Elemental Mapping and Characterization of Petroleum-Rich Rock Samples by Laser-Induced Breakdown Spectroscopy (LIBS)
Abstract
:1. Introduction
2. Methods
2.1. Samples and Preparation
2.2. Instrumentation
2.3. Calibration Standards Preparation
3. Results and Discussion
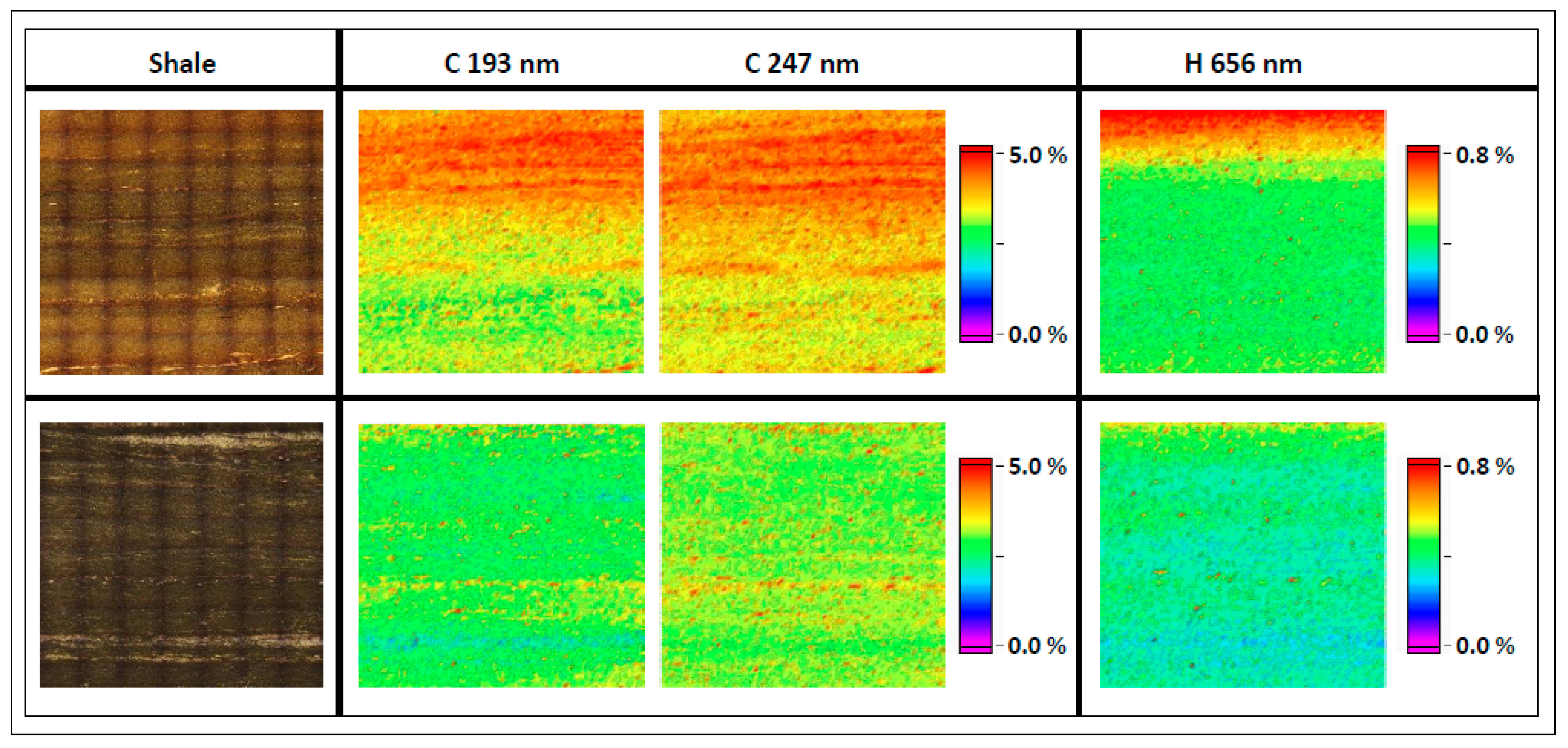
3.1. Elemental Mapping for Hydrocarbon Rich Samples

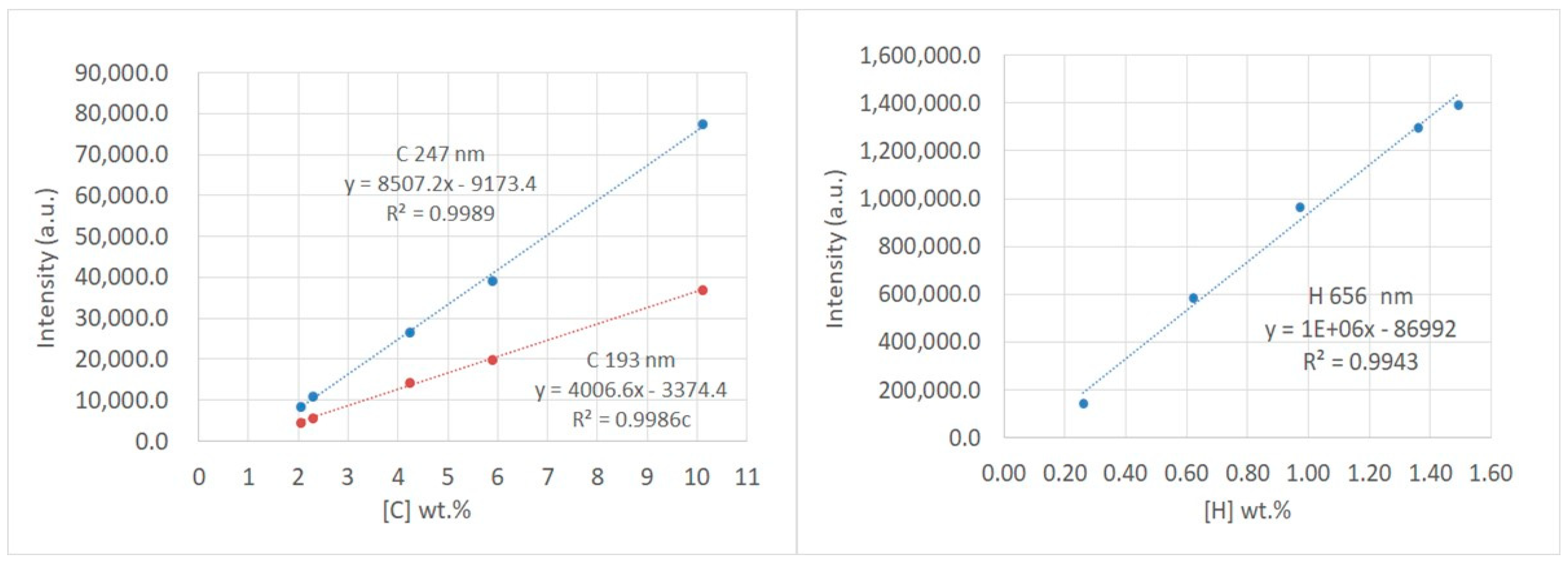
3.2. Calibration Method—Core and Shale
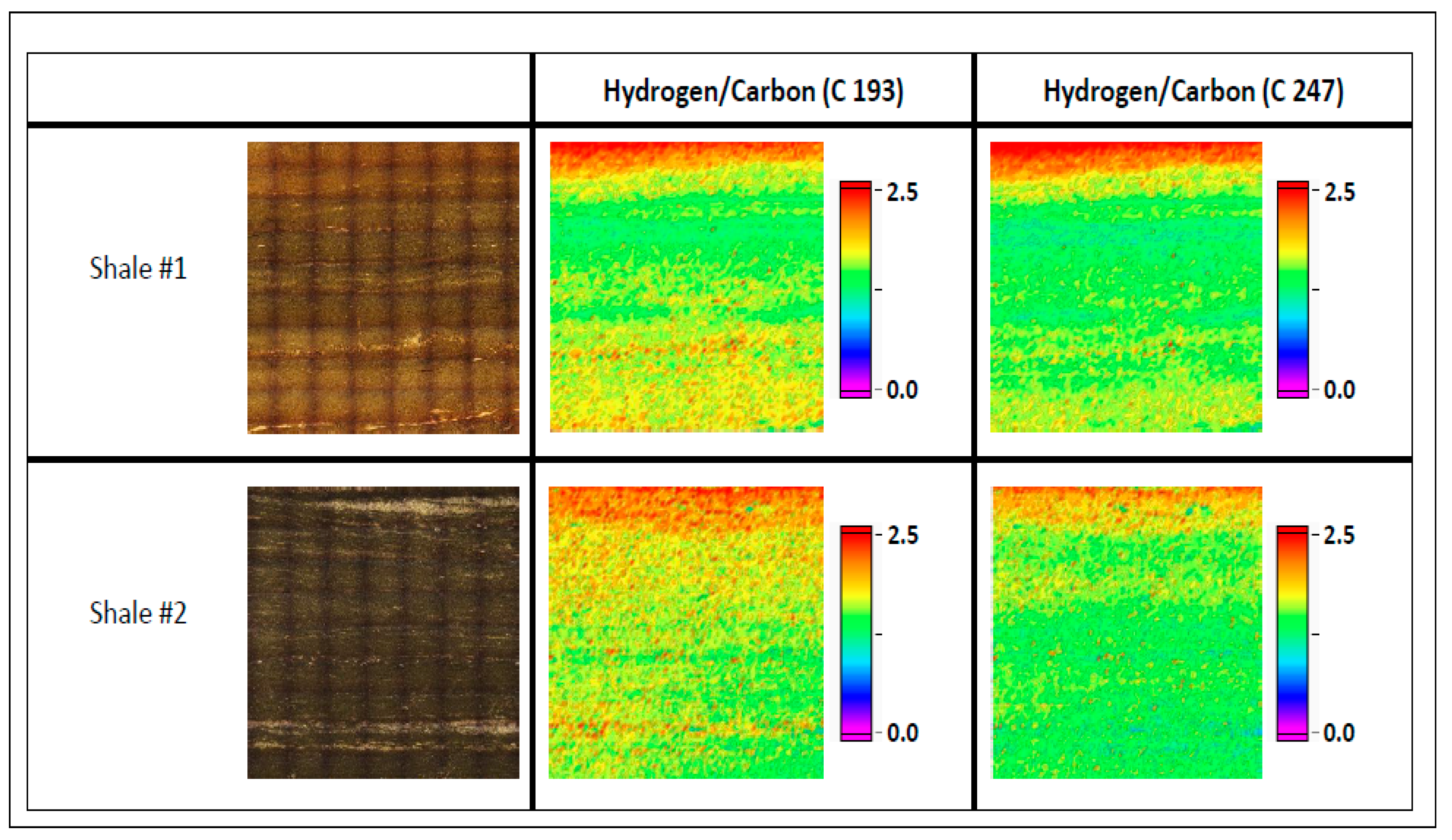
3.3. H/C Molar Ratio Mapping—Shale
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.; Speight, J.G.; Loyalka, S.K. Handbook of Alternative Fuel Technologies, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Al-Saddique, M.A.; Hamada, G.M.; Al-Awad, M.N. Recent advances in coring and core analysis technology new techniques to improve reservoir evaluation. Eng. J. Univ. Qatar 2000, 13, 29–52. [Google Scholar]
- Forbes, P. The status of core analysis. J. Pet. Sci. Eng. 1998, 19, 1–6. [Google Scholar] [CrossRef]
- Paul, F.W.; Longeron, D. Advances in Core Evaluation II: Reservoir Appraisal; Gordon and Breach Science Publisher: Philadelphia, PA, USA, 1991. [Google Scholar]
- Rezaee, R. Fundamentals of Gas Shale Reservoirs; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Nadkarni, K.R.A. Elemental Analysis of Fossil Fuels and Related Materials; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar] [CrossRef]
- Washburn, K.E. Rapid geochemical and mineralogical characterization of shale by laser-induced breakdown spectroscopy. Org. Geochem. 2015, 83, 114–117. [Google Scholar] [CrossRef]
- Birdwell, J.E.; Washburn, K.E. Rapid Analysis of Kerogen Hydrogen-to-Carbon Ratios in Shale and Mudrocks by Laser-Induced Breakdown Spectroscopy. Energy Fuels 2015, 29, 6999–7004. [Google Scholar] [CrossRef]
- Sanghapi, H.K.; Jain, J.; Bol’shakov, A.; Lopano, C.; McIntyre, D.; Russo, R. Determination of elemental composition of shale rocks by laser induced breakdown spectroscopy. Spectrochim. Acta Part B Spectrosc. 2016, 122, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Khajehzadeh, N.; Kauppinen, T.K. Fast mineral identification using elemental LIBS technique. FAC-PapersOnLine 2015, 28, 119–124. [Google Scholar] [CrossRef]
- Harmon, R.S.; DeLucia, F.C.; McManus, C.E.; McMillan, N.J.; Jenkins, T.F.; Walsh, M.E.; Miziolek, A. Laser-induced breakdown spectroscopy—An emerging chemical sensor technology for real-time field-portable, geochemical, mineralogical, and environmental applications. Appl. Geochem. 2006, 21, 730–747. [Google Scholar] [CrossRef]
- Gottfried, J.L.; Harmon, R.S.; De Lucia, F.C.; Miziolek, A.W. Multivariate analysis of laser-induced breakdown spectroscopy chemical signatures for geomaterial classification. Spectrochim. Acta Part B Spectrosc. 2009, 64, 1009–1019. [Google Scholar] [CrossRef]
- Harmon, R.S.; Remus, J.; McMillan, N.J.; McManus, C.; Collins, L.; Gottfried, J.L., Jr.; DeLucia, F.C.; Miziolek, A.W. LIBS analysis of geomaterials: Geochemical fingerprinting for the rapid analysis and discrimination of minerals. Appl. Geochem. 2009, 24, 1125–1141. [Google Scholar] [CrossRef]
- Tucker, J.M.; Dyar, M.D.; Schaefer, M.W.; Clegg, S.M.; Wiens, R.C. Optimization of laser-induced breakdown spectroscopy for rapid geochemical analysis. Chem. Geol. 2010, 277, 137–148. [Google Scholar] [CrossRef]
- McMillan, N.J.; Rees, S.; Kochelek, K.; McManus, C. Geological Applications of Laser-Induced Breakdown Spectroscopy. Geostand. Geoanalytical Res. 2014, 38, 329–343. [Google Scholar] [CrossRef]
- Hark, R.R.; Harmon, R. Geochemical fingerprint using LIBS. In Laser-Induced Breakdown Spectroscopy; Musazzi, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 329–343. [Google Scholar]
- Jain, J.; Quarles, C.D.; Moore, J.; Hartzler, D.A.; McIntyre, D.; Crandall, D. Elemental mapping and geochemical characterization of gas producing shales by laser-induced breakdown spectroscopy. Spectrochim. Acta Part B Spectrosc. 2018, 150, 1–8. [Google Scholar] [CrossRef]
- Tognoni, E.; Cristoforetti, G. Signal and noise in Laser-Induced Breakdown Spectroscopy: An introductory review. Opt. Laser Technol. 2016, 79, 164–172. [Google Scholar] [CrossRef]
- Russo, R.E.; Mao, X.L.; Yoo, J.; Gonzalez, J.J. Laser Ablation. In Laser-Induced Breakdown Spectroscopy, 1st ed.; Singh, J.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 49–82. [Google Scholar] [CrossRef]
- Fa, S.; Krebs, H. Calculations and experiments of material removal and kinetic energy during pulsed laser ablation of metals. Appl. Surf. Sci. 1996, 96–98, 61–65. [Google Scholar] [CrossRef]
- Wauquier, J.-P. Petroleum Refining, V.1: Crude Oil. Petroleum Products. Process Flowsheets; Éditions Technip: Paris, France, 1995. [Google Scholar]
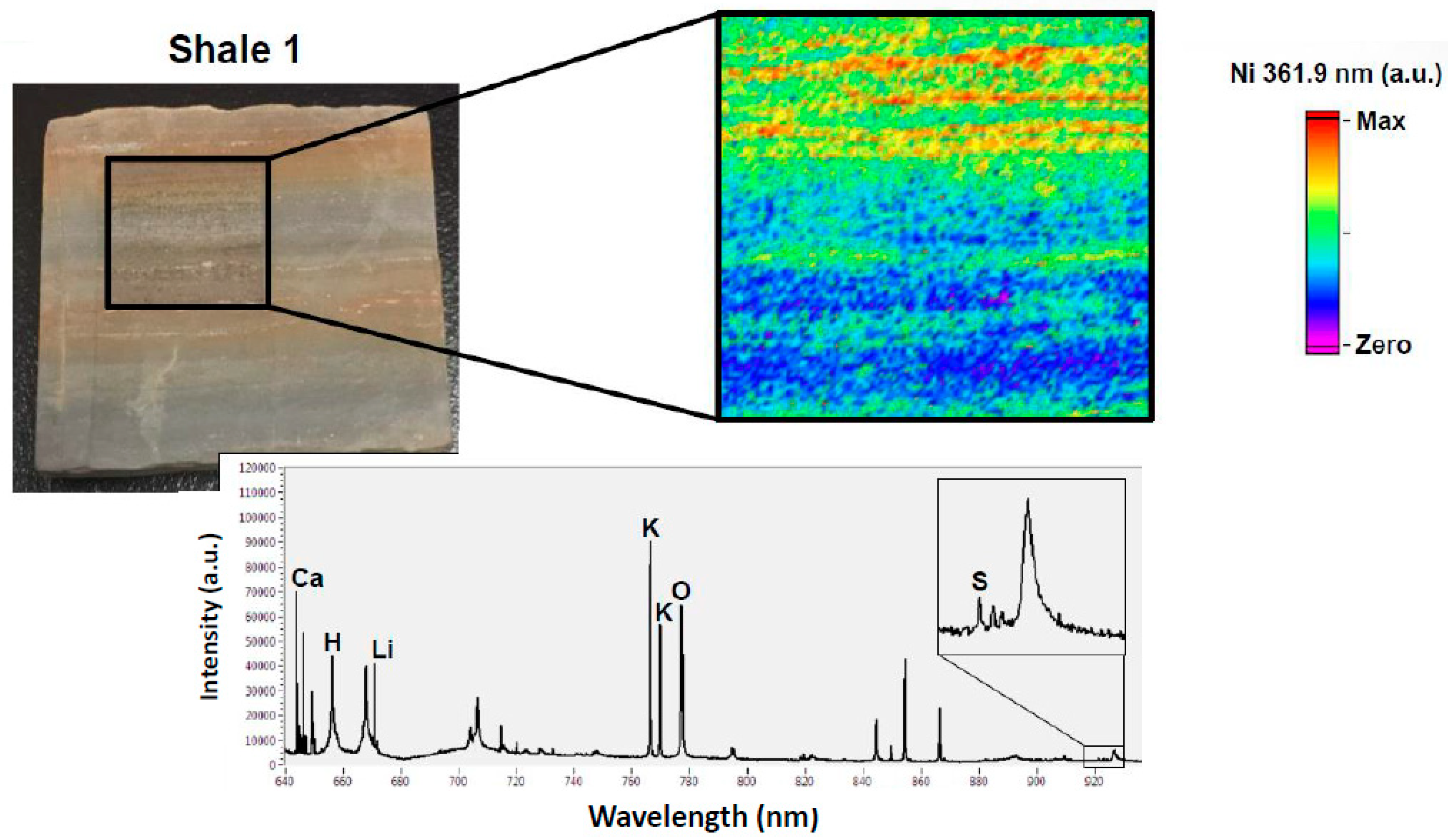

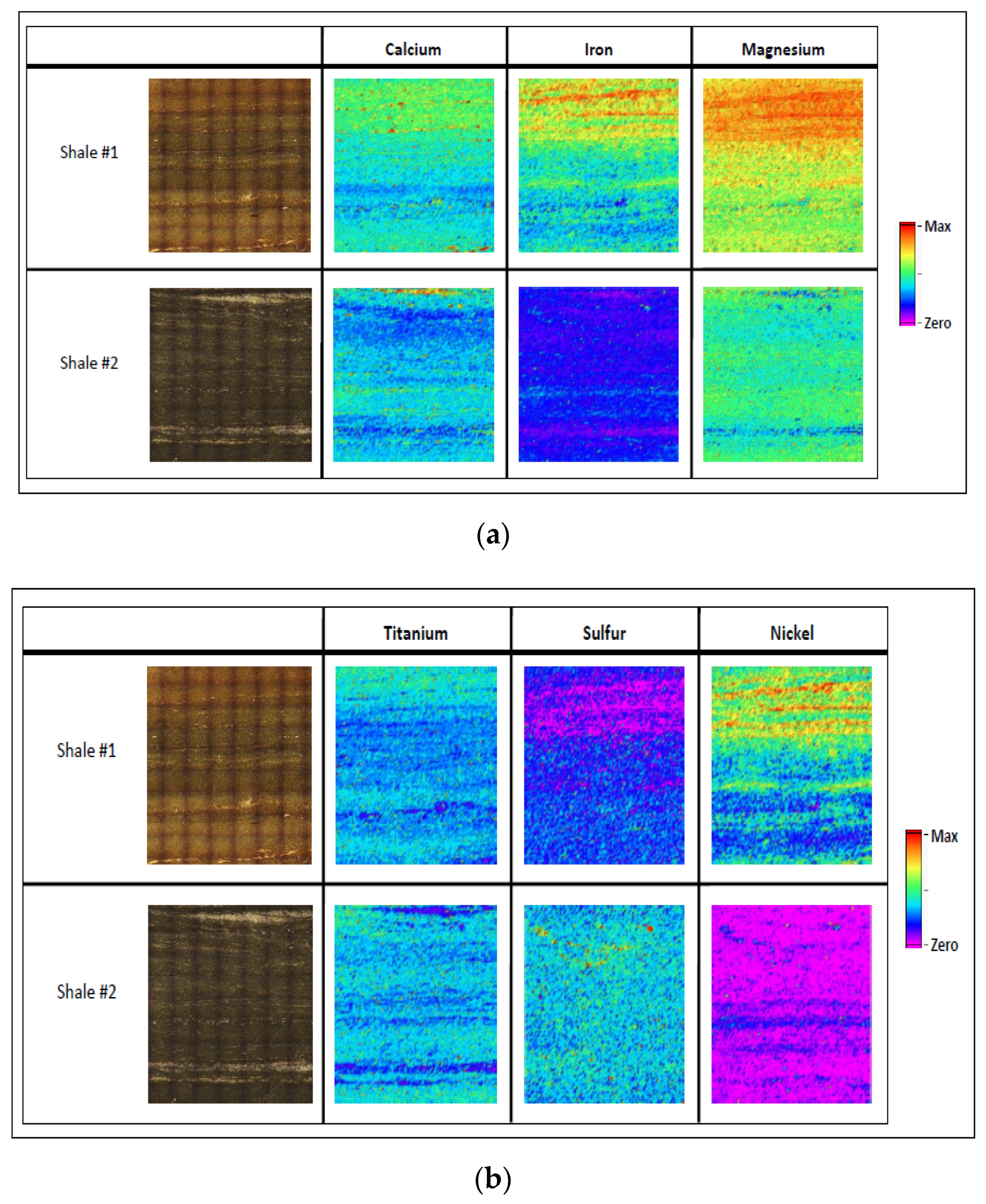

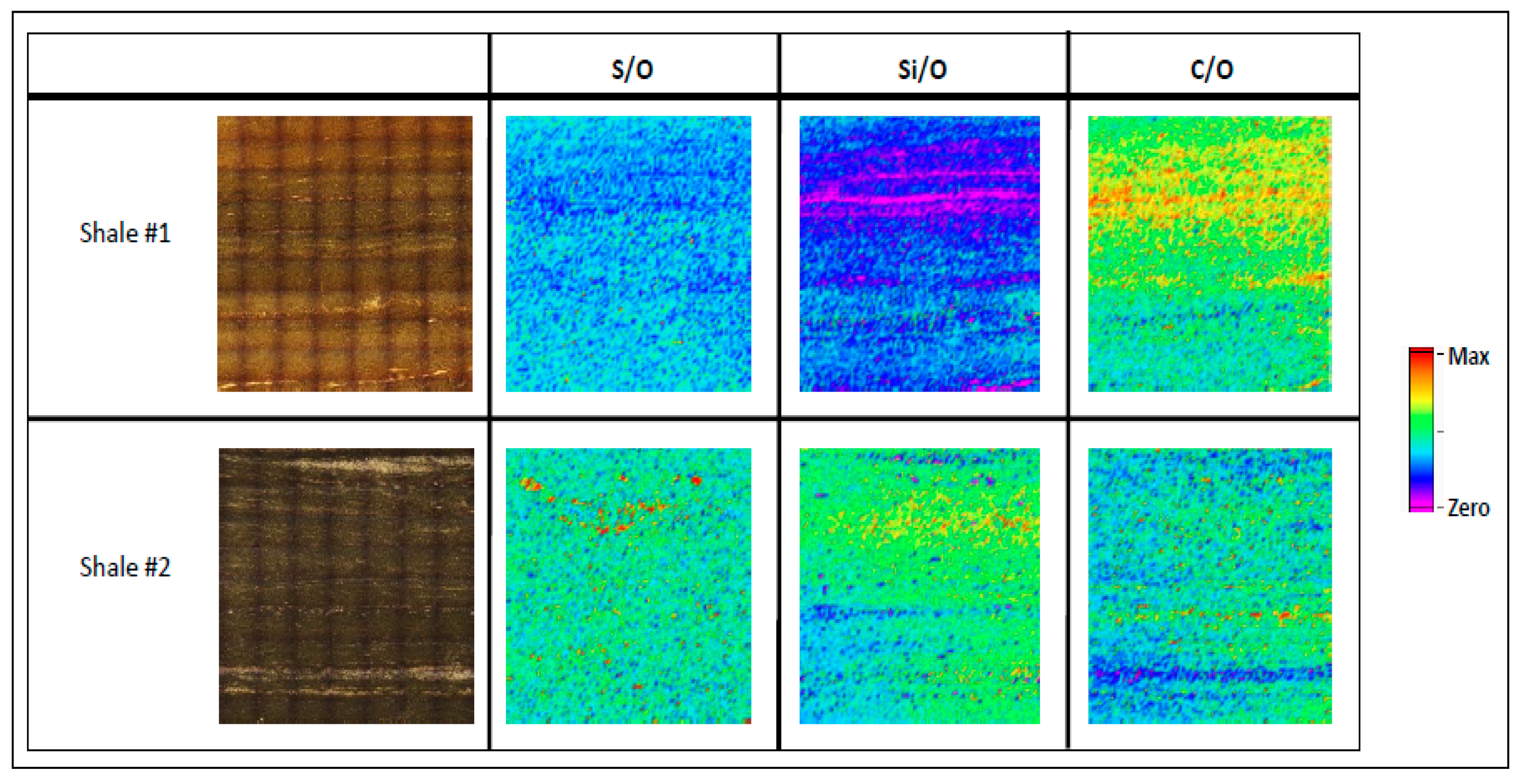
| Core and Shale Maps | Calibration Samples | |
|---|---|---|
| Laser spot size | 50 µm | 50 µm |
| Laser energy | 6.75 mJ | 6.75 mJ |
| Laser repetition rate | 10 Hz | 10 Hz |
| Gas environment | 0.5 L/min He | 0.5 L/min He |
| Gate delay | 0.25 µs | 0.25 µs |
| Laser pulses | 5 pulses per location (accumulated) | 5 pulses per location (accumulated) |
| Analyzed area | 12 mm × 12 mm (144 mm2) | 2 mm × 2 mm (4 mm2) |
| Mapping method type | Grid points | Grid points |
| Mapping method size | 100 × 100 (10,000 data points) | 5 × 5 (25 data points) |
| Time per sample | 585 min | 1 min 32 s |
| Sample | Reference Values | C 193 | %BIAS | C 247 | %BIAS |
| Shale 1 | 3.64 wt.% C | 3.59% | −1.4 | 3.78% | 3.8 |
| Shale 2 | 2.78 wt.% C | 2.71% | −2.5 | 3.04% | 9.4 |
| Sample | Reference Values | H 656 | %BIAS | ||
| Shale 1 | <1.0 wt.% H | 0.48% | n/a | ||
| Shale 2 | <1.0 wt.% H | 0.38% | n/a |
| H/C 193 | Minimum | Maximum | |
| Shale 1 | 1.60 ± 0.29 | 0.99 | 3.39 |
| Shale 2 | 1.69 ± 0.31 | 0.90 | 3.74 |
| H/C 247 | Minimum | Maximum | |
| Shale 1 | 1.52 ± 0.33 | 0.81 | 3.34 |
| Shale 2 | 1.50 ± 0.30 | 0.75 | 3.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quarles, C.D., Jr.; Miao, T.; Poirier, L.; Gonzalez, J.J.; Lopez-Linares, F. Elemental Mapping and Characterization of Petroleum-Rich Rock Samples by Laser-Induced Breakdown Spectroscopy (LIBS). Fuels 2022, 3, 353-364. https://doi.org/10.3390/fuels3020022
Quarles CD Jr., Miao T, Poirier L, Gonzalez JJ, Lopez-Linares F. Elemental Mapping and Characterization of Petroleum-Rich Rock Samples by Laser-Induced Breakdown Spectroscopy (LIBS). Fuels. 2022; 3(2):353-364. https://doi.org/10.3390/fuels3020022
Chicago/Turabian StyleQuarles, Charles Derrick, Jr., Toni Miao, Laura Poirier, Jhanis Jose Gonzalez, and Francisco Lopez-Linares. 2022. "Elemental Mapping and Characterization of Petroleum-Rich Rock Samples by Laser-Induced Breakdown Spectroscopy (LIBS)" Fuels 3, no. 2: 353-364. https://doi.org/10.3390/fuels3020022
APA StyleQuarles, C. D., Jr., Miao, T., Poirier, L., Gonzalez, J. J., & Lopez-Linares, F. (2022). Elemental Mapping and Characterization of Petroleum-Rich Rock Samples by Laser-Induced Breakdown Spectroscopy (LIBS). Fuels, 3(2), 353-364. https://doi.org/10.3390/fuels3020022







