The Prevalence of Helicobacter pylori Infection in the Adult Population of Russia: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sources and Search
2.2. Study Selection
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
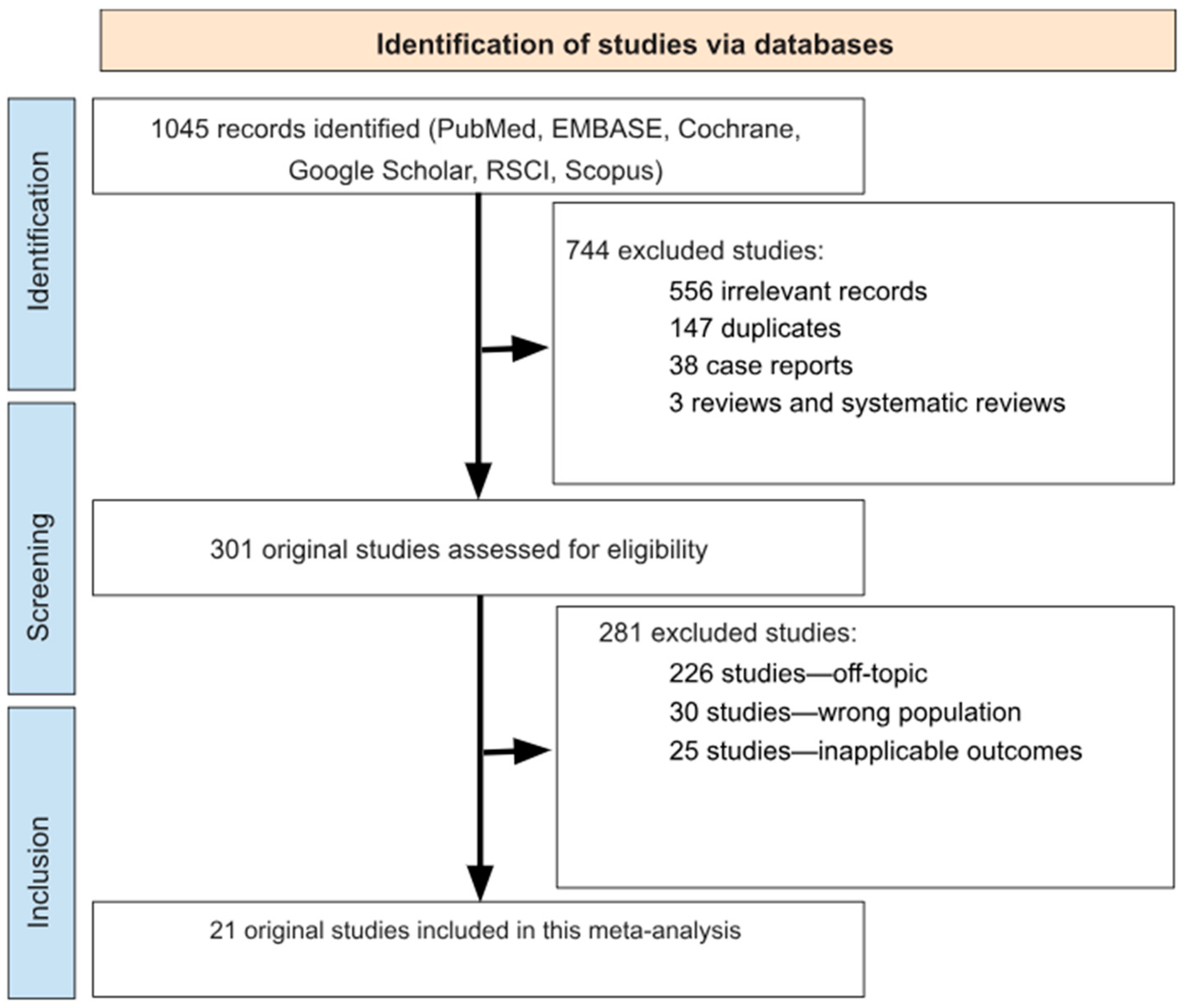
3.1. Search Results
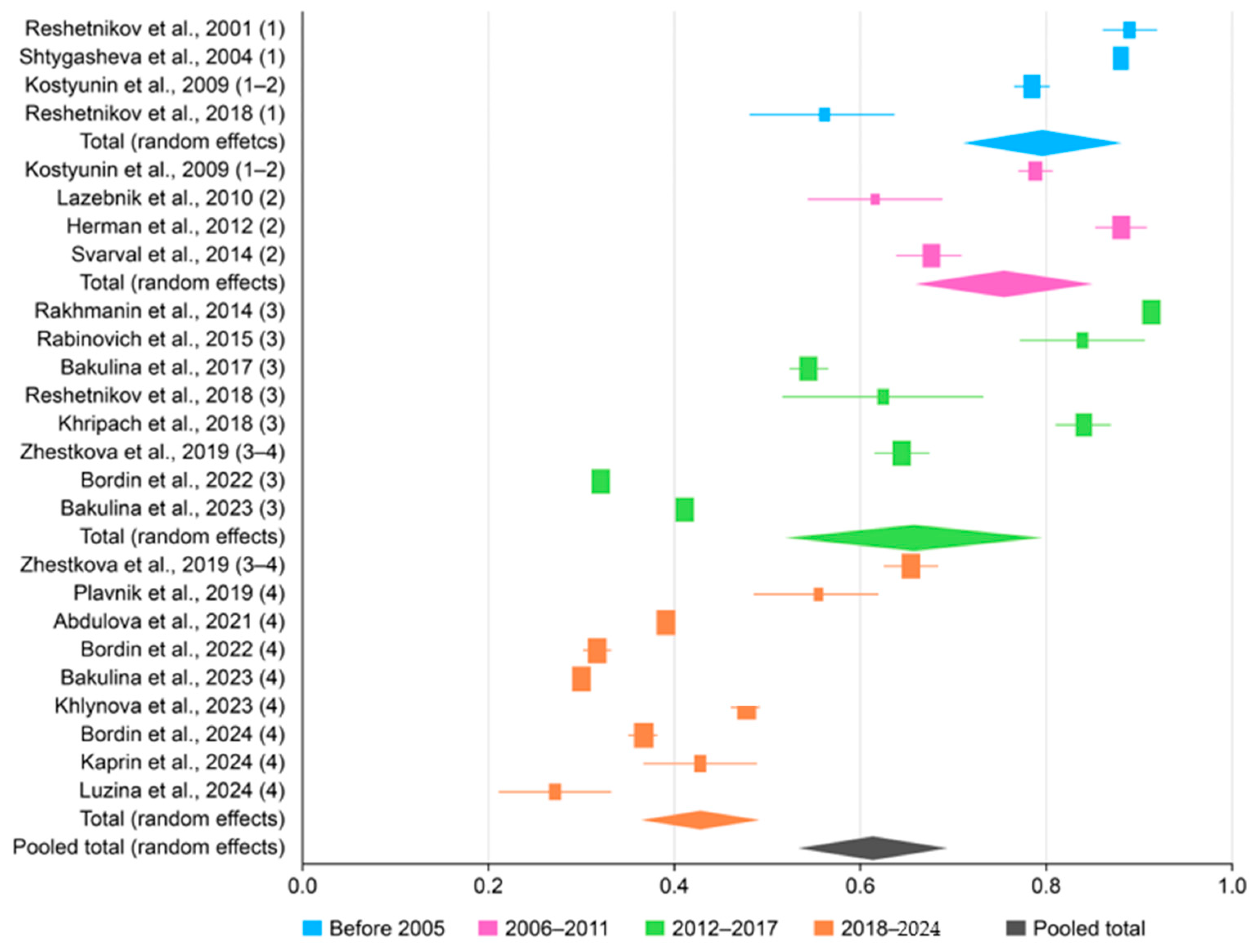
3.2. The Prevalence of H. pylori in Russia
3.3. Meta-Regression
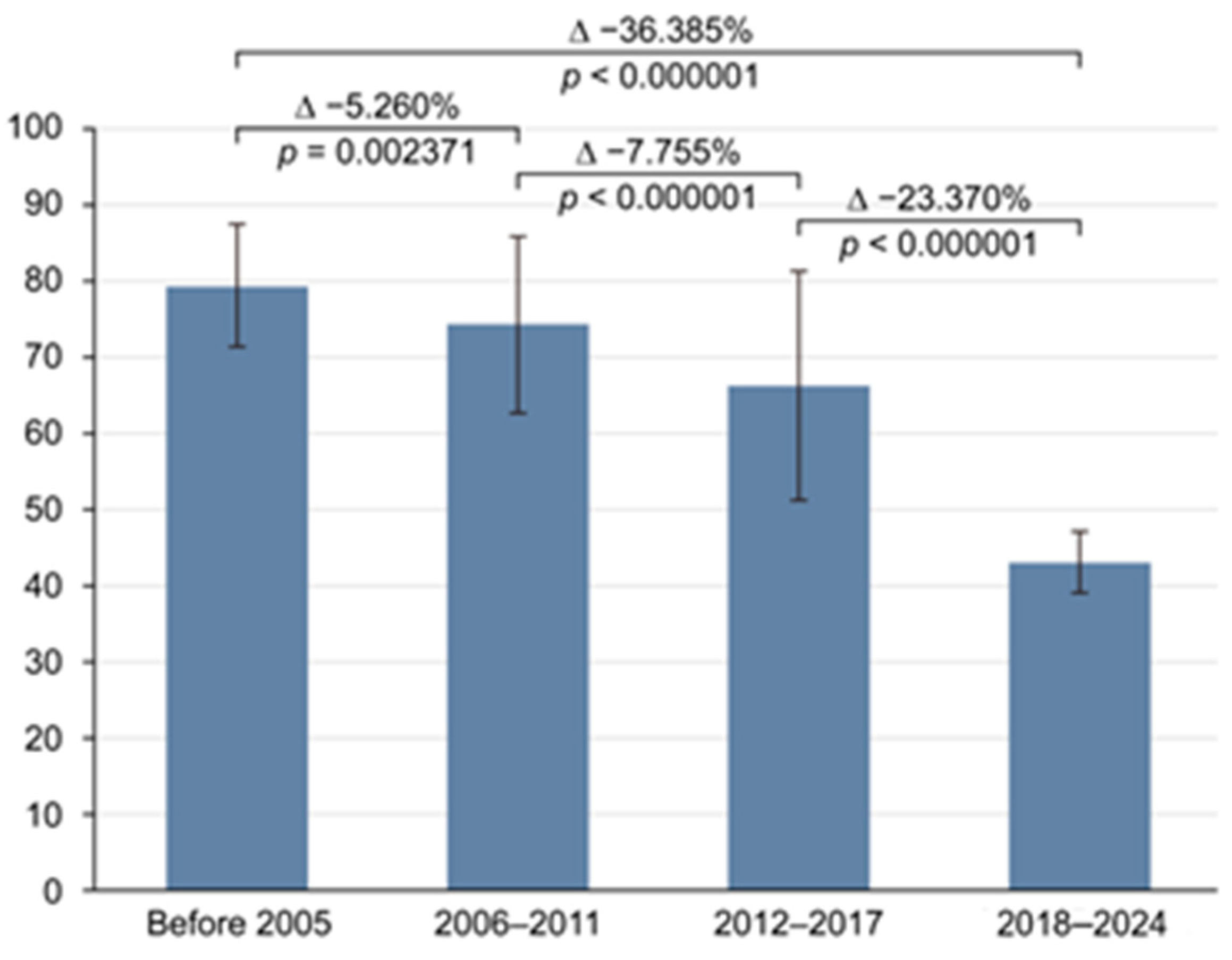
3.4. Subgroup Analysis
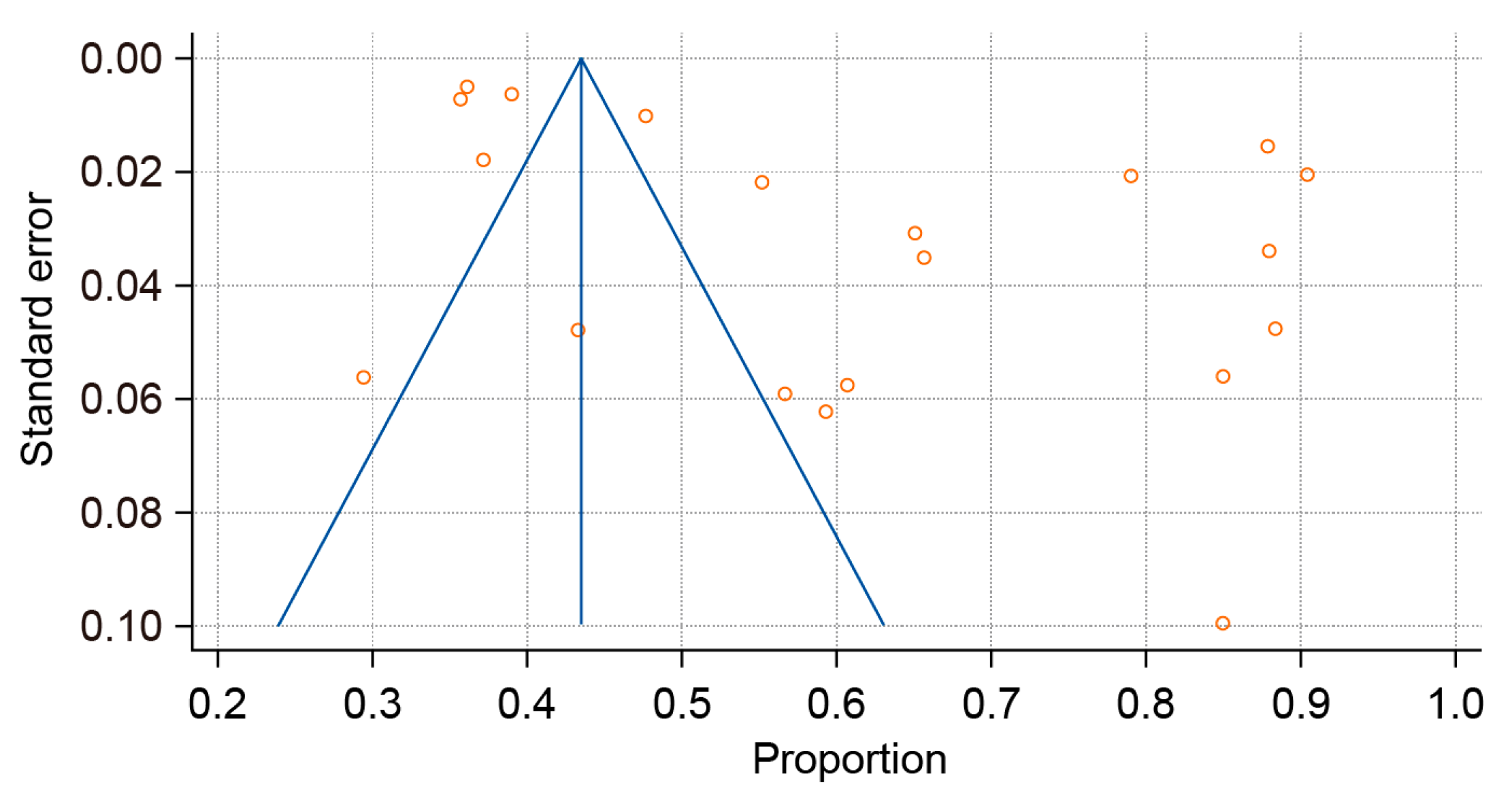
3.5. Sensitivity Analysis
4. Discussion
4.1. Main Findings
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC | Gastric cancer |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| RSCI | Russian Science Citation Index |
| NOS | Newcastle–Ottawa scale |
| H. Pylori | Helicobacter pylori |
| CI | Confidence interval |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| WHO | World Health Organization |
| IARC | International Agency for Research on Cancer |
| MALT | Mucosa-associated lymphoid tissue |
| Hp-EuReg | Helicobacter pylori European Registry |
| ET | Eradication therapy |
| PPI | Proton pump inhibitor |
References
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. European Helicobacter and Microbiota Study group. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Katelaris, P.; Hunt, R.; Bazzoli, F.; Cohen, H.; Fock, K.M.; Gemilyan, M.; Malfertheiner, P.; Mégraud, F.; Piscoya, A.; Quach, D.; et al. Helicobacter pylori World Gastroenterology Organization Global Guideline. J. Clin. Gastroenterol. 2023, 57, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; Franceschi, S.; Vignat, J.; Forman, D.; de Martel, C. Global burden of gastric cancer attributable to Helicobacter pylori. Int. J. Cancer 2015, 136, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Gullo, I.; Grillo, F.; Mastracci, L.; Vanoli, A.; Carneiro, F.; Saragoni, L.; Limarzi, F.; Ferro, J.; Parente, P.; Fassan, M. Precancerous lesions of the stomach, gastric cancer and hereditary gastric cancer syndromes. Pathologica 2020, 112, 166–185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 13 October 2024).
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.H.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M.H. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef]
- Li, Y.; Choi, H.; Leung, K.; Jiang, F.; Graham, D.Y.; Leung, W.K. Global prevalence of Helicobacter pylori infection between 1980 and 2022: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 553–564. [Google Scholar] [CrossRef]
- Suzuki, H.; Mori, H. World trends for H. pylori eradication therapy and gastric cancer prevention strategy by H. pylori test-and-treat. J. Gastroenterol. 2018, 53, 354–361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bordin, D.; Morozov, S.; Plavnik, R.; Bakulina, N.; Voynovan, I.; Skibo, I.; Isakov, V.; Bakulin, I.; Andreev, D.; Maev, I. Helicobacter pylori infection prevalence in ambulatory settings in 2017–2019 in RUSSIA: The data of real-world national multicenter trial. Helicobacter 2022, 27, e12924. [Google Scholar] [CrossRef]
- Zlokachestvennye Novoobrazovaniia v Rossii v 2023 g. (Zabolevaemost’ i Smertnost’)/pod Red. AD Kaprina, VV Starinskogo, GV Petrovoi. Moscow: MNIOI im. P.A. Gertsena—Filial FGBU “NMITs radiologii” Minzdrava Rossii, 2024. Available online: https://oncology-association.ru/wp-content/uploads/2024/08/zis-2023-elektronnaya-versiya.pdf (accessed on 14 October 2024).
- Merabishvili, V.M.; Rumyantsev, P.O.; Yurkova, J.P.; Artemeva, A.S.; Belyaev, A.M. The state of cancer care in Russia: Epidemiology and survival in patients with the most common and life-threatening solid malignant tumors. Part 1 (population study). Russ. J. Oncol. 2023, 28, 99–109. [Google Scholar] [CrossRef]
- Cai, T.; Li, Y.; Li, X.M.; Chen, B.; Liang, L.X.; Yuan, L.Z.; Hu, H.; Zhang, M.L.; Deng, A.J.; Liu, X.M.; et al. A population-based study of Helicobacter pylori: Does asymptomatic infection mean no gastroscopic lesions? Postgrad. Med. J. 2024, 100, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Malaty, H.M.; Paykov, V.; Bykova, O.; Ross, A.; Anneger, J.F.; Graham, D.Y. Helicobacter pylori and socioeconomic factors in Russia. Helicobacter 1996, 1, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Bakulina, N.V.; Simanenkov, V.I.; Bakulin, I.G.; Ilchishina, T.A. Prevalence of Helicobacter pylori infection among physicians. Exp. Klin. Gastroenterol. 2017, 12, 20–24. (In Russian) [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Reshetnikov, O.V.; Häivä, V.M.; Granberg, C.; Kurilovich, S.A.; Babin, V.P. Seroprevalence of Helicobacter pylori infection in Siberia. Helicobacter 2001, 6, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Shtygasheva, O.V.; Tsukanov, V.V. Prevalence of Helicobacter pylori infection and frequency of dyspeptic complaints in the population of Khakassia. Russ. J. Gastroenterol. Hepatol. Coloproctol. 2004, 14, 33–36. (In Russian) [Google Scholar]
- Kostyunin, K.Y.; Ogarkov, O.B.; Sukhanov, A.V.; Serebrennikova, E.N.; Gutnikova, M.Y.; Tsinserling, V.A. Study of Helicobacter pylori gastritis in the Irkutsk region: The role and place of the morphological method. Baikal Med. J. 2009, 85, 78–82. (In Russian) [Google Scholar]
- Lazebnik, L.B.; Vasiliev, Y.V.; Shcherbakov, P.L.; Khomeriki, S.G.; Masharova, A.A.; Bordin, D.S.; Kasyanenko, V.I.; Dubtsova, E.A. Helicobacter pylori: Prevalence, diagnosis, treatment. Eksp. Klin. Gastroenterol. 2010, 2, 3–7. (In Russian) [Google Scholar]
- German, S.V.; Zykova, I.E.; Modestova, A.V.; Ermakov, N.V. Epidemiological features of pyloric Helicobacter infection in Moscow. Gig. Sanit. 2011, 1, 44–48. (In Russian) [Google Scholar]
- Rakhmanin, I.; Zykova, I.E.; Fedichkina, T.P.; Solenova, L.G.; German, S.V.; Modestova, A.V.; Kislitsin, V.A. The study of spatial distribution of Helicobacter pylori infection rate in able-bodied population of Moscow in the course of medical examination of the manufacturing contingents. Gig. Sanit. 2013, 5, 79–82. (In Russian) [Google Scholar] [PubMed]
- Svarval, A.V.; Ferman, R.S.; Zhebrun, A.B. Study of the dynamics of the prevalence of infection caused by Helicobacter pylori among various age groups of the population of St. Petersburg in 2007–2011. Infect. Immun. 2014, 2, 741–746. (In Russian) [Google Scholar] [CrossRef]
- Rabinovich, E.I.; Povolotskaya, S.V. Prevalence of Helicobacter pylori infection among residents of the Ural region living in areas contaminated with radionuclides. Infect. Dis. 2015, 13, 279–280. (In Russian) [Google Scholar]
- Reshetnikov, O.V.; Krotov, S.A.; Kurilovich, S.A.; Denisova, D.V.; Malyutina, S.K. Prevalence of Helicobacter pylori in population studies in Novosibirsk (1994–2015). Exp. Klin. Gastroenterol. 2018, 7, 20–24. (In Russian) [Google Scholar]
- Khripach, L.V.; Knjazeva, T.D.; Yudin, S.M.; German, S.V.; Zykova, I.E. Comparative analysis of serum antibody responses to H. pylori and to recombinant CagA in the cohort of working-age Moscow adults. Gig. I Sanit. 2018, 97, 785–790. (In Russian) [Google Scholar] [CrossRef]
- Zhestkova, T.V.; Butov, M.A.; Lymar, Y.Y.; Papkov, S.V. Prevalence of Helicobacter pylori infection among residents of the Ryazan region. I.P. Pavlov. Russ. Med. Biol. Her. 2019, 27, 35–40. (In Russian) [Google Scholar] [CrossRef]
- Plavnik, R.G.; Bakulina, N.V.; Mareeva, D.V.; Bordin, D.S. Epidemiology of Helicobacter pylori: Clinical and laboratory parallels. Effective Pharmacotherapy. Gastroenterology 2019, 15, 16–20. (In Russian) [Google Scholar] [CrossRef]
- Abdulova, M.S.; Igonina, N.A.; Torshina, I.G.; Chashchikhina, E.V.; Kondrasheva, E.A.; Gasilova, N.A.; Lipilina, P.A.; Nasonenko, I.V.; Aksenova, A.V.; Doludenko, I.I. Assessment of the estimated population prevalence of Helicobacter pylori infection and the frequency of achieving eradication after treatment based on the results of the 13C-urease breath test in individuals who applied to the federal INVITRO laboratory network (2019–2020, n = 42,843). Eksperimental’naya I Klin. Gastroenterol. [Exp. Clin. Gastroenterol.] 2021, 2, 47–51. (In Russian) [Google Scholar]
- Bakulina, N.V.; Tikhonov, S.V.; Savilova, I.V.; Zharkov, A.V.; Ponomarenko, V.A. Dynamics of the prevalence of Helicobacter pylori infection from 2015 to 2023. Her. North-West. State Med. Univ. Named After I.I. Mechnikov 2023, 15, 41–51. [Google Scholar] [CrossRef]
- Khlynova, R.I.; Khromtsova, O.M.; Khlinov, I.B.; Berdnikov, R.B.; Petrov, V.M.; Moroz, G.A.; Abduragimova, L.Z. Prevalence of Helicobacter pylori-associated diseases in the Ural Federal District. Ural. Med. J. 2023, 22, 14–22. (In Russian) [Google Scholar] [CrossRef]
- Bordin, D.S.; Kuznetsova, E.S.; Stauver, E.E.; Nikol’skaya, K.A.; Chebotareva, M.V.; Voynovan, I.N.; Neyasova, N.A. Epidemiology of Helicobacter pylori infection in the Russian Federation from 1990 to 2023: A systematic review. Russ. Med. Inq. 2024, 8, 260–267. (In Russian) [Google Scholar] [CrossRef]
- Kaprin, A.D.; Sergeeva, N.S.; Pirogov, S.S.; Alentov, I.I.; Yutsevich, O.K.; Ryabtseva, V.I.; Minibaeva, G.F.; Marshutina, N.V.; Karmakova, T.A. Detection Rate of Helicobacter pylori Infection and Atrophic Gastritis Using Serological Markers “GastroPanel®” Among Employees of the National Medical Research Radiological Centre of the Ministry of Health of the Russian Federation. Russ. J. Gastroenterol. Hepatol. Coloproctol. 2024, 34, 57–71. (In Russian) [Google Scholar] [CrossRef]
- Luzina, E.V.; Lareva, N.V.; Zhigzhitova, E.B.; Zhilina, N.A. Prevalence and risk factors of Helicobacter pylori infection in the Transbaikal region. Sib. Med. Rev. 2024, 2, 30–35. (In Russian) [Google Scholar]
- Liou, J.M.; Malfertheiner, P.; Smith, S.I.; El-Omar, E.M.; Wu, M.S. 40 years after the discovery of Helicobacter pylori: Towards elimination of H pylori for gastric cancer prevention. Lancet 2024, 403, 2570–2572. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Wang, A.Y.; Wallace, M.B.; Hwang, J.H. AGA Clinical Practice Update on Screening and Surveillance in Individuals at Increased Risk for Gastric Cancer in the United States: Expert Review. Gastroenterology 2025, 168, 405–416.e1. [Google Scholar] [CrossRef] [PubMed]
- Kayali, S.; Manfredi, M.; Gaiani, F.; Bianchi, L.; Bizzarri, B.; Leandro, G.; Di Mario, F.; De’ Angelis, G.L. Helicobacter pylori, transmission routes and recurrence of infection: State of the art. Acta Biomed. 2018, 89, 72–76. [Google Scholar] [CrossRef]
- Elbehiry, A.; Abalkhail, A.; Anajirih, N.; Alkhamisi, F.; Aldamegh, M.; Alramzi, A.; AlShaqi, R.; Alotaibi, N.; Aljuaid, A.; Alzahrani, H.; et al. Helicobacter pylori: Routes of Infection, Antimicrobial Resistance, and Alternative Therapies as a Means to Develop Infection Control. Diseases 2024, 12, 311. [Google Scholar] [CrossRef]
- Yuan, C.; Adeloye, D.; Luk, T.T.; Huang, L.; He, Y.; Xu, Y.; Ye, X.; Yi, Q.; Song, P.; Rudan, I.; et al. The global prevalence of and factors associated with Helicobacter pylori infection in children: A systematic review and meta-analysis. Lancet Child Adolesc. Health 2022, 6, 185–194. [Google Scholar] [CrossRef]
- Xie, L.; Liu, G.W.; Liu, Y.N.; Li, P.Y.; Hu, X.N.; He, X.Y.; Huan, R.B.; Zhao, T.L.; Guo, H.J. Prevalence of Helicobacter pylori infection in China from 2014–2023: A systematic review and meta-analysis. World J. Gastroenterol. 2024, 30, 4636–4656. [Google Scholar] [CrossRef]
- Strachunskii, L.S.; Ivashkin, V.T.; Lapina, T.L.; Dekhnich, N.N.; Simanenkov, V.I.; Zakharova, N.V.; Tkachev, A.V.; Abdulkhakov, R.A.; Nikolaeva, N.N.; Osipenko, M.F. Management of peptic ulcer disease in outpatient settings: Results of a multicenter Russian pharmacoepidemiological study. Ross. Zhurnal Gastroenterol. Gepatologii Koloproktol. [Russ. J. Gastroenterol. Hepatol. Coloproctol.] 2005, 6, 16–21. (In Russian) [Google Scholar]
- Ivashkin, V.T.; Maev, I.V.; Lapina, T.L.; Sheptulin, A.A. Recommendations of the Russian Gastroenterological Association for the diagnosis and treatment of Helicobacter pylori infection in adults. Ross. Zhurnal Gastroenterol. Gepatologii I Koloproktol. [Russ. J. Gastroenterol. Hepatol. Coloproctol.] 2012, 22, 87–89. (In Russian) [Google Scholar]
- Andreev, D.N.; Khurmatullina, A.R.; Bordin, D.S.; Maev, I.V. Trends in the prevalence of Helicobacter pylori infection among adults in Moscow: A systematic review and meta-analysis. Ter. Arkhiv 2025, 97, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Andreev, D.N.; Khurmatullina, A.R.; Maev, I.V.; Bordin, D.S.; Zaborovskiy, A.V.; Abdulkhakov, S.R.; Kucheryavyy, Y.A.; Sokolov, F.S.; Beliy, P.A. Helicobacter pylori Antibiotic Resistance in Russia: A Systematic Review and Meta-Analysis. Antibiotics 2025, 14, 524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McNicholl, A.G.; O’Morain, C.A.; Megraud, F.; Gisbert, J.P. As Scientific Committee of the Hp-Eureg on Behalf of the National Coordinators. Protocol of the European Registry on the management of Helicobacter pylori infection (Hp-EuReg). Helicobacter 2019, 24, e12630. [Google Scholar] [CrossRef] [PubMed]
- Bordin, D.S.; Voynovan, I.N.; Embutnieks, Y.V.; Nyssen, O.P.; Megraud, F.; O Morain, C.; Perez-Gisbert, J. [European registry on Helicobacter pylori management (Hp-EuReg) as a tool to evaluate and improve clinical practice in Moscow]. Ter. Arkh. 2020, 92, 12–18. (In Russian) [Google Scholar] [CrossRef]
- Bordin, D.S.; Abdulkhakov, S.R.; Andreev, D.N.; Voynovan, I.; Bakulin, I.G.; Bakulina, N.V.; Baryshnikova, N.V.; Ilchishina, T.A.; Starostin, B.D.; Vologzhanina, L.G.; et al. Effectiveness of the first-line eradication therapy in 14 cities in Russia: Results for the period 2013–2022 of the European registry on Helicobacter pylori management (HpEuReg). United Eur. Gastroenterol. J. 2024, 12, 812–813. [Google Scholar] [CrossRef]
- Ko, S.W.; Kim, Y.J.; Chung, W.C.; Lee, S.J. Bismuth supplements as the first-line regimen for Helicobacter pylori eradication therapy: Systemic review and meta-analysis. Helicobacter 2019, 24, e12565. [Google Scholar] [CrossRef]
- Zagari, R.M.; Dajti, E.; Cominardi, A.; Frazzoni, L.; Fuccio, L.; Eusebi, L.H.; Vestito, A.; Lisotti, A.; Galloro, G.; Romano, M.; et al. Standard Bismuth Quadruple Therapy versus Concomitant Therapy for the First-Line Treatment of Helicobacter pylori Infection: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 3258. [Google Scholar] [CrossRef]
- Ivashkin, V.T.; Lapina, T.L.; Maev, I.V.; Drapkina, O.M.; Kozlov, R.S.; Sheptulin, A.A.; Trukhmanov, A.S.; Ab-dulkhakov, S.R.; Alekseeva, O.P.; Alekseenko, S.A.; et al. Clinical Practice Guidelines of Russian Gastroentero-logical Association, Scientific Society for the Clinical Study of Human Microbiome, Russian Society for the Prevention of Non-Communicable Diseases, Interregional Association for Clinical Microbiology and Antimi-crobial Chemotherapy for H. pylori Diagnostics and Treatment in Adults. Russ. J. Gastroenterol. Hepatol. Colo-Proctol. 2022, 32, 72–93. [Google Scholar] [CrossRef]
- Khatkov, I.E.; Abdulkhakov, S.R.; Alekseenko, S.A.; Amelina, I.D.; Andreev, D.N.; Artamonova, E.V.; Bakulina, N.V.; Besova, N.S.; Bolotina, L.V.; Bordin, D.S.; et al. Russian consensus on the prevention, diagnosis, and treatment of gastric cancer. Zlokachestvennye Opukholi [Malig. Tumors] 2023, 13, 56–68. (In Russian) [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Study, Year | Methodology of H. pylori Diagnostic | Geographical Location | Total Number of Patients, n | Total Number of Patients with H. pylori | Mean Age, Years | Period of Inquiry | NOS Criteria Score |
|---|---|---|---|---|---|---|---|
| Reshetnikov et al., 2001 [17] | Serology | Novosibirsk | 438 | 387 | 44.36 ± 16.38 | 1994–1995 | 8 |
| Shtygasheva et al., 2004 [18] | Four methods (morphological, urease breath test, polymerase chain reaction in biopsy, serological testing) | Khakassia Republic | 4217 | 3625 | not available | 2004 | 3 |
| Kostyunin et al., 2009 [19] | Examination of gastrobiopathology (histologically and cytologically) | Irkutsk Region | 2324 | 1836 | 44.36 ± 0.33 | 2001–2006 | 6 |
| Lazebnik et al., 2010 [20] | 13C-urea breath test | Moscow | 300 | 182 | 56.6 ± 15.3 | 2006 | 7 |
| Herman et al., 2012 [21] | Serology | Moscow | 863 | 759 | not available | 2011 | 6 |
| Rakhmanin et al., 2014 [22] | Serology | Moscow | 2414 | 2182 | not available | 2013 | 5 |
| Svarval et al., 2014 [23] | Serology | Saint Petersburg | 1057 | 688 | not available | 2007–2011 | 7 |
| Rabinovich et al., 2015 [24] | Serology | Ural Federal District | 100 | 85 | 58.6 ± 15 | 2015 | 7 |
| Bakulina et al., 2017 [15] | 13C-urea breath test | Multicenter Research | 2098 (of these, 127 patients in Moscow; 74 in Kazan; 448 in Saint Petersburg; 26 in Novosibirsk; and 10 in other cities) | 1157 (of these, 61 patients in Moscow; 38 in Kazan; 227 in Saint Petersburg; 17 in Novosibirsk; and 10 in other cities) | 48 ± 13.5 | 2016–2017 | 7 |
| Reshetnikov et al., 2018 [25] | Serology | Novosibirsk | 168 | 97 | not available | 2003–2005 | 5 |
| 90 | 56 | 2013–2015 | |||||
| Khripach et al., 2018 [26] | Serology | Moscow | 319 | 271 | 42 ± 14.07 | 2017 | 7 |
| Zhestkova et al., 2019 [27] | Serology | Ryazan Region | 809 | 531 | 57.38 ± 11.63 | 2017–2018 | 8 |
| Plavnik et al., 2019 [28] | 13C-urea breath test | Kazan and Moscow | 286 | 162 | 38 ± 12.5 | 2019 | 7 |
| Abdulova et al., 2021 [29] | 13C-urea breath test | Multicenter Research | 26,127 | 10,190 | 42.9 ± 17.8 | 2019–2020 | 7 |
| Bordin et al., 2022 [10] | 13C-urea breath test | Multicenter Research | 10,225 | 3669 | 43.65 ± 15.5 | 2017 | 9 |
| 9650 | 3431 | 2019 | |||||
| Bakulina et al., 2023 [30] | 13C-urea breath test | Saint Petersburg | 8553 | 3537 | 43.76 ± 15.73 | 2015–2017 | 8 |
| 33,990 | 11,821 | 2018–2023 | |||||
| Khlynova et al., 2023 [31] | 13C-urea breath test | Ural Federal District | 9939 | 4733 | 42.95 ± 17.77 | 2018–2022 | 7 |
| Bordin et al., 2024 [32] | 13C-urea breath test | Moscow | 3124 | 1162 | not available | 2022 | 6 |
| Kaprin et al., 2024 [33] | Serology | Moscow | 434 | 188 | 48.5 ± 0.6 | 2024 | 7 |
| Luzina et al., 2024 [34] | Detection of H. pylori antigen in feces by single-stage immunochromatographic analysis | Zabaykalsky Krai | 316 | 93 | 46.83 ± 14.66 | 2019–2023 | 7 |
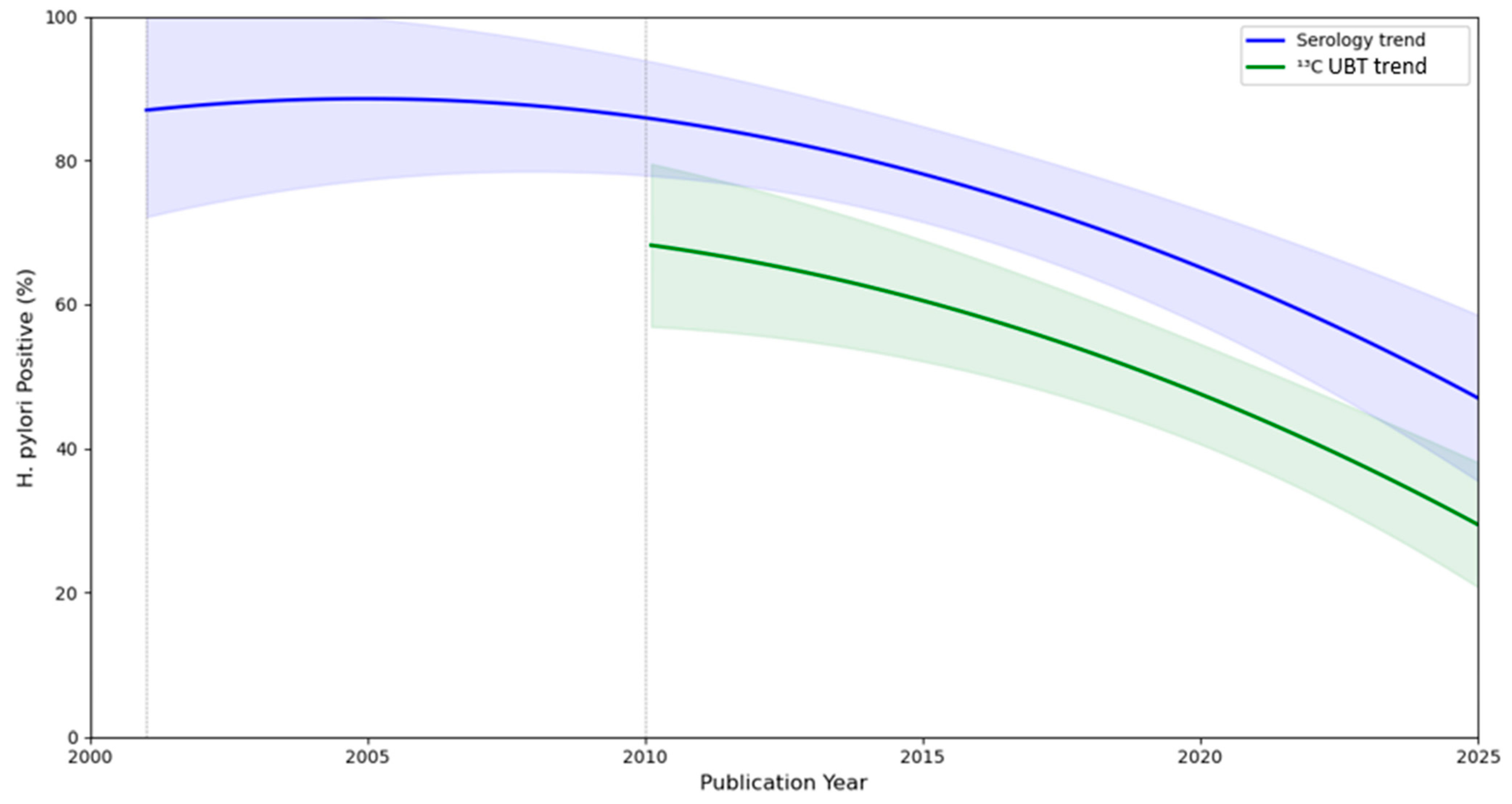
| Diagnostic Method | Before 2005, % | 2005–2011, % | 2012–2017, % | 2018–2024, % |
|---|---|---|---|---|
| Serology | 74.625 (95% CI: 40.693–96.927) | 77.533 (95% CI: 52.005–95.139) | 78.677 (95% CI: 61.839–86.248) | 54.650 (95% CI: 32.892–75.514) |
| 13C-urea breath test | No data | 60.667 (95% CI: 54.889–66.231) | 45.404 (95% CI: 27.197–64.272) | 41.097 (95% CI: 37.038–45.218) |
| Study | Sampling Population | ROBINS-I Risk of Bias (Participant Selection) [52] |
|---|---|---|
| Reshetnikov et al., 2001 [17] | General population | Low risk |
| Shtygasheva et al., 2004 [18] | Multiple population groups | Moderate risk |
| Kostyunin et al., 2009 [19] | Gastroenterology patients | High risk |
| Lazebnik et al., 2010 [20] | General population | Low risk |
| Herman et al., 2012 [21] | General population | Low risk |
| Rakhmanin et al., 2014 [22] | General population | Low risk |
| Svarval et al., 2014 [23] | Gastroenterology patients | High risk |
| Rabinovich et al., 2015 [24] | Multiple population groups | Moderate risk |
| Bakulina et al., 2017 [15] | Healthcare workers | High risk |
| Reshetnikov et al., 2018 [25] | General population | Low risk |
| Khripach et al., 2018 [26] | General population | Low risk |
| Zhestkova et al., 2019 [27] | Multiple population groups | Moderate risk |
| Plavnik et al., 2019 [28] | General population | Low risk |
| Abdulova et al., 2021 [29] | General population | Low risk |
| Bordin et al., 2022 [10] | General population | Low risk |
| Bakulina et al., 2023 [30] | General population | Low risk |
| Khlynova et al., 2023 [31] | Gastroenterology patients | High risk |
| Bordin et al., 2024 [32] | General population | Low risk |
| Kaprin et al., 2024 [33] | Healthcare workers | High risk |
| Luzina et al., 2024 [34] | General population | Low risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreev, D.N.; Khurmatullina, A.R.; Maev, I.V.; Bordin, D.S.; Abdulkhakov, S.R.; Kucheryavyy, Y.A.; Beliy, P.A.; Sokolov, F.S. The Prevalence of Helicobacter pylori Infection in the Adult Population of Russia: A Systematic Review and Meta-Analysis. Epidemiologia 2025, 6, 47. https://doi.org/10.3390/epidemiologia6030047
Andreev DN, Khurmatullina AR, Maev IV, Bordin DS, Abdulkhakov SR, Kucheryavyy YA, Beliy PA, Sokolov FS. The Prevalence of Helicobacter pylori Infection in the Adult Population of Russia: A Systematic Review and Meta-Analysis. Epidemiologia. 2025; 6(3):47. https://doi.org/10.3390/epidemiologia6030047
Chicago/Turabian StyleAndreev, Dmitrii N., Alsu R. Khurmatullina, Igor V. Maev, Dmitry S. Bordin, Sayar R. Abdulkhakov, Yury A. Kucheryavyy, Petr A. Beliy, and Filipp S. Sokolov. 2025. "The Prevalence of Helicobacter pylori Infection in the Adult Population of Russia: A Systematic Review and Meta-Analysis" Epidemiologia 6, no. 3: 47. https://doi.org/10.3390/epidemiologia6030047
APA StyleAndreev, D. N., Khurmatullina, A. R., Maev, I. V., Bordin, D. S., Abdulkhakov, S. R., Kucheryavyy, Y. A., Beliy, P. A., & Sokolov, F. S. (2025). The Prevalence of Helicobacter pylori Infection in the Adult Population of Russia: A Systematic Review and Meta-Analysis. Epidemiologia, 6(3), 47. https://doi.org/10.3390/epidemiologia6030047







