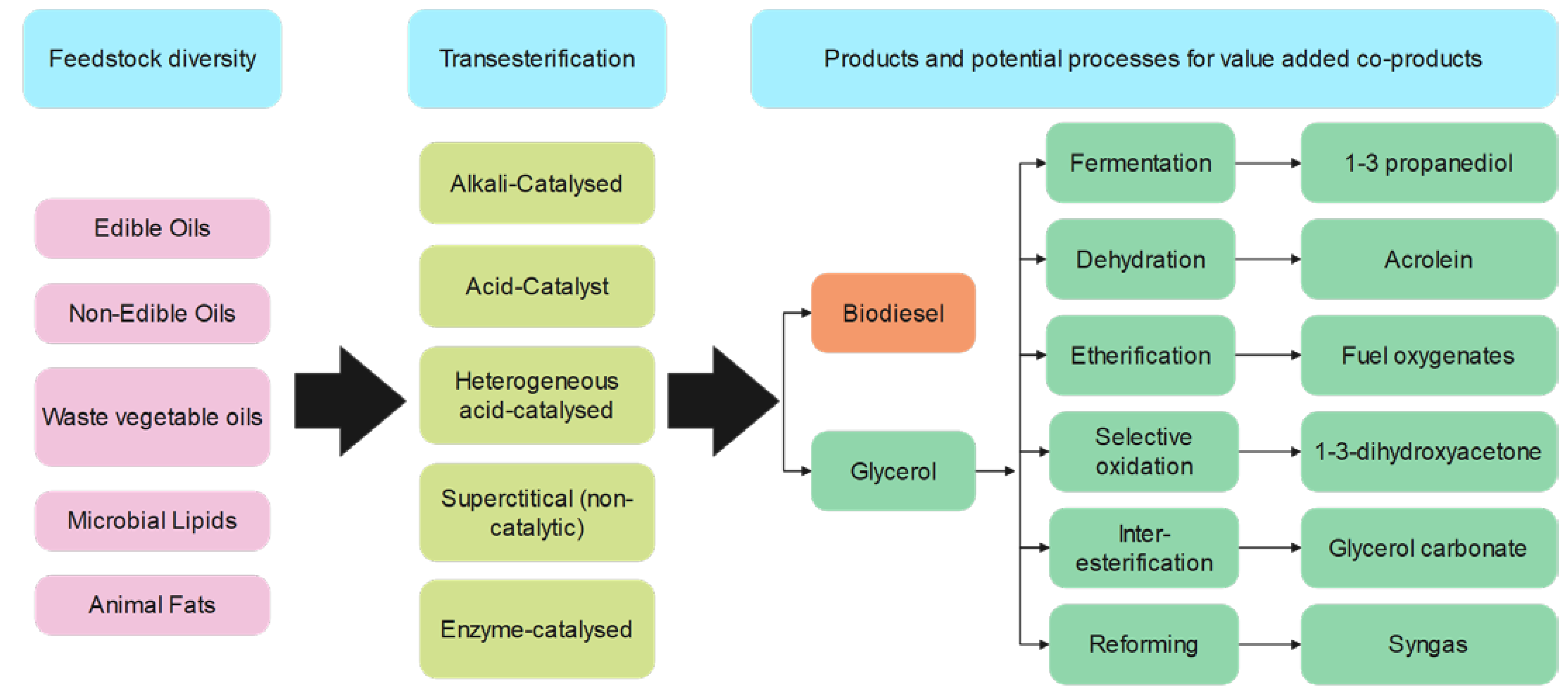
2.2. Biodiesel Production Technologies
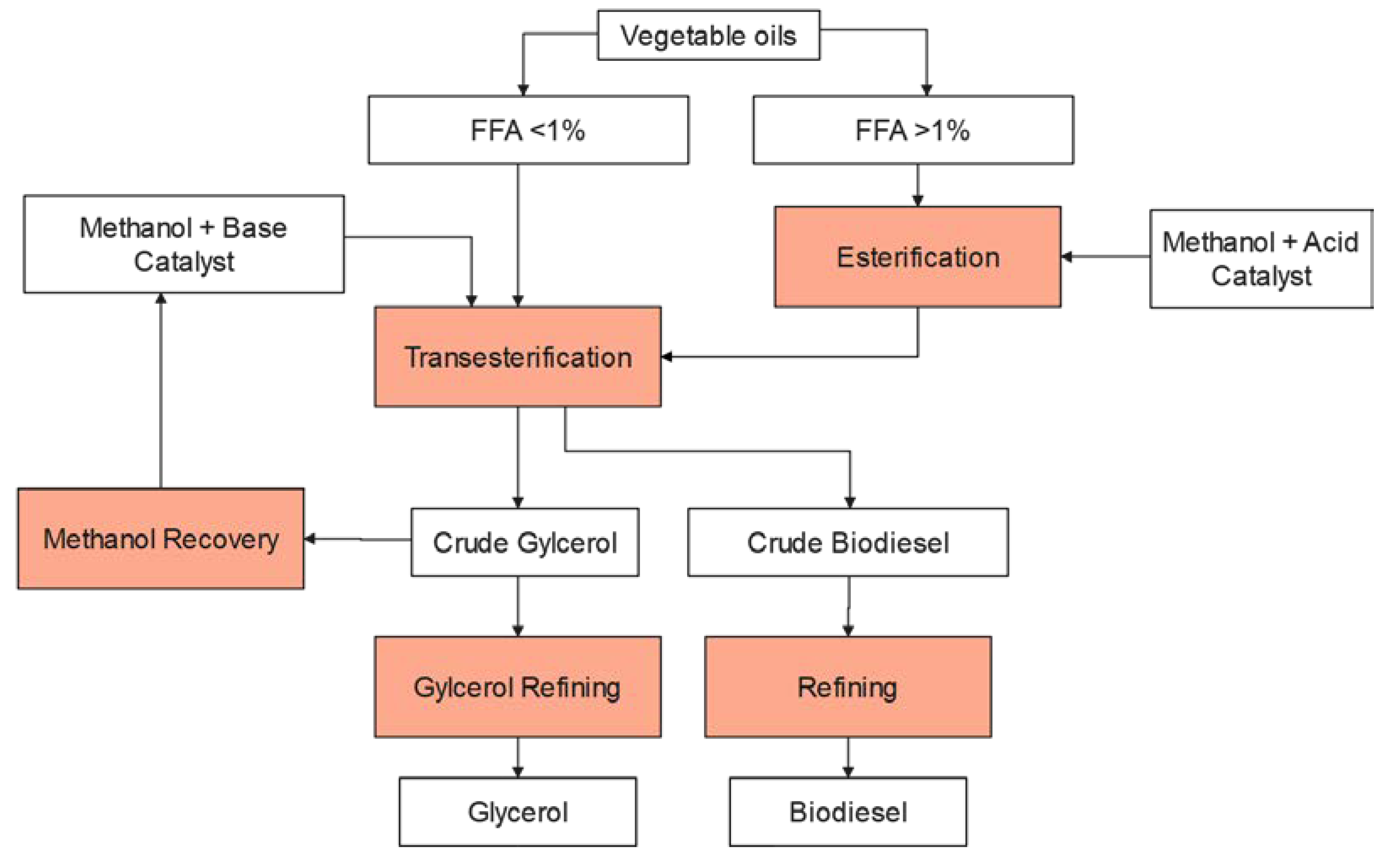
2.3. Transesterification Process
2.3.1. Esterification Process
2.3.2. Transesterification Process
2.3.3. Biodiesel Purification
2.4. Effect of Process Parameter
2.4.1. Temperature
2.4.2. Alcohol to Oil Ratio
2.4.3. Catalyst Concentration
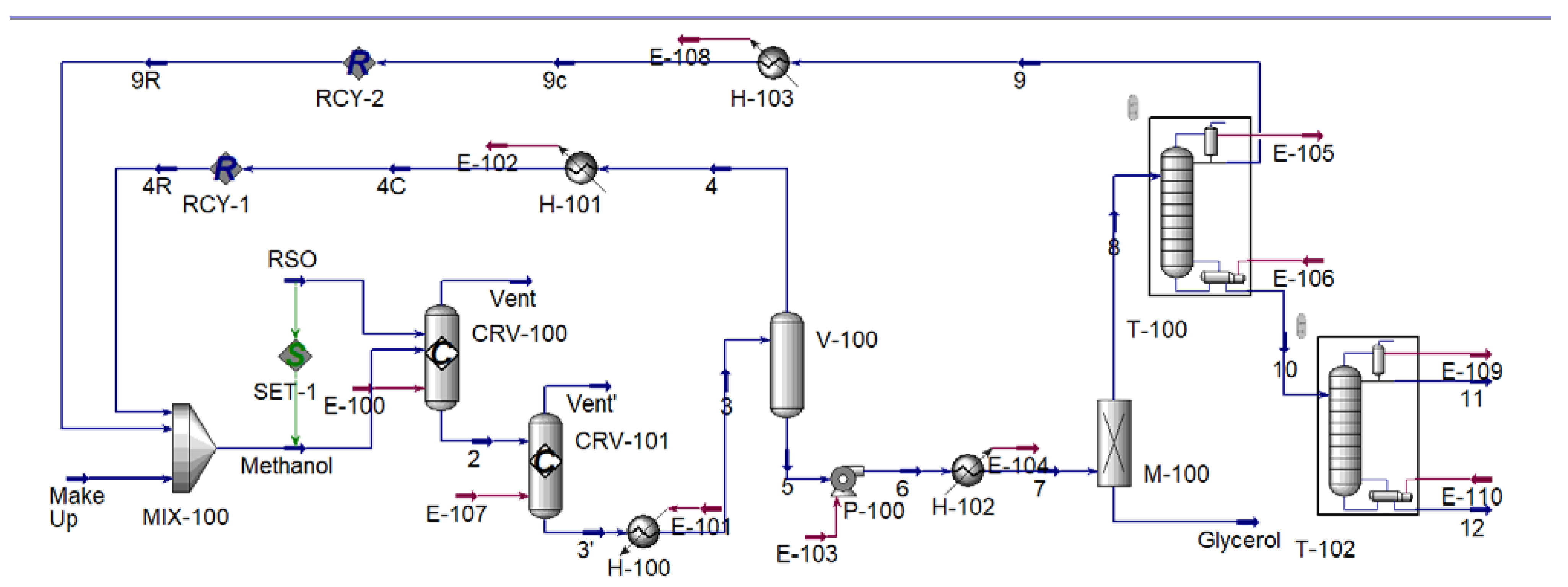
2.5. Simulation Methodology
3. Results and Discussion
3.1. Process Simulation
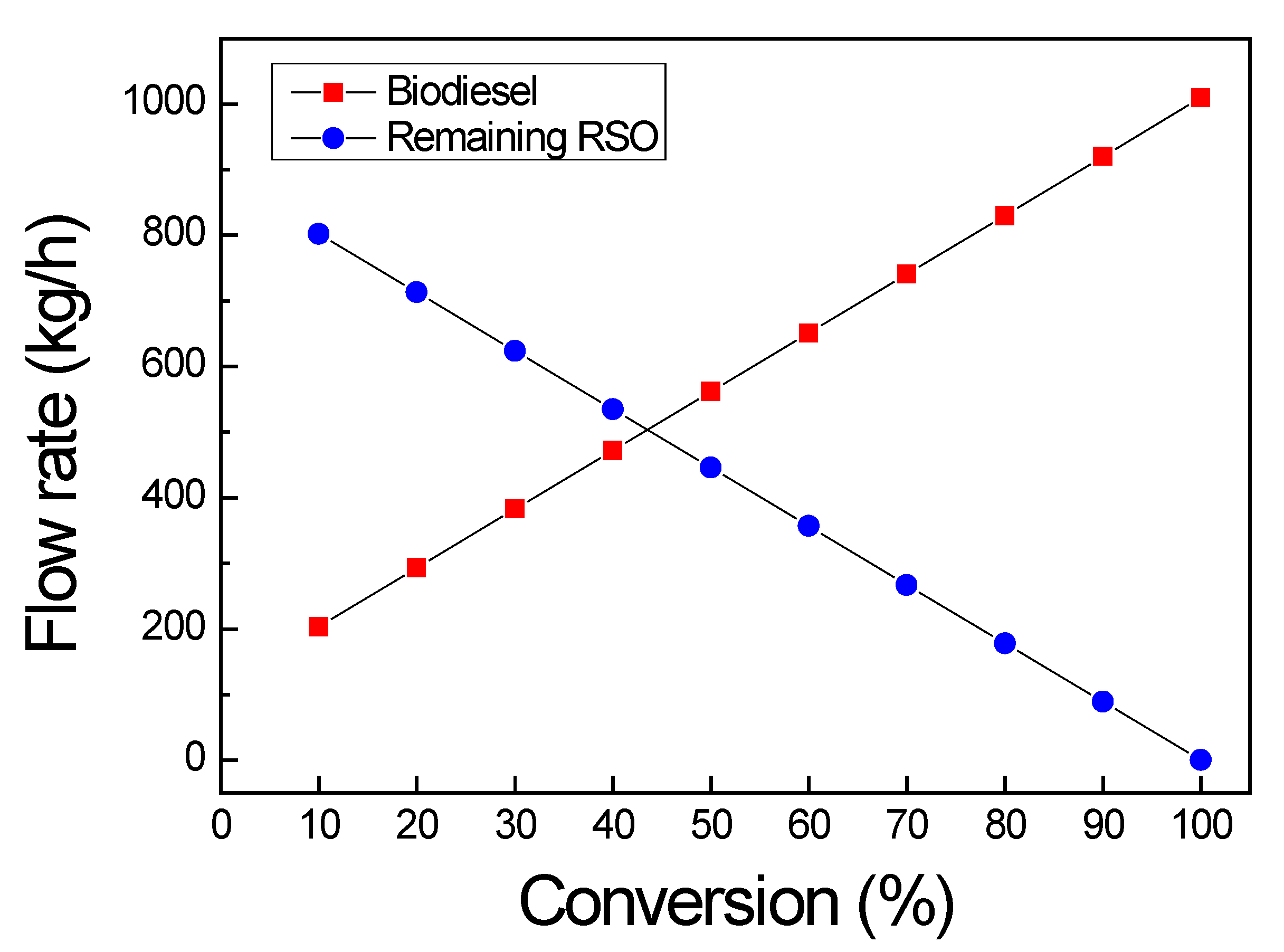
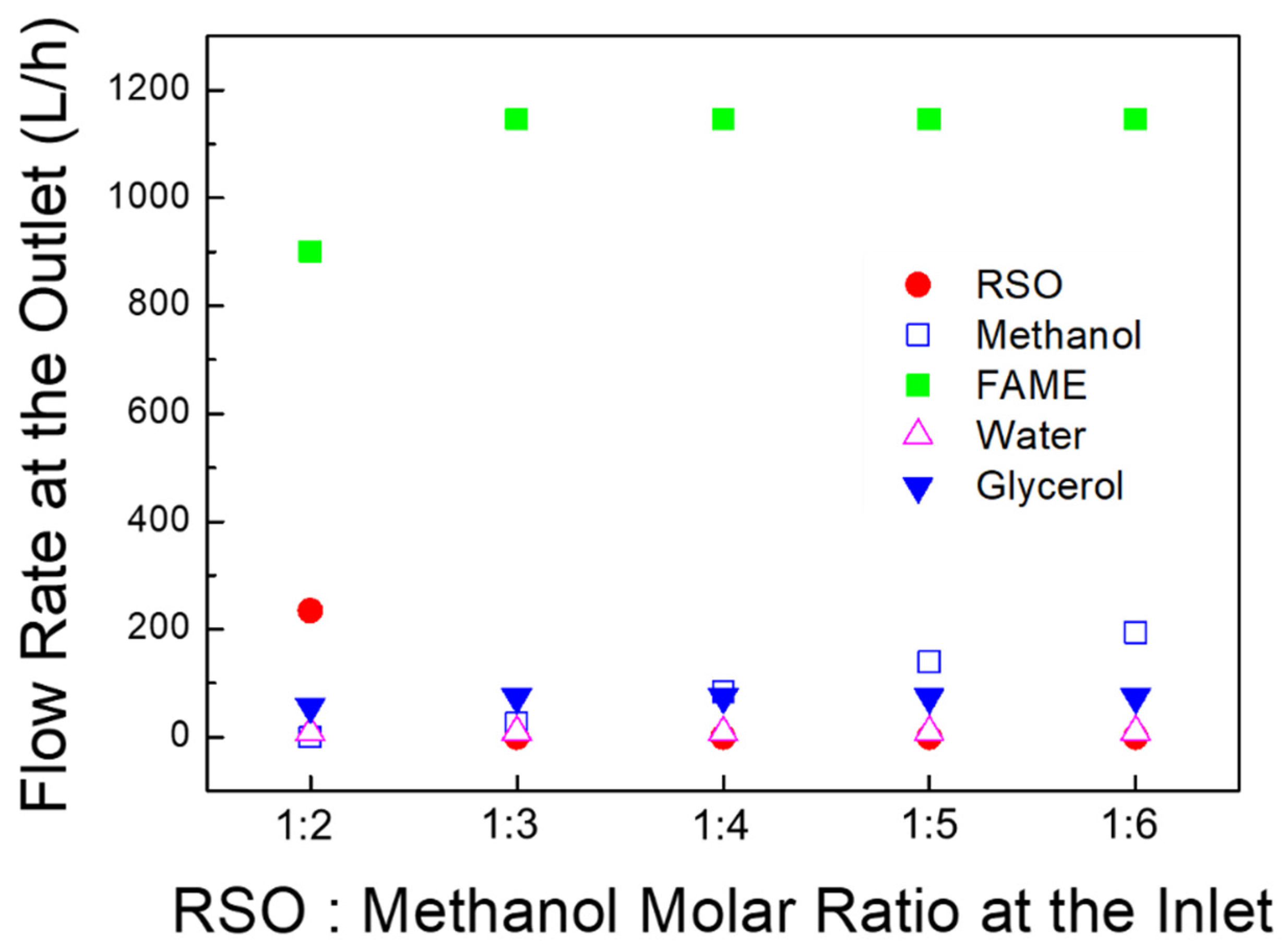
3.1.1. Material Balance
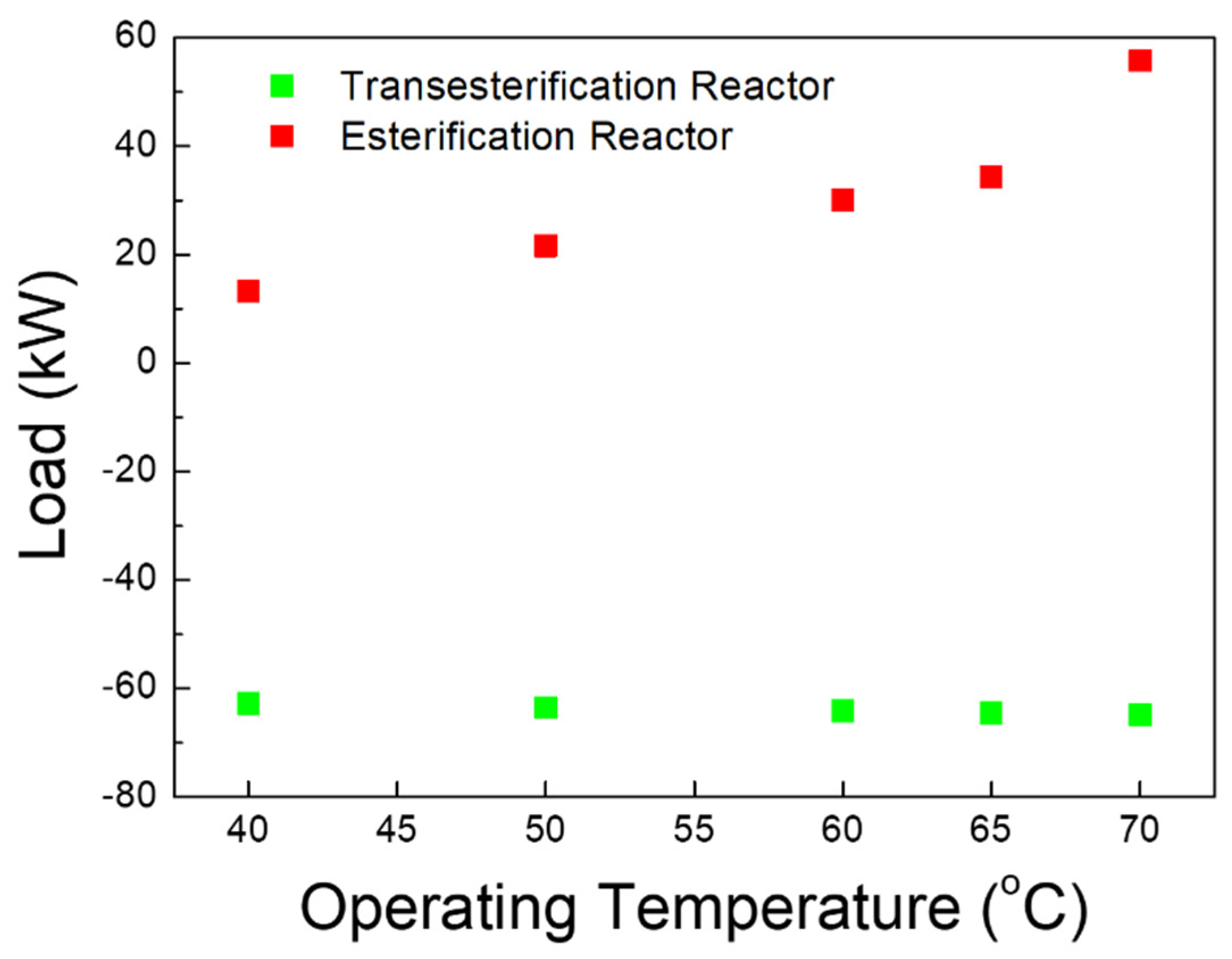
3.1.2. Energy Balance
3.1.3. Biodiesel Properties
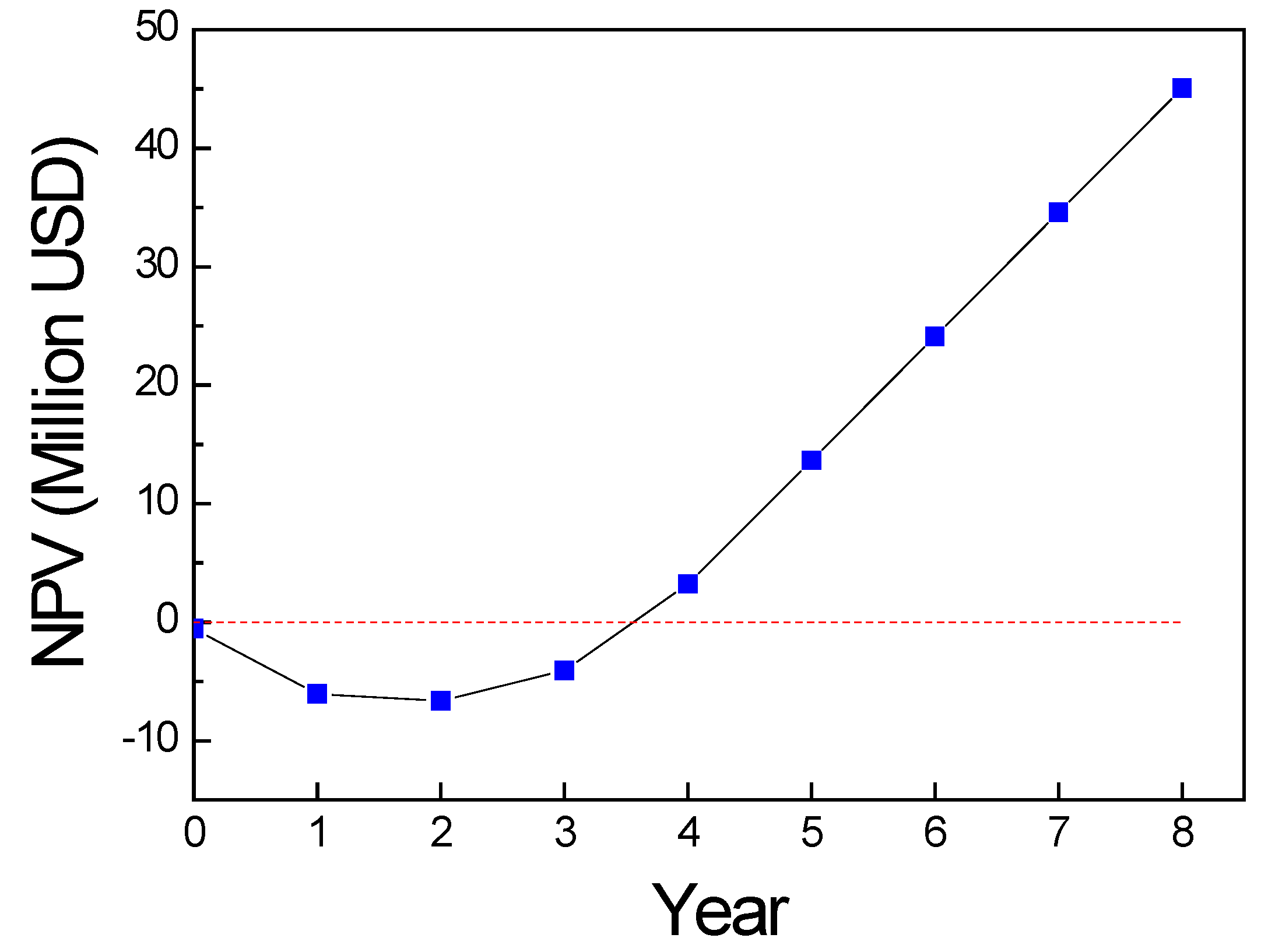
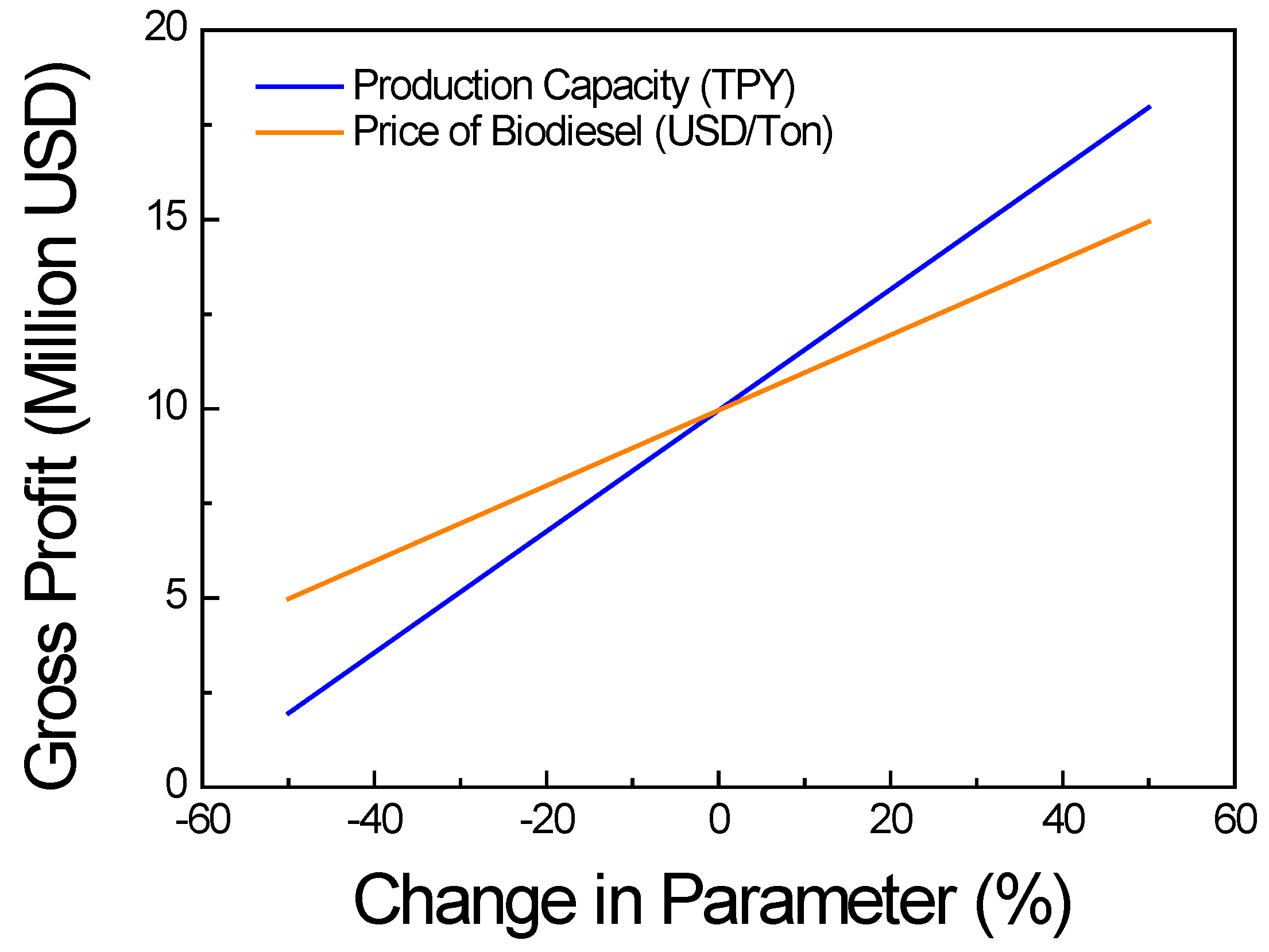
3.1.4. Economic Analysis
- = lower discount rate chosen
- = higher discount rate chosen
- NPVa = Net Present Value at
- NPVb = Net Present Value at
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of fossil fuel energy consumption and environmental impacts in european countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef]
- Shahzad, U. The Need for Renewable Energy Sources. Int. J. Inf. Technol. Electr. Eng. 2012, 15, 16–18. [Google Scholar]
- Altin, R.; Çetinkaya, S.; Yücesu, H.S. Potential of using vegetable oil fuels as fuel for diesel engines. Energy Convers. Manag. 2001, 42, 529–538. [Google Scholar] [CrossRef]
- Galloni, E.; Scala, F.; Fontana, G. Influence of fuel bio-alcohol content on the performance of a turbo-charged, PFI, spark-ignition engine. Energy 2019, 170, 85–92. [Google Scholar] [CrossRef]
- Yang, L.; Ge, X.; Wan, C.; Yu, F.; Li, Y. Progress and perspectives in converting biogas to transportation fuels. Renew. Sustain. Energy Rev. 2014, 40, 1133–1152. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O.; Adeoye, A.S.; Onifade, D.V. Modelling and optimisation of biodiesel production from Euphorbia lathyris using ASPEN Hysys. SN Appl. Sci. 2019, 1, 1452. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.O.; Madzimbamuto, T.F.; Ikhu-Omoregbe, D.I.O. A review of current technology for biodiesel production: State of the art. Biomass Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Trirahayu, D.A. Simulation of Rice Bran Oil Transesterification Process for Biodiesel Production. In International Seminar of Science and Applied Technology (ISSAT 2020); Atlantis Press: Bandung, Indonesia, 2020; Volume 198, pp. 384–387. [Google Scholar] [CrossRef]
- Trirahayu, D.A. Process simulation of propylene production from crude palm oil by hydrodeoxygenation and propane dehydrogenation. J. Phys. Conf. Ser. 2020, 1450, 012009. [Google Scholar]
- Trirahayu, D.A. Process simulation of glycerol production from corn oil via transesterification. IOP Conf. Ser. Mater. Sci. Eng. 2020, 830, 6–10. [Google Scholar] [CrossRef]
- Trirahayu, D.A. Process Simulation of Glycerol Conversion to Formic Acid Using Hydrothermal Oxidation. In International Conference on Innovation in Science and Technology (ICIST 2020); Atlantis Press: Padang, Indonesia, 2021; Volume 208, pp. 179–182. [Google Scholar]
- Abidin, A.Z.; Choliq, N.S.; Yemensia, E.V.; Hastuti, R. Study on Environmental Health Aspect of Plastic Refinery in MASARO Cirebon Unit in Indonesia. In Proceedings of the 2020 4th International Conference on Green Energy and Applications (ICGEA), Singapore, 7–9 March 2020; pp. 116–120. [Google Scholar] [CrossRef]
- Abidin, A.Z.; Yemensia, E.V.; Wijaya, K.W.; Rahardjo, A.P. Circular Economy on Non-Biodegradable Waste Management with MASARO Technology. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1143, 012052. [Google Scholar] [CrossRef]
- Abduh, M.Y.; Manurung, R.; Heeres, H.J. Techno-Economic Analysis for Small Scale Production of Rubber Seed Oil and Biodiesel in Palangkaraya, Indonesia. J. Clean Energy Technol. 2017, 5, 268–273. [Google Scholar] [CrossRef]
- Aryasomayajula Venkata Satya Lakshmi, S.B.; Subramania Pillai, N.; Khadhar Mohamed, M.S.B.; Narayanan, A. Biodiesel production from rubber seed oil using calcined eggshells impregnated with Al2O3 as heterogeneous catalyst: A comparative study of RSM and ANN optimization. Braz. J. Chem. Eng. 2020, 37, 351–368. [Google Scholar] [CrossRef]
- Bharadwaj, A.V.S.L.S.; Singh, M.; Niju, S.; Begum, K.M.M.S.; Anantharaman, N. Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst-optimization and modeling studies. Green Processing Synth. 2019, 8, 430–442. [Google Scholar] [CrossRef]
- Gimbun, J.; Ali, S.; Kanwal, C.C.S.C.; Shah, L.A.; Muhamad, N.H.; Cheng, C.K.; Nurdin, S. Biodiesel production from rubber seed oil using activated cement clinker as catalyst. Procedia Eng. 2013, 53, 13–19. [Google Scholar] [CrossRef]
- Samart, C.; Karnjanakom, S.; Chaiya, C.; Reubroycharoen, P.; Sawangkeaw, R.; Charoenpanich, M. Statistical optimization of biodiesel production from para rubber seed oil by SO3H-MCM-41 catalyst. Arab. J. Chem. 2019, 12, 2028–2036. [Google Scholar] [CrossRef]
- Onoji, S.E.; Iyuke, S.E.; Igbafe, A.I.; Daramola, M.O. Transesterification of Rubber Seed Oil to Biodiesel over a Calcined Waste Rubber Seed Shell Catalyst: Modeling and Optimization of Process Variables. Energy Fuels 2017, 31, 6109–6119. [Google Scholar] [CrossRef]
- Zamberi, M.M.; Ani, F.N. Biodiesel production from high FFA rubber seed oil using waste cockles. ARPN J. Eng. Appl. Sci. 2016, 11, 7782–7787. [Google Scholar]
- Sebastian, J.; Muraleedharan, C.; Santhiagu, A. A comparative study between chemical and enzymatic transesterification of high free fatty acid contained rubber seed oil for biodiesel production. Cogent Eng. 2016, 3, 1178370. [Google Scholar] [CrossRef]
- El-Enin, S.A.A.; Attia, N.K.; El-Ibiari, N.N.; El-Diwani, G.I.; El-Khatib, K.M. In-situ transesterification of rapeseed and cost indicators for biodiesel production. Renew. Sustain. Energy Rev. 2013, 18, 471–477. [Google Scholar] [CrossRef]
- Nguyen, T.; Do, L.; Sabatini, D.A. Biodiesel production via peanut oil extraction using diesel-based reverse-micellar microemulsions. Fuel 2010, 89, 2285–2291. [Google Scholar] [CrossRef]
- Tupufia, S.C.; Jeon, Y.J.; Marquis, C.; Adesina, A.A.; Rogers, P.L. Enzymatic conversion of coconut oil for biodiesel production. Fuel Processing Technol. 2013, 106, 721–726. [Google Scholar] [CrossRef]
- Zahan, K.A.; Kano, M. Biodiesel production from palm oil, its by-products, and mill effluent: A review. Energies 2018, 11, 2132. [Google Scholar] [CrossRef]
- Degirmenbasi, N.; Coskun, S.; Boz, N.; Kalyon, D.M. Biodiesel synthesis from canola oil via heterogeneous catalysis using functionalized CaO nanoparticles. Fuel 2015, 153, 620–627. [Google Scholar] [CrossRef]
- Chakraborty, R.; Das, S.; Bhattacharjee, S.K. Optimization of biodiesel production from Indian mustard oil by biological tri-calcium phosphate catalyst derived from Turkey bone ash. Clean Technol. Environ. Policy 2015, 17, 455–463. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; Cortés, Á.G. High oleic safflower oil as a feedstock for stable biodiesel and biolubricant production. Ind. Crops Prod. 2021, 170, 113701. [Google Scholar] [CrossRef]
- Thirumarimurugan, M.; Sivakumar, V.M.; Xavier, A.M.; Prabhakaran, D.; Kannadasan, T. Preparation of Biodiesel from Sunflower Oil by Transesterification. Int. J. Biosci. Biochem. Bioinform. 2012, 2, 441–444. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Das, L.M.; Babu, M.K.G.; Naik, S.N. Biodiesel development from high acid value polanga seed oil and performance evaluation in a CI engine. Fuel 2007, 86, 448–454. [Google Scholar] [CrossRef]
- Chandrashekar, L.A.; Mahesh, N.S.; Gowda, B.; Hall, W. Life cycle assessment of biodiesel production from pongamia oil in rural Karnataka. Agric. Eng. Int. CIGR J. 2012, 14, 67–77. [Google Scholar]
- Keera, S.T.; El Sabagh, S.M.; Taman, A.R. Castor oil biodiesel production and optimization. Egypt. J. Pet. 2018, 27, 979–984. [Google Scholar] [CrossRef]
- Shah, M.; Ali, S.; Tariq, M.; Khalid, N.; Ahmad, F.; Khan, M.A. Catalytic conversion of jojoba oil into biodiesel by organotin catalysts, spectroscopic and chromatographic characterization. Fuel 2014, 118, 392–397. [Google Scholar] [CrossRef]
- Usta, N.; Aydoǧan, B.; On, A.H.; Uǧuzdǒan, E.; Özkal, S.G. Properties and quality verification of biodiesel produced from tobacco seed oil. Energy Convers. Manag. 2011, 52, 2031–2039. [Google Scholar] [CrossRef]
- Patel, R.L.; Sankhavara, C.D. Biodiesel production from Karanja oil and its use in diesel engine: A review. Renew. Sustain. Energy Rev. 2017, 71, 464–474. [Google Scholar] [CrossRef]
- Anwar, F.; Rashid, U.; Ashraf, M.; Nadeem, M. Okra (Hibiscus esculentus) seed oil for biodiesel production. Appl. Energy 2010, 87, 779–785. [Google Scholar] [CrossRef]
- Eevera, T.; Pazhanichamy, K. Cotton seed oil: A feasible oil source for biodiesel production. Energy Sources Part A Recovery Util. Environ. Eff. 2013, 35, 1118–1128. [Google Scholar] [CrossRef]
- Ulfah, M.; Mulyazmi, M.; Burmawi, B.; Praputri, E.; Sundari, E.; Firdaus, F. Biodiesel production methods of rubber seed oil: A review. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Banda Aceh, Indonesia, 2018; p. 334. [Google Scholar] [CrossRef]
- Krishnakumar, U.; Sivasubramanian, V.; Selvaraju, N. Physico-Chemical Properties of the Biodiesel Extracted from Rubber Seed Oil Using Solid Metal Oxide Catalysts. Int. J. Eng. Res. Appl. 2013, 3, 2206–2209. Available online: www.ijera.com (accessed on 2 June 2022).
- Tabatabaei, M.M.A.; Dehhaghi, M.; Panahi, H.K.S.; Mollahosseini, A.; Hosseini, M.; Soufiyan, M.M. Reactor technologies for biodiesel production and processing: A review. Prog. Energy Combust. Sci. J. 2019, 74, 239–303. [Google Scholar]
- Yusuf, N.N.A.N.; Kamarudin, S.K.; Yaakob, Z. Overview on the production of biodiesel from Jatropha curcas L. by using heterogenous catalysts. Biofuels Bioprod. Biorefining 2012, 6, 246–256. [Google Scholar] [CrossRef]
- Pasha, M.K.; Dai, L.; Liu, D.; Guo, M.; Du, W. An overview to process design, simulation and sustainability evaluation of biodiesel production. Biotechnol. Biofuels 2021, 14, 129. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production-A review. Clean Energy 2019, 3, 2–23. [Google Scholar] [CrossRef]
- Salam, K.A.; Velasquez-Orta, S.B.; Harvey, A.P. A sustainable integrated in situ transesterification of microalgae for biodiesel production and associated co-products-A review. Renew. Sustain. Energy Rev. 2016, 65, 1179–1198. [Google Scholar] [CrossRef]
- Lee, D. Preparation of a sulfonated carbonaceous material from lignosulfonate and its usefulness as an esterification catalyst. Molecules 2013, 18, 8168–8180. [Google Scholar] [CrossRef]
- Zaccheria, F.; Brini, S.; Psaro, R.; Scotti, N.; Ravasio, N. Esterification of acidic oils over a versatile amorphous solid catalyst. ChemSusChem 2009, 2, 535–537. [Google Scholar] [CrossRef]
- Lin, L.; Ying, D.; Chaitep, S.; Vittayapadung, S. Biodiesel production from crude rice bran oil and properties as fuel. Appl. Energy 2009, 86, 681–688. [Google Scholar] [CrossRef]
- Mazaheri, H.; Ong, H.C.; Masjuki, H.H.; Amini, Z.; Harrison, M.D.; Wang, C.T.; Kusumo, F.; Alwia, A. Rice bran oil based biodiesel production using calcium oxide catalyst derived from Chicoreus brunneus shell. Energy 2018, 144, 10–19. [Google Scholar] [CrossRef]
- Evangelista, J.P.C.; Chellappa, T.; Coriolano, A.C.F.; Fernandes, V.J.; Souza, L.D.; Araujo, A.S. Synthesis of alumina impregnated with potassium iodide catalyst for biodiesel production from rice bran oil. Fuel Processing Technol. 2012, 104, 90–95. [Google Scholar] [CrossRef]
- Jariah, N.F.; Hassan, M.A.; Taufiq-Yap, Y.H.; Roslan, A.M. Technological advancement for efficiency enhancement of biodiesel and residual glycerol refining: A mini review. Processes 2021, 9, 1198. [Google Scholar] [CrossRef]
- Saleh, J.; Tremblay, A.Y.; Dubé, M.A. Glycerol removal from biodiesel using membrane separation technology. Fuel 2010, 89, 2260–2266. [Google Scholar] [CrossRef]
- Trisnaliani, L.; Zaki, A. Separation of Glycerol from Biodiesel Oil Products Using High Voltage Electrolysis Method. Indones. J. Fundam. Appl. Chem. 2018, 3, 7–11. [Google Scholar] [CrossRef]
- Dhar, B.R.; Kirtania, K. Excess Methanol Recovery in Biodiesel Production Process Using a Distillation Column: A Simulation Study. Chem. Eng. Res. Bull. 2009, 13, 55–60. [Google Scholar] [CrossRef]
- Baroutian, S.; Aroua, M.K.; Raman, A.A.A.; Sulaiman, N.M.N. Methanol recovery during transesterification of palm oil in a TiO2/Al2O3 membrane reactor: Experimental study and neural network modeling. Sep. Purif. Technol. 2010, 76, 58–63. [Google Scholar]
- Verma, P.; Sharma, M.P. Review of process parameters for biodiesel production from different feedstocks. Renew. Sustain. Energy Rev. 2016, 62, 1063–1071. [Google Scholar] [CrossRef]
- Ahmad, J.; Yusup, S.; Bokhari, A.; Kamil, R.N.M. Study of fuel properties of rubber seed oil based biodiesel. Energy Convers. Manag. 2014, 78, 266–275. [Google Scholar] [CrossRef]
- Mathew, G.M.; Raina, D.; Narisetty, V.; Kumar, V.; Saran, S.; Pugazhendi, A.; Sindhu, R.; Pandey, A.; Binod, P. Recent advances in biodiesel production: Challenges and solutions. Sci. Total Environ. 2021, 794, 148751. [Google Scholar] [CrossRef]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef]

| Vegetable Oil | Oleic Acid C18:1 | Linoleic Acid C18:2 | Linolenic Acid C18:3 | Palmitic Acid C16:0 | Stearic Acid C18:0 |
|---|---|---|---|---|---|
| Edible Oil | |||||
| Rapeseed oil | 53–70 | 15–30 | 5–13 | 2.5–6.5 | 0.8–3.0 |
| Peanut oil | 33 | 13.3 | 0.2 | 26.5 | 2.9 |
| Palm Oil | 39 | 11 | - | 45 | 4 |
| Soybean oil | 23.4 | 53.2 | 7.8 | 11.0 | 4.0 |
| Corn Oil | 30–50 | 34–56 | 0.5–1.5 | 8–10 | 1–4 |
| Sunflower Oil | 20.6 | 66.2 | 0.8 | 4.8 | 5.7 |
| Non-edible Oil | |||||
| Polanga Oil | 34.09 | 38.26 | 0.3 | 12.01 | 12.95 |
| Castor Bean Oil | 36–64 | 18–45 | 2.4–3.4 | 10–17 | 5–10 |
| Rubber Seed Oil (RSO) | 12.8–24.9 | 18.9–39.6 | 8–18.2 | 6.5–10.2 | 6.6–9.9 |
| Karanja Oil | 51.6–72.2 | 11.8–16.5 | 0–2.65 | 9.8–11.65 | 6.2–7.5 |
| Cotton Seed Oil | 13.3–21.7 | 46.7–58.2 | 0 | 11.7–26.4 | 0.9–5.0 |
| Animal oils and fats | |||||
| Chicken fat | 34.6 | 30.9 | 2.9 | 19.8 | 6.1 |
| Lamb meat (oil) | 35.0 | 36.0 | - | 10.1 | 6.0 |
| Fish waste (oil) | 17.3 | 1.7 | 2.9 | 10.1 | 6.0 |
| Beef tallow | 46.4 | 2.7 | 0 | 24.8 | 20.6 |
| Microbial lipid | |||||
| Fungi | 30.1–41.3 | 8.7–23.3 | 0.1–0.6 | 20.1–36.0 | 10.7–23.6 |
| Algae | 13.6–17.2 | 33.7–40.8 | 11.3–18.5 | 24.5–36.4 | 1.0–2.1 |
| Microalgae | 7.8–14.9 | 6.8–8.3 | 15.4–25.0 | 10.8–16.7 | 2.3–2.6 |
| Yeast | 3.5–38.6 | 2.7–14.6 | - | 2.8–24.1 | 4.6–7.7 |
| Waste Cooking Oil | 46.0 | 3.9 | 0.3 | 24.6 | 18.4 |
| Production Process | Advantage | Disadvantage |
|---|---|---|
| Direct use and blending |
|
|
| Microemulsion |
|
|
| Pyrolysis (Thermal Cracking) |
|
|
| Transesterification |
|
|
| RSO:Methanol | Catalyst | Temperature | Reaction Time | Yield | Ref |
|---|---|---|---|---|---|
| 1:12 | Eggshell-Al2O3 3% | 65 | 4 h | 98.9 | [16] |
| 1:12 | Eggsshell 4% | 65 | 3 h | 99.7 | [17] |
| 1:4 | Cement cklinker 5% | 65 | 4 h | 96.9 | [18] |
| 1:16 | SO3H-MCM-41 14.5% | 129.6 | 48 h | 83.10 | [19] |
| 1:16 | Water cockle shell 9% | 60–64 | 3 h | 88.06 | [20] |
| 1:6 | KOH | 55 | ~1 h | 96.8 | [57] |
| Parameters | Values |
|---|---|
| RSO flowrate (L/h) | 1100 |
| Methanol to oil ratio | 6:1 |
| Reaction temperature (°C) | 65 |
| Conversion (%) | 10–100 |
| RSO compositions (%-mole) | |
| 24.33 |
| 32.78 |
| 12.37 |
| 3.27 |
| 27.25 |
| Code | Description |
|---|---|
| MIX-100 | Mixer |
| CRV-100 | Esterification Reactor |
| CRV-101 | Transesterification Reactor |
| V-100 | Flash drum |
| M-100 | Membrane |
| T-100 and T-102 | Distillation Tower |
| P-100 | Pump |
| H-100, H-101, H-102, and H-103 | Heat Exchanger |
| Type | Equipment | Consumption (kW) | Total (kW) |
|---|---|---|---|
| Electricity | E-103 | 0.23 | 0.222 |
| Heating stream | E-100 | 34.42 | 319.38 |
| E-101 | 25.93 | ||
| E-106 | 172.40 | ||
| E-110 | 86.63 | ||
| Cooling stream | E-102 | 11.86 | 208.69 |
| E-104 | 44.92 | ||
| E-105 | 77.31 | ||
| E-107 | 64.48 | ||
| E-108 | 5.02 | ||
| E-109 | 5.10 |
| Properties | ASTM D 6751 Standards | EN 14214 Standards | Onoji et al. [20] | Ahmad et al. [57] | This Study |
|---|---|---|---|---|---|
| Water & sediment, max | <0.05 | <0.05 | 0.0062 | 0.042 | 0.01 |
| Viscosity (cSt) @ 40 °C | 1.9–6.0 | 3.5–5.0 | 4.32 | 3.89 | 1.811 |
| Density @15 °C (kg/m3) | 870–900 | 860–900 | 876 | 885 | 880.6 |
| Ester content | >96.5 | 96.7 | 96.8 | 99.93 |
| Parameter | Value |
|---|---|
| RSO Price (USD/ton) | 550 |
| Biodiesel Price (USD/ton) | 2000 |
| Glycerol price (USD/ton) | 2100 |
| Biodiesel production capacity (TPY) | 8000 |
| Glycerol production (TPY) | 750 |
| Total capital cost (million USD) | 5.50 |
| Production cost (million USD/year) | 1.80 |
| Raw materials cost (million USD/year) | 4.24 |
| Biodiesel revenue (million USD/year) | 16 |
| Glycerol revenue (million USD/year) | 1.6 |
| Parameter | Value |
|---|---|
| PBP | 1.60 years |
| ROROI | 144% |
| IRR | 28% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trirahayu, D.A.; Abidin, A.Z.; Putra, R.P.; Hidayat, A.S.; Safitri, E.; Perdana, M.I. Process Simulation and Design Considerations for Biodiesel Production from Rubber Seed Oil. Fuels 2022, 3, 563-579. https://doi.org/10.3390/fuels3040034
Trirahayu DA, Abidin AZ, Putra RP, Hidayat AS, Safitri E, Perdana MI. Process Simulation and Design Considerations for Biodiesel Production from Rubber Seed Oil. Fuels. 2022; 3(4):563-579. https://doi.org/10.3390/fuels3040034
Chicago/Turabian StyleTrirahayu, Dhyna Analyes, Akhmad Zainal Abidin, Ridwan P. Putra, Achmad Syarif Hidayat, Erwina Safitri, and Muhammad Iqbal Perdana. 2022. "Process Simulation and Design Considerations for Biodiesel Production from Rubber Seed Oil" Fuels 3, no. 4: 563-579. https://doi.org/10.3390/fuels3040034
APA StyleTrirahayu, D. A., Abidin, A. Z., Putra, R. P., Hidayat, A. S., Safitri, E., & Perdana, M. I. (2022). Process Simulation and Design Considerations for Biodiesel Production from Rubber Seed Oil. Fuels, 3(4), 563-579. https://doi.org/10.3390/fuels3040034