Development of Porous Polyacrylonitrile Composite Fibers: New Precursor Fibers with High Thermal Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PAN Fibers
2.3. Characterization
2.3.1. Field Emission Scanning Electron Microscope (FE-SEM)
2.3.2. High Resolution Transmission Electron Microscopy (HR-TEM)
2.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. X-ray Diffraction (XRD)
2.3.6. Thermogravimetric Analysis (TGA)
3. Results
3.1. Ternary Phase Diagram
3.2. Morphological Analysis of PAN-Based Fibers
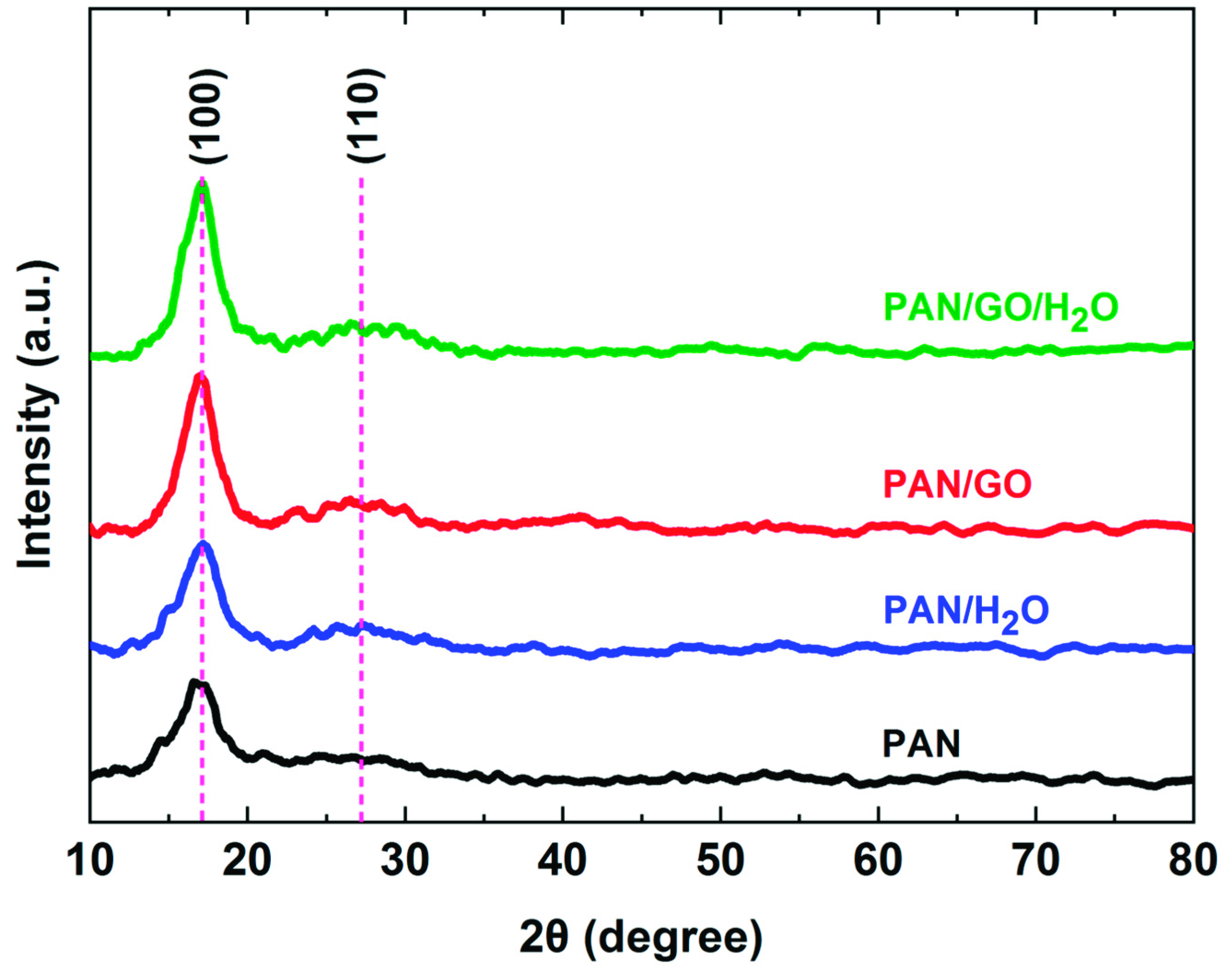
3.3. Structural Analysis of PAN-Based Fibers
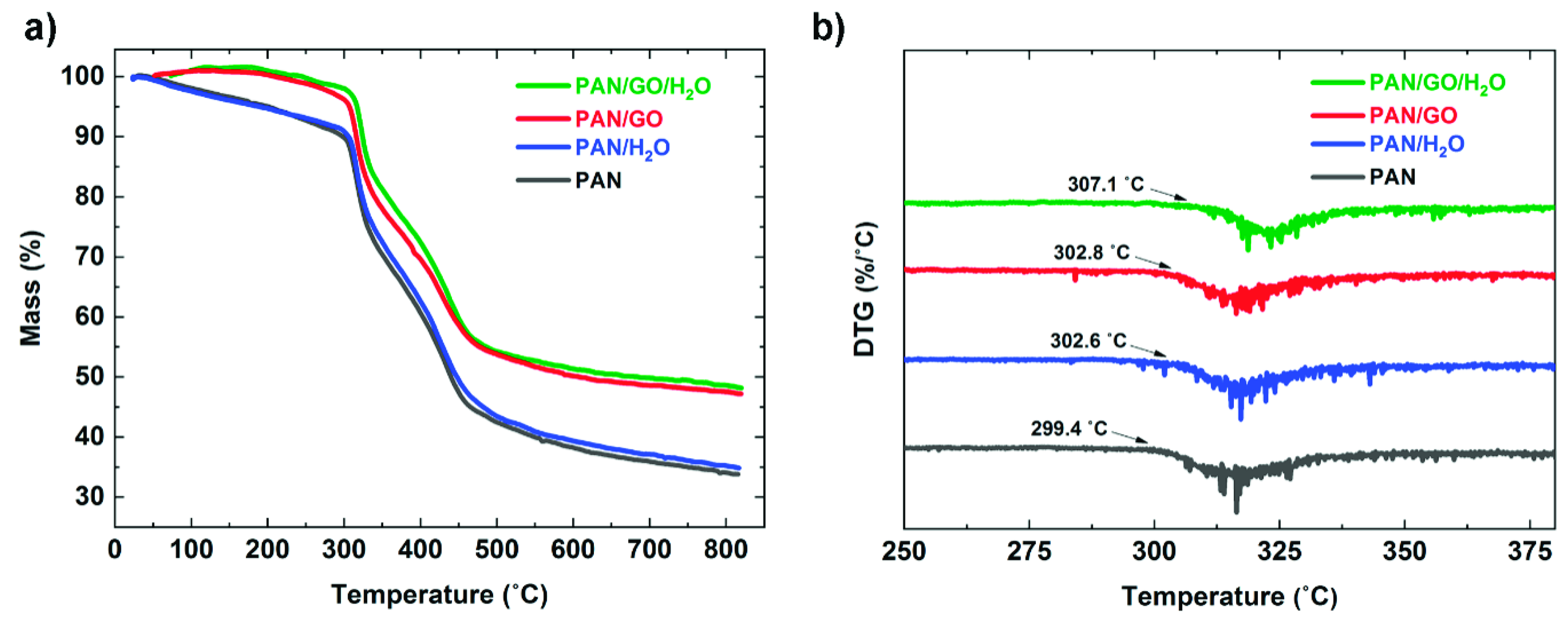
3.4. Thermal Behavior of PAN-Based Fibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edie, D.D. The effect of processing on the structure and properties of carbon fibers. Carbon 1998, 36, 345–362. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Li, Q.; Shafei, S.; Huson, M.G.; Naebe, M. Carbon fibre surface modification using functionalized nanoclay: A hierarchical interphase for fibre-reinforced polymer composites. Compos. Sci. Technol. 2017, 148, 49–58. [Google Scholar] [CrossRef]
- Wangxi, Z.; Jie, L.; Gang, W. Evolution of structure and properties of PAN precursors during their conversion to carbon fibers. Carbon 2003, 41, 2805–2812. [Google Scholar] [CrossRef]
- Morris, E.A.; Weisenberger, M.C.; Abdallah, M.G.; Vautard, F.; Grappe, H.; Ozcan, S.; Paulauskas, F.L.; Eberle, C.; Jackson, D.; Mecham, S.J.; et al. High performance carbon fibers from very high molecular weight polyacrylonitrile precursors. Carbon 2016, 101, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Fox, B. Making stronger carbon-fiber precursors. Science 2019, 366, 1314–1315. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, S.K.; Yang, K.S.; Aminabhavi, T.M. Polyacrylonitrile-based nanofibers-A state of the art review. Prog. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Functional materials by electrospinning of polymers. Prog. Polym. Sci. 2013, 38, 963–991. [Google Scholar] [CrossRef]
- Abolhasani, M.M.; Naebe, M.; Amiri, M.H.; Shirvanimoghaddam, K.; Anwar, S.; Michels, J.J.; Asadi, K. Hierarchically structured porous piezoelectric polymer nanofibers for energy harvesting. Adv. Sci. 2020, 7, 2000517. [Google Scholar] [CrossRef]
- Azimi, S.; Golabchi, A.; Nekookar, A.; Rabbani, S.; Amiri, M.H.; Asadi, K.; Abolhasani, M.M. Self-powered cardiac pacemaker by piezoelectric polymer nanogenerator implant. Nano Energy 2021, 83, 105781. [Google Scholar] [CrossRef]
- Anwar, S.; Amiri, M.H.; Jiang, S.; Abolhasani, M.M.; Rocha, P.R.F.; Asadi, K. Piezoelectric nylon-11 fibers for electronic textiles, energy harvesting and sensing. Adv. Funct. Mater. 2020, 31, 2004326. [Google Scholar] [CrossRef]
- Baqeri, M.; Abolhasani, M.M.; Mozdianfard, M.R.; Guo, Q.; Oroumei, A.; Naebe, M. Influence of processing conditions on polymorphic behavior, crystallinity, and morphology of electrospun poly(VInylidene fluoride) nanofibers. Appl. Polym. Sci. 2015, 132, 30. [Google Scholar] [CrossRef]
- Jia, Z.; Lu, C.; Liu, Y.; Zhou, P.; Wang, L. Lignin/Polyacrylonitrile composite hollow fibers prepared by wet-spinning method. ACS Sustain. Chem. Eng. 2016, 4, 2838–2842. [Google Scholar] [CrossRef]
- Fashandi, H.; Abolhasani, M.M.; Sandoghdar, P.; Zohdi, N.; Li, Q.; Naebe, M. Morphological changes towards enhancing piezoelectric properties of PVDF electrical generators using cellulose nanocrystals. Cellulose 2016, 23, 3625–3637. [Google Scholar] [CrossRef]
- Abolhasani, M.M.; Shirvanimoghaddam, K.; Naebe, M. PVDF/graphene composite nanofibers with enhanced piezoelectric performance for development of robust nanogenerators. Compos. Sci. Technol. 2017, 138, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.K.; Mishra, P.; Verma, K.; Mondal, A.; Chaudhary, R.G.; Abolhasani, M.M.; Loganathan, S. Electrospinning production of nanofibrous membranes. Environ. Chem. Lett. 2018, 17, 767–800. [Google Scholar] [CrossRef]
- Abolhasani, M.M.; Naebe, M.; Shirvanimoghaddam, K.; Fashandi, H.; Joordens, H.K.M.; Pipertzis, A.; Anwar, S.; Berger, R.; Floudas, G.; Michels, J.; et al. Thermodynamic approach to tailor porosity in piezoelectric polymer fibers for application in nanogenerators. Nano Energy 2019, 62, 594–600. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, L.; Gao, A.; Tong, Y.; Shi, F.; Xu, L. The structure and properties of polyacrylonitrile nascent composite fibers with grafted multi walled carbon nanotubes prepared by wet spinning method. Polymers 2019, 11, 422. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Shang, L.; Xiao, L.; Zhang, M.; Li, M.; Ao, Y. Structural changes of polyacrylonitrile fibers in the process of wet spinning. Appl. Polym. Sci. 2020, 137, 48905. [Google Scholar] [CrossRef]
- Abolhasani, M.M.; Azimi, S.; Fashandi, H. Enhanced ferroelectric properties of electrospun poly (vinylidene fluoride) nanofibers by adjusting processing parameters. RSC Adv. 2015, 5, 61277–61283. [Google Scholar] [CrossRef]
- Abolhasani, M.M.; Jalaei, A.; Tavana, R.; Kashani, F.Z. Processing and performance properties of amino silicone-based softener on various textile substrates. Polym. Bull. 2020, 77, 2557–2572. [Google Scholar] [CrossRef]
- Abolhasani, M.M.; Shirvanimoghaddam, K.; Khayyam, H.; Moosavi, S.M.; Zohdi, N.; Naebe, M. Towards predicting the piezoelectricity and physiochemical properties of the electrospun P (VDF-TrFE) nanogenrators using an artificial neural network. Polym. Test. 2018, 66, 178–188. [Google Scholar] [CrossRef]
- Soleymani, H.; Noormohammadi, M.; Kashi, M.A.; Amiri, M.H.; Michels, J.J.; Asadi, K.; Abolhasani, M.M. Self-Poled Sausage-Like PVDF nanowires produced by confined phase inversion as novel piezoelectric nanogenerators. Adv. Mater. Interfaces 2021, 8, 2001734. [Google Scholar] [CrossRef]
- Chen, J.; Ge, H.-Y.; Dong, X.-G.; Wang, C.-G. The formation of polyacrylonitrile nascent fibers in wet-spinning process. J. Appl. Polym. Sci. 2007, 106, 692–696. [Google Scholar] [CrossRef]
- Fakhrhoseini, S.M.; Khayyam, H.; Naebe, M. Chemically enhanced wet-spinning process to accelerate thermal stabilization of polyacrylonitrile fibers. Macromol. Mater. Eng. 2018, 303, 1700557. [Google Scholar] [CrossRef]
- Al Faruque, M.A.; Remadevi, R.; Razal, J.; Wang, X.; Naebe, M. Investigation on structure and characteristics of alpaca-based wet-spun polyacrylonitrile composite fibers by utilizing natural textile waste. Appl. Polym. Sci. 2019, 137, 48370. [Google Scholar] [CrossRef]
- Yang, H.-S.; Kim, Y.-M.; Choi, H.; Jang, J.; Youk, J.H.; Lee, B.-S.; Yu, W.-R. Electrochemical wet-spinning process for fabricating strong PAN fibers via an in situ induced plasticizing effect. Polymer 2020, 202, 2641. [Google Scholar] [CrossRef]
- Liu, Y.; Kumar, S. Recent progress in fabrication, structure, and properties of carbon fibers. Polym. Rev. 2012, 52, 234–258. [Google Scholar] [CrossRef]
- Liu, Y.; Chae, H.G.; Kumar, S. Gel-spun carbon nanotubes/polyacrylonitrile composite fibers. Part III: Effect of stabilization conditions on carbon fiber properties. Carbon 2011, 49, 4487–4496. [Google Scholar] [CrossRef]
- Gao, Q.; Ma, H.; Bao, W.; Gao, C.; Ge, M. Polyacrylonitrile/electroconductive TiO2 nanoparticles composite fibers via wet-spinning. Fibers Polym. 2016, 17, 1048–1054. [Google Scholar] [CrossRef]
- Hiremath, N.; Mays, J.; Bhat, G. Recent developments in carbon fibers and carbon nanotube-based fibers: A Review. Polym. Rev. 2016, 57, 339–368. [Google Scholar] [CrossRef]
- Elagib, T.H.H.; Hassan, E.A.M.; Fan, C.; Han, K.; Yu, M. Microwave pre-oxidation for polyacrylonitrile precursor coated with nano-carbon black. Polym. Eng. Sci. 2019, 59, 457–464. [Google Scholar] [CrossRef]
- Chang, H.; Luo, J.; Liu, H.C.; Zhang, S.; Park, J.G.; Liang, Z.; Kumar, S. Stabilization study of polyacrylonitrile/cellulose nanocrystals composite fibers. ACS Appl. Polym. Mater. 2019, 1, 1015–1021. [Google Scholar] [CrossRef]
- Liu, H.C.; Luo, J.; Chang, H.; Davijani, A.A.B.; Wang, P.-H.; Kumar, S. Polyacrylonitrile sheath and polyacrylonitrile/lignin core bi-component carbon fibers. Carbon 2019, 149, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Ge, Q.; Guo, L.; Yang, Z. Nitrogen doped carbon fibers derived from carbonization of electrospun polyacrylonitrile as efficient metal-free HER electrocatalyst. Int. J. Hydrog. Energy 2020, 45, 4035–4042. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Jiang, Z.-H.; Lu, B.-R.; Liu, J.-T.; Cao, F.-H.; Li, H.; Yu, Z.-L.; Yu, S.-H. MoS2 nanoplates assembled on electrospun polyacrylonitrile-metal organic framework-derived carbon fibers for lithium storage. Nano Energy 2019, 61, 104–110. [Google Scholar] [CrossRef]
- Dai, Z.; Ren, P.-G.; Jin, Y.-L.; Zhang, H.; Ren, F.; Zhang, Q. Nitrogen-sulphur Co-doped graphenes modified electrospun lignin/polyacrylonitrile-based carbon nanofiber as high performance supercapacitor. Power Sources 2019, 437, 226937. [Google Scholar] [CrossRef]
- Benko, A.; Nocuń, M.; Gajewska, M.; Błażewicz, M. Addition of carbon nanotubes to electrospun polyacrylonitrile as a way to obtain carbon nanofibers with desired properties. Polym. Degrad. Stab. 2019, 161, 260–276. [Google Scholar] [CrossRef]
- Cao, Z.; Li, W.; Sou, X.; Liu, Y.; Lu, C. Structures and cyclization behaviors of gel-spun cellulose/polyacrylonitrile composite fibers. Polym. Test. 2020, 81, 106276. [Google Scholar] [CrossRef]
- Chien, A.T.; Liu, H.C.; Newcomb, B.A.; Xiang, C.; Tour, J.M.; Kumar, S. Polyacrylonitrile fibers containing graphene oxide nanoribbons. ACS Appl. Mater. Interfaces 2015, 7, 5281–5288. [Google Scholar] [CrossRef] [PubMed]
- Mottaleb, M.M.A.; Mohamed, A.; Karim, S.A.; Osman, T.A.; Khattab, A. Preparation, characterization, and mechanical properties of polyacrylonitrile (PAN)/graphene oxide (GO) nanofibers. Mech. Adv. Mater. Struct. 2018, 27, 346–351. [Google Scholar] [CrossRef]
- Zhao, R.; Tian, M.; Zhao, Y.; Chen, S.; Han, G. Wet-spinning assembly of continuous and macroscopic graphene oxide/polyacrylonitrile reinforced composite fibers with enhanced mechanical properties and thermal stability. Appl. Polym. Sci. 2019, 136, 46950. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, D.; Zhang, L.; Li, M.; Liu, M.; Fu, S. Simultaneously electrochemical exfoliation and functionalization of graphene nanosheets: Multifunctional reinforcements in thermal, flame-retardant, and mechanical properties of polyacrylonitrile composite fibers. Polym. Compos. 2020, 41, 1561–1573. [Google Scholar] [CrossRef]
- Papkov, D.; Goponenko, A.; Compton, O.C.; An, Z.; Moravsky, A.; Li, X.-Z.; Nguyen, S.T.; Dzenis, Y.A. Improved graphitic structure of continuous carbon nanofibers via graphene oxide templating. Adv. Funct. Mater. 2013, 23, 5763–5770. [Google Scholar] [CrossRef]
- Gulgunje, P.V.; Newcomb, B.A.; Gupta, K.; Chae, H.G.; Tsotsis, T.K.; Kumar, S. Low-density and high-modulus carbon fibers from polyacrylonitrile with honeycomb structure. Carbon 2015, 95, 710–714. [Google Scholar] [CrossRef]
- Tan, L.; Pan, D.; Pan, N. Thermodynamic study of a water-dimethylformamide-polyacrylonitrile ternary system. Appl. Polym. Sci. 2008, 110, 3439–3447. [Google Scholar] [CrossRef] [Green Version]
- Arbab, S.; Noorpanah, P.; Mohammadi, N.; Soleimani, M. Designing index of void structure and tensile properties in wet-spun polyacrylonitrile (PAN) fiber. I. Effect of dope polymer or nonsolvent concentration. Appl. Polym. Sci. 2008, 109, 3461–3469. [Google Scholar] [CrossRef]
- Takahashi, M.; Nukushina, Y.; Kosugi, S. Effect of fiber-forming conditions on the microstructure of acrylic fiber. Text. Res. 1964, 34, 87–97. [Google Scholar] [CrossRef]
- Saxena, S.; Tyson, T.A.; Shukla, S.; Negusse, E.; Chen, H.; Bai, J. Investigation of structural and electronic properties of graphene oxide. Appl. Phys. Lett. 2011, 99, 013104. [Google Scholar] [CrossRef] [Green Version]
- Korobeinyk, A.V.; Whitby, R.L.D.; Mikhalovsky, S.V. High temperature oxidative resistance of polyacrylonitrile-methylmethacrylate copolymer powder converting to a carbonized monolith. Eur. Polym. J. 2012, 48, 97–104. [Google Scholar] [CrossRef]
- Haris, M.R.H.M.; Kathiresan, S.; Mohan, S. FT-IR and FT-Raman spectra and normal coordinate analysis of poly methyl methacrylate. Der Pharma Chem. 2010, 2, 316–323. [Google Scholar]
- Singhal, A.; Dubey, A.; Bhardwaj, Y.K.; Jain, D.; Choudhury, S.; Tyagi, A.Y. UV-shielding transparent PMMA/In2O3 nanocomposite films based on In2O3 nanoparticles. RSC Adv. 2013, 3, 43. [Google Scholar] [CrossRef]
- Tucureanu, V.; Matei, A.; Mihalache, I.; Danila, M.; Popescu, M.; Bita, B. Synthesis and characterization of YAG:Ce,Gd and YAG:Ce,Gd/PMMA nanocomposites for optoelectronic applications. Mater. Sci. 2014, 50, 1883–1890. [Google Scholar] [CrossRef]
- Jin, J.; Ogale, A.A. Carbon fibers derived from wet-spinning of equi-component lignin/polyacrylonitrile blends. Appl. Polym. Sci. 2018, 135, 45903. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, M.; Huang, Z.-H.; Kang, F. Preparation of graphene/carbon hybrid nanofibers and their performance for NO oxidation. Carbon 2015, 87, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Wang, H.; Xue, L.; Fan, L.; Zhu, Z. Effect of microstructure on the mechanical properties of PAN-based carbon fibers during high-temperature graphitization. Mater. Sci. 2008, 43, 4316–4322. [Google Scholar] [CrossRef]
- Gutmann, P.; Will, J.M.; Kurt, S.; Xu, Y.; Horn, S. Carbonization of polyacrylonitrile-based fibers under defined tensile load: Influence on shrinkage behavior, microstructure, and mechanical properties. Polym. Degrad. Stab. 2019, 163, 174–184. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.C.; Ahn, W.; Kim, C.Y. Reaction mechanisms of polyacrylonitrile on thermal treatment. Polym. Eng. Sci. 1993, 33, 1452–1457. [Google Scholar] [CrossRef]
- Zhao, W.; Lu, Y.; Wang, J.; Chen, Q.; Zhou, L.; Jiang, J.; Chen, L. Improving crosslinking of stabilized polyacrylonitrile fibers and mechanical properties of carbon fibers by irradiating with γ-ray. Polym. Degrad. Stab. 2016, 133, 16–26. [Google Scholar] [CrossRef]
- Kaur, J.; Millington, K.; Cai, J.Y. Rheology of polyacrylonitrile-based precursor polymers produced from controlled (RAFT) and conventional polymerization: Its role in solution spinning. Appl. Polym. Sci. 2016, 133, 48. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Uddin, M.E.; Kim, N.H.; Lau, A.K.T.; Lee, J.H. In-situ synthesis and characterization of electrically conductive polypyrrole/graphene nanocomposites. Polymer 2010, 51, 5921–5928. [Google Scholar] [CrossRef]
- Ouyang, Q.; Wang, X.; Wang, X.; Huang, J.; Huang, X.; Chen, Y. Simultaneous DSC/TG analysis on the thermal behavior of PAN polymers prepared by aqueous free-radical polymerization. Polym. Degrad. Stab. 2016, 130, 320–327. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Yang, J.; Ji, M.; Yu, J.; Wang, M.; Chai, X.; Yang, B.; Zhu, C.; Xu, J. Preparation, stabilization and carbonization of a novel polyacrylonitrile-based carbon fiber precursor. Polymers 2019, 11, 1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Quan, L.; Gao, A.; Tong, Y.; Shi, F.; Xu, L. Thermal analysis and crystal structure of poly(acrylonitrile-co-itaconic acid) copolymers synthesized in water. Polymers 2020, 12, 221. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Li, G.; Zhang, J.; Huang, F.; Lu, K.; Wei, Q. PAN nanofibers reinforced with MMT/GO hybrid nanofillers. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Qiao, M.; Kong, H.; Ding, X.; Zhang, L.; Yu, M. Effect of graphene oxide coatings on the structure of polyacrylonitrile fibers during pre-oxidation process. RSC Adv. 2019, 9, 28146–28152. [Google Scholar] [CrossRef] [Green Version]
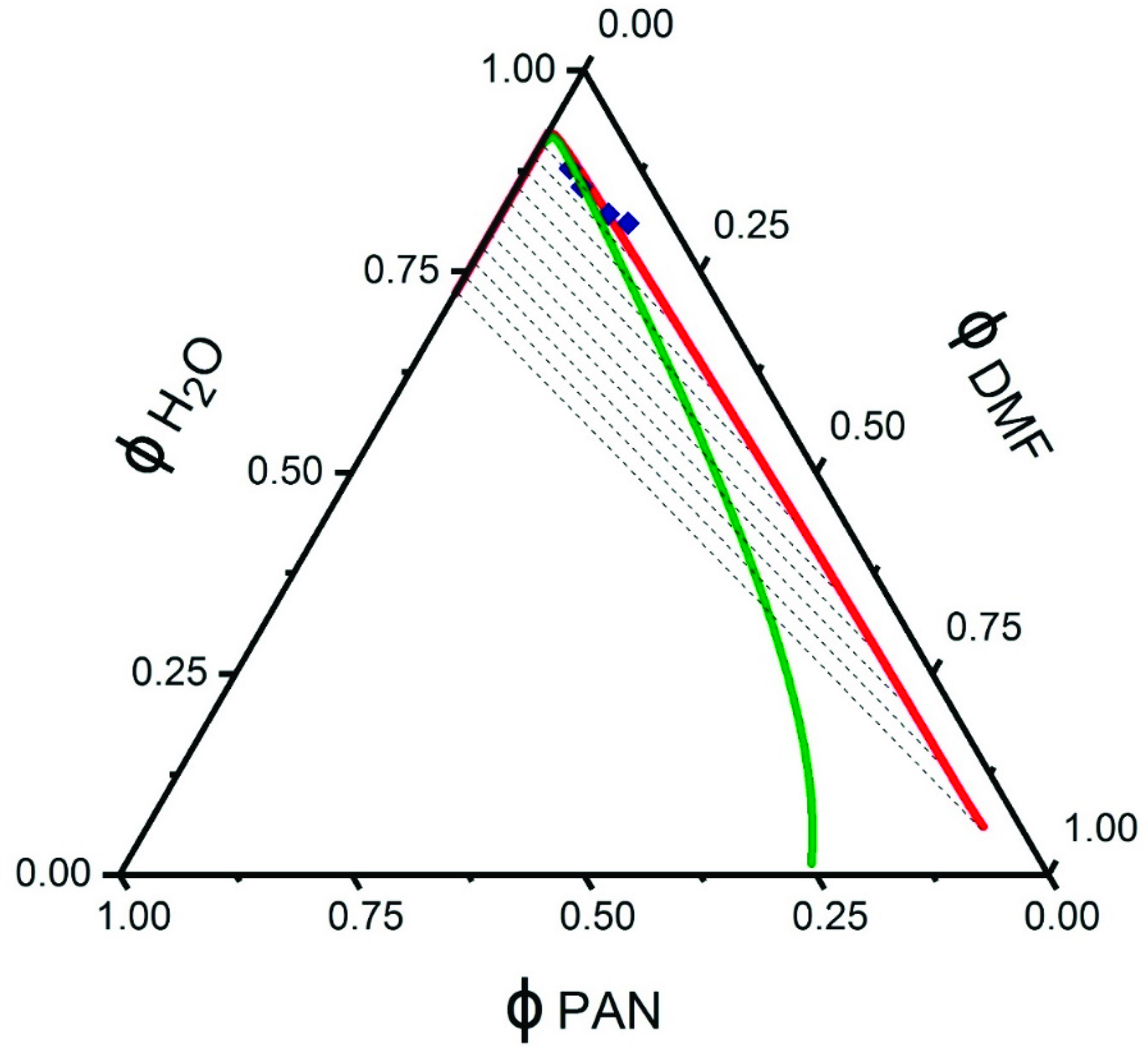
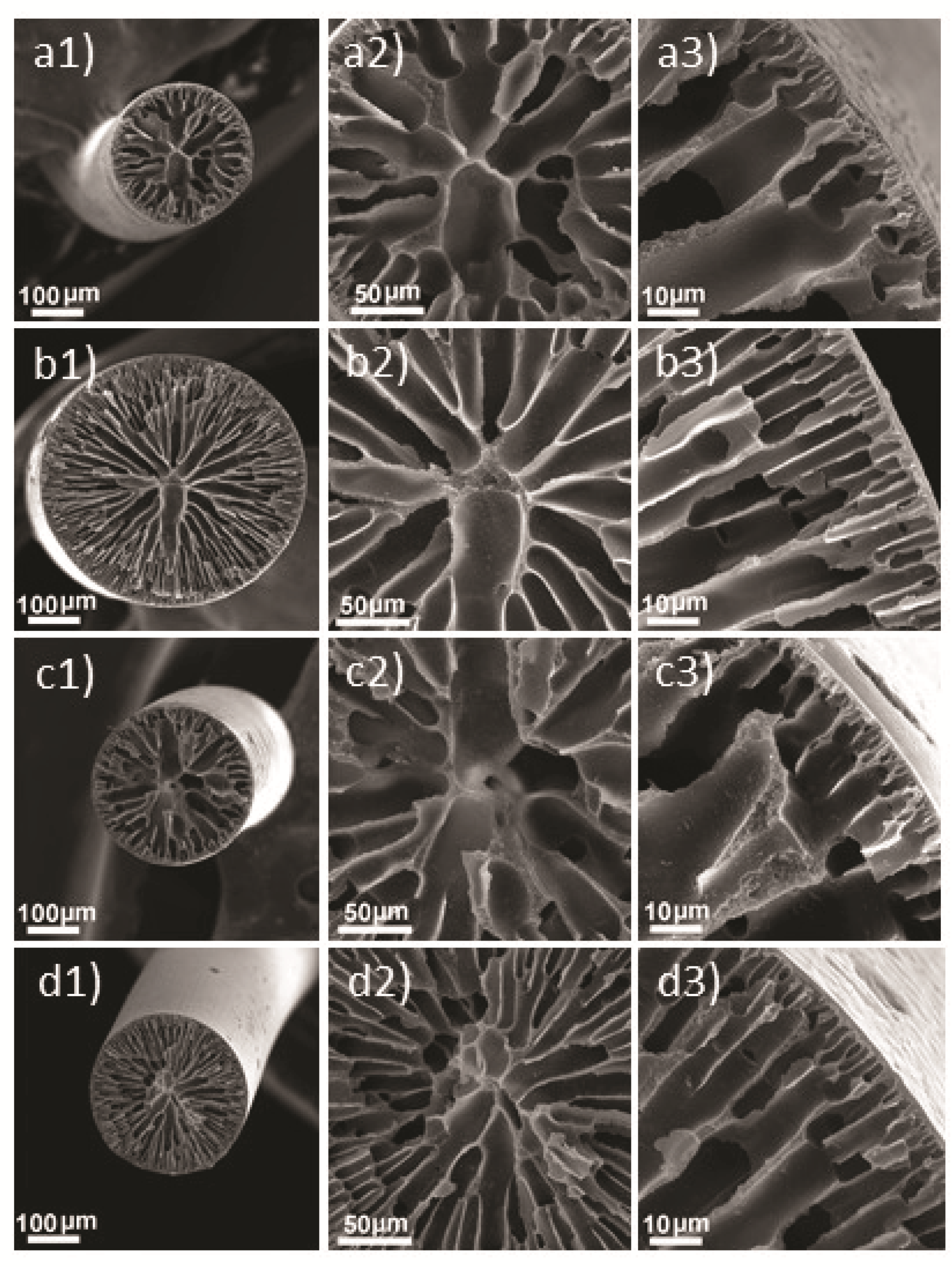
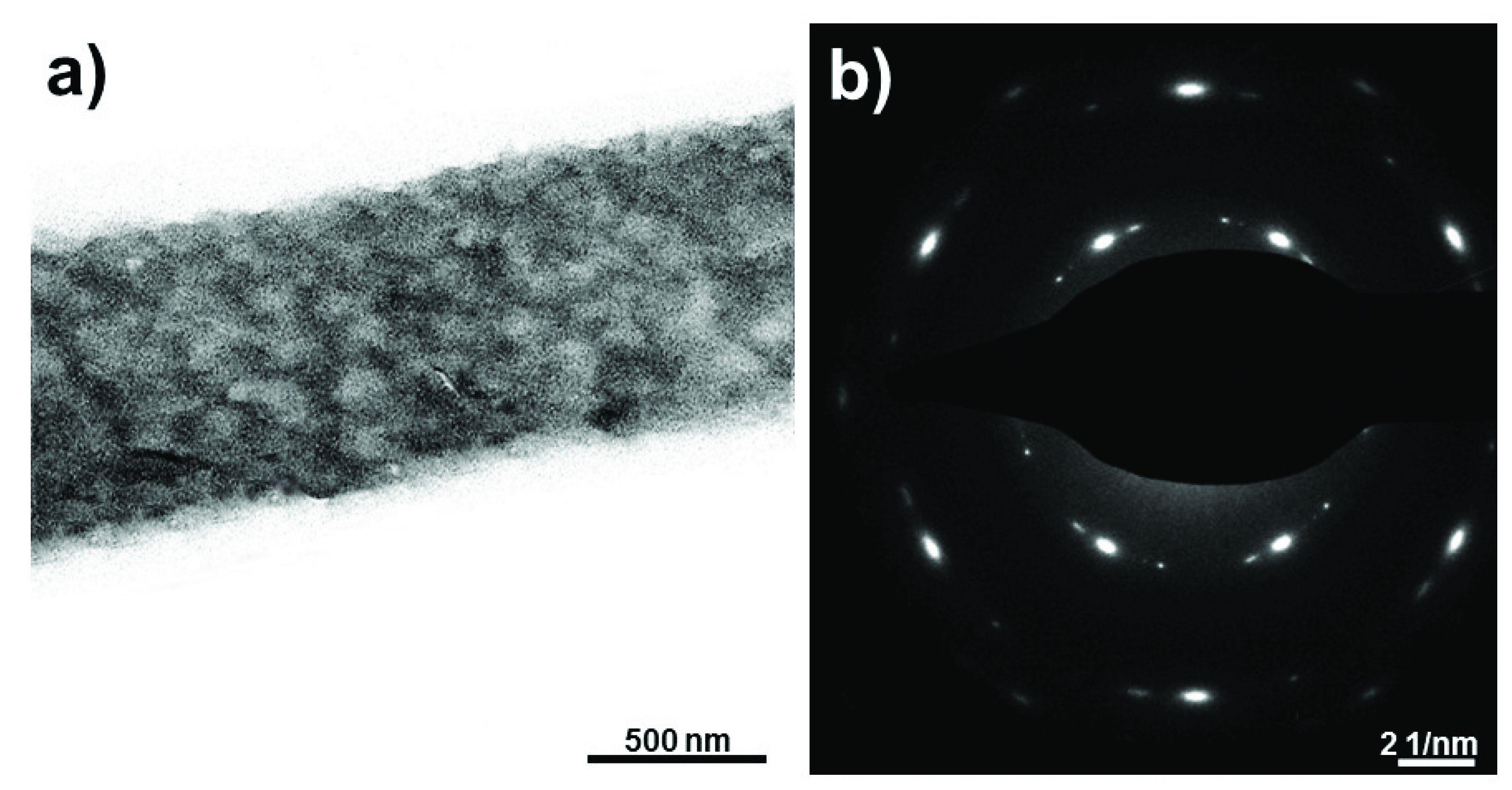
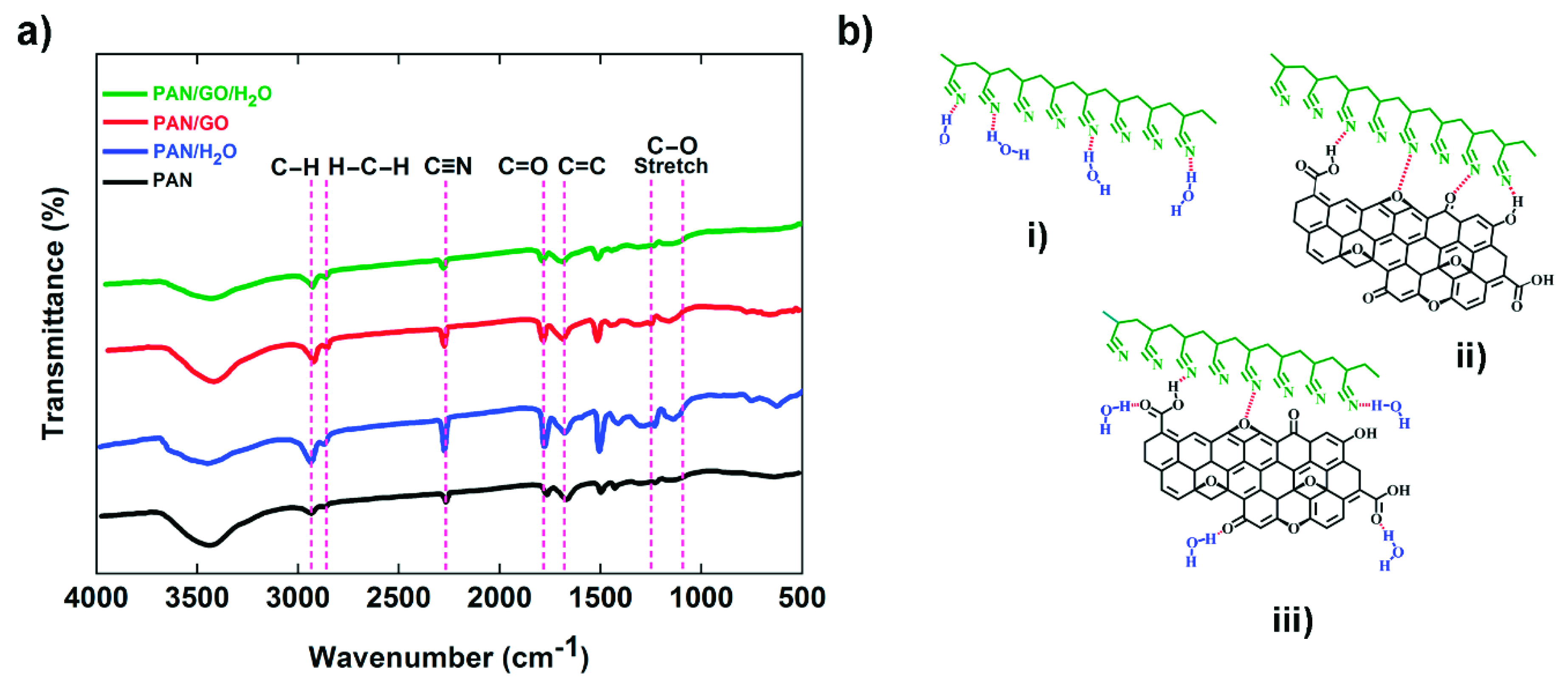

| Dope | GO (wt%) | H2O (wt%) | Sample Code |
|---|---|---|---|
| A | 0 | 0 | PAN |
| B | 0 | 4.1 | PAN/H2O |
| C | 1 | 0 | PAN/GO |
| D | 1 | 4.1 | PAN/GO/H2O |
| Sample Fiber | Ti (°C) | TP (°C) | Tf (°C) | ∆T (°C) | ∆H (Wg−1) | ∆H/∆T (Wg−1·°C−1) |
|---|---|---|---|---|---|---|
| PAN | 271.5 | 308.4 | 328.1 | 56.6 | 7.26 | 0.128 |
| PAN/H2O | 270.1 | 297.6 | 310.6 | 40.5 | 3.57 | 0.088 |
| PAN/GO | 260.6 | 295.1 | 310.2 | 49.6 | 3.24 | 0.065 |
| PAN/GO/H2O | 254.9 | 294.6 | 309.4 | 54.5 | 3.41 | 0.062 |
| Sample Fiber | Td (°C) | Wight Loss at Td (%) | Residual Weight at 800 °C (%) |
|---|---|---|---|
| PAN | 299.4 | 10.1 | 33.8 |
| PAN/H2O | 302.6 | 9.5 | 35.0 |
| PAN/GO | 302.8 | 5.1 | 47.2 |
| PAN/GO/H2O | 307.1 | 2.2 | 48.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samimi-Sohrforozani, E.; Azimi, S.; Abolhasani, A.; Malekian, S.; Arbab, S.; Zendehdel, M.; Abolhasani, M.M.; Yaghoobi Nia, N. Development of Porous Polyacrylonitrile Composite Fibers: New Precursor Fibers with High Thermal Stability. Electron. Mater. 2021, 2, 454-465. https://doi.org/10.3390/electronicmat2040031
Samimi-Sohrforozani E, Azimi S, Abolhasani A, Malekian S, Arbab S, Zendehdel M, Abolhasani MM, Yaghoobi Nia N. Development of Porous Polyacrylonitrile Composite Fibers: New Precursor Fibers with High Thermal Stability. Electronic Materials. 2021; 2(4):454-465. https://doi.org/10.3390/electronicmat2040031
Chicago/Turabian StyleSamimi-Sohrforozani, Ehsan, Sara Azimi, Alireza Abolhasani, Samira Malekian, Shahram Arbab, Mahmoud Zendehdel, Mohammad Mahdi Abolhasani, and Narges Yaghoobi Nia. 2021. "Development of Porous Polyacrylonitrile Composite Fibers: New Precursor Fibers with High Thermal Stability" Electronic Materials 2, no. 4: 454-465. https://doi.org/10.3390/electronicmat2040031
APA StyleSamimi-Sohrforozani, E., Azimi, S., Abolhasani, A., Malekian, S., Arbab, S., Zendehdel, M., Abolhasani, M. M., & Yaghoobi Nia, N. (2021). Development of Porous Polyacrylonitrile Composite Fibers: New Precursor Fibers with High Thermal Stability. Electronic Materials, 2(4), 454-465. https://doi.org/10.3390/electronicmat2040031









