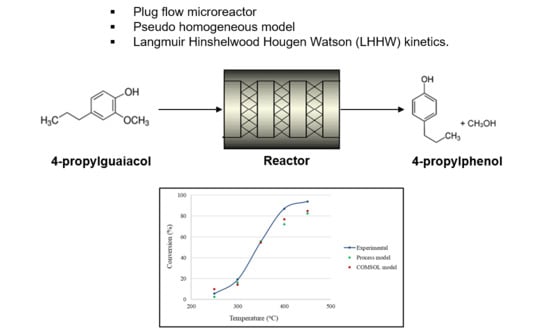
Process Simulation Modelling of the Catalytic Hydrodeoxygenation of 4-Propylguaiacol in Microreactors
Abstract
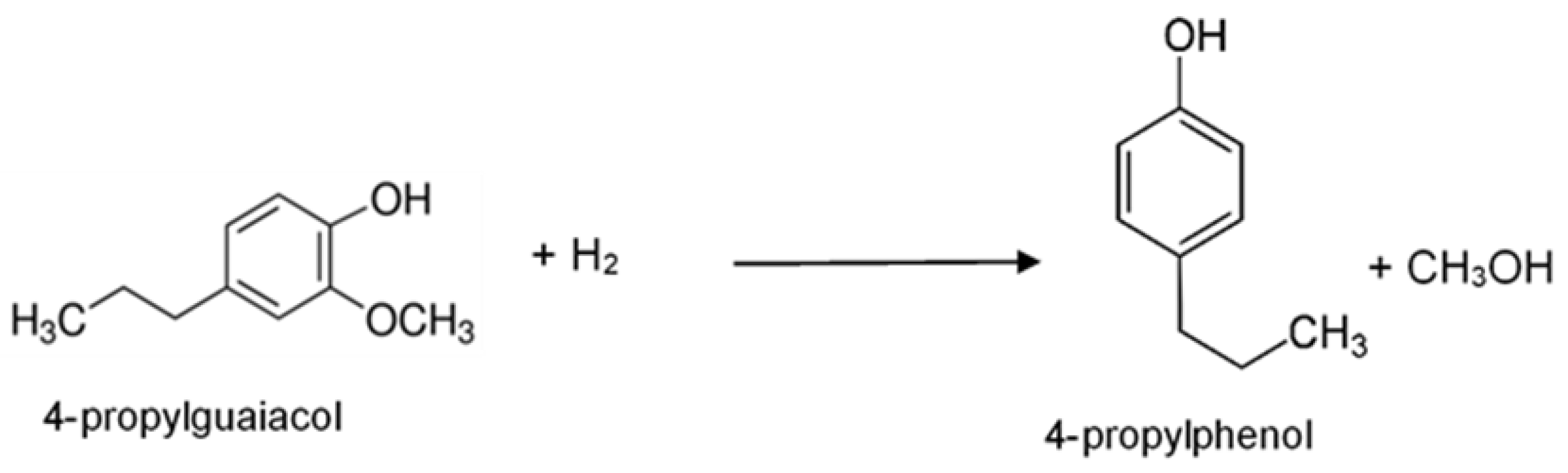
:1. Introduction
2. Results and Discussion
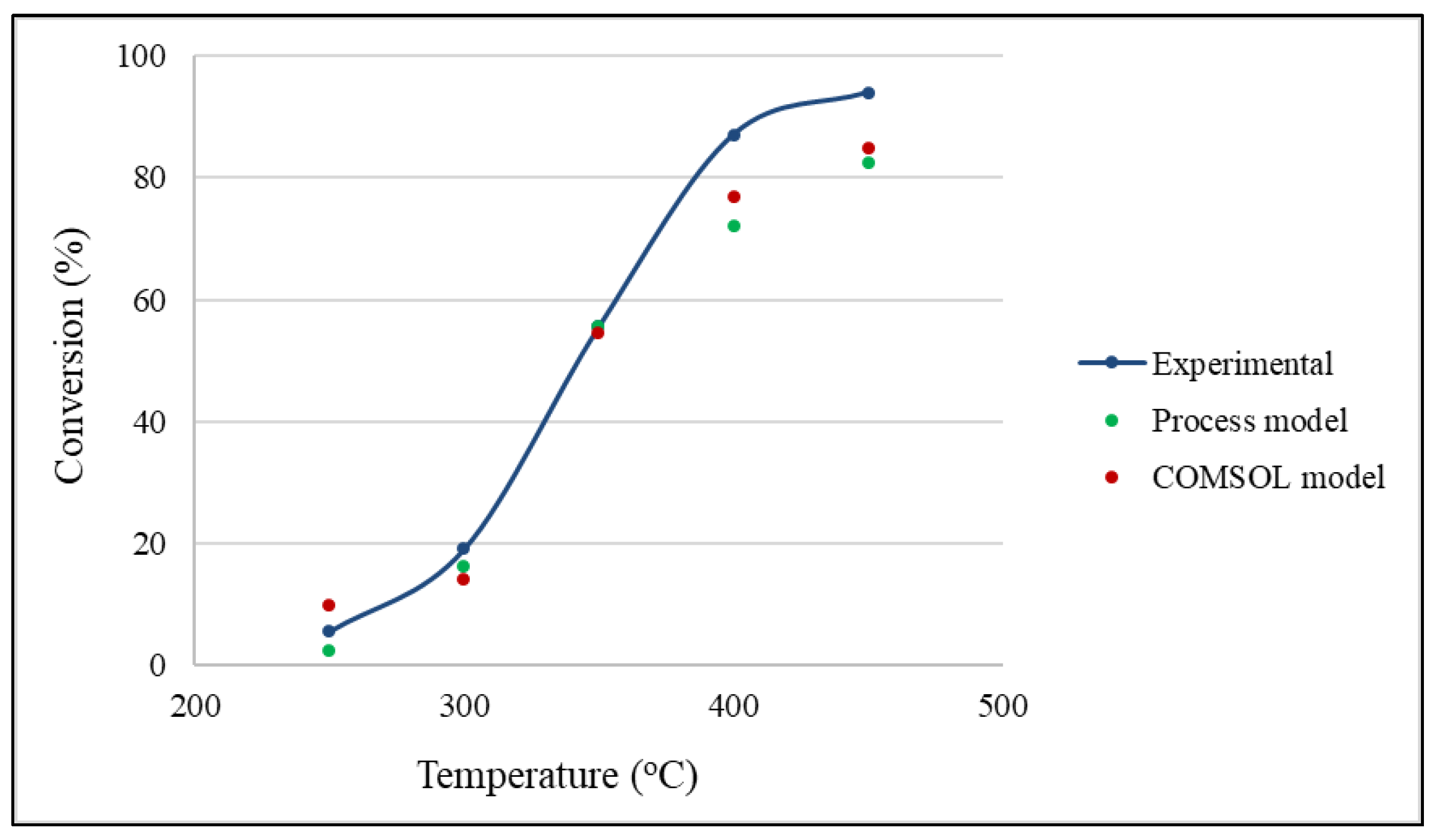
2.1. Effects of Temperature
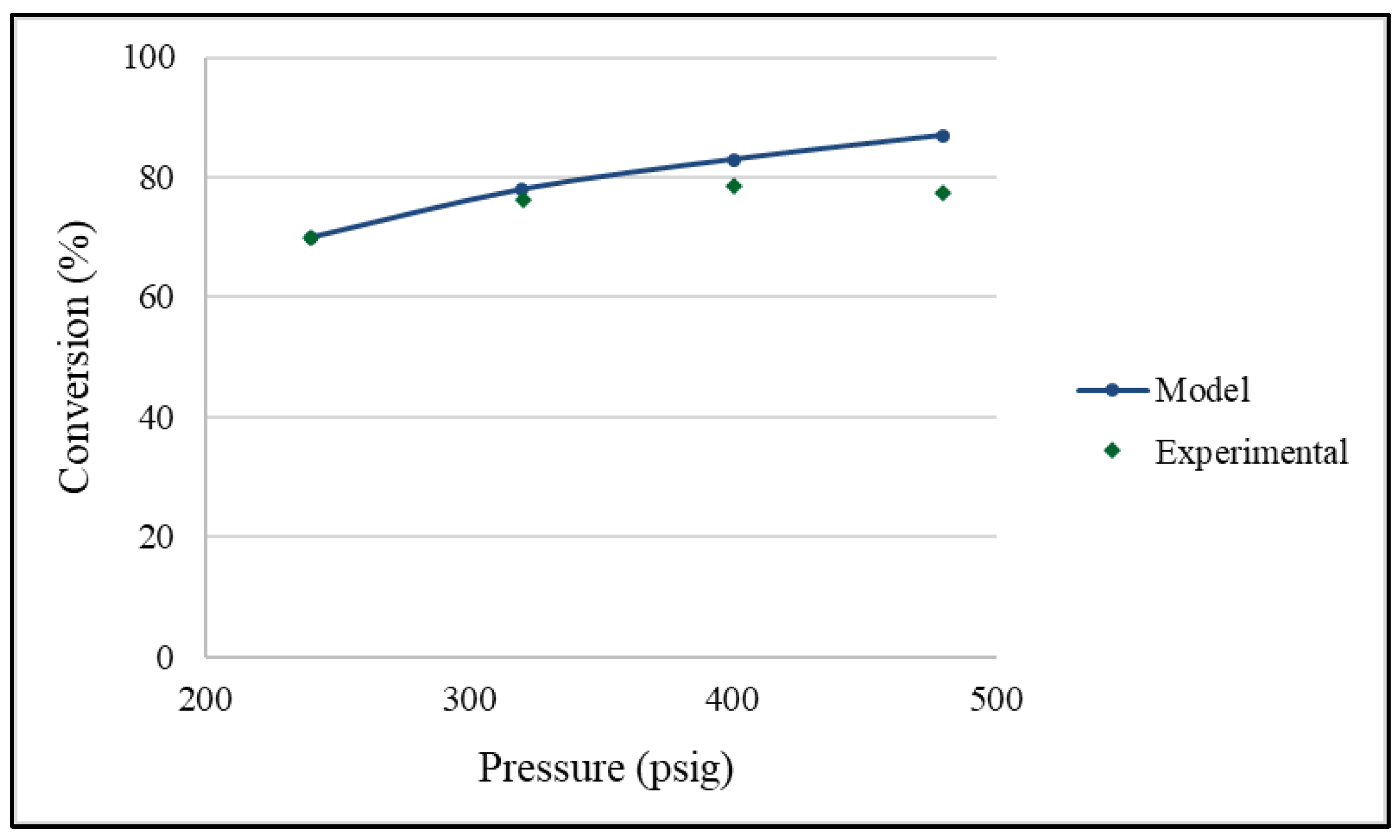
2.2. Effects of Pressure
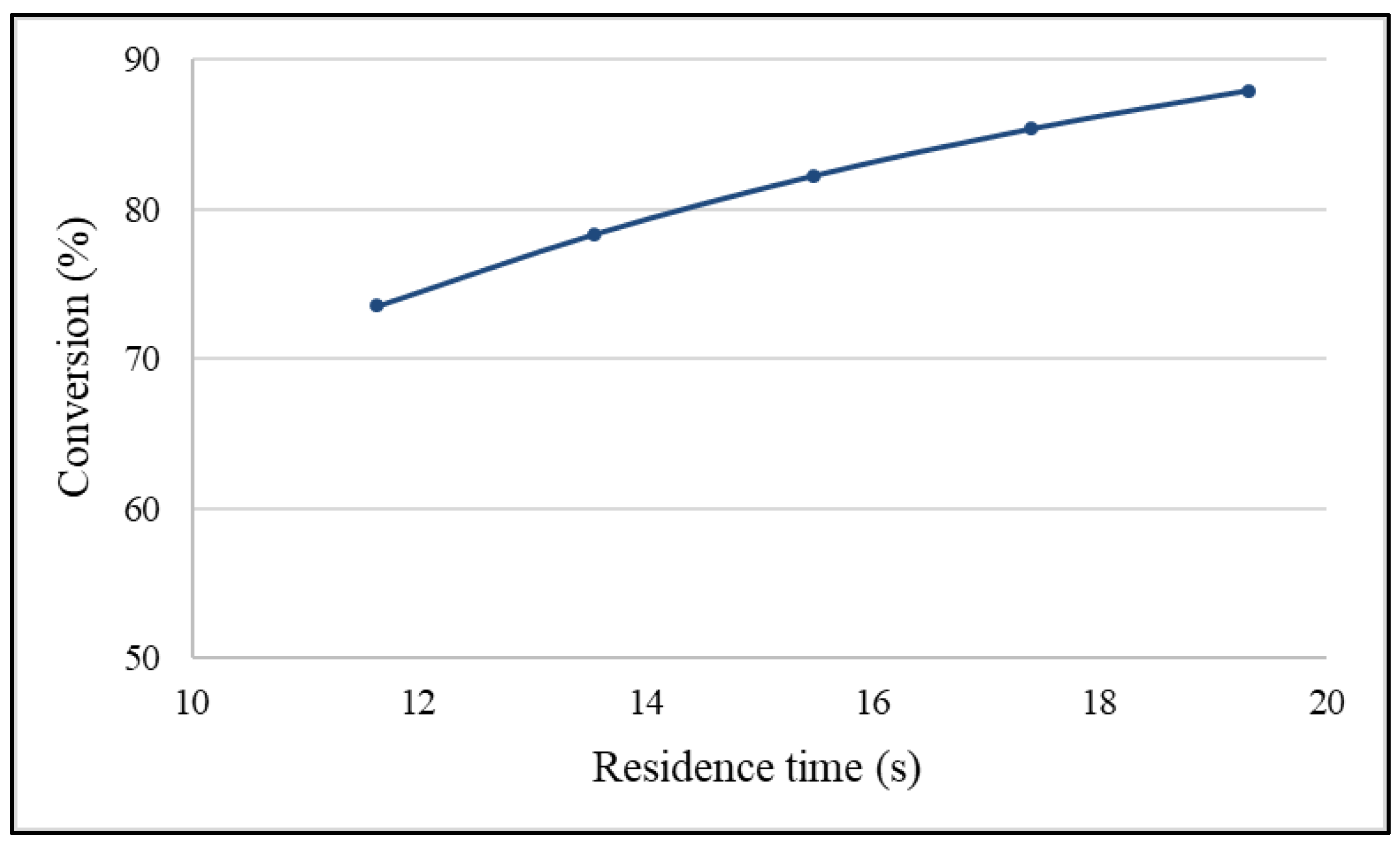
2.3. Effects of Residence Time
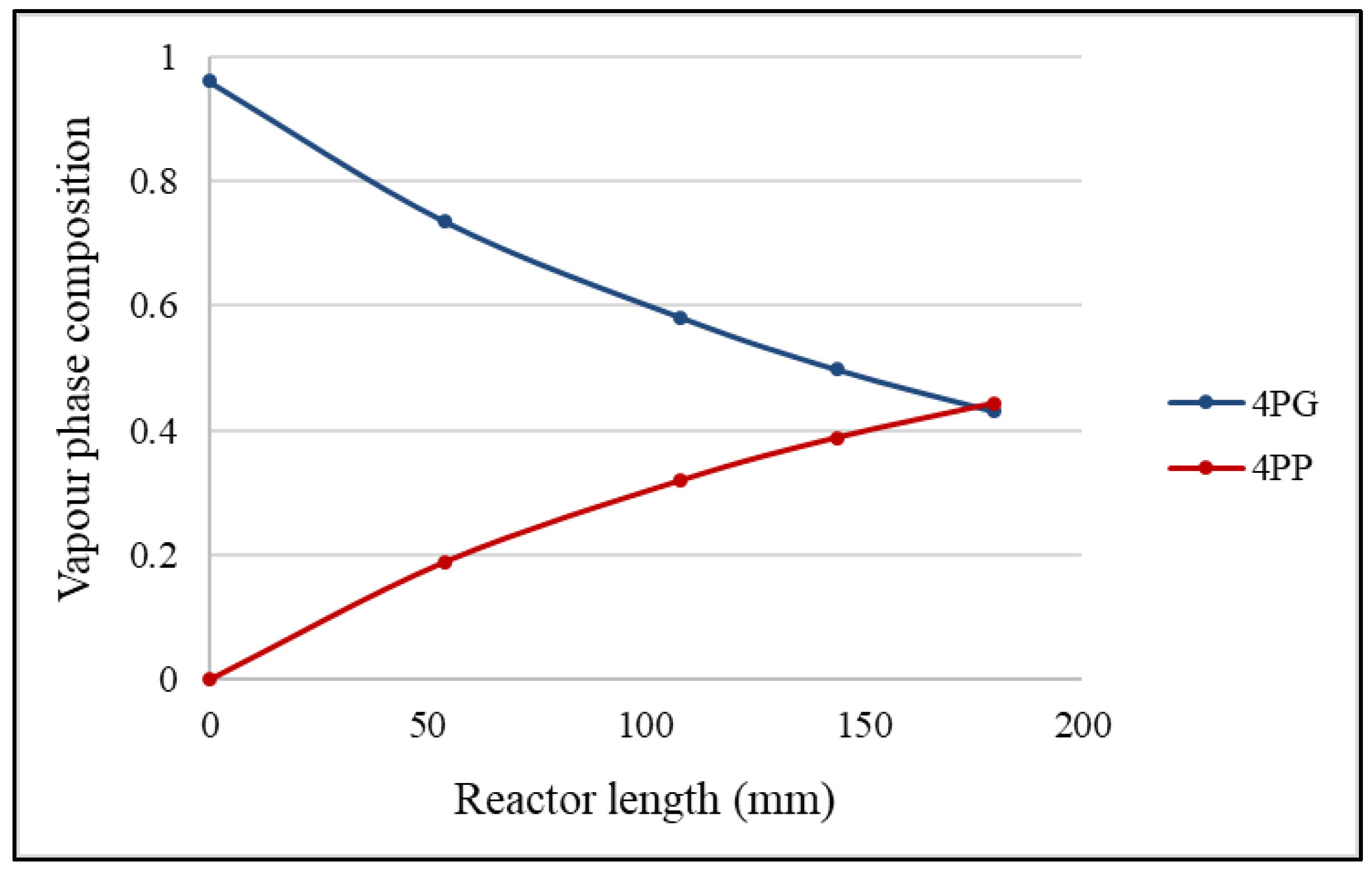
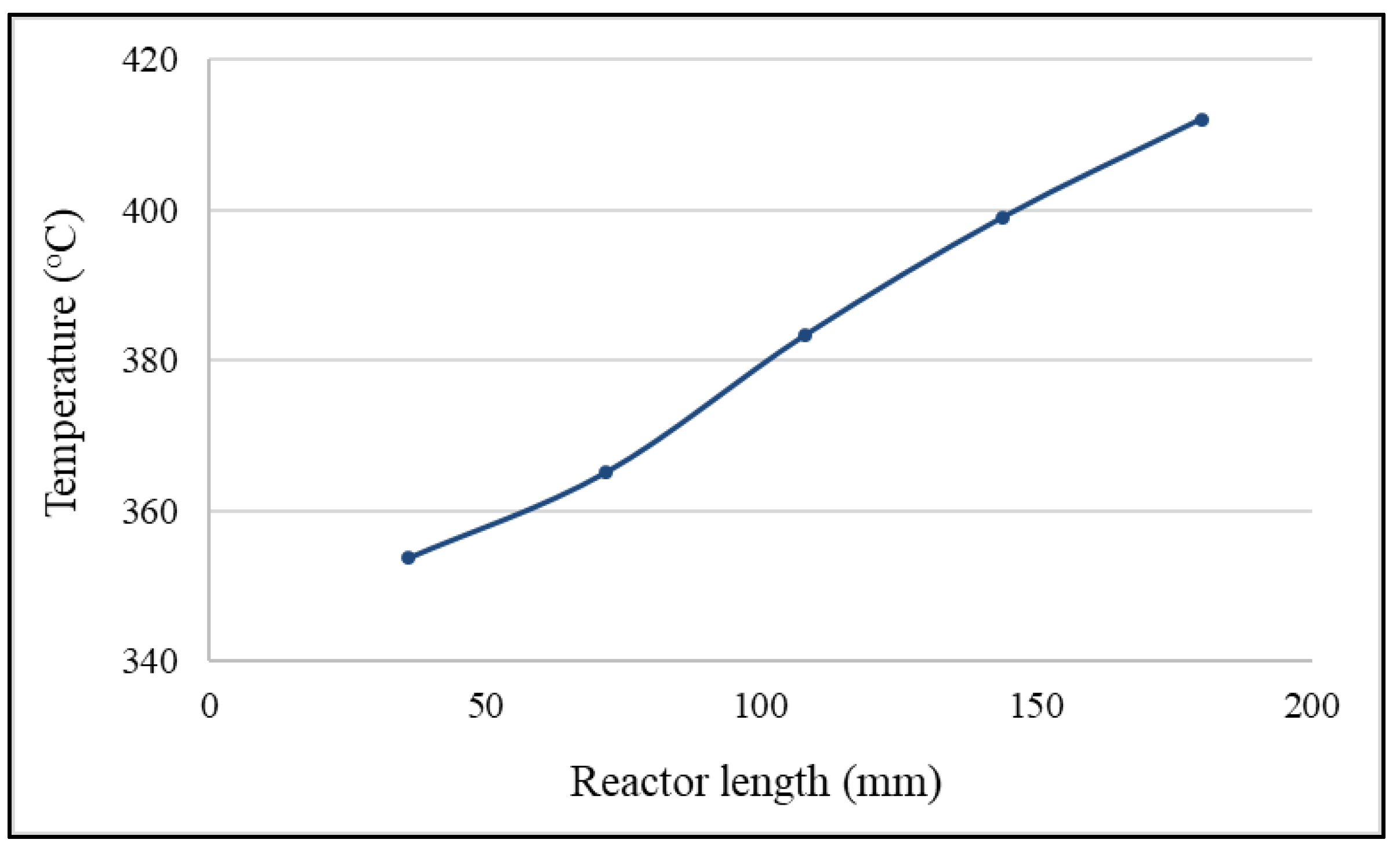
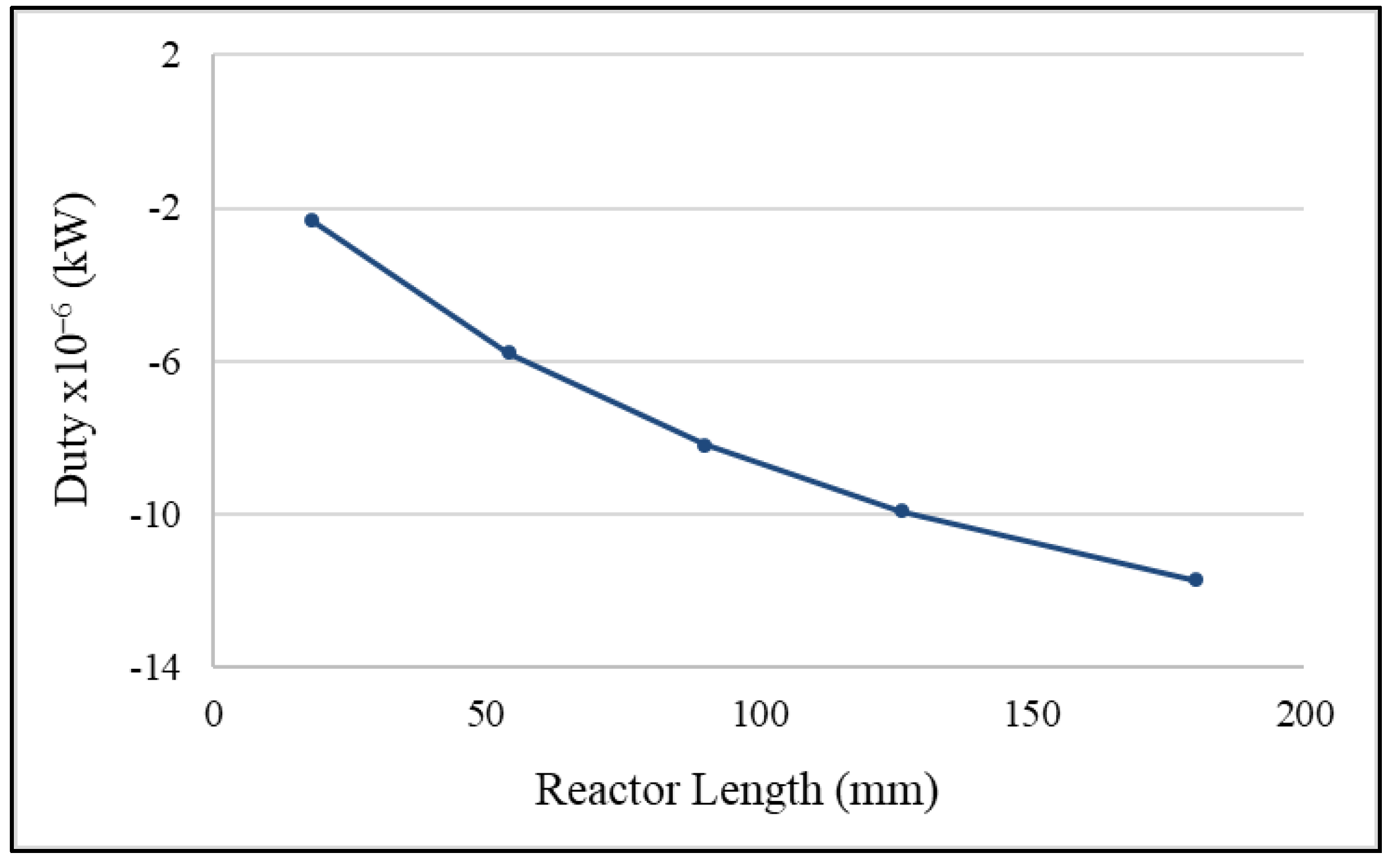
2.4. Effects of Adiabatic Conditions
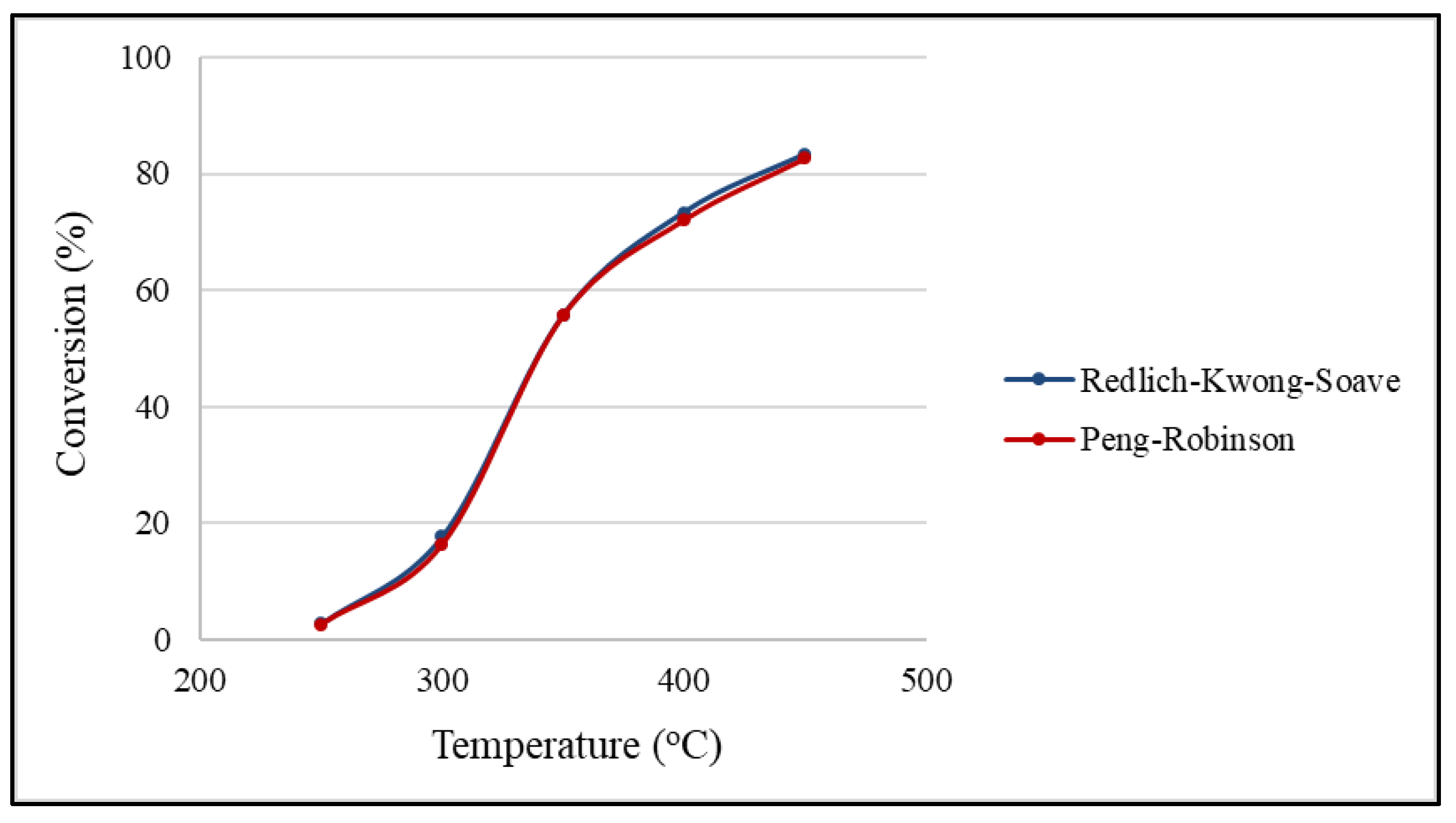
2.5. Effects of Equation of State
3. Modelling Methodology
3.1. Process Description and Mathematical Formulae
3.2. Reaction Pathway and Kinetics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| reaction rate for HDO of 4-Propylguaiacol (mol·g−1 h−1) | |
| reaction rate constant (mol·g−1 h−1) | |
| equilibrium constant for hydrogen (L·mol−1) | |
| equilibrium constant for 4-Propylguaiacol (L·mol−1) | |
| concentration of hydrogen (mol·L−1) | |
| concentration of 4-propylguaiacol (mol·L−1) | |
| activation energy (J·mol−1) | |
| F | molar flow rate (mol·s−1) |
| ideal gas constant (J·mol−1 K−1) | |
| bed voidage | |
| a | attraction parameter (atm·L2·mol−2) |
| b | van der Waals covolume (L·mol−1) |
| v | molar volume (m3·mol−1) |
| T | Temperature (°C) |
| P | Pressure (Psig) |
References
- Hafeez, S.; Pallari, E.; Manos, G.; Constantinou, A. Catalytic conversion and chemical recovery. In Plastics to Energy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 147–172. [Google Scholar]
- Hallale, N.; Liu, F. Refinery hydrogen management for clean fuels production. Adv. Environ. Res. 2001, 6, 81–98. [Google Scholar] [CrossRef]
- Joshi, N.; Lawal, A. Hydrodeoxygenation of 4-propylguaiacol (2-methoxy-4-propylphenol) in a microreactor: Performance and kinetic studies. Ind. Eng. Chem. Res. 2013, 52, 4049–4058. [Google Scholar] [CrossRef]
- Bridgwater, A.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Nimmanwudipong, T.; Runnebaum, R.C.; Block, D.E.; Gates, B.C. Catalytic reactions of guaiacol: Reaction network and evidence of oxygen removal in reactions with hydrogen. Catal. Lett. 2011, 141, 779–783. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, Y.-M.; Lee, I.-G.; Jeon, J.-K.; Jung, S.-C.; Chung, J.D.; Choi, W.G.; Park, Y.-K. Recent advances in the catalytic hydrodeoxygenation of bio-oil. Korean J. Chem. Eng. 2016, 33, 3299–3315. [Google Scholar] [CrossRef]
- Hafeez, S.; Aristodemou, E.; Manos, G.; Al-Salem, S.; Constantinou, A. Computational fluid dynamics (CFD) and reaction modelling study of bio-oil catalytic hydrodeoxygenation in microreactors. React. Chem. Eng. 2020, 5, 1083–1092. [Google Scholar] [CrossRef]
- Shu, R.; Lin, B.; Zhang, J.; Wang, C.; Yang, Z.; Chen, Y. Efficient catalytic hydrodeoxygenation of phenolic compounds and bio-oil over highly dispersed Ru/TiO2. Fuel Process. Technol. 2019, 184, 12–18. [Google Scholar] [CrossRef]
- Zerva, C.; Karakoulia, S.A.; Kalogiannis, K.G.; Margellou, A.; Iliopoulou, E.F.; Lappas, A.A.; Papayannakos, N.; Triantafyllidis, K.S. Hydrodeoxygenation of phenol and biomass fast pyrolysis oil (bio-oil) over Ni/WO3-ZrO2 catalyst. Catal. Today 2021, 366, 57–67. [Google Scholar] [CrossRef]
- Tran, Q.K.; Ly, H.V.; Kwon, B.; Kim, S.-S.; Kim, J. Catalytic hydrodeoxygenation of guaiacol as a model compound of woody bio-oil over Fe/AC and Ni/γ-Al2O3 catalysts. Renew. Energy 2021, 173, 886–895. [Google Scholar] [CrossRef]
- Lv, Q.; Yue, H.; Xu, Q.; Zhang, C.; Zhang, R. Quantifying the exergetic performance of bio-fuel production process including fast pyrolysis and bio-oil hydrodeoxygenation. J. Renew. Sustain. Energy 2018, 10, 043107. [Google Scholar] [CrossRef]
- Bagnato, G.; Sanna, A. Process and techno-economic analysis for fuel and chemical production by hydrodeoxygenation of bio-oil. Catalysts 2019, 9, 1021. [Google Scholar] [CrossRef] [Green Version]
- Shemfe, M.B.; Fidalgo, B.; Gu, S. Heat integration for bio-oil hydroprocessing coupled with aqueous phase steam reforming. Chem. Eng. Res. Des. 2016, 107, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Hafeez, S.; Manos, G.; Al-Salem, S.; Aristodemou, E.; Constantinou, A. Liquid fuel synthesis in microreactors. React. Chem. Eng. 2018, 3, 414–432. [Google Scholar] [CrossRef]
- Constantinou, A.; Ghiotto, F.; Lam, K.F.; Gavriilidis, A. Stripping of acetone from water with microfabricated and membrane gas–liquid contactors. Analyst 2014, 139, 266–272. [Google Scholar] [CrossRef]
- Sun, X.; Constantinou, A.; Gavriilidis, A. Stripping of acetone from isopropanol solution with membrane and mesh gas–liquid contactors. Chem. Eng. Process. Process Intensif. 2011, 50, 991–997. [Google Scholar] [CrossRef]
- Todić, B.; Ordomsky, V.V.; Nikačević, N.M.; Khodakov, A.Y.; Bukur, D.B. Opportunities for intensification of Fischer–Tropsch synthesis through reduced formation of methane over cobalt catalysts in microreactors. Catal. Sci. Technol. 2015, 5, 1400–1411. [Google Scholar] [CrossRef]
- Fortunate, O.; Kishore, N. Computational Fluid Dynamics Investigation on Catalytic Hydrodeoxygenation of a Bio-Oil Model Compound in a Fluidized Bed Reactor. J. Therm. Sci. Eng. Appl. 2021, 13, 061018. [Google Scholar] [CrossRef]
- Gollakota, A.R.; Subramanyam, M.D.; Kishore, N.; Gu, S. CFD simulations on the effect of catalysts on the hydrodeoxygenation of bio-oil. RSC Adv. 2015, 5, 41855–41866. [Google Scholar] [CrossRef] [Green Version]
- Subramanyam, M.D.; Gollakota, A.R.; Kishore, N. CFD simulations of catalytic hydrodeoxygenation of bio-oil using Pt/Al2O3 in a fixed bed reactor. RSC Adv. 2015, 5, 90354–90366. [Google Scholar] [CrossRef]
- Ng, K.S.; Martinez-Hernandez, E. Techno-economic assessment of an integrated bio-oil steam reforming and hydrodeoxygenation system for polygeneration of hydrogen, chemicals, and combined heat and power production. In Towards Sustainable Chemical Processes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 69–98. [Google Scholar]
- Nowakowska, M.; Herbinet, O.; Dufour, A.; Glaude, P.-A. Kinetic study of the pyrolysis and oxidation of guaiacol. J. Phys. Chem. A 2018, 122, 7894–7909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, S.; Wang, X.; He, X.; Liu, L.; Zhong, X.; Zhuang, G.; Wang, J.-G. NiFe/γ-Al2O3: A universal catalyst for the hydrodeoxygenation of bio-oil and its model compounds. Catal. Commun. 2013, 41, 34–37. [Google Scholar] [CrossRef]
- Su-Ping, Z. Study of hydrodeoxygenation of bio-oil from the fast pyrolysis of biomass. Energy Sources 2003, 25, 57–65. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Chrysikou, L.P.; Meletidis, G.; Terzis, G.; Auersvald, M.; Kubička, D.; Bezergianni, S. Bio-based refinery intermediate production via hydrodeoxygenation of fast pyrolysis bio-oil. Renew. Energy 2021, 168, 593–605. [Google Scholar] [CrossRef]
- Patil, M.L.; Lali, A.M.; Dalai, A.K. Catalytic hydrodeoxygenation of bio-oil model compound for production of fuel grade oil. Asia Pac. J. Chem. Eng. 2019, 14, e2317. [Google Scholar] [CrossRef]
- Ausavasukhi, A.; Huang, Y.; To, A.T.; Sooknoi, T.; Resasco, D.E. Hydrodeoxygenation of m-cresol over gallium-modified beta zeolite catalysts. J. Catal. 2012, 290, 90–100. [Google Scholar] [CrossRef]
- Taghvaei, H.; Rahimpour, M.R. Catalytic hydrodeoxygenation of bio-oil using in situ generated hydrogen in plasma reactor: Effects of allumina supported catalysts and plasma parameters. Process Saf. Environ. Prot. 2019, 121, 221–228. [Google Scholar] [CrossRef]
- Ghanbari, M.; Ahmadi, M.; Lashanizadegan, A. A comparison between Peng-Robinson and Soave-Redlich-Kwong cubic equations of state from modification perspective. Cryogenics 2017, 84, 13–19. [Google Scholar] [CrossRef]
- Hafeez, S.; Aristodemou, E.; Manos, G.; Al-Salem, S.; Constantinou, A. Modelling of packed bed and coated wall microreactors for methanol steam reforming for hydrogen production. RSC Adv. 2020, 10, 41680–41692. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Langmuir–Hinshelwood kinetics–A theoretical study. Catal. Commun. 2008, 9, 82–84. [Google Scholar] [CrossRef]
- Ohtani, B. Photocatalysis by inorganic solid materials: Revisiting its definition, concepts, and experimental procedures. Adv. Inorg. Chem. 2011, 63, 395–430. [Google Scholar] [CrossRef]
- Al-Malah, K.I. Aspen Plus: Chemical Engineering Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Peng, D.-Y.; Robinson, D.B. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Fogler, H. Chapter 10: Catalysis and Catalytic Reactors. Elem. Chem. React. Eng. 2016, 5, 454–456. [Google Scholar]

| Reactor Conditions | Conversion (%) |
|---|---|
| Isothermal (400 °C) | 55.60 |
| Adiabatic | 66.10 |
| T (°C) | g−1 h−1) | K4PG (L·mol−1) | KH2 (L·mol−1) | R2 |
|---|---|---|---|---|
| 250 | 22.21 ± 0.77 | 0.02 ± 0.002 | 0.3 ± 0.004 | 0.97 |
| 300 | 41.40 ± 4.21 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.96 |
| 350 | 77.78 ± 0.01 | 0.6 ± (9.7 × 10−5) | 0.02 ± (1.6 × 10−6) | 0.96 |
| Intrinsic Constant | Value |
|---|---|
| Pre-exponential factors | |
| molg−1 h−1) | 5.29 ± 0.66 |
| Lmol−1) | 2.750 ± 0.14 |
| Lmol−1) | 1.6 ± 0.13 |
| Activation energies and heats of adsorption | |
| (kJmol−1) | 33.86 ± 2.70 |
| (kJmol−1) | 91.85 ± 2.69 |
| (kJmol−1) | −72.69 ± 2.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafeez, S.; Mahmood, S.; Aristodemou, E.; Al-Salem, S.M.; Manos, G.; Constantinou, A. Process Simulation Modelling of the Catalytic Hydrodeoxygenation of 4-Propylguaiacol in Microreactors. Fuels 2021, 2, 272-285. https://doi.org/10.3390/fuels2030016
Hafeez S, Mahmood S, Aristodemou E, Al-Salem SM, Manos G, Constantinou A. Process Simulation Modelling of the Catalytic Hydrodeoxygenation of 4-Propylguaiacol in Microreactors. Fuels. 2021; 2(3):272-285. https://doi.org/10.3390/fuels2030016
Chicago/Turabian StyleHafeez, Sanaa, Sabbir Mahmood, Elsa Aristodemou, Sultan M. Al-Salem, George Manos, and Achilleas Constantinou. 2021. "Process Simulation Modelling of the Catalytic Hydrodeoxygenation of 4-Propylguaiacol in Microreactors" Fuels 2, no. 3: 272-285. https://doi.org/10.3390/fuels2030016
APA StyleHafeez, S., Mahmood, S., Aristodemou, E., Al-Salem, S. M., Manos, G., & Constantinou, A. (2021). Process Simulation Modelling of the Catalytic Hydrodeoxygenation of 4-Propylguaiacol in Microreactors. Fuels, 2(3), 272-285. https://doi.org/10.3390/fuels2030016