Biomonitoring Environmental Exposure in Syrian Refugees in Lebanon
Abstract
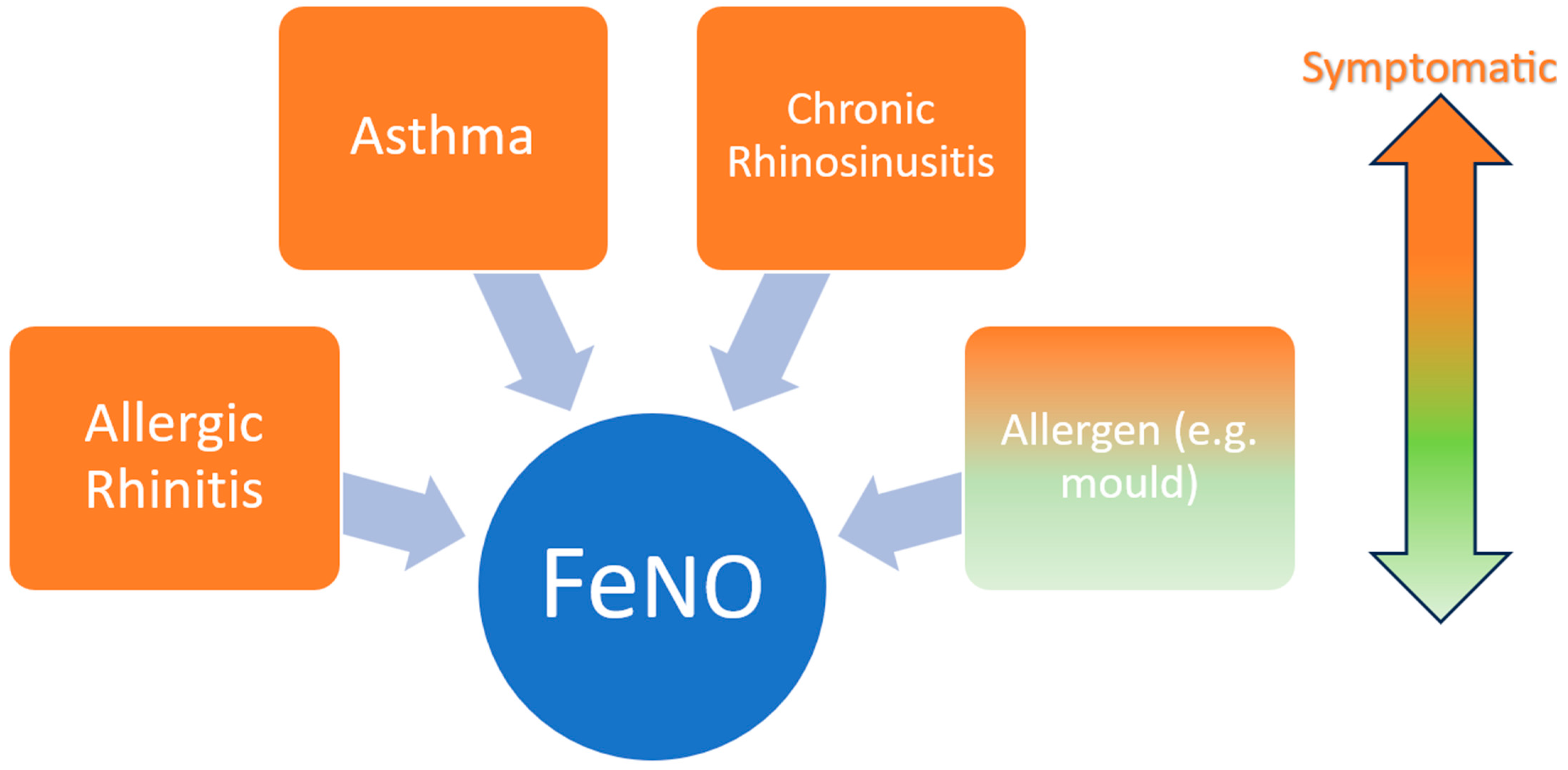
1. Introduction
2. Materials and Methods
3. Results and Discussion
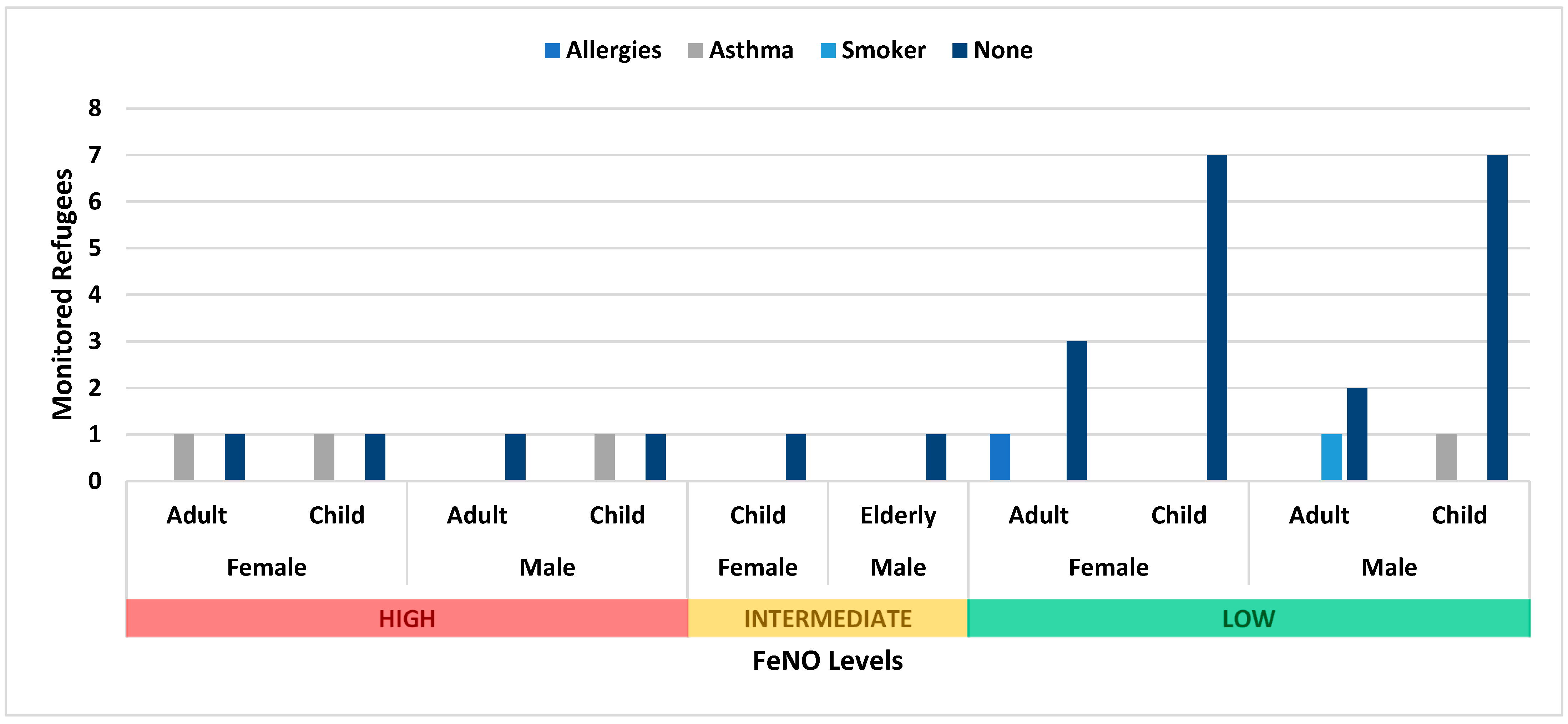
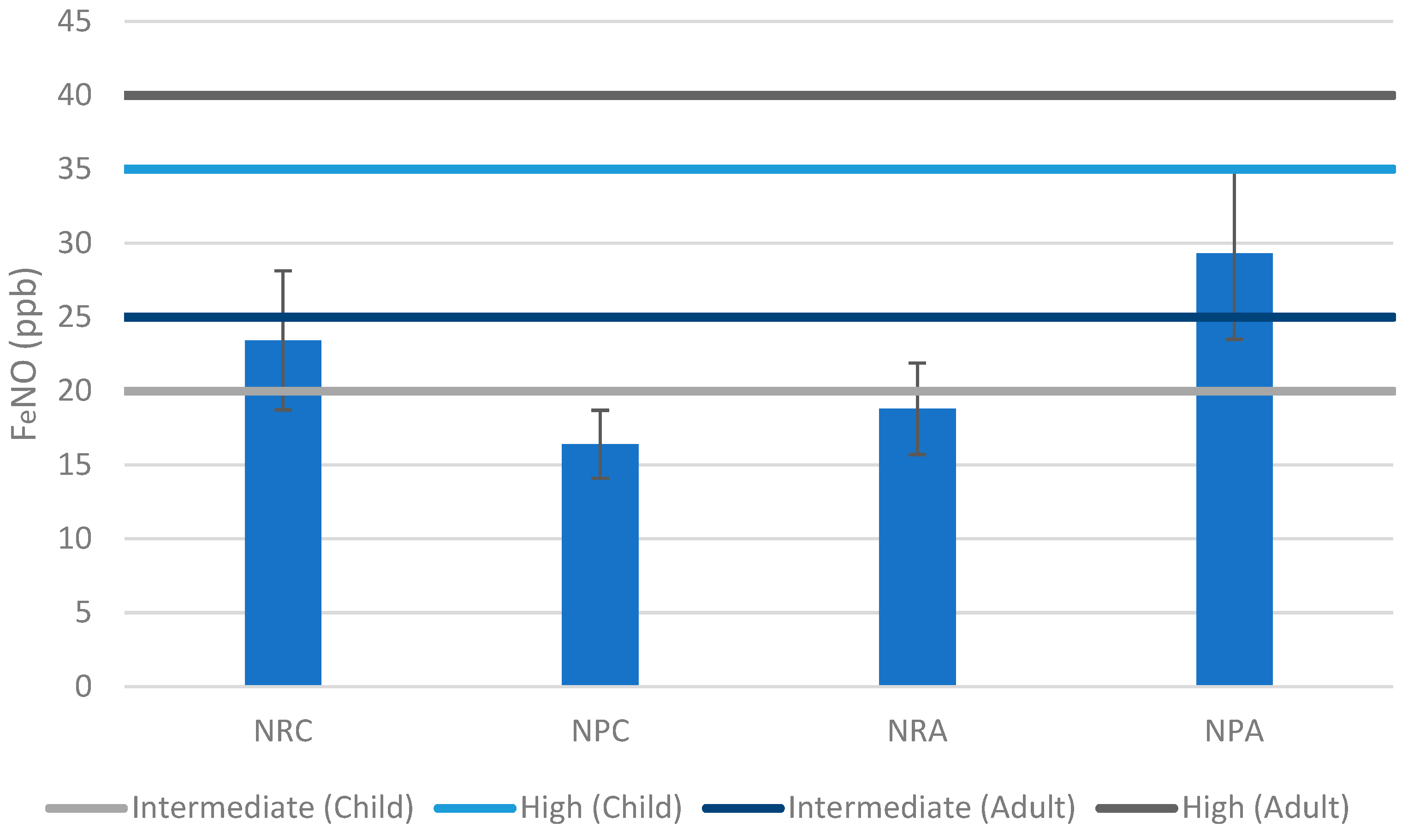
3.1. Non-Residential Shelters
3.2. Non-Permanent Shelters
4. Conclusions, Limitations, and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lambert, H. Temporary refuge from war: Customary international law and the Syrian conflict. Int. Comp. Law Q. 2017, 66, 723. [Google Scholar] [CrossRef]
- UNHCR. 3RP Regional Refugee & Resilience Plan 2017–2018; UNHCR: Geneva, Switzerland, 2017. [Google Scholar]
- UNHCR. Refugee Statistics. Available online: https://www.unhcr.org/refugee-statistics/download/?url=2bxU2f (accessed on 17 December 2022).
- John Hopkins Bloomberg School of Public Health; Mèdecins Du Monde; International Medical Corps; American University of Beirut; UNHCR. Humanitarian Aid and Civil Protection Syrian refugee and Affected Host Population Health Access Survey in Lebanon. 2015. Available online: https://data.unhcr.org/en/documents/details/44869 (accessed on 24 April 2022).
- Blanchet, K.; Fouad, F.M.; Pherali, T. Syrian refugees in Lebanon: The search for universal health coverage. Confl. Health 2016, 10, 12. [Google Scholar] [CrossRef]
- El-Khatib, Z.; Scales, D.; Vearey, J.; Forsberg, B.C. Syrian refugees, between rocky crisis in Syria and hard inaccessibility to healthcare services in Lebanon and Jordan. Confl. Health 2013, 7, 6–8. [Google Scholar] [CrossRef][Green Version]
- Amnesty International. Agonizing Choices: Syrian Refugees in Need of Health Care in Lebanon; Amnesty International: London, UK, 2014. [Google Scholar]
- UNHCR. Health Access and Utilization Survey among Syrian Refugees in Lebanon; UNHCR: Geneva, Switzerland, 2022. [Google Scholar]
- Li, Y.; Feng, L.; Chen, B.; Kim, H.; Yi, S.; Guo, Y.L.; Wu, C. Association of urban particle numbers and sources with lung function among children with asthma or allergies. Sci. Total Environ. 2016, 542, 841–844. [Google Scholar] [CrossRef]
- Lin, L.; Tsai, M.; Chen, M.; Ng, S.; Hsieh, C.; Lin, C.; Lu, F.L.; Hsieh, W.; Chen, P. Childhood exposure to phthalates and pulmonary function. Sci. Total Environ. 2018, 615, 1282–1289. [Google Scholar] [CrossRef]
- Alaouie, M.; Troisi, G.M.; Saliba, N.; Shaib, H.; Hajj, R.; El Hajj, R.; Malak, S.; Jakarian, C.; Jaafar, W. Fungal Exposure and Shelter Assessment in Syrian Refugee Settlements in Lebanon. Aerobiology 2023, 1, 19–36. [Google Scholar] [CrossRef]
- WHO. Regional Office for Europe Human Biomonitoring: Facts and Figures; WHO: Geneva, Switzerland, 2015; p. 88. [Google Scholar]
- Sexton, K.; Needham, L.L.; Pirkle, J.L. Human Biomonitoring of Environmental Chemicals; National Academies Press: Washington, DC, USA, 2004; Volume 92, p. 38. [Google Scholar]
- Hanson, N.; Halling, M.; Norin, H. Biomarkers for Environmental Monitoring Suggestions for Norwegian Monitoring Programs; Norwegian Environment Agency: Trondheim, Norway, 2013. [Google Scholar]
- National Research Council. Biological Markers in Environmental Health Research; Committee on Biological Markers of the National Research Council; National Research Council: Washington, DC, USA, 1987; Volume 74, pp. 3–9. [Google Scholar]
- Lam, P.K.S.; Gray, J.S. The use of biomarkers in environmental monitoring programmes. Mar. Pollut. Bull. 2003, 46, 182–186. [Google Scholar] [CrossRef]
- Paredi, P.; Kharitonov, S.A.; Barnes, P.J. Analysis of Expired Air for Oxidation Products. Am. J. Respir. Crit. Care Med. 2002, 166, S31–S37. [Google Scholar] [CrossRef]
- US Consumer Product Safety Commission. Indoor Air Pollution: An Introduction for Health Professionals; DIANE Publishing: Darby, PA, USA, 1996. [Google Scholar]
- Horvath, I.; Loukides, S.; Wodehouse, T.; Kharitonov, S.A.; Cole, P.J.; Barnes, P.J. Increased levels of exhaled carbon monoxide in bronchiectasis: A new marker of oxidative stress. Thorax 1998, 53, 867–870. [Google Scholar] [CrossRef]
- Uasuf, C.G.; Jatakanon, A.; James, A.; Kharitonov, S.A.; Wilson, N.M.; Barnes, P.J. Exhaled carbon monoxide in childhood asthma. J. Pediatr. 1999, 135, 569–574. [Google Scholar] [CrossRef]
- Nathan, C.; Xie, Q. Nitric oxide synthases: Roles, tolls, and controls. Cell 1994, 78, 915–918. [Google Scholar] [CrossRef]
- Taylor, D.R.; Pijnenburg, M.W.; Smith, A.D.; Jongste, J.C.D. Exhaled nitric oxide measurements: Clinical application and interpretation. Thorax 2006, 61, 817. [Google Scholar] [CrossRef]
- Kelekci, S.; Sen, V.; Yolbas, I.; Uluca, Ü.; Tan, I.; Gürkan, M.F. FeNO levels in children with asthma and other diseases of the lung. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 3078–3082. [Google Scholar]
- Pignatti, P.; Visca, D.; Loukides, S.; Märtson, A.; Alffenaar, J.C.; Migliori, G.B.; Spanevello, A. A snapshot of exhaled nitric oxide and asthma characteristics: Experience from high to low income countries. Pulmonology 2022, 28, 44–58. [Google Scholar] [CrossRef]
- Tang, K.; Shao, X.; Liu, F.; Zhu, B.; Dong, Z.; Xu, W.; Yang, Q. Correlation between nitric oxide content in exhaled breath condensate and the severity of acute respiratory distress syndrome. Int. J. Clin. Exp. Pathol. 2017, 10, 7350. [Google Scholar]
- Nguyen-Thi-Bich, H.; Duong-Thi-Ly, H.; Thom, V.T.; Pham-Thi-Hong, N.; Dinh, L.D.; Le-Thi-Minh, H.; Craig, T.J.; Duong-Quy, S. Study of the correlations between fractional exhaled nitric oxide in exhaled breath and atopic status, blood eosinophils, FCER2 mutation, and asthma control in Vietnamese children. J. Asthma Allergy 2016, 9, 163–170. [Google Scholar]
- Brzozowska, A.; Majak, P.; Jerzyńska, J.; Smejda, K.; Bobrowska-Korzeniowska, M.; Stelmach, W.; Koczkowska, M.; Stelmach, I. Exhaled nitric oxide correlates with IL-2, MCP-1, PDGF-BB and TIMP-2 in exhaled breath condensate of children with refractory asthma. Adv. Dermatol. Allergol./Postępy Dermatol. Alergol. 2015, 32, 107–113. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 inflammation in asthma—Present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef]
- Dunican, E.M.; Fahy, J.V. The role of type 2 inflammation in the pathogenesis of asthma exacerbations. Ann. Am. Thorac. Soc. 2015, 12, S144–S149. [Google Scholar] [CrossRef]
- Busse, W.W.; Kraft, M.; Rabe, K.F.; Deniz, Y.; Rowe, P.J.; Ruddy, M.; Castro, M. Understanding the key issues in the treatment of uncontrolled persistent asthma with type 2 inflammation. Eur. Respir. J. 2021, 58, 2003393. [Google Scholar] [CrossRef]
- Kosoy, I.; Lew, E.; Ledanois, O.; Derrickson, W. Characterization of uncontrolled, severe asthma patients with type 2 inflammation (T2): Results from a physician survey across countries from Latin American, Eurasian Middle East regions and China. J. Asthma 2022, 59, 1021–1029. [Google Scholar] [CrossRef]
- CIRCASSIA. Clinical Guidelines for The Interpretation of FeNO Levels. 2020. Available online: https://www.niox.com/en-us/feno-asthma/interpreting-feno/ (accessed on 24 April 2022).
- Centre of Excellence in Severe Asthma. Inflammation Biomarkers in the Assessment and Management of Severe Asthma—Tools and Interpretation. 2019. Available online: https://www.severeasthma.org.au/biomarkers-recommendation/ (accessed on 24 April 2022).
- Chiappori, A.; De Ferrari, L.; Folli, C.; Mauri, P.; Riccio, A.M.; Canonica, G.W. Biomarkers and severe asthma: A critical appraisal. Clin. Mol. Allergy 2015, 13, 20. [Google Scholar] [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.; Plummer, A.L.; Taylor, D.R. An Official ATS Clinical Practice Guideline: Interpretation of Exhaled Nitric Oxide Levels (FeNO) for Clinical Applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef]
- CIRCASSIA. NIOX VERO Airway Inflammation Monitor; User Manual; Circassia AB: Uppsala, Sweden, 2016. [Google Scholar]
- National Institute for Health and Care Excellence. Asthma: Diagnosis, Monitoring and Chronic Asthma Management; National Institute for Health and Care Excellence: London, UK, March 2017. [Google Scholar]
- Kumar, R.; Gupta, N.; Goel, N. Correlation of atopy and FeNO in allergic rhinitis: An Indian study. Indian J. Chest Dis. Allied Sci. 2013, 55, 79–83. [Google Scholar]
- Czubaj-Kowal, M.; Nowicki, G.J.; Kurzawa, R.; Polak, M.; Ślusarska, B. Factors Influencing the Concentration of Exhaled Nitric Oxide (FeNO) in School Children Aged 8–9-Years-Old in Krakow, with High FeNO Values ≥ 20 ppb. Medicina 2022, 58, 146. [Google Scholar] [CrossRef]
- Murugesan, N.; Saxena, D.; Dileep, A.; Adrish, M.; Hanania, N.A. Update on the role of FeNO in asthma management. Diagnostics 2023, 13, 1428. [Google Scholar] [CrossRef]
- Serbina, N.V.; Salazar-Mather, T.P.; Biron, C.A.; Kuziel, W.A.; Pamer, E.G. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 2003, 19, 59–70. [Google Scholar] [CrossRef]
- Such, J.; Francés, R.; Pérez-Mateo, M. Nitric oxide in patients with cirrhosis and bacterial infections. Metab. Brain Dis. 2002, 17, 303–309. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Lin, J.; Xie, G.; Lv, C.; Zhang, M. Sex differences of small airway function and fractional exhaled nitric oxide in patients with mild asthma. Ann. Allergy Asthma Immunol. 2023, 130, 187–198.e3. [Google Scholar] [CrossRef]
- Olivieri, M.; Corradi, M.; Malerba, M. Gender and exhaled nitric oxide. CHEST J. 2007, 132, 1410. [Google Scholar] [CrossRef][Green Version]
- Janahi, I.; Saadoon, A.; Tuffaha, A.; Panneerselvam, B. Effects of age, gender, and environmental exposures on exhaled nitric oxide level in healthy 12 to 18 years Qatari children. Ann. Thorac. Med. 2012, 7, 98–103. [Google Scholar] [CrossRef]
- Zhang, H.; Shu, L.; Cai, X.; Wang, Z.; Jiao, X.; Liu, F.; Hou, P.; Wang, L.; Shan, L.; Chen, N. Gender and age affect the levels of exhaled nitric oxide in healthy children. Exp. Ther. Med. 2013, 5, 1174–1178. [Google Scholar] [CrossRef]
- Escamilla-Gil, J.M.; Fernandez-Nieto, M.; Acevedo, N. Understanding the cellular sources of the fractional exhaled nitric oxide (FeNO) and its role as a biomarker of type 2 inflammation in asthma. BioMed Res. Int. 2022, 2022, 5753524. [Google Scholar] [CrossRef]

| Type of Shelter | Gender | Children (<18) | Reported Conditions | Adults (18 < 60) | Reported Conditions | Elderly (>60) | Reported Conditions |
|---|---|---|---|---|---|---|---|
| Non-residential | F | 8 | Asthma (1) | ||||
| 6 | Asthma (1) | 1 | Allergies (1) | ||||
| M | 6 | Smoker (1) | |||||
| Asthma (1) Allergies (1) | 3 | Smoker (2) | 2 | Asthma (2) | |||
| Non-permanent | F | 10 | Asthma (1) | 6 | Asthma (1) Allergies (1) | 0 | None |
| M | 10 | Asthma (2) | 4 | Smoker (2) | 1 | None |
| FeNO Levels | T2 Inflammation | Symptomatic | Asymptomatic | Reference |
|---|---|---|---|---|
| Low | Unlikely | 6 | 34 | |
| Intermediate | Likely | 0 | 3 | [32] |
| High | Significant | 6 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaouie, M.; Troisi, G.M. Biomonitoring Environmental Exposure in Syrian Refugees in Lebanon. Epidemiologia 2024, 5, 309-317. https://doi.org/10.3390/epidemiologia5020021
Alaouie M, Troisi GM. Biomonitoring Environmental Exposure in Syrian Refugees in Lebanon. Epidemiologia. 2024; 5(2):309-317. https://doi.org/10.3390/epidemiologia5020021
Chicago/Turabian StyleAlaouie, Malek, and Gera M. Troisi. 2024. "Biomonitoring Environmental Exposure in Syrian Refugees in Lebanon" Epidemiologia 5, no. 2: 309-317. https://doi.org/10.3390/epidemiologia5020021
APA StyleAlaouie, M., & Troisi, G. M. (2024). Biomonitoring Environmental Exposure in Syrian Refugees in Lebanon. Epidemiologia, 5(2), 309-317. https://doi.org/10.3390/epidemiologia5020021








