1. Introduction
The World Stroke Organization (WSO) 2025 stroke burden estimates revealed that stroke remains the second leading cause of death (about 7 million) and the third leading cause of death and disability globally, with over 160 million years of healthy life lost each year due to stroke-related death and disability. Ischemic stroke incidence constitutes 65% of all incident strokes and over 7.8 million new cases each year. Almost 70 million people who are alive have experienced ischemic stroke [
1]. Ischemic stroke is a stroke caused by sudden arterial occlusion due to a thrombus that forms directly in the area of occlusion (thrombotic ischemic stroke) or that forms in another area of the circulation, causing arterial obstruction (embolic ischemic stroke). The care of ischemic stroke requires integrated and comprehensive care, including the prevention of complications that can affect stroke outcomes, with the largest service costs based on data from the World Health Organization (WHO) over the past two decades [
1]. Annually, 3.6 million people die from ischemic stroke, with the estimated global cost of stroke being over USD 890 billion per year, which is projected to almost double by 2050 [
2].
Complications that are often encountered in the care of acute stroke patients include infections, among others, which need to be addressed, considering that this incident affects the high cost of stroke services [
3]. A previous study indicated that infectious complications were independently associated with prolonged hospital stay (OR = 1.78) [
4]. Even though the prevalence of infection after stroke decreased, which was reported from 30% to 9.14% (8.23–10.10%), the mortality remained at 15.91% [
5]. Therefore, one crucial part of the early management of stroke patients is the prevention of infection after stroke [
4].
Most infections after stroke occur in the first three to seven days after the stroke in patients who experience complications during treatment or up to the seventh day in 15% of acute ischemic stroke (AIS) patients [
5]. Previous research has shown that age, diabetes mellitus, disturbance of consciousness, the National Institutes of Health Stroke Scale (NIHSS) score at admission, invasive surgery, and chronic obstructive pulmonary disease (COPD) were risk factors for infections after AIS [
6].
The presence of these various factors is a challenge to the management of infection as a complication after AIS. Alternatives for preventing infections after stroke that have been studied include the administration of immunomodulators in experimental animals and preventive antibiotics, which have produced varying outcomes. Since the use of antibiotics might be non-optimal, prophylactic antibiotics did not always improve outcomes or reduce the overall incidence of infection after stroke [
7]. In addition, being mainly unmanaged and relying on antibiotics for treatment would lead to the development of antibiotic resistance [
8].
Relying on antibiotics for treatment occurred during the coronavirus disease 2019 (COVID-19) pandemic. Healthcare has transformed to involve the abundant use of antibiotics, increasing by more than 75%. Though antibiotics do not cure COVID-19, the overuse of antibiotics for treating COVID-19 patients was out of concern that bacterial coinfection would occur as a result of panic about an unknown disease, which has similar symptoms to pneumonia and a higher death rate [
8,
9]. A meta-analysis study showed that the COVID-19 pandemic has had an impact on the accelerated emergence and transmission of antibiotic resistance across healthcare settings [
8].
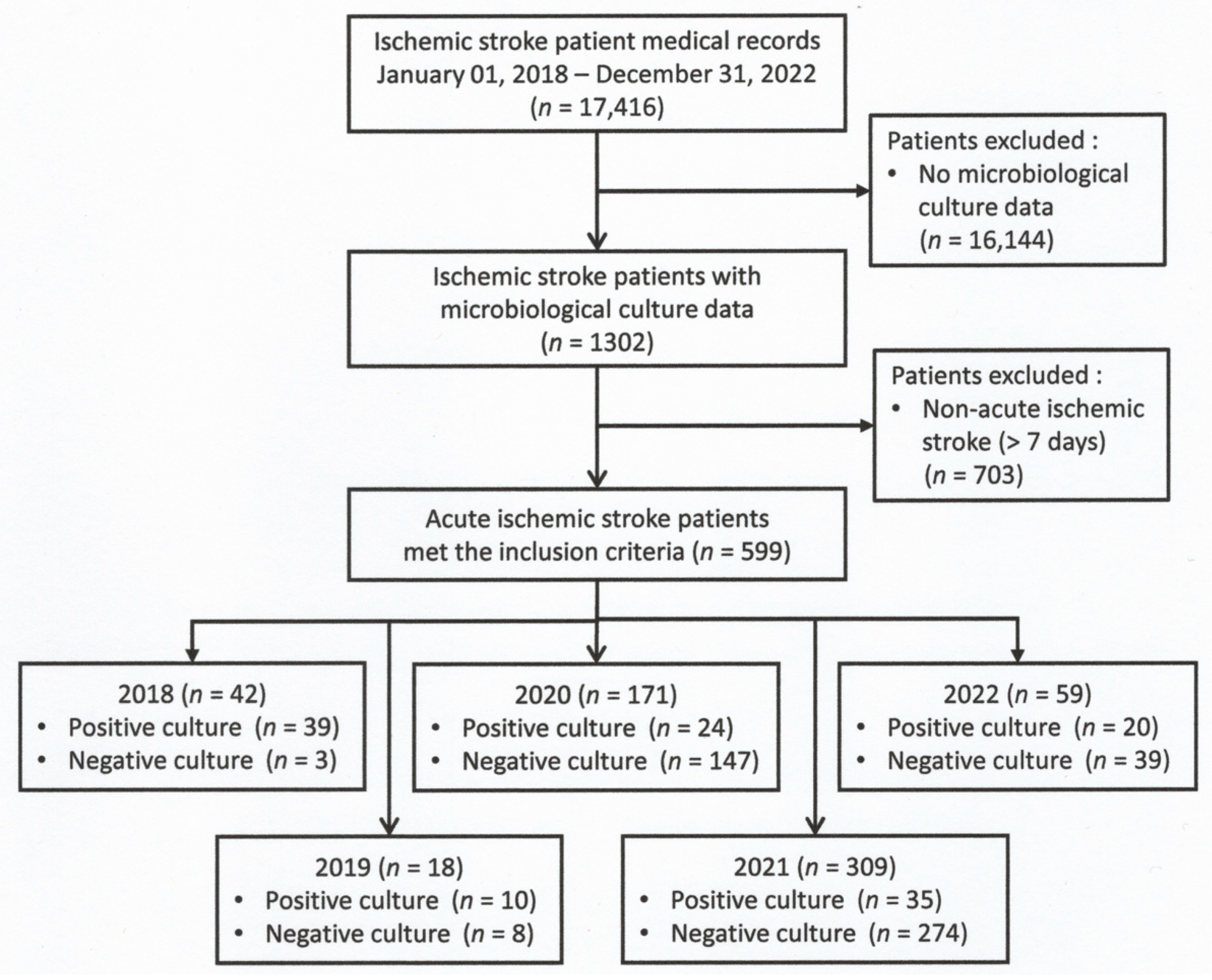
The aim of this study was to determine whether the profile of pathogenic agents of infections after AIS was also influenced by the COVID-19 pandemic period, during which antibiotics were used generously. This study, over five years, was expected to result in a dynamic profile of pathogenic agents of infections after AIS, for which there were limited studies.
4. Discussion
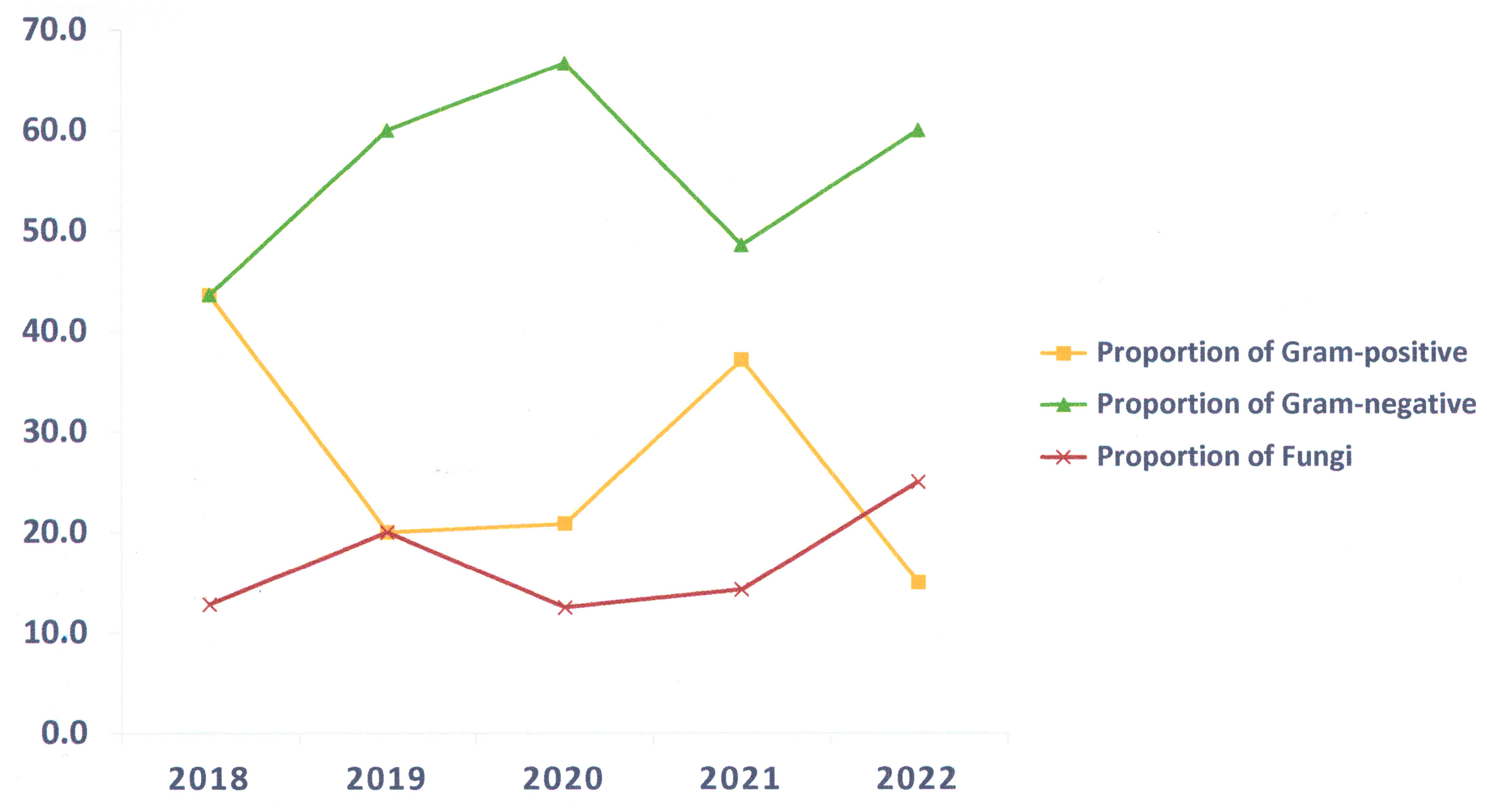
Microbiologic cultures were frequently positive during the non-COVID-19 period (33–92%) and decreased to 11–14% during the COVID-19 period. Previous studies revealed that during the COVID-19 pandemic, the proportion of patients with bacterial or secondary infections was relatively low [
20]. In addition, microbiologic culture was commonly used to determine whether the patient had a secondary infection. The prevalence in this study was 21.4%, lower than in previous studies, since the prevalence was not based only on clinical symptoms but also on culture positivity, which is the gold standard for infection diagnosis.
Sputum samples were the most common microbiological culture samples in five years, denoting that pneumonia was the most common clinical manifestation of infections after AIS. Previous studies in intensive care and general wards revealed that pneumonia is mainly diagnosed in the first days after stroke [
21]. The pneumonia in this study was higher compared to the pneumonia infection rate in most previous studies, which ranged from 1% to 33%. It is believed that pneumonia in stroke patients can occur as a result of dysphagia, which leads to pulmonary aspiration within the first 72 h. This condition can significantly increase the risk of mortality [
22]. Therefore, the use of NGT in this study was associated with a decrease in microbiology culture positivity rates of 46% in AIS during the five years.
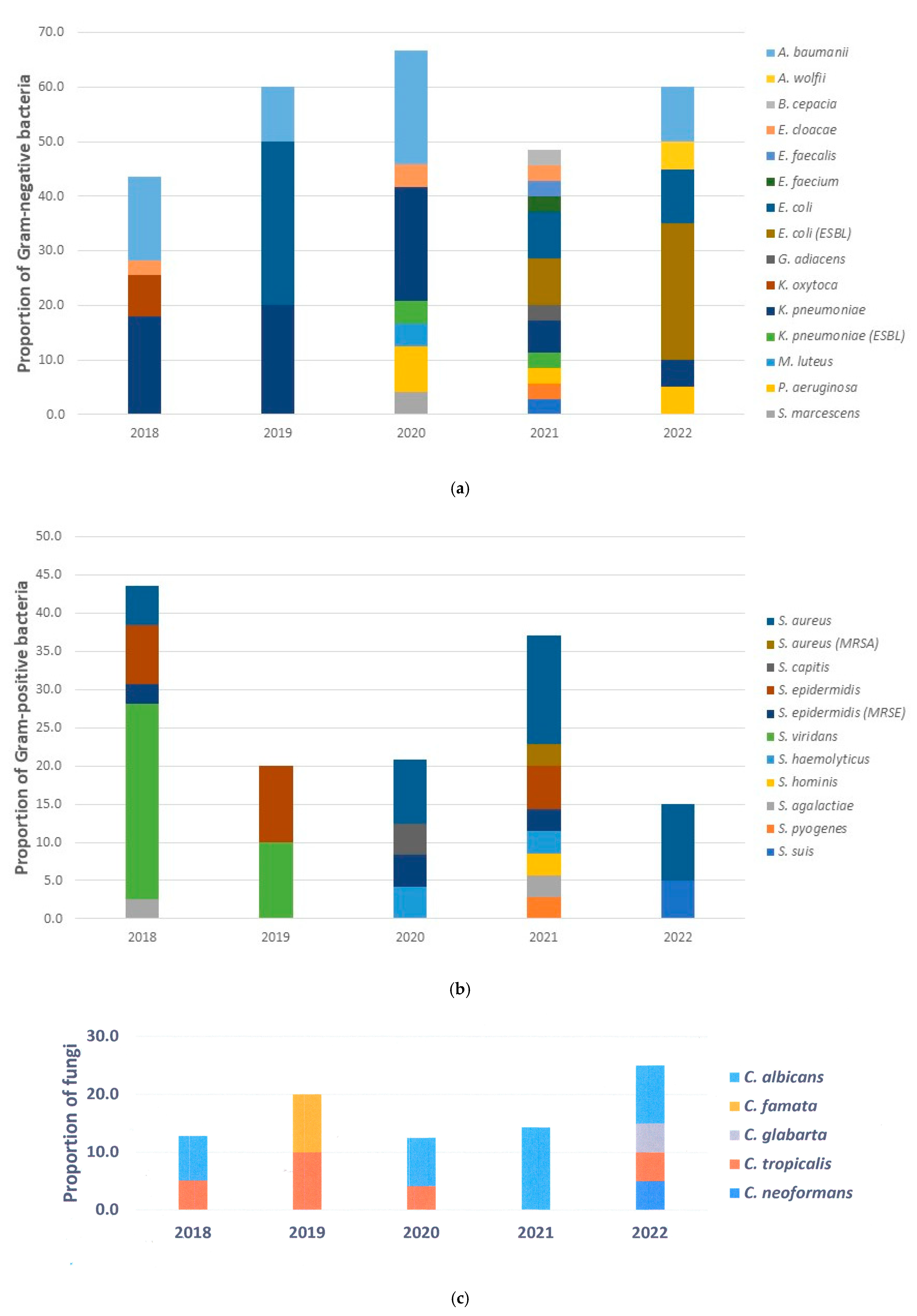
The most prevalent cause of infection in this study was bacteria. Most pathogens were Gram-negative bacteria such as
K. pneumoniae,
E. coli, and
A. baumannii, while
S. aureus was the most common cause of Gram-positive bacteria. These pathogens are mostly nosocomial infections, which is consistent with previous studies showing that infections caused by Gram-negative bacteria and
S. aureus are frequently observed in hospitals.
Streptococcus species were also identified in our study, with
S. viridans being a predominant species. Nowadays,
Streptococcus species remain the most ecumenically identified pathogen in community-acquired pneumonia in the region. This type of pneumonia develops in stroke patients with dysphagia, which can induce aspiration and aggravate the patient’s condition and further complicate their recovery [
13].
In the early stages of the global COVID-19 outbreak, healthcare providers often resorted to an empirical antibiotic treatment of hospitalized patients, especially those infected with COVID-19. Unfortunately, this approach leads to antibiotic resistance [
8]. This study suggested that bacterial strains were more diverse during the COVID-19 period, and bacteria developed resistance to various antimicrobial agents, including β-lactamase antibiotics for Gram-negative bacteria and methicillin for Gram-positive bacteria.
This study showed that fungal infections caused by
C. albicans and
C. tropicalis represent only a minority of the causes of infection in AIS. All
Candida species were cultured from the sputum sample, whilst
Cryptococcus neoformans was isolated from the cerebrospinal fluid.
Candida species are naturally occurring microorganisms in the human body that are generally harmless and are often detected in sputum samples as uninvolved third parties. However,
Candida infections, whether endogenous or from the hospital environment, are increasing in immunocompromised post-stroke patients. To address this problem and develop feasible solutions, molecular biology research is needed [
23].
While the human microbiome does not usually contain
C. neoformans, patients with symptomatic cryptococcosis usually have a well-defined immunocompromised status, including advanced cancers, DM, sarcoidosis, organ transplantation, HIV, and prolonged corticosteroid therapy. In this study, the AIS patient with Cryptococcosis has HIV as a condition that predisposes immunocompromised patients with a normal leukocyte count. However, the normal leukocyte count does not imply that the function of the leucocyte and its subsets was normal, as we did not have data on those. Based on the previous study, it is now estimated that approximately 20% of patients with Cryptococcosis do not have HIV infection [
24].
Intensive and stroke unit care was associated with a decrease in microbiology culture positivity rates in AIS during the five years, both in the non-COVID-19 and COVID-19 periods. It has been shown that the rapid transfer of patients to an intensive care or stroke unit can improve functional outcomes, reduce the need for institutional care and disability, and reduce complications such as dysphagia screening and better management, also reducing mortality by more than 20% compared to regular ward care [
25].
In the non-COVID-19 period, urinary catheterization was associated with an increase in pathogen detection, while antibiotic administration was associated with a decrease in the microbiological culture positivity rate. As expected, leukocytosis upon admission was associated with a higher microbiology culture positivity rate in the non-COVID-19 period. In contrast, leukocytosis and steroids were associated with lower pathogen detection in microbiological culture in the COVID-19 period. Leukocytes are recruited and activated to destroy the pathogen. During the COVID-19 period, viral infections dominated among patients. Therefore, the positivity rate of the microbiological culture was low. Cytokines produced by dendritic cells, macrophages, and other cell types during the innate immune response are involved in the leukocyte reactions. If the initial immune response has not cleared the viral infection, the administration of steroids is thought to downregulate immune-mediated lung injury and the cytokine storm. When properly administered, systemic steroids have been reported to increase the survival rate of COVID-19 patients and modulate the immune response, particularly in sepsis, by reversing septic shock [
20].
TPN has been associated with a decrease in the positivity rate of microbiological culture in AIS in the COVID-19 period. TPN restores clinical development and the immunologic response against pathogens directly related to malnutrition. Malnutrition is associated with poorer outcomes, higher mortality, and poor prognosis in stroke patients. Patients with stroke often have dysphagia, which promotes malnutrition. During the COVID-19 period, it is recommended that patients consider starting tube feeding within 24 h of admission to the hospital [
26,
27]. However, in previous studies, TPN use can disrupt gut mucosal and epithelial barrier function, which might affect the gut immune response, for instance, gut microbiome shifting and the dysregulation of T-cells and cytokines. Therefore, there is a possibility of gaining access to blood vessels [
28].
During the COVID-19 period, strokes were often more severe, and there was a greater need for early tracheostomy to prevent aspiration. Infection control procedures were heavily utilized, and healthcare workers were advised to use personal protective equipment and practice hand hygiene, as these measures can reduce infection and cross-contamination. In our study, tracheostomy was reduced by 20% of pathogen growth in microbiologic culture in AIS during the COVID-19 period. Though DSA was not associated with microbiology culture positivity rates in AIS during both the non-COVID-19 and COVID-19 periods, DSA was associated with a decrease in microbiology culture positivity rates in AIS of 22% over the five years, suggesting that DSA has been useful to identify the blockages in cerebral arteries and for direct treatment, enabling early recovery.
This study has several drawbacks. This study collected data from the first 7 days of AIS patients’ admission, without further follow-up until patients’ discharge. Hence, it remains unclear whether infections after AIS in this study influenced the length of hospital stay, as in a previous study [
4]. Due to the retrospective design, some data could not be collected, for instance, the size or location of the brain lesion or detailed clinical and other examinations of the patient. In addition, the microbiologic culture results were bacteria and fungi but not anaerobic, atypical, or fastidious bacteria due to an equipment limitation. Because this study was conducted at a single center, the sample size was small. Even though this study was performed in a large neurology referral center, the results may not be generalizable to primary stroke units and other facilities. It is suggested that future studies need to determine the prevalence and risk factors associated with infections after AIS in different settings and address the long-term influence of infections in AIS. The prevalence and risk factors associated with infection after ischemic stroke subtypes, non-ischemic stroke, or subsets of acute stroke patients are all important and challenging issues and deserve to be evaluated in further studies.