COVID-19 Vaccination Strategy in China: A Case Study
Abstract
:1. Introduction
2. Methodology
3. Results
3.1. Case Presentation
3.1.1. The Origin of the Novel Coronavirus Infection
3.1.2. Sociodemographic Characteristics of COVID-19 Cases
3.1.3. The Chinese Healthcare System
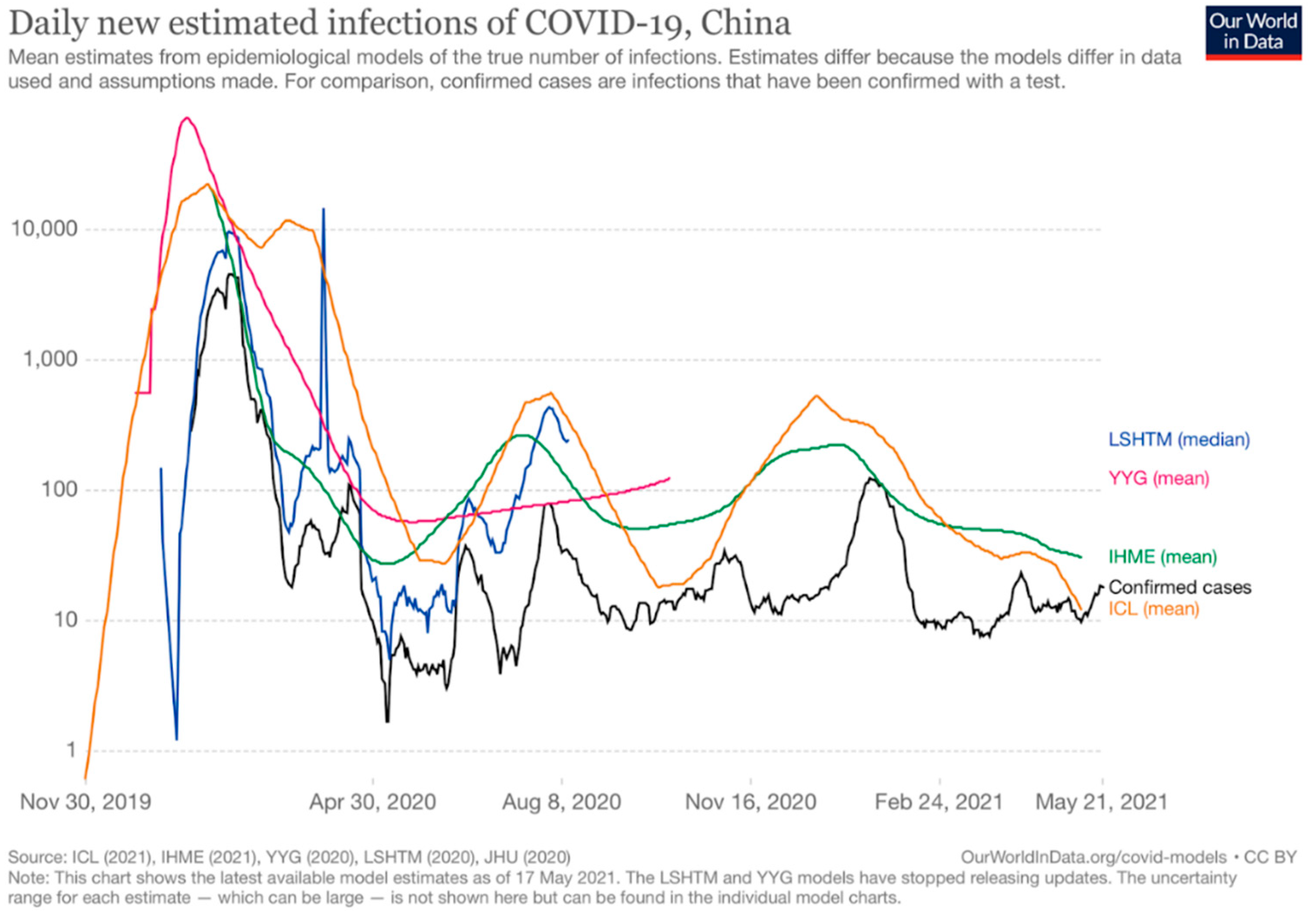
3.1.4. Chinese Epidemiological Situation
3.2. Management and Impacts of the COVID-19 Outbreak
3.2.1. Non-Pharmaceutical Interventions
3.2.2. Impact of the Media
3.2.3. Economic Impact
3.2.4. Psychological Impacts
3.3. Vaccination Strategy
3.3.1. Acceptance of COVID-19 Vaccination in China
3.3.2. Available Vaccines and the Characteristics
The General Vaccine Development and Approval Process
Current Available Vaccines
3.3.3. National Vaccination Strategy
“Three-Steps” and “Two-Emphases” Vaccination Strategy and Prioritization of Target Groups
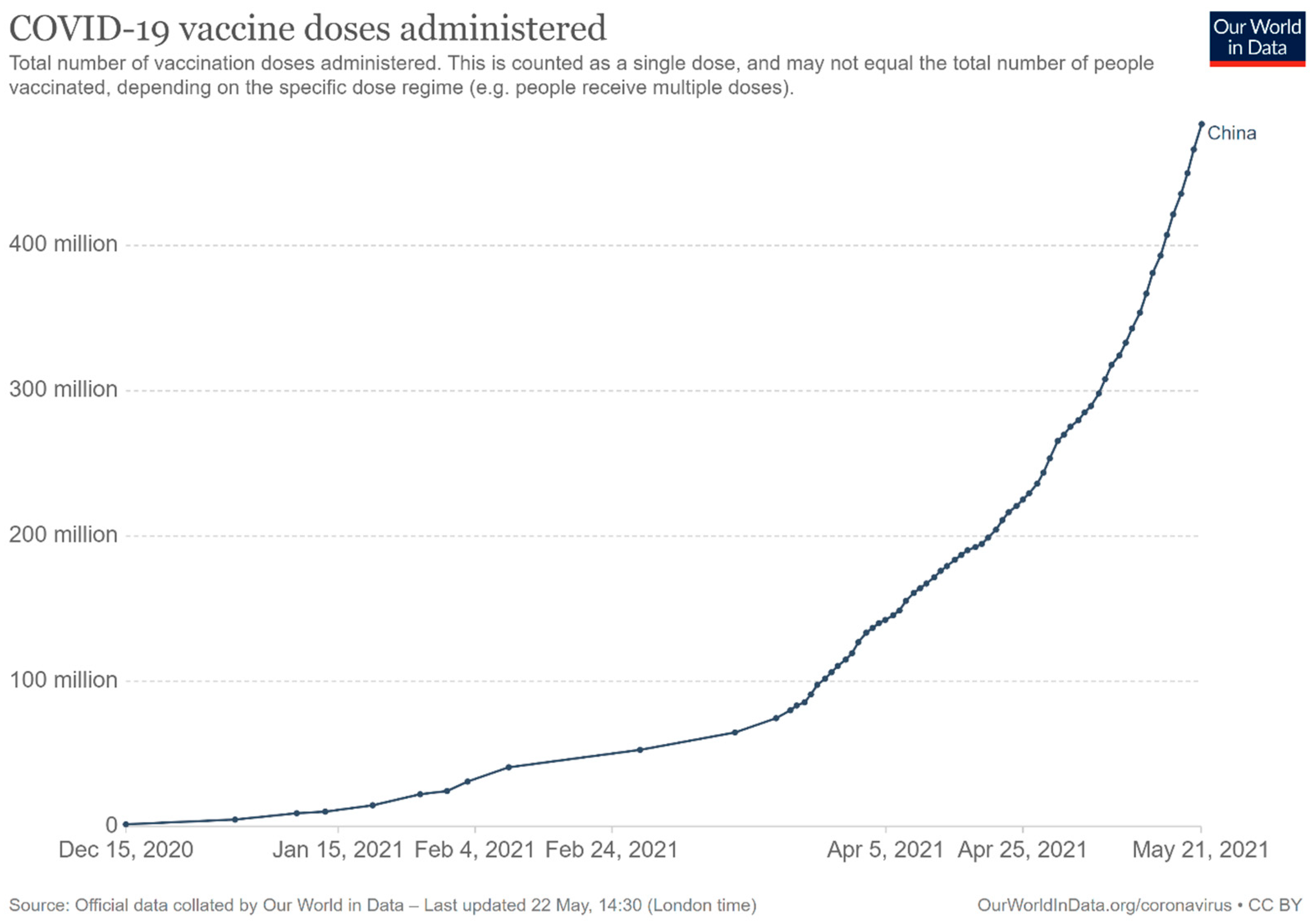
Vaccine Supply Volume and Its Dynamics
Vaccination Procedure for Individuals
Clinical Management of Potential Adverse Events
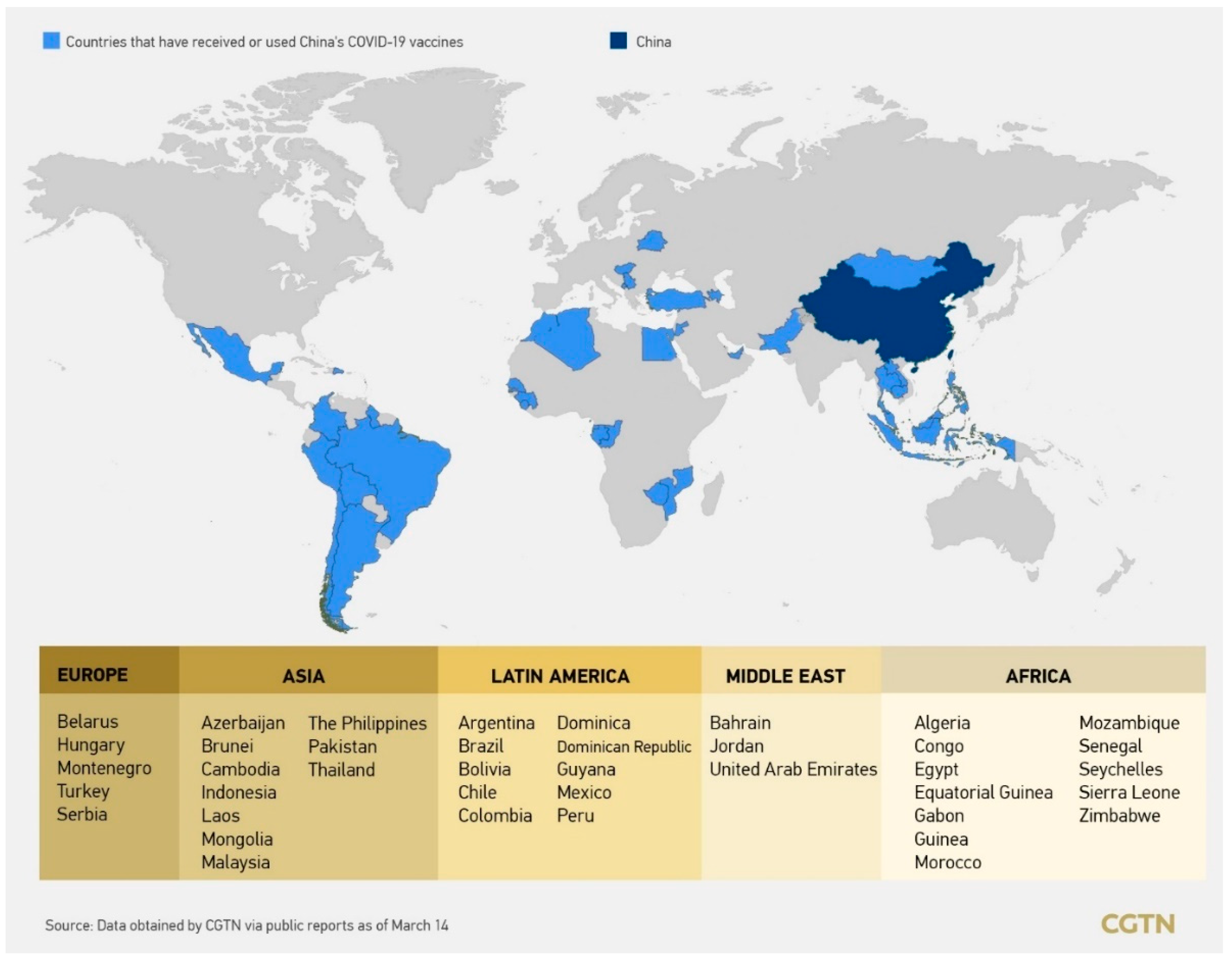
3.3.4. Vaccine Delivery Outside China
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, Q.; Wang, B.; Mao, J.; Fu, J.; Shang, S.; Shu, Q.; Zhang, T. Epidemiological analysis of COVID-19 and practical experience from China. J. Med. Virol. 2020, 92, 755–769. [Google Scholar] [CrossRef] [Green Version]
- Les Etapes de la Propagation du Coronavirus dans le Monde. Available online: https://www.letemps.ch/monde/etapes-propagation-coronavirus-monde (accessed on 7 May 2021).
- Nombre de Personnes Infectées par le Coronavirus (COVID-19) dans le Monde au 17 Mai 2021, Selon le Pays. Available online: https://fr.statista.com/statistiques/1091585/morts-infections-coronavirus-monde/ (accessed on 20 April 2021).
- Nombre de Personnes Infectées par le Coronavirus (COVID-19) en Chine au 17 Mai 2021, Selon la Région. Available online: https://fr.statista.com/statistiques/1101778/infections-coronavirus-chine-region/ (accessed on 20 April 2021).
- Nombre de Personnes Décédées à Cause du Coronavirus (COVID-19) en Chine au 17 Mai 2021, Selon la Région. Available online: https://fr.statista.com/statistiques/1101794/morts-coronavirus-chine-region/ (accessed on 20 April 2021).
- Nombre de Personnes Décédées à Cause du Coronavirus (COVID-19) dans le Monde au 17 Mai 2021, Selon le Pays ou Territoire. Available online: https://fr.statista.com/statistiques/1101324/morts-coronavirus-monde/ (accessed on 20 April 2021).
- Regmi, K.; Lwin, C.M. Impact of non-pharmaceutical interventions for reducing transmission of COVID-19: A systematic review and meta-analysis protocol. BMJ Open 2020, 10, e041383. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 24 August 2021).
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Li, Y.-H.; Guan, X.-H.; Hou, L.-H.; Wang, W.-J.; Li, J.-X.; Wu, S.-P.; Wang, B.-S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Andreadakis, Z.; Kumar, A.; Román, R.G.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar]
- Russia’s Fast-Track Coronavirus Vaccine Draws Outrage over Safety. Available online: https://www.nature.com/articles/d41586-020-02386-2 (accessed on 24 August 2021).
- U.K. Becomes first Country to Approve Pfizer-BioNTech Covid-19 Vaccine. Available online: https://www.nbcnews.com/news/world/u-k-becomes-first-country-approve-pfizer-biontech-covid-19-n1249651 (accessed on 24 August 2021).
- COVID-19 Vaccine Market Dashboard. Available online: https://www.unicef.org/supply/covid-19-vaccine-market-dashboard (accessed on 10 May 2021).
- Press Conference of the Joint Prevention and Control Mechanism of the State Council (31 December 2020). Available online: http://www.gov.cn/xinwen/gwylflkjz143/index.htm (accessed on 30 April 2021).
- Press Conference of the Joint Prevention and Control Mechanism of the State Council (19 December 2020). Available online: http://www.gov.cn/xinwen/gwylflkjz140/index.htm (accessed on 30 April 2021).
- Global No.1 Business Data Plateform. Available online: https://www.statista.com (accessed on 22 May 2021).
- Our World in Data. Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations?country=~CHN (accessed on 22 May 2021).
- Kpozehouen, E.B.; Chen, X.; Zhu, M.; Macintyre, C.R. Using Open-Source Intelligence to Detect Early Signals of COVID-19 in China: Descriptive Study. MIR Public Health Surveill. 2020, 6, e18939. [Google Scholar] [CrossRef]
- Zaugg, J. Comment la Chine a Laissé Echapper le Coronavirus. Available online: https://www.letemps.ch/monde/chine-laisse-echapper-coronavirus (accessed on 26 April 2021).
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irving, A.T.; Ahn, M.; Goh, G.; Anderson, D.E.; Wang, L.-F. Lessons from the host defences of bats, a unique viral reservoir. Nature 2021, 589, 363–370. [Google Scholar] [CrossRef]
- Campbell, J.; Atwood, K.; Perez, E. US Explores Possibility that Coronavirus Spread Started in Chinese Lab, Not a Market. Available online: https://edition.cnn.com/2020/04/15/politics/us-intelligence-virus-started-chinese-lab/index.html (accessed on 26 April 2021).
- Roberts, M. Covid: WHO Says ‘Extremely Unlikely’ Virus Leaked from Lab in China. Available online: https://www.bbc.com/news/world-asia-china-55996728 (accessed on 26 April 2021).
- China Demographics. Available online: https://www.worldometers.info/demographics/china-demographics/ (accessed on 10 April 2021).
- Illmer, A.; Wang, Y.; Wong, T. Wuhan lockdown: A Year of China’s Fight against the Covid Pandemic. Available online: https://www.bbc.com/news/world-asia-china-55628488 (accessed on 2 May 2021).
- Geng, M.-J.; Wang, L.-P.; Ren, X.; Yu, J.-X.; Chang, Z.-R.; Zheng, C.-J.; An, Z.-J.; Li, Y.; Yang, X.-K.; Zhao, H.-T.; et al. Risk factors for developing severe COVID-19 in China: An analysis of disease surveillance data. Infect. Dis. Poverty 2021, 10, 48. [Google Scholar] [CrossRef]
- The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 2019, 41, 145.
- Case Fatality Rate of COVID-19 vs. Median Age of the Population. Available online: https://ourworldindata.org/grapher/case-fatality-rate-of-covid-19-vs-median-age?yScale=log&time=earliest..latest&country=~CHN (accessed on 8 May 2021).
- Mi, Y.N.; Huang, T.T.; Zhang, J.X.; Qin, Q.; Gong, Y.X.; Liu, S.Y.; Xue, H.M.; Ning, C.H.; Cao, L.; Cao, Y.X. Estimating the instant case fatality rate of COVID-19 in China. Int. J. Infect. Dis. 2020, 97, 1–6. [Google Scholar] [CrossRef]
- Revised Death Toll Shows Wuhan Is Moving on. Available online: http://global.chinadaily.com.cn/a/202004/18/WS5e9a5457a3105d50a3d171dc.html (accessed on 3 May 2021).
- The World Bank in China. Available online: https://www.worldbank.org/en/country/china/overview#1 (accessed on 24 April 2021).
- Top 15 Countries by GDP in 2020. Available online: https://globalpeoservices.com/top-15-countries-by-gdp-in-2020/ (accessed on 24 April 2021).
- Liu, X.; Wang, J.L. An introduction to China’s health care system. J. Public Health Policy 1991, 12, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Mills, A.; Wang, L.; Han, Q. What can we learn from China’s health system reform? BMJ 2019, 365, l2349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qingyue, M.; Hongwei, Y.; Wen, C.; Qiang, S.; Xiaoyun, L. People’s Republic of China Health System Review; World Health Organization, Regional Office for the Western Pacific: Manila, Philippines, 2015; Volume 5, p. 7. [Google Scholar]
- Fu, W.; Zhao, S.; Zhang, Y.; Chai, P.; Goss, J. Research in health policy making in China: Out-of-pocket payments in Healthy China 2030. BMJ 2018, 360, k234. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, Z.; Ma, Q.; Fang, G.; Yang, J. The development and reform of public health in China from 1949 to 2019. Glob. Health 2019, 15, 45. [Google Scholar] [CrossRef]
- Health Care Quality in the Far East: China, Japan, Philippines, South Korea and Taiwan. Available online: https://www.aetnainternational.com/en/about-us/explore/living-abroad/culture-lifestyle/health-care-quality-in-the-far-east.html (accessed on 2 May 2021).
- Zhu, W.; Wang, Y.; Xiao, K.; Zhang, H.; Tian, Y.; Clifford, S.P.; Xu, J.; Huang, J. Establishing and Managing a Temporary Coronavirus Disease 2019 Specialty Hospital in Wuhan, China. Anesthesiology 2020, 132, 1339–1345. [Google Scholar] [CrossRef] [Green Version]
- No Country Should Make ‘Fatal’ Mistake of Ignoring COVID-19: Tedros. Available online: https://news.un.org/en/story/2020/02/1058221 (accessed on 28 August 2021).
- Our World in Data. Daily New Estimated Infections of COVID-19, China. 2021. Available online: https://ourworldindata.org/grapher/daily-new-estimated-infections-of-covid-19 (accessed on 22 May 2021).
- Covid-19: China’s Qingdao to Test Nine Million in Five Days. Available online: https://www.bbc.com/news/world-asia-54504785 (accessed on 2 May 2021).
- Covid-19: China Tests Entire City of Kashgar in Xinjiang. Available online: https://www.bbc.com/news/world-asia-china-54687533 (accessed on 2 May 2021).
- Bo, Y.; Guo, C.; Lin, C.; Zeng, Y.; Li, H.B.; Zhang, Y.; Hossain, M.S.; Chan, J.W.M.; Yeung, D.W.; Kwok, K.O. Effectiveness of non-pharmaceutical interventions on COVID-19 transmission in 190 countries from 23 January to 13 April 2020. Int. J. Infect. Dis. 2021, 102, 247–253. [Google Scholar] [CrossRef]
- Lai, S.; Ruktanonchai, N.W.; Zhou, L.; Prosper, O.; Luo, W.; Floyd, J.R.; Wesolowski, A.; Santillana, M.; Zhang, C.; Du, X. Effect of non-pharmaceutical interventions to contain COVID-19 in China. Nature 2020, 585, 410–413. [Google Scholar] [CrossRef]
- Zhang, Y.; Diao, X.; Chen, K.Z.; Robinson, S.; Fan, S. Impact of COVID-19 on China’s macroeconomy and agri-food system–an economy-wide multiplier model analysis. China Agric. Econ. Rev. 2020, 12, 387–407. [Google Scholar] [CrossRef]
- Sun, J.; Shi, Z.; Xu, H. Non-pharmaceutical interventions used for COVID-19 had a major impact on reducing influenza in China in 2020. J. Travel Med. 2020, 27, taaa064. [Google Scholar] [CrossRef]
- Liu, Y.; Morgenstern, C.; Kelly, J.; Lowe, R.; Munday, J.; Villabona-Arenas, C.J.; Gibbs, H.; Pearson, C.A.B.; Prem, K.; Leclerc, Q.J.; et al. The impact of non-pharmaceutical interventions on SARS-CoV-2 transmission across 130 countries and territories. BMC Med. 2021, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.; Khosrawipour, V.; Kocbach, P.; Mikolajczyk, A.; Schubert, J.; Bania, J.; Khosrawipour, T. The positive impact of lockdown in Wuhan on containing the COVID-19 outbreak in China. J. Travel Med. 2020, 27, taaa037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, Q.; Jin, H.; Sun, Z.; Kao, Q.; Chen, J. Non-pharmaceutical intervention strategies for outbreak of COVID-19 in Hangzhou, China. Public Health 2020, 182, 185. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shen, F. Exploring the impacts of media use and media trust on health behaviors during the COVID-19 pandemic in China. J. Health Psychol. 2021, 1359105321995964. [Google Scholar] [CrossRef]
- Hou, Z.; Du, F.; Jiang, H.; Zhou, X.; Lin, L. Assessment of public attention, risk perception, emotional and behavioural responses to the COVID-19 outbreak: Social media surveillance in China. Lancet 2020. under review. [Google Scholar] [CrossRef]
- Lin, L.; Savoia, E.; Agboola, F.; Viswanath, K. What have we learned about communication inequalities during the H1N1 pandemic: A systematic review of the literature. BMC Public Health 2014, 14, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Chen, Z.; Bao, G. Role of media coverage in mitigating COVID-19 transmission: Evidence from China. Technol. Forecast. Soc. Change 2021, 163, 120435. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, Y.; Chang, H. Media use and health behavior in H1N1 flu crisis: The mediating role of perceived knowledge and fear. Atl. J. Commun. 2015, 23, 67–80. [Google Scholar] [CrossRef]
- Prati, G.; Pietrantoni, L.; Zani, B. Compliance with recommendations for pandemic influenza H1N1 2009: The role of trust and personal beliefs. Health Educ. Res. 2011, 26, 761–769. [Google Scholar] [CrossRef] [Green Version]
- Korda, H.; Itani, Z. Harnessing social media for health promotion and behavior change. Health Promot. Pract. 2013, 14, 15–23. [Google Scholar] [CrossRef]
- Gui, L. Media framing of fighting COVID-19 in China. Sociol. Health Illn. 2021, 43, 966–970. [Google Scholar] [CrossRef]
- Chang, X.; Liu, M.; Jin, Z.; Wang, J. Studying on the impact of media coverage on the spread of COVID-19 in Hubei Province, China. Math. Biosci. Eng. 2020, 17, 3147–3159. [Google Scholar] [CrossRef]
- Fabre, G. China’s Digital Transformation. Why is Artificial Intelligence a Priority for Chinese R&D? 2018. Available online: https://halshs.archives-ouvertes.fr/halshs-01818508v2 (accessed on 10 May 2021).
- Luo, S.; Xin, M.; Wang, S.; Zhao, J.; Zhang, G.; Li, L.; Li, L.; Lau, J.T.-f. Behavioral intention of receiving COVID-19 vaccination, social media exposures, and peer discussions in China. Epidemiol. Infect. 2021, 1–33. [Google Scholar]
- Yan, J.; Feng, L.; Denisov, A.; Steblyanskaya, A.; Oosterom, J.-P. Correction: Complexity theory for the modern Chinese economy from an information entropy perspective: Modeling of economic efficiency and growth potential. PLoS ONE 2020, 15, e0230165. [Google Scholar] [CrossRef] [Green Version]
- Vasiev, M.; Bi, K.; Denisov, A.; Bocharnikov, V. How COVID-19 Pandemics Influences Chinese Economic Sustainability. Foresight STI Gov. 2020, 14, 7–22. [Google Scholar]
- Gössling, S.; Scott, D.; Hall, C.M. Pandemics, tourism and global change: A rapid assessment of COVID-19. J. Sustain. Tour. 2020, 29, 1–20. [Google Scholar] [CrossRef]
- Liu, K. COVID-19 and the Chinese economy: Impacts, policy responses and implications. Int. Rev. Appl. Econ. 2021, 35, 308–330. [Google Scholar] [CrossRef]
- Pan, D.; Yang, J.; Zhou, G.; Kong, F. The influence of COVID-19 on agricultural economy and emergency mitigation measures in China: A text mining analysis. PLoS ONE 2020, 15, e0241167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Song, H.; Wen, L.; Liu, C. Forecasting tourism recovery amid COVID-19. Ann. Tour. Res. 2021, 87, 103149. [Google Scholar] [CrossRef]
- Hoque, A.; Shikha, F.A.; Hasanat, M.W.; Arif, I.; Hamid, A.B.A. The effect of Coronavirus (COVID-19) in the tourism industry in China. Asian J. Multidiscip. Stud. 2020, 3, 52–58. [Google Scholar]
- Bakar, N.A.; Rosbi, S. Effect of Coronavirus disease (COVID-19) to tourism industry. Int. J. Adv. Sci. Eng. Inf. Technol. 2020, 7, 189–193. [Google Scholar] [CrossRef] [Green Version]
- China’s U.S. Embassy Has Begun Accepting Non-Chinese Vaccine Records. Available online: https://www.reuters.com/world/china/chinas-us-embassy-has-begun-accepting-non-chinese-vaccine-records-2021-04-20/ (accessed on 21 June 2021).
- Int’l Students Ask un to Help Them Return to Their Universities in China after Over a Year of Distance Learning. Available online: https://collegenews.org/intl-students-ask-un-to-help-them-return-to-their-universities-in-china-after-over-a-year-of-distance-learning/ (accessed on 1 May 2021).
- Xinhua. Report: China to See Increased Domestic Tourism in 2021. Available online: https://www.chinadailyhk.com/article/158413 (accessed on 1 May 2021).
- Zhou, Q.; Hu, Z.; Bian, G.; Yu, H.; Li, X.; Lu, Y.; Yu, C.; Li, X.; Yao, Q.; Zhou, W. Mental health and psychosocial function of general population during the COVID-19 epidemic in China. J. Transl. Med. 2020, 10, e103. [Google Scholar] [CrossRef]
- Xiang, Y.-T.; Yang, Y.; Li, W.; Zhang, L.; Zhang, Q.; Cheung, T.; Ng, C.H. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry 2020, 7, 228–229. [Google Scholar] [CrossRef] [Green Version]
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.Z.; Ahmed, O.; Aibao, Z.; Hanbin, S.; Siyu, L.; Ahmad, A. Epidemic of COVID-19 in China and associated psychological problems. Asian J. Psychiatr. 2020, 51, 102092. [Google Scholar] [CrossRef]
- Morris, S.E.; Moment, A.; Thomas, J.d. Caring for bereaved family members during the COVID-19 pandemic: Before and after the death of a patient. J. Pain Symptom. Manag. 2020, 60, e70–e74. [Google Scholar] [CrossRef]
- Menichetti Delor, J.P.; Borghi, L.; Cao di San Marco, E.; Fossati, I.; Vegni, E. Phone follow up to families of COVID-19 patients who died at the hospital: Families’ grief reactions and clinical psychologists’ roles. Int. J. Psychol. 2021, 56, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, W.; Zhao, X.; Zhang, W. Recommended psychological crisis intervention response to the 2019 novel coronavirus pneumonia outbreak in China: A model of West China Hospital. Precis. Clin. Med. 2020, 3, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yang, Y.; Liu, Z.-H.; Zhao, Y.-J.; Zhang, Q.; Zhang, L.; Cheung, T.; Xiang, Y.-T. Progression of mental health services during the COVID-19 outbreak in China. Int. J. Biol. Sci. 2020, 16, 1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldwell, T.M.; Rodgers, B.; Jorm, A.F.; Christensen, H.; Jacomb, P.A.; Korten, A.E.; Lynskey, M.T. Patterns of association between alcohol consumption and symptoms of depression and anxiety in young adults. Addiction 2002, 97, 583–594. [Google Scholar] [CrossRef]
- Morita, T.; Tanimoto, T.; Hori, A.; Kanazawa, Y. Alcohol use disorder due to social isolation after a nuclear disaster in Fukushima. BMJ Case Rep. 2015, 2015, bcr2015209971. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Bouey, J. Public mental health crisis during COVID-19 pandemic, China. Emerg. Infect. Dis. 2020, 26, 1616. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ren, Y.; Yan, F.; Li, Y.; Xu, X.; Yu, X.; Qu, W.; Wang, Z.; Tian, B.; Yang, F.; et al. Psychological Impact and Predisposing Factors of the Coronavirus Disease 2019 (COVID-19) Pandemic on General Public in China. Lancet Psychiatry 2020. under review. [Google Scholar] [CrossRef]
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef]
- Greenberg, M.E.; Lai, M.H.; Hartel, G.F.; Wichems, C.H.; Gittleson, C.; Bennet, J.; Dawson, G.; Hu, W.; Leggio, C.; Washington, D.; et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N. Engl. J. Med. 2009, 361, 2405–2413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.; Henningsen, K.H.; Brehaut, J.C.; Hoe, E.; Wilson, K. Acceptance of a pandemic influenza vaccine: A systematic review of surveys of the general public. Infect. Drug Resist. 2011, 4, 197–207. [Google Scholar]
- Mereckiene, J.; Cotter, S.; Weber, J.T.; Nicoll, A.; D’Ancona, F.; Lopalco, P.L.; Johansen, K.; Wasley, A.M.; Jorgensen, P.; Lévy-Bruhl, D.; et al. Influenza A (H1N1) pdm09 vaccination policies and coverage in Europe. Eurosurveillance 2012, 17, 20064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubé, E.; Laberge, C.; Guay, M.; Bramadat, P.; Roy, R.; Bettinger, J.A. Vaccine hesitancy. Hum. Vaccin. Immunother. 2013, 9, 1763–1773. [Google Scholar] [CrossRef]
- MacDonald, N.E. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- Soares, P.; Rocha, J.V.; Moniz, M.; Gama, A.; Laires, P.A.; Pedro, A.R.; Dias, S.; Leite, A.; Nunes, C. Factors associated with COVID-19 vaccine hesitancy. Vaccines 2021, 9, 300. [Google Scholar] [CrossRef]
- Wang, J.; Jing, R.; Lai, X.; Zhang, H.; Lyu, Y.; Knoll, M.D.; Fang, H. Acceptance of COVID-19 Vaccination during the COVID-19 Pandemic in China. Vaccines 2020, 8, 482. [Google Scholar] [CrossRef]
- Wang, J.; Lu, X.; Lai, X.; Lyu, Y.; Zhang, H.; Fenghuang, Y.; Jing, R.; Li, L.; Yu, W.; Fang, H. The changing acceptance of COVID-19 vaccination in different epidemic phases in China: A longitudinal study. Vaccines 2021, 9, 191. [Google Scholar] [CrossRef]
- National Medical Product Administration. Vaccine Administration Law of the People’s Republic of China. Available online: https://www.nmpa.gov.cn/xxgk/fgwj/flxzhfg/20190702121701506.html (accessed on 29 April 2021).
- National Medical Products Administration. Guideline for Clinical Evaluation of Novel Coronavirus Preventive Vaccines (Interim). Available online: https://www.nmpa.gov.cn/yaopin/ypggtg/20200814230916157.html (accessed on 29 April 2021).
- National Medical Products Administration. Technical Guidance for Clinical Research on Novel Coronavirus Preventive Vaccines (Interim). Available online: https://www.nmpa.gov.cn/yaopin/ypggtg/20200814230916157.html (accessed on 29 April 2021).
- WHO Target Product Profiles for COVID-19 Vaccines. Available online: https://www.who.int/publications/m/item/who-target-product-profiles-for-covid-19-vaccines (accessed on 30 April 2021).
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef]
- Baraniuk, C. What do we know about China’s COVID-19 vaccines? BMJ 2021, 373, n912. [Google Scholar] [CrossRef] [PubMed]
- Baptista, E. Covid-19 Vaccines Made by China’s Sinopharm, CanSino Release Efficacy Data. Available online: https://www.scmp.com/news/china/science/article/3122980/covid-19-vaccines-made-chinas-sinopharm-cansino-release-efficacy (accessed on 3 May 2021).
- National Health Commission. Technical Guideline for the Inoculation of COVID-19 Vaccines. Available online: http://www.nhc.gov.cn/xcs/yqfkdt/202103/c2febfd04fc5498f916b1be080905771.shtml (accessed on 30 April 2021).
- Press Conference of the Joint Prevention and Control Mechanism of the State Council (15 March 2021). Available online: http://www.gov.cn/xinwen/gwylflkjz151/index.htm (accessed on 30 April 2021).
- Shenzhen Kangtai Biologics COVID-9 Inactivated Vaccine Was Approved for Emergency Use. Available online: http://www.xinhuanet.com/2021-05/15/c_1127448669.htm (accessed on 21 May 2021).
- Lu, C. First Recombination Covid-19 Vaccine was Approved for Emergency Use in China. Available online: http://www.stdaily.com/index/kejixinwen/2021-03/15/content_1090899.shtml (accessed on 10 May 2021).
- Mallapaty, S. China’s COVID vaccines are going global—but questions remain. Nature 2021, 593, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-n.; Huang, Y.; Wang, W.; Jing, Q.-l.; Zhang, C.-h.; Qin, P.-z.; Guan, W.-j.; Gan, L.; Li, Y.-l.; Liu, W.-h.; et al. Efficacy of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: A test-negative case-control real-world study. Emerg. Microbes Infect. 2021, 1–32. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Overview of the National Monitoring Information on Adverse Events of COVID-19 Vaccination (by 30 April 2021). Available online: http://www.chinacdc.cn/jkzt/ymyjz/ymyjjz_6758/202105/t20210528_230911.html (accessed on 21 August 2021).
- Riad, A.; Sağıroğlu, D.; Üstün, B.; Pokorná, A.; Klugarová, J.; Attia, S.; Klugar, M. Prevalence and Risk Factors of CoronaVac Side Effects: An Independent Cross-Sectional Study among Healthcare Workers in Turkey. J. Clin. Med. 2021, 10, 2629. [Google Scholar] [CrossRef]
- Zhang, M.-X.; Zhang, T.-T.; Shi, G.-F.; Cheng, F.-M.; Zheng, Y.-M.; Tung, T.-H.; Chen, H.-X. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev. Vaccin. 2021, 1–8. [Google Scholar]
- Saeed, B.Q.; Al-Shahrabi, R.; Alhaj, S.S.; Alkokhardi, Z.M.; Adrees, A.O. Side Effects and Perceptions Following Sinopharm COVID-19 Vaccination. Int. J. Infect. Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Press Conference of the Joint Prevention and Control Mechanism of the State Council (11 April 2021). Available online: http://www.gov.cn/xinwen/gwylflkjz154/index.htm (accessed on 30 April 2021).
- Yang, J.; Zheng, W.; Shi, H.; Yan, X.; Dong, K.; You, Q.; Zhong, G.; Gong, H.; Chen, Z.; Jit, M.; et al. Who should be prioritized for COVID-19 vaccination in China? A descriptive study. BMC Med. 2021, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Production Capacity Continues to Increase, Helping Countries Fight the Pandemic. Chinese Vaccines are Becoming a Global Public Product. Available online: http://www.gov.cn/xinwen/2021-01/26/content_5582537.htm (accessed on 1 May 2021).
- Can the Second Shot of COVID-19 Vaccine be Administered on time? Can the Delivery and Production Capacity Catches Up? A Authoritative Response. Available online: http://www.xinhuanet.com/politics/2021-04/30/c_1127399279.htm (accessed on 1 May 2021).
- Kangtai Biotech’s COVID-19 Vaccine Was Approved for Emergency Use, with a Designed Annual Production Capacity of 200 Million Doses, Which Can Be Increased to 600 Million Doses. Available online: http://www.eeo.com.cn/2021/0521/488988.shtml (accessed on 21 May 2021).
- Press Conference of the Joint Prevention and Control Mechanism of the State Council (29 April 2021). Available online: http://www.gov.cn/xinwen/gwylflkjz156/index.htm (accessed on 2 May 2021).
- COVID-19 Vaccination Guide for Foreigners in China. Available online: http://english.nmpa.gov.cn/2021-04/25/c_614203.htm (accessed on 30 April 2021).
- Centers for Disease Control and Prevention. Possible Side Effects after Getting a COVID-19 Vaccine. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html (accessed on 23 August 2021).
- COVAX: Ensuring Global Equitable Access to COVID-19 Vaccines. Available online: https://www.unicef.org/supply/covax-ensuring-global-equitable-access-covid-19-vaccines (accessed on 4 May 2021).
- Shumei, L.; Xuanmin, L. China Joins Covax, to Provide Vaccines to Developing Countries First. Available online: https://www.globaltimes.cn/content/1202940.shtml (accessed on 4 May 2021).
- Mancini, D.P.; Shepherd, C. WHO Approves Emergency Use of China’s Sinopharm Covid Vaccine. Available online: https://www.ft.com/content/7220cbdd-4b8c-49f1-9cf7-5146a691adb6 (accessed on 7 May 2021).
- China’s Vaccine Map: Countries Using Chinese Vaccines. Available online: https://news.cgtn.com/news/2021-03-14/China-continues-to-contribute-to-global-vaccine-distribution-YCquwiznFK/index.html (accessed on 4 May 2021).
- Lain, M.; Sudhi, R.S.; James, P. World turns to China for Vaccines after India, U.S. Stumble. Available online: https://www.bloomberg.com/news/articles/2021-05-06/the-world-turns-to-china-for-vaccines-after-india-u-s-stumble (accessed on 8 May 2021).
- Huaxia. China Vows COVID-19 Vaccine at Fair, Reasonable Prices. Available online: http://www.xinhuanet.com/english/2021-02/04/c_139721463.htm (accessed on 4 May 2021).
- Gemünden, M.; Thiel, J. COVAX needs a political future. CSS Policy Perspect. 2021, 9, 4. [Google Scholar]
- Department of Health and Social Care. UK COVID-19 Vaccines Delivery Plan. Available online: https://www.gov.uk/government/publications/uk-covid-19-vaccines-delivery-plan/uk-covid-19-vaccines-delivery-plan#executive-summary-and-scope (accessed on 10 May 2021).
- The First Ones to Have the COVID-19 Vaccine within the “Ten Katef” Vaccine Campaign. Available online: https://www.gov.il/en/departments/news/16122020-01 (accessed on 10 May 2021).
- Rebecca, H.; Pijar, A. Indonesia Coronavirus: The Vaccination Drive Targeting Younger People. Available online: https://www.bbc.com/news/world-asia-55620356 (accessed on 10 May 2021).
- Egypt Aims for Deal to Produce Sinovac COVID-19 Vaccines. Available online: https://www.reuters.com/article/uk-health-coronavirus-egypt-china-idUSKBN2BE26R (accessed on 10 May 2021).
- Egypt Signs Agreement with China to Manufacture Sinovac Vaccine Locally. Available online: http://www.xinhuanet.com/english/2021-04/22/c_139898938.htm (accessed on 10 May 2021).

| Longitudinal Samples | Cross-sectional Samples | |||||
|---|---|---|---|---|---|---|
| March 2020 (Severe Epidemic Phase) | November–December 2020 (Well-Contained Phase) | March 2020 (Severe Epidemic Phase) | November–December 2020 (Well-Contained Phase) | |||
| N (%) | N (%) | p Value | N (%) | N (%) | p Value | |
| Overall respondents | 791 (100) | 791 (100) | 2058 (100) | 2013 (100) | ||
| COVID-19 vaccination is an effective way to prevent and control COVID-19 | ||||||
| Yes | 718 (90.8) | 746 (94.3) | 1842 (89.5) | 1874 (93.1) | ||
| No | 73 (9.2) | 45 (5.7) | 0.007 | 216 (10.5) | 139 (6.9) | <0.001 |
| Accept vaccination if the COVID-19 vaccine is successfully developed and approved for listing in the future | ||||||
| Yes | 727 (91.9) | 701 (88.6) | 1879 (91.3) | 1782 (88.5) | ||
| No | 64 (8.1) | 90 (11.4) | 0.03 | 179 (8.7) | 231 (11.5) | 0.003 |
| Vaccine accept group | 727 (100) | 701 (100) | 1879 (100) | 1782 (100) | ||
| Want to receive vaccination as soon as possible when the vaccine is available | ||||||
| Yes, as soon as possible | 424 (58.3) | 161 (23.0) | 980 (52.2) | 441 (24.7) | ||
| No, delay vaccination until I confirm the vaccine’s safety | 303 (41.7) | 540 (77.0) | <0.001 | 899 (47.8) | 1341 (75.3) | <0.001 |
| Vaccine | Type | Developer | Registration Number/Identifier (Country of Recruitment; Date of Registration) | Protection Efficacy | Storage Requirement during Transport [100] | Dose | Authorization Status (Date) | ||
|---|---|---|---|---|---|---|---|---|---|
| Clinical Trial Phase 1 | Clinical Trial Phase 2 | Clinical Trial Phase 3 | |||||||
| BBIBP-CorV | Inactivated vaccine | Beijing Institute of Biological Products Co., LTD./Sinopharm | ChiCTR2000032459 (China; 29.04.2020) | NCT04560881 (Argentina; 23.09.2020) NCT04795414 (China; 12.03.2021) NCT04510207 (Bahrain, Egypt, Jordan, United Arab Emirates; 12.08.2020) | 78.1% [101] | 2–8 °C | 2 | Conditional marketing authorization (30.12.2020) | |
| Corona Vac (PiCoVacc) | Inactivated vaccine | Sinovac Biotech | NCT04352608 (China; 20.04.2020) NCT04383574 (China; 12.05.2020) NCT04551547 (China; 16.09.2020) | NCT04456595 (Brazil; 02.07.2020) | Brazil: symptomatic prevention: 50.4%; mild cases prevention: 78%; Severe cases prevention: 100% Turkey: 83.5% Indonesia: 65.3% (confidence interval not reported) the United Arab Emirates: 86% [102] | Room temperature | 2 | Conditional marketing authorization (05.02.2021) | |
| New Crown COVID-19 | Inactivated vaccine | Wuhan Institute of Biological Products/Sinopharm | ChiCTR2000031809 (China; 11.04.2020) | ChiCTR2000034780 (The Union Arab Emirates; 18.07.2020) ChiCTR2000039000 (Morocco; 13.10.2020) NCT04612972 (Peru; 03.11.2020) NCT04510207 (Bahrain, Egypt, Jordan, United Arab Emirates; 12.08.2020) | 72.8% [101] | 2–8 °C | 2 | Conditional marketing authorization (25.02.2021) | |
| Ad5-nCoV | Viral vector vaccine | CanSino Biologics Inc. & Academy of Military Medical Sciences, PLA of China | ChiCTR2000030906 (China; 17.03.2020) NCT04313127 (China; 18.03.2020) | ChiCTR2000031781 (China; 10.04.2020) NCT04341389 (China; 10.04.2020) | ChiCTR2100044249 (Pakistan, Russia, Argentina, Chile, Mexico; 12.03.2021) NCT04526990 (Pakistan, Russia, Argentina, Chile, Mexico; 26.08.2020) | 65% for preventing symptoms, 90% effective at preventing severe symptoms [103] | 2–8 °C | 1 | Conditional marketing authorization (25.02.2021) |
| ZF2001 | Recombinant protein subunit vaccine | Anhui Zhifei Longcom Biopharmaceutical | ChiCTR2000035691 (China; 16.08.2020) NCT04445194 (China; 24.07.2020) | NCT04466085 (China; 10.07.2020) | ChiCTR2000040153 (China; 22.11.2020) NCT04646590 (China, Ecuador, Indonesia, Pakistan, Uzbekistan; 30.11.2020) | Not available at the time of writing | 2–8 °C | 2–3 | Emergency use authorization (10.03.2021) |
| KCONVAC | Inactivated vaccine | Shenzhen Kangtai Biological Products Co., LTD. & Beijing Minhai Biotechnology Co., LTD. | ChiCTR2000038804 (China; 02.10.2020) NCT04758273 (China; 17.02.2021) | ChiCTR2000039462 (China; 28.10.2020) NCT04756323 (China; 16.02.2021) | NCT04852705 (21.04.2021) | Not available at the time of writing | 2–8 °C | 2 | Emergency use authorization (07.05.2021) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamadi, M.; Lin, Y.; Vulliet, M.V.S.; Flahault, A.; Rozanova, L.; Fabre, G. COVID-19 Vaccination Strategy in China: A Case Study. Epidemiologia 2021, 2, 402-425. https://doi.org/10.3390/epidemiologia2030030
Mohamadi M, Lin Y, Vulliet MVS, Flahault A, Rozanova L, Fabre G. COVID-19 Vaccination Strategy in China: A Case Study. Epidemiologia. 2021; 2(3):402-425. https://doi.org/10.3390/epidemiologia2030030
Chicago/Turabian StyleMohamadi, Marjan, Yuling Lin, Mélissa Vuillet Soit Vulliet, Antoine Flahault, Liudmila Rozanova, and Guilhem Fabre. 2021. "COVID-19 Vaccination Strategy in China: A Case Study" Epidemiologia 2, no. 3: 402-425. https://doi.org/10.3390/epidemiologia2030030
APA StyleMohamadi, M., Lin, Y., Vulliet, M. V. S., Flahault, A., Rozanova, L., & Fabre, G. (2021). COVID-19 Vaccination Strategy in China: A Case Study. Epidemiologia, 2(3), 402-425. https://doi.org/10.3390/epidemiologia2030030








