Energy Homeostasis and Kisspeptin System, Roles of Exercise and Outcomes with a Focus on Male Reproductive Health
Abstract
1. Introduction
Methods
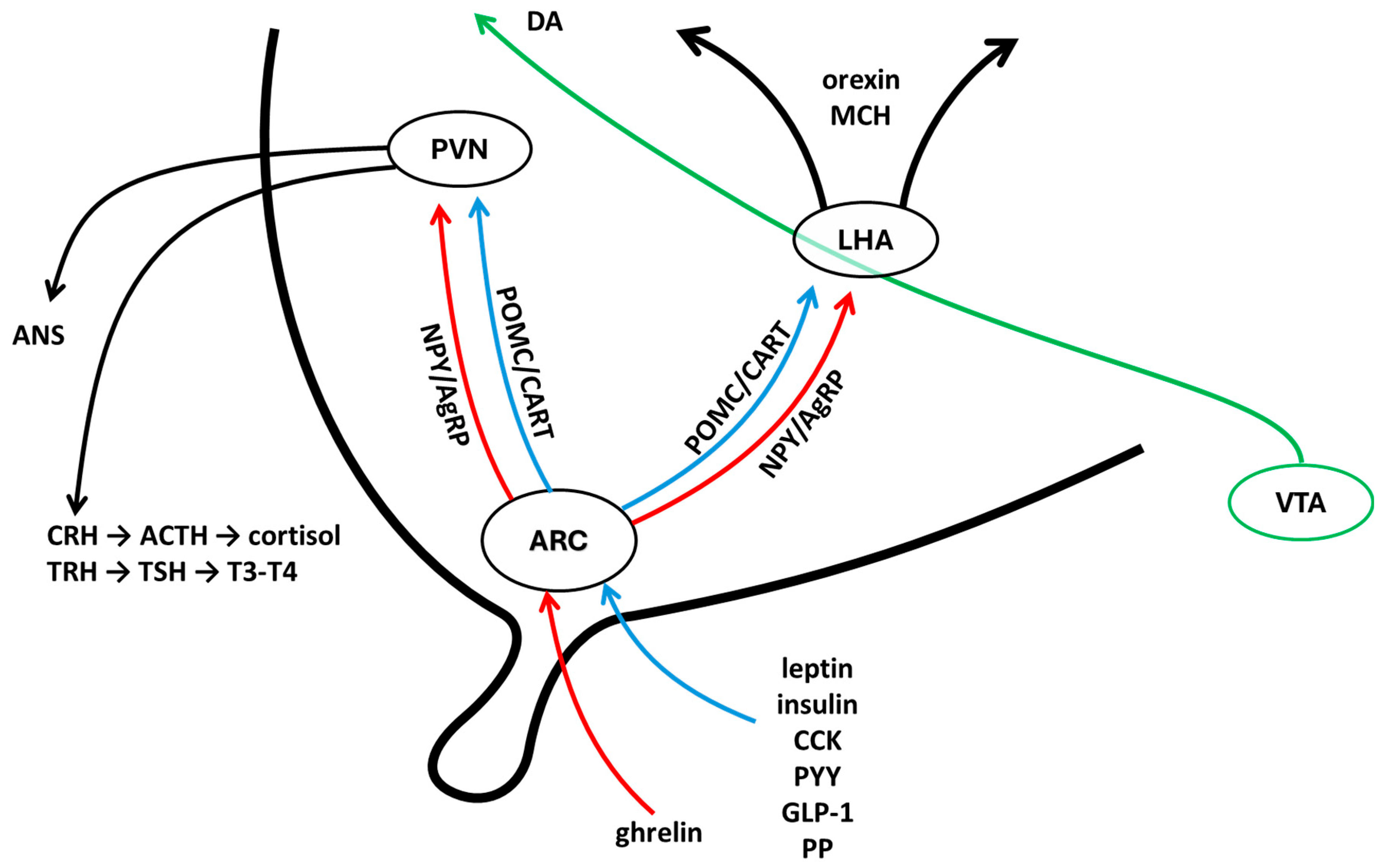
2. Energy Homeostasis, KS and Reproduction at the Central Level
3. Energy Homeostasis, KS and Reproduction at Periphery
4. Energy Homeostasis, KS and Exercise: The Outcomes on Reproduction
4.1. Animal Models
4.2. Studies in Humans
- Is There a Link Between the KS and Doping?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Infertility. Available online: https://www.who.int/news-room/fact-sheets/detail/infertility (accessed on 15 July 2025).
- WHO Obesity. Available online: https://www.who.int/health-topics/obesity/#tab=tab_1 (accessed on 15 July 2025).
- Valassi, E.; Scacchi, M.; Cavagnini, F. Neuroendocrine Control of Food Intake. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 158–168. [Google Scholar] [CrossRef]
- Chianese, R.; Coccurello, R.; Viggiano, A.; Scafuro, M.; Fiore, M.; Coppola, G.; Operto, F.F.; Fasano, S.; Laye, S.; Pierantoni, R.; et al. Impact of Dietary Fats on Brain Functions. Curr. Neuropharmacol. 2018, 16, 1059–1085. [Google Scholar] [CrossRef]
- Kaprara, A.; Huhtaniemi, I.T. The hypothalamus-pituitary-gonad axis: Tales of mice and men. Metabolism 2018, 86, 3–17. [Google Scholar] [CrossRef]
- Donato, J.J.; Cravo, R.M.; Frazão, R.; Elias, C.F. Hypothalamic Sites of Leptin Action Linking Metabolism and Reproduction. Neuroendocrinology 2011, 93, 9–18. [Google Scholar] [CrossRef]
- Leshan, R.L.; Björnholm, M.; Münzberg, H.; Myers, M.G. Leptin Receptor Signaling and Action in the Central Nervous System. Obesity 2006, 14 (Suppl. S5), 208S–212S. [Google Scholar] [CrossRef]
- Navarro, V.M.; Kaiser, U.B. Metabolic Influences on Neuroendocrine Regulation of Reproduction. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 335–341. [Google Scholar] [CrossRef]
- Evans, M.C.; Campbell, R.E.; Anderson, G.M. Physiological Regulation of Leptin as an Integrative Signal of Reproductive Readiness. Curr. Opin. Pharmacol. 2022, 67, 102321. [Google Scholar] [CrossRef]
- Pereira, S.; Cline, D.L.; Glavas, M.M.; Covey, S.D.; Kieffer, T.J. Tissue-Specific Effects of Leptin on Glucose and Lipid Metabolism. Endocr. Rev. 2021, 42, 1–28. [Google Scholar] [CrossRef]
- Li, W.; Liu, Q.; Deng, X.; Chen, Y.; Liu, S.; Story, M. Association between Obesity and Puberty Timing: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2017, 14, 1266. [Google Scholar] [CrossRef]
- Vilmann, L.S.; Thisted, E.; Baker, J.L.; Holm, J.-C. Development of Obesity and Polycystic Ovary Syndrome in Adolescents. Horm. Res. Paediatr. 2012, 78, 269–278. [Google Scholar] [CrossRef]
- Rivera, H.M.; Stincic, T.L. Estradiol and the Control of Feeding Behavior. Steroids 2018, 133, 44–52. [Google Scholar] [CrossRef]
- Allan, C.A.; McLachlan, R.I. Androgens and Obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 224–232. [Google Scholar] [CrossRef]
- Pinilla, L.; Aguilar, E.; Dieguez, C.; Millar, R.P.; Tena-Sempere, M. Kisspeptins and Reproduction: Physiological Roles and Regulatory Mechanisms. Physiol. Rev. 2012, 92, 1235–1316. [Google Scholar] [CrossRef]
- Dudek, M.; Ziarniak, K.; Sliwowska, J.H. Kisspeptin and Metabolism: The Brain and Beyond. Front. Endocrinol. 2018, 9, 145. [Google Scholar] [CrossRef]
- Navarro, V.M. Metabolic Regulation of Kisspeptin—The Link between Energy Balance and Reproduction. Nat. Rev. Endocrinol. 2020, 16, 407–420. [Google Scholar] [CrossRef]
- Kotani, M.; Detheux, M.; Vandenbogaerde, A.; Communi, D.; Vanderwinden, J.-M.; Le Poul, E.; Brézillon, S.; Tyldesley, R.; Suarez-Huerta, N.; Vandeput, F.; et al. The Metastasis Suppressor Gene KiSS-1 Encodes Kisspeptins, the Natural Ligands of the Orphan G Protein-Coupled Receptor GPR54. J. Biol. Chem. 2001, 276, 34631–34636. [Google Scholar] [CrossRef]
- Seminara, S.B.; Messager, S.; Chatzidaki, E.E.; Thresher, R.R.; Acierno, J.S.; Shagoury, J.K.; Bo-Abbas, Y.; Kuohung, W.; Schwinof, K.M.; Hendrick, A.G.; et al. The GPR54 Gene as a Regulator of Puberty. N. Engl. J. Med. 2003, 349, 1614–1627. [Google Scholar] [CrossRef]
- de Roux, N.; Genin, E.; Carel, J.-C.; Matsuda, F.; Chaussain, J.-L.; Milgrom, E. Hypogonadotropic Hypogonadism Due to Loss of Function of the KiSS1-Derived Peptide Receptor GPR54. Proc. Natl. Acad. Sci. USA 2003, 100, 10972–10976. [Google Scholar] [CrossRef] [PubMed]
- Silveira, L.G.; Noel, S.D.; Silveira-Neto, A.P.; Abreu, A.P.; Brito, V.N.; Santos, M.G.; Bianco, S.D.C.; Kuohung, W.; Xu, S.; Gryngarten, M.; et al. Mutations of the KISS1 Gene in Disorders of Puberty. J. Clin. Endocrinol. Metab. 2010, 95, 2276–2280. [Google Scholar] [CrossRef] [PubMed]
- Topaloglu, A.K.; Tello, J.A.; Kotan, L.D.; Ozbek, M.N.; Yilmaz, M.B.; Erdogan, S.; Gurbuz, F.; Temiz, F.; Millar, R.P.; Yuksel, B. Inactivating KISS1 Mutation and Hypogonadotropic Hypogonadism. N. Engl. J. Med. 2012, 366, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Teles, M.G.; Bianco, S.D.C.; Brito, V.N.; Trarbach, E.B.; Kuohung, W.; Xu, S.; Seminara, S.B.; Mendonca, B.B.; Kaiser, U.B.; Latronico, A.C. A GPR54-Activating Mutation in a Patient with Central Precocious Puberty. N. Engl. J. Med. 2008, 358, 709–715. [Google Scholar] [CrossRef]
- Wakabayashi, Y.; Nakada, T.; Murata, K.; Ohkura, S.; Mogi, K.; Navarro, V.M.; Clifton, D.K.; Mori, Y.; Tsukamura, H.; Maeda, K.-I.; et al. Neurokinin B and Dynorphin A in Kisspeptin Neurons of the Arcuate Nucleus Participate in Generation of Periodic Oscillation of Neural Activity Driving Pulsatile Gonadotropin-Releasing Hormone Secretion in the Goat. J. Neurosci. 2010, 30, 3124–3132. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.M.; Gottsch, M.L.; Chavkin, C.; Okamura, H.; Clifton, D.K.; Steiner, R.A. Regulation of Gonadotropin-Releasing Hormone Secretion by Kisspeptin/Dynorphin/Neurokinin B Neurons in the Arcuate Nucleus of the Mouse. J. Neurosci. 2009, 29, 11859–11866. [Google Scholar] [CrossRef]
- Quennell, J.H.; Howell, C.S.; Roa, J.; Augustine, R.A.; Grattan, D.R.; Anderson, G.M. Leptin Deficiency and Diet-Induced Obesity Reduce Hypothalamic Kisspeptin Expression in Mice. Endocrinology 2011, 152, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Luque, R.M.; Kineman, R.D.; Tena-Sempere, M. Regulation of Hypothalamic Expression of KiSS-1 and GPR54 Genes by Metabolic Factors: Analyses Using Mouse Models and a Cell Line. Endocrinology 2007, 148, 4601–4611. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Navarro, V.M.; Fernández-Fernández, R.; Nogueiras, R.; Tovar, S.; Roa, J.; Vazquez, M.J.; Vigo, E.; Casanueva, F.F.; Aguilar, E.; et al. Changes in Hypothalamic KiSS-1 System and Restoration of Pubertal Activation of the Reproductive Axis by Kisspeptin in Undernutrition. Endocrinology 2005, 146, 3917–3925. [Google Scholar] [CrossRef]
- Castellano, J.M.; Navarro, V.M.; Fernández-Fernández, R.; Roa, J.; Vigo, E.; Pineda, R.; Dieguez, C.; Aguilar, E.; Pinilla, L.; Tena-Sempere, M. Expression of Hypothalamic KiSS-1 System and Rescue of Defective Gonadotropic Responses by Kisspeptin in Streptozotocin-Induced Diabetic Male Rats. Diabetes 2006, 55, 2602–2610. [Google Scholar] [CrossRef]
- Fu, L.-Y.; van den Pol, A.N. Kisspeptin Directly Excites Anorexigenic Proopiomelanocortin Neurons but Inhibits Orexigenic Neuropeptide Y Cells by an Indirect Synaptic Mechanism. J. Neurosci. 2010, 30, 10205–10219. [Google Scholar] [CrossRef]
- Villa, P.A.; Ruggiero-Ruff, R.E.; Jamieson, B.B.; Campbell, R.E.; Coss, D. Obesity Alters POMC and Kisspeptin Neuron Cross Talk Leading to Reduced Luteinizing Hormone in Male Mice. J. Neurosci. 2024, 44, e0222242024. [Google Scholar] [CrossRef]
- Backholer, K.; Smith, J.T.; Rao, A.; Pereira, A.; Iqbal, J.; Ogawa, S.; Li, Q.; Clarke, I.J. Kisspeptin Cells in the Ewe Brain Respond to Leptin and Communicate with Neuropeptide Y and Proopiomelanocortin Cells. Endocrinology 2010, 151, 2233–2243. [Google Scholar] [CrossRef]
- Cravo, R.M.; Frazao, R.; Perello, M.; Osborne-Lawrence, S.; Williams, K.W.; Zigman, J.M.; Vianna, C.; Elias, C.F. Leptin Signaling in Kiss1 Neurons Arises after Pubertal Development. PLoS ONE 2013, 8, e58698. [Google Scholar] [CrossRef]
- Egan, O.K.; Inglis, M.A.; Anderson, G.M. Leptin Signaling in AgRP Neurons Modulates Puberty Onset and Adult Fertility in Mice. J. Neurosci. 2017, 37, 3875–3886. [Google Scholar] [CrossRef]
- Smith, J.T.; Acohido, B.V.; Clifton, D.K.; Steiner, R.A. KiSS-1 Neurones Are Direct Targets for Leptin in the Ob/Ob Mouse. J. Neuroendocr. 2006, 18, 298–303. [Google Scholar] [CrossRef]
- True, C.; Kirigiti, M.A.; Kievit, P.; Grove, K.L.; Smith, M.S. Leptin Is Not the Critical Signal for Kisspeptin or Luteinising Hormone Restoration during Exit from Negative Energy Balance. J. Neuroendocrinol. 2011, 23, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Smith, J.T. Novel Actions of Kisspeptin Signaling Outside of GnRH-Mediated Fertility: A Potential Role in Energy Balance. Domest. Anim. Endocrinol. 2020, 73, 106467. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Wang, L.; Goebel-Stengel, M.; Taché, Y. Centrally Injected Kisspeptin Reduces Food Intake by Increasing Meal Intervals in Mice. Neuroreport 2011, 22, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Tanaka, K.; Nishimura, H.; Nishimura, K.; Sonoda, S.; Ueno, H.; Motojima, Y.; Yoshimura, M.; Maruyama, T.; Yamamoto, Y.; et al. Centrally Administered Kisspeptin Suppresses Feeding via Nesfatin-1 and Oxytocin in Male Rats. Peptides 2019, 112, 114–124. [Google Scholar] [CrossRef]
- Talbi, R.; Laran-Chich, M.-P.; Magoul, R.; El Ouezzani, S.; Simonneaux, V. Kisspeptin and RFRP-3 Differentially Regulate Food Intake and Metabolic Neuropeptides in the Female Desert Jerboa. Sci. Rep. 2016, 6, 36057. [Google Scholar] [CrossRef]
- Tolson, K.P.; Garcia, C.; Yen, S.; Simonds, S.; Stefanidis, A.; Lawrence, A.; Smith, J.T.; Kauffman, A.S. Impaired Kisspeptin Signaling Decreases Metabolism and Promotes Glucose Intolerance and Obesity. J. Clin. Investig. 2014, 124, 3075–3079. [Google Scholar] [CrossRef]
- Padilla, S.L.; Perez, J.G.; Ben-Hamo, M.; Johnson, C.W.; Sanchez, R.E.A.; Bussi, I.L.; Palmiter, R.D.; de la Iglesia, H.O. Kisspeptin Neurons in the Arcuate Nucleus of the Hypothalamus Orchestrate Circadian Rhythms and Metabolism. Curr. Biol. 2019, 29, 592–604.e4. [Google Scholar] [CrossRef]
- Velasco, I.; León, S.; Barroso, A.; Ruiz-Pino, F.; Heras, V.; Torres, E.; León, M.; Ruohonen, S.T.; García-Galiano, D.; Romero-Ruiz, A.; et al. Gonadal Hormone-Dependent vs. -Independent Effects of Kisspeptin Signaling in the Control of Body Weight and Metabolic Homeostasis. Metabolism 2019, 98, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Nestor, C.C.; Bedenbaugh, M.N.; Hileman, S.M.; Coolen, L.M.; Lehman, M.N.; Goodman, R.L. Regulation of GnRH Pulsatility in Ewes. Reproduction 2018, 156, R83–R99. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Si, L.; Shu, W.; Zhang, X.; Wei, C.; Wei, M.; Cheng, L.; Chen, Z.; Qiao, Y.; Yang, S. Exogenous Melatonin Regulates Puberty and the Hypothalamic GnRH-GnIH System in Female Mice. Brain Sci. 2022, 12, 1550. [Google Scholar] [CrossRef]
- Bohlen, T.M.; Silveira, M.A.; Buonfiglio, D.D.C.; Ferreira-Neto, H.C.; Cipolla-Neto, J.; Donato, J.; Frazao, R. A Short-Day Photoperiod Delays the Timing of Puberty in Female Mice via Changes in the Kisspeptin System. Front. Endocrinol. 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Si, L.; Zhang, X.; Wei, C.; Shu, W.; Wei, M.; Cheng, L.; Chen, Z.; Qiao, Y.; Yang, S. Therapeutic Effects of Melatonin in Female Mice with Central Precocious Puberty by Regulating the Hypothalamic Kiss-1/Kiss1R System. Behav. Brain Res. 2024, 461, 114783. [Google Scholar] [CrossRef]
- Avendaño, M.S.; Vazquez, M.J.; Tena-Sempere, M. Disentangling puberty: Novel neuroendocrine pathways and mechanisms for the control of mammalian puberty. Hum. Reprod. Updat. 2017, 23, 737–763. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.J.; Toro, C.A.; Castellano, J.M.; Ruiz-Pino, F.; Roa, J.; Beiroa, D.; Heras, V.; Velasco, I.; Dieguez, C.; Pinilla, L.; et al. SIRT1 Mediates Obesity- and Nutrient-Dependent Perturbation of Pubertal Timing by Epigenetically Controlling Kiss1 Expression. Nat. Commun. 2018, 9, 4194. [Google Scholar] [CrossRef]
- Frisch, R.E.; Revelle, R. Height and Weight at Menarche and a Hypothesis of Menarche. Arch. Dis. Child. 1971, 46, 695–701. [Google Scholar] [CrossRef]
- Marino, M.; Di Pietro, P.; D’Auria, R.; Lombardi, M.; Pastorino, G.M.G.; Troisi, J.; Operto, F.F.; Carrizzo, A.; Vecchione, C.; Viggiano, A.; et al. Adult Neurogenesis Is Regulated by the Endocannabinoid and Kisspeptin Systems. Int. J. Mol. Sci. 2025, 26, 3977. [Google Scholar] [CrossRef]
- Majarune, S.; Nima, P.; Sugimoto, A.; Nagae, M.; Inoue, N.; Tsukamura, H.; Uenoyama, Y. Ad Libitum Feeding Triggers Puberty Onset Associated with Increases in Arcuate Kiss1 and Pdyn Expression in Growth-Retarded Rats. J. Reprod. Dev. 2019, 65, 397–406. [Google Scholar] [CrossRef]
- D’Angelo, S.; Mele, E.; Di Filippo, F.; Viggiano, A.; Meccariello, R. Sirt1 Activity in the Brain: Simultaneous Effects on Energy Homeostasis and Reproduction. Int. J. Environ. Res. Public Health 2021, 18, 1243. [Google Scholar] [CrossRef]
- Ruggiero, M.; Motti, M.L.; Meccariello, R.; Mazzeo, F. Resveratrol and Physical Activity: A Successful Combination for the Maintenance of Health and Wellbeing? Nutrients 2025, 17, 837. [Google Scholar] [CrossRef] [PubMed]
- Leon, S.; Talbi, R.; McCarthy, E.A.; Ferrari, K.; Fergani, C.; Naule, L.; Choi, J.H.; Carroll, R.S.; Kaiser, U.B.; Aylwin, C.F.; et al. Sex-Specific Pubertal and Metabolic Regulation of Kiss1 Neurons via Nhlh2. eLife 2021, 10, e69765. [Google Scholar] [CrossRef] [PubMed]
- Hudson, A.D.; Kauffman, A.S. Metabolic Actions of Kisspeptin Signaling: Effects on Body Weight, Energy Expenditure, and Feeding. Pharmacol. Ther. 2022, 231, 107974. [Google Scholar] [CrossRef]
- Meccariello, R.; Fasano, S.; Pierantoni, R. Kisspeptins, New Local Modulators of Male Reproduction: A Comparative Overview. Gen. Comp. Endocrinol. 2020, 299, 113618. [Google Scholar] [CrossRef]
- Meccariello, R. The Kisspeptin System in Male Reproduction. Endocrines 2022, 3, 168–174. [Google Scholar] [CrossRef]
- Sharma, A.; Thaventhiran, T.; Minhas, S.; Dhillo, W.S.; Jayasena, C.N. Kisspeptin and Testicular Function—Is it Necessary? Int. J. Mol. Sci. 2020, 21, 2958. [Google Scholar] [CrossRef]
- Ricci, G.; Guillou, F.; Catizone, A.; Mele, V.G.; Moggio, M.; Chioccarelli, T.; Diano, N.; Meccariello, R.; Pierantoni, R.; Fasano, S.; et al. KISS1R and ANKRD31 Cooperate to Enhance Leydig Cell Gene Expression via the Cytoskeletal-Nucleoskeletal Pathway. Front. Cell Dev. Biol. 2022, 10, 877270. [Google Scholar] [CrossRef]
- Ramzan, F.; Qureshi, I.Z.; Ramzan, M.H. Dose-Dependent Degeneration of Leydig Cells Following Kisspeptin-10 Administration: An Ultrastructural Study. Protein Pept. Lett. 2022, 29, 64–70. [Google Scholar] [CrossRef]
- Petrucci, L.; Maranesi, M.; Verini Supplizi, A.; Dall’Aglio, C.; Mandara, M.T.; Quassinti, L.; Bramucci, M.; Miano, A.; Gobbetti, A.; Catone, G.; et al. Kisspeptin/GnRH1 system in Leydig cells of horse (Equus caballus): Presence and function. Theriogenology 2020, 152, 1–7. [Google Scholar] [CrossRef]
- Marino, M.; D’Auria, R.; Mele, E.; Pastorino, G.M.G.; Di Pietro, P.; D’Angelo, S.; Della Rocca, N.; Operto, F.F.; Vecchione, C.; Fasano, S.; et al. The Interplay between Kisspeptin and Endocannabinoid Systems Modulates Male Hypothalamic and Gonadic Control of Reproduction in Vivo. Front. Endocrinol. 2023, 14, 1269334. [Google Scholar] [CrossRef]
- Ciaramella, V.; Meccariello, R.; Chioccarelli, T.; Sirleto, M.; Fasano, S.; Pierantoni, R.; Chianese, R. Anandamide Acts via Kisspeptin in the Regulation of Testicular Activity of the Frog, Pelophylax Esculentus. Mol. Cell. Endocrinol. 2016, 420, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Ciaramella, V.; Fasano, S.; Pierantoni, R.; Meccariello, R. Kisspeptin Drives Germ Cell Progression in the Anuran Amphibian Pelophylax Esculentus: A Study Carried out in Ex Vivo Testes. Gen. Comp. Endocrinol. 2015, 211, 81–91. [Google Scholar] [CrossRef]
- Chianese, R.; Ciaramella, V.; Fasano, S.; Pierantoni, R.; Meccariello, R. Kisspeptin Regulates Steroidogenesis and Spermiation in Anuran Amphibian. Reproduction 2017, 154, 403–414. [Google Scholar] [CrossRef]
- Santoro, A.; Mele, E.; Marino, M.; Viggiano, A.; Nori, S.L.; Meccariello, R. The Complex Interplay between Endocannabinoid System and the Estrogen System in Central Nervous System and Periphery. Int. J. Mol. Sci. 2021, 22, 972. [Google Scholar] [CrossRef] [PubMed]
- Barchi, M.; Innocenzi, E.; Giannattasio, T.; Dolci, S.; Rossi, P.; Grimaldi, P. Cannabinoid Receptors Signaling in the Development, Epigenetics, and Tumours of Male Germ Cells. Int. J. Mol. Sci. 2019, 21, 25. [Google Scholar] [CrossRef]
- Leeners, B.; Geary, N.; Tobler, P.N.; Asarian, L. Ovarian Hormones and Obesity. Hum. Reprod. Update 2017, 23, 300–321. [Google Scholar] [CrossRef]
- Mahboobifard, F.; Pourgholami, M.H.; Jorjani, M.; Dargahi, L.; Amiri, M.; Sadeghi, S.; Tehrani, F.R. Estrogen as a Key Regulator of Energy Homeostasis and Metabolic Health. Biomed. Pharmacother. 2022, 156, 113808. [Google Scholar] [CrossRef]
- Mazzeo, F.; Meccariello, R. Cannabis and Paternal Epigenetic Inheritance. Int. J. Environ. Res. Public Health 2023, 20, 5663. [Google Scholar] [CrossRef] [PubMed]
- Leisegang, K.; Sengupta, P.; Agarwal, A.; Henkel, R. Obesity and Male Infertility: Mechanisms and Management. Andrologia 2021, 53, e13617. [Google Scholar] [CrossRef] [PubMed]
- Tajar, A.; Forti, G.; O’Neill, T.W.; Lee, D.M.; Silman, A.J.; Finn, J.D.; Bartfai, G.; Boonen, S.; Casanueva, F.F.; Giwercman, A.; et al. Characteristics of Secondary, Primary, and Compensated Hypogonadism in Aging Men: Evidence from the European Male Ageing Study. J. Clin. Endocrinol. Metab. 2010, 95, 1810–1818. [Google Scholar] [CrossRef]
- Andreozzi, F.; Mannino, G.C.; Mancuso, E.; Spiga, R.; Perticone, F.; Sesti, G. Plasma Kisspeptin Levels Are Associated with Insulin Secretion in Nondiabetic Individuals. PLoS ONE 2017, 12, e0179834. [Google Scholar] [CrossRef]
- Asare-Anane, H.; Ofori, E.K.; Kwao-Zigah, G.; Ateko, R.O.; Annan, B.D.R.T.; Adjei, A.B.; Quansah, M. Lower Circulating Kisspeptin and Primary Hypogonadism in Men with Type 2 Diabetes. Endocrinol. Diabetes Metab. 2019, 2, e00070. [Google Scholar] [CrossRef]
- Izzi-Engbeaya, C.; Comninos, A.N.; Clarke, S.A.; Jomard, A.; Yang, L.; Jones, S.; Abbara, A.; Narayanaswamy, S.; Eng, P.C.; Papadopoulou, D.; et al. The Effects of Kisspeptin on β-Cell Function, Serum Metabolites and Appetite in Humans. Diabetes Obes. Metab. 2018, 20, 2800–2810. [Google Scholar] [CrossRef]
- Celik, O.; Yildiz, B.O. Obesity and Physical Exercise. Minerva Endocrinol. 2021, 46, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gil, A.M.; Elizondo-Montemayor, L. The Role of Exercise in the Interplay between Myokines, Hepatokines, Osteokines, Adipokines, and Modulation of Inflammation for Energy Substrate Redistribution and Fat Mass Loss: A Review. Nutrients 2020, 12, 1899. [Google Scholar] [CrossRef]
- Rocliffe, P.; Adamakis, M.; O’Keeffe, B.T.; Walsh, L.; Bannon, A.; Garcia-Gonzalez, L.; Chambers, F.; Stylianou, M.; Sherwin, I.; Mannix-McNamara, P.; et al. The Impact of Typical School Provision of Physical Education, Physical Activity and Sports on Adolescent Mental Health and Wellbeing: A Systematic Literature Review. Adolesc. Res. Rev. 2024, 9, 339–364. [Google Scholar] [CrossRef]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Global Trends in Insufficient Physical Activity among Adolescents: A Pooled Analysis of 298 Population-Based Surveys with 1·6 Million Participants. Lancet Child Adolesc. Health 2020, 4, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise Interventions for Cognitive Function in Adults Older than 50: A Systematic Review with Meta-Analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Andreato, L.V.; Esteves, J.V.; Coimbra, D.R.; Moraes, A.J.P.; de Carvalho, T. The Influence of High-Intensity Interval Training on Anthropometric Variables of Adults with Overweight or Obesity: A Systematic Review and Network Meta-Analysis. Obes. Rev. 2019, 20, 142–155. [Google Scholar] [CrossRef]
- Baptista, F.M.; Andias, R.; Rocha, N.P.; Silva, A.G. A Practice Guide for Physical Therapists Prescribing Physical Exercise for Older Adults. J. Aging Phys. Act. 2024, 32, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shi, Q.; Nong, K.; Li, S.; Yue, J.; Huang, J.; Dong, B.; Beauchamp, M.; Hao, Q. Exercise for Sarcopenia in Older People: A Systematic Review and Network Meta-Analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 1199–1211. [Google Scholar] [CrossRef]
- Davenport, M.H.; Meah, V.L.; Ruchat, S.-M.; Davies, G.A.; Skow, R.J.; Barrowman, N.; Adamo, K.B.; Poitras, V.J.; Gray, C.E.; Jaramillo Garcia, A.; et al. Impact of Prenatal Exercise on Neonatal and Childhood Outcomes: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2018, 52, 1386–1396. [Google Scholar] [CrossRef]
- Mottola, M.F.; Davenport, M.H.; Ruchat, S.-M.; Davies, G.A.; Poitras, V.J.; Gray, C.E.; Garcia, A.J.; Barrowman, N.; Adamo, K.B.; Duggan, M.; et al. 2019 Canadian Guideline for Physical Activity throughout Pregnancy. Br. J. Sports Med. 2018, 52, 1339–1346. [Google Scholar] [CrossRef]
- Ribeiro, M.M.; Andrade, A.; Nunes, I. Physical Exercise in Pregnancy: Benefits, Risks and Prescription. J. Perinat. Med. 2022, 50, 4–17. [Google Scholar] [CrossRef]
- Gallotta, M.C.; Franciosi, E.; Giorgi, M.; Guidetti, L.; Cerbara, E.; Pes, G.; Silvestri, F.; Curzi, D. Benefits of Inclusive Sport Training on Fitness and Health of Athletes with and without Intellectual Disability. Sci. Rep. 2024, 14, 21203. [Google Scholar] [CrossRef]
- Diz, S.; Jacinto, M.; Costa, A.M.; Monteiro, D.; Matos, R.; Antunes, R. Physical Activity, Quality of Live and Well-Being in Individuals with Intellectual and Developmental Disability. Healthcare 2024, 12, 654. [Google Scholar] [CrossRef]
- Collado-Mateo, D.; Lavín-Pérez, A.M.; Peñacoba, C.; Del Coso, J.; Leyton-Román, M.; Luque-Casado, A.; Gasque, P.; Fernández-Del-Olmo, M.Á.; Amado-Alonso, D. Key Factors Associated with Adherence to Physical Exercise in Patients with Chronic Diseases and Older Adults: An Umbrella Review. Int. J. Environ. Res. Public. Health. 2021, 18, 2023. [Google Scholar] [CrossRef] [PubMed]
- Araujo, D.N.; Ribeiro, C.T.; Maciel, A.C.; Bruno, S.S.; Fregonezi, G.A.; Dias, F.A. Physical Exercise for the Treatment of Non-Ulcerated Chronic Venous Insufficiency. Cochrane Database Syst. Rev. 2023, 6, CD010637. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.J.; Fegers-Wustrow, I.; Halle, M.; Haykowsky, M.J.; Chung, E.H.; Kovacic, J.C. Exercise for Primary and Secondary Prevention of Cardiovascular Disease: JACC Focus Seminar 1/4. J. Am. Coll. Cardiol. 2022, 80, 1091–1106. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines on Physical Activity and Sedentary Behaviour. 2020. Available online: https://www.who.int/publications/i/item/9789240015128 (accessed on 15 July 2025).
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and Adiponectin Receptors in Insulin Resistance, Diabetes, and the Metabolic Syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Bouassida, A.; Chamari, K.; Zaouali, M.; Feki, Y.; Zbidi, A.; Tabka, Z. Review on Leptin and Adiponectin Responses and Adaptations to Acute and Chronic Exercise. Br. J. Sports Med. 2010, 44, 620–630. [Google Scholar] [CrossRef]
- Martinez-Huenchullan, S.F.; Tam, C.S.; Ban, L.A.; Ehrenfeld-Slater, P.; Mclennan, S.V.; Twigg, S.M. Skeletal Muscle Adiponectin Induction in Obesity and Exercise. Metabolism 2020, 102, 154008. [Google Scholar] [CrossRef]
- Sagheb, M.M.; Azarpira, N.; Yaghobi, R. The Effect of Leptin and Adiponectin on KiSS-1 and KissR mRNA Expression in Rat Islets of Langerhans and CRI-D2 Cell Line. Int. J. Endocrinol. Metab. 2014, 12, e15297. [Google Scholar] [CrossRef]
- Wen, J.-P.; Liu, C.; Bi, W.-K.; Hu, Y.-T.; Chen, Q.; Huang, H.; Liang, J.-X.; Li, L.-T.; Lin, L.-X.; Chen, G. Adiponectin Inhibits KISS1 Gene Transcription through AMPK and Specificity Protein-1 in the Hypothalamic GT1-7 Neurons. J. Endocrinol. 2012, 214, 177–189. [Google Scholar] [CrossRef]
- Mlyczyńska, E.; Kieżun, M.; Kurowska, P.; Dawid, M.; Pich, K.; Respekta, N.; Daudon, M.; Rytelewska, E.; Dobrzyń, K.; Kamińska, B.; et al. New Aspects of Corpus Luteum Regulation in Physiological and Pathological Conditions: Involvement of Adipokines and Neuropeptides. Cells 2022, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, X.; Song, G.; Schmidt, S.F.; Sun, L.; Chen, J.; Pan, X.; Zhao, H.; Yan, Y. Adipose Kiss1 Controls Aerobic Exercise-Related Adaptive Responses in Adipose Tissue Energy Homeostasis. FASEB J. 2024, 38, e23743. [Google Scholar] [CrossRef]
- Arisha, A.H.; Moustafa, A. Potential Inhibitory Effect of Swimming Exercise on the Kisspeptin-GnRH Signaling Pathway in Male Rats. Theriogenology 2019, 133, 87–96. [Google Scholar] [CrossRef]
- Xu, R.; Feng, J.; Liang, C.; Song, G.; Yan, Y. Effects of High-Fat Diet and Treadmill Running on the Hypothalamic Kiss-1-GPR54 Signaling Pathway in Male Growing Rats. Hormones 2022, 21, 641–652. [Google Scholar] [CrossRef]
- Kacar, E.; Bulmus, O.; Ercan, Z.; Kavuran, I.B.; Zorlu, G.; Tan, F.; Serhatlioglu, I.; Kelestimur, H. Treadmill Exercise Has Healing Effects on Obesity-Induced Sexual Behavior Disorder through Kisspeptin and kiss1R Expression in Male Rats. Cell. Mol. Biol. 2023, 69, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Song, C.; Gao, H.; Ma, T.; Li, T.; Ma, Q.; Yao, T.; Wang, M.; Li, J.; Yi, X.; et al. Leptin and Inflammatory Factors Play a Synergistic Role in the Regulation of Reproduction in Male Mice through Hypothalamic Kisspeptin-Mediated Energy Balance. Reprod. Biol. Endocrinol. 2021, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Pereira, B.C.; da Rocha, A.L.; Pauli, J.R.; Ropelle, E.R.; de Souza, C.T.; Cintra, D.E.; Sant’Ana, M.R.; da Silva, A.S.R. Excessive Eccentric Exercise Leads to Transitory Hypothalamic Inflammation, Which May Contribute to the Low Body Weight Gain and Food Intake in Overtrained Mice. Neuroscience 2015, 311, 231–242. [Google Scholar] [CrossRef]
- Khajehnasiri, N.; Khazali, H.; Sheikhzadeh, F. Various Responses of Male Pituitary-Gonadal Axis to Different Intensities of Long-Term Exercise: Role of Expression of KNDY-related Genes. J. Biosci. 2018, 43, 569–574. [Google Scholar] [CrossRef]
- Yi, X.; Gao, H.; Chen, D.; Tang, D.; Huang, W.; Li, T.; Ma, T.; Chang, B. Effects of Obesity and Exercise on Testicular Leptin Signal Transduction and Testosterone Biosynthesis in Male Mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R501–R510. [Google Scholar] [CrossRef] [PubMed]
- Rosety, M.Á.; Díaz, A.J.; Rosety, J.M.; Pery, M.T.; Brenes-Martín, F.; Bernardi, M.; García, N.; Rosety-Rodríguez, M.; Ordoñez, F.J.; Rosety, I. Exercise Improved Semen Quality and Reproductive Hormone Levels in Sedentary Obese Adults. Nutr. Hosp. 2017, 34, 603–607. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Sapra, K.J.; Kim, S.D.; Chen, Z.; Louis, G.M.B. Semen Quality and Pregnancy Loss in a Contemporary Cohort of Couples Recruited before Conception: Data from the Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertil. Steril. 2017, 108, 613–619. [Google Scholar] [CrossRef]
- Rafiee, B.; Morowvat, M.H.; Rahimi-Ghalati, N. Comparing the Effectiveness of Dietary Vitamin C and Exercise Interventions on Fertility Parameters in Normal Obese Men. Urol. J. 2016, 13, 2635–2639. [Google Scholar]
- Wise, L.A.; Wang, T.R.; Ulrichsen, S.P.; Krivorotko, D.; Mikkelsen, E.M.; Kuriyama, A.S.; Laursen, A.S.D.; Jørgensen, M.D.; Eisenberg, M.L.; Rothman, K.J.; et al. A Prospective Study of Male Physical Activity and Fecundability. Hum. Reprod. 2025, 40, 360–371. [Google Scholar] [CrossRef]
- Bird, S.R.; Goebel, C.; Burke, L.M.; Greaves, R.F. Doping in Sport and Exercise: Anabolic, Ergogenic, Health and Clinical Issues. Ann. Clin. Biochem. 2016, 53, 196–221. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, F.; Raiola, G. An Investigation of Drugs Abuse in Sport Performance. J. Hum. Sport Exerc. 2018, 13, S309–S319. [Google Scholar] [CrossRef]
- Ihalainen, J.K.; Mikkonen, R.S.; Ackerman, K.E.; Heikura, I.A.; Mjøsund, K.; Valtonen, M.; Hackney, A.C. Beyond Menstrual Dysfunction: Does Altered Endocrine Function Caused by Problematic Low Energy Availability Impair Health and Sports Performance in Female Athletes? Sports Med. 2024, 54, 2267–2289. [Google Scholar] [CrossRef]
- McQuilliam, S.J.; Clark, D.R.; Erskine, R.M.; Brownlee, T.E. Free-Weight Resistance Training in Youth Athletes: A Narrative Review. Sports Med. 2020, 50, 1567–1580. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Strauss, J.A.; Hackney, A.C. Understanding the female athlete: Molecular mechanisms underpinning menstrual phase differences in exercise metabolism. Eur. J. Appl. Physiol. 2023, 123, 423–450. [Google Scholar] [CrossRef]
- Mazzeo, F.; Volpe, A.R. From Gene Doping to Athlete Biological Passport. Sport. Sci. 2016, 9, 97–103. [Google Scholar]
- Kim, G.L.; Dhillon, S.S.; Belsham, D.D. Kisspeptin Directly Regulates Neuropeptide Y Synthesis and Secretion via the ERK1/2 and P38 Mitogen-Activated Protein Kinase Signaling Pathways in NPY-Secreting Hypothalamic Neurons. Endocrinology 2010, 151, 5038–5047. [Google Scholar] [CrossRef] [PubMed]
- Yeager, K.K.; Agostini, R.; Nattiv, A.; Drinkwater, B. The Female Athlete Triad: Disordered Eating, Amenorrhea, Osteoporosis. Med. Sci. Sports Exerc. 1993, 25, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.B.; Rieger, E.; Touyz, S.W.; De la Garza García Lic, Y. Muscle Dysmorphia and the DSM-V Conundrum: Where Does It Belong? A Review Paper. Int. J. Eat. Disord. 2010, 43, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Carter, S.; Constantini, N.; Lebrun, C.; Meyer, N.; Sherman, R.; Steffen, K.; Budgett, R.; et al. The IOC Consensus Statement: Beyond the Female Athlete Triad--Relative Energy Deficiency in Sport (RED-S). Br. J. Sports Med. 2014, 48, 491–497. [Google Scholar] [CrossRef]
- Montesano, P.; Di Silvestro, M.; Cipriani, G.; Mazzeo, F. Overtraining Syndrome, Stress and Nutrition in Football Amateur Athletes. J. Hum. Sport Exerc. 2019, 14, S957–S969. [Google Scholar] [CrossRef]
- Adelowo, O.E.; Akindele, B.M.; Adegbola, C.A.; Oyedokun, P.A.; Akhigbe, T.M.; Akhigbe, R.E. Unraveling the Complexity of the Impact of Physical Exercise on Male Reproductive Functions: A Review of Both Sides of a Coin. Front. Physiol. 2024, 15, 1492771. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, F.; Tafuri, D. Hormone and Metabolic Modulators, Combined Pill and Progestogen Only Pill in Sport: Study of Their Use. Acta Medica Mediterr. 2020, 36, 443–446. [Google Scholar]
- Mazzeo, F. Anabolic Steroid Use in Sports and in Physical Activity: Overview and Analysis. Sport Mont 2018, 16, 113–118. [Google Scholar] [CrossRef]
- Bhasin, S.; Storer, T.W.; Berman, N.; Callegari, C.; Clevenger, B.; Phillips, J.; Bunnell, T.J.; Tricker, R.; Shirazi, A.; Casaburi, R. The Effects of Supraphysiologic Doses of Testosterone on Muscle Size and Strength in Normal Men. N. Engl. J. Med. 1996, 335, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Luque, R.M.; Córdoba-Chacón, J.; Gahete, M.D.; Navarro, V.M.; Tena-Sempere, M.; Kineman, R.D.; Castaño, J.P. Kisspeptin Regulates Gonadotroph and Somatotroph Function in Nonhuman Primate Pituitary via Common and Distinct Signaling Mechanisms. Endocrinology 2011, 152, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, C.N.; Comninos, A.N.; Narayanaswamy, S.; Bhalla, S.; Abbara, A.; Ganiyu-Dada, Z.; Busbridge, M.; Ghatei, M.A.; Bloom, S.R.; Dhillo, W.S. Acute and Chronic Effects of Kisspeptin-54 Administration on GH, Prolactin and TSH Secretion in Healthy Women. Clin. Endocrinol. 2014, 81, 891–898. [Google Scholar] [CrossRef][Green Version]
- Foradori, C.D.; Whitlock, B.K.; Daniel, J.A.; Zimmerman, A.D.; Jones, M.A.; Read, C.C.; Steele, B.P.; Smith, J.T.; Clarke, I.J.; Elsasser, T.H.; et al. Kisspeptin Stimulates Growth Hormone Release by Utilizing Neuropeptide Y Pathways and Is Dependent on the Presence of Ghrelin in the Ewe. Endocrinology 2017, 158, 3526–3539. [Google Scholar] [CrossRef]
- WADA Investigations into the Metabolism and Analysis of Kisspeptin and Analogs for Doping Controls by Means of LC-MS. Available online: https://www.wada-ama.org/en/resources/scientific-research/investigations-metabolism-and-analysis-kisspeptin-and-analogs-doping (accessed on 15 July 2025).
- Mazzeo, F. Current Concept of Obesity. Sport. Sci. 2016, 9, 42–48. [Google Scholar]
- Marques, P.; De Sousa Lages, A.; Skorupskaite, K.; Rozario, K.S.; Anderson, R.A.; George, J.T. Physiology of GnRH and Gonadotrophin Secretion. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Pinto, F.M.; Cejudo-Román, A.; Ravina, C.G.; Fernández-Sánchez, M.; Martín-Lozano, D.; Illanes, M.; Tena-Sempere, M.; Candenas, M.L. Characterization of the Kisspeptin System in Human Spermatozoa. Int. J. Androl. 2012, 35, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Montesano, P.; Mazzeo, F. Improvement in Soccer Learning and Methodology for Young Athletes. J. Phys. Educ. Sport 2019, 19, 795–801. [Google Scholar] [CrossRef]
- Mazzeo, F.; Altavilla, G.; D’elia, F.; Raiola, G. Development of Doping in Sports: Overview and Analysis. J. Phys. Educ. Sport 2018, 18, 1669–1677. [Google Scholar] [CrossRef]
- Colpaert, T.; Risseeuw, M.; Deventer, K.; Van Eenoo, P. Investigating the detection of the novel doping-relevant peptide kisspeptin-10 in urine using liquid chromatography high-resolution mass spectrometry. Biomed. Chromatogr. 2024, 38, e5946. [Google Scholar] [CrossRef] [PubMed]
| Population Group | Recommended Physical Activity | Examples of Activities | Notes |
|---|---|---|---|
| Children and Adolescents (5–17 years) | At least 60 min/day of moderate-to-vigorous physical activity, mostly aerobic. 3 days/week: vigorous activity + muscle- and bone-strengthening exercises. | Active play, running, swimming, cycling, team sports (soccer, volleyball) [79,80] | Limit sedentary time, especially screen time. |
| Adults (18–64 years) | 150–300 min/week of moderate aerobic activity or 75–150 min/week of vigorous activity. 2 days/week: muscle-strengthening exercises. | Brisk walking, swimming, running, intense cycling [81,82] | Replace sedentary time with activity of any intensity. |
| Older Adults (65+ years) | 150–300 min/week of moderate aerobic activity or 75–150 min of vigorous activity. 2 days/week: muscle-strengthening. 3 days/week: balance exercises (e.g., multicomponent). | Walking, pilates, dancing, gardening, light weight exercises, fall-prevention exercises [83,84] | Adapt intensity to physical abilities. |
| Pregnant and Postpartum Women | 150 min/week of moderate aerobic activity. Muscle-strengthening and stretching exercises. May continue vigorous activity if already habitual. | Walking, swimming, prenatal yoga, pelvic floor exercises [85,86,87] | Avoid high-risk activities (falling/overexertion). Consult a doctor. |
| People with Disabilities | Same recommendations as their age group, adapted to individual abilities. Consult a specialist for personalized activities. | Adapted sports (e.g., wheelchair basketball, mixed ability rugby), water exercises, stretching [88,89] | Ensure inclusive and safe opportunities. |
| People with Chronic Conditions (e.g., diabetes, hypertension, cancer) | Same recommendations as adults/older adults, adjusted for health conditions. Consult a doctor to plan activities. | Walking, swimming, cycling, light resistance exercises [90,91,92] | Physical activity improves disease management. |
| Description | Implications for Sport | |
|---|---|---|
| Primary Role | Central regulation of the hypothalamic–pituitary–gonadal (HPG) axis, which controls reproduction through the release of GnRH (Gonadotropin-Releasing Hormone). | Influences the production of sex hormones (testosterone in men, estrogen in women), important for muscle development, bone density, energy, and libido, all relevant factors for athletic performance [113]. GnRH: Section S2 of the World Anti-Doping Agency (WADA) Prohibited List [114]. |
| Response to Exercise | Physical exercise can influence kisspeptin levels. The response may vary depending on the intensity, duration, and type of exercise, as well as the individual’s energy status. | Intense and/or chronic exercise, especially in combination with caloric restriction (common in some sports), can suppress the kisspeptin system, leading to a reduction in sex hormones [113]. This can have negative consequences on performance, recovery, and general health (e.g., bone density, menstrual cycle in women) [115]. |
| Energy Balance | The kisspeptin system is sensitive to energy status. Low energy levels (caloric deficit) can inhibit the activity of kisspeptin neurons. | Athletes in sports that require low body weight or who follow restrictive diets are at risk of overthrow of the kisspeptin system, with potential negative impacts on reproduction and metabolic health [116]. |
| Gender Differences | There are differences in the regulation of the kisspeptin system between men and women, as well as in the response to exercise. | Female athletes may be more susceptible to exercise-induced hormonal imbalances due to the complex interaction between the kisspeptin system, the menstrual cycle, and energy balance [117]. |
| Potential Applications | Understanding the role of kisspeptin in sport could lead to strategies to optimize hormonal health and athletic performance; for example, through targeted nutritional or training interventions. | Monitoring kisspeptin and related hormone levels could be useful in identifying athletes at risk of hormonal imbalances. Interventions to maintain adequate energy balance and modulate training intensity could preserve the function of the kisspeptin system and hormonal health [118,119]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggiero, M.; Vicidomini, A.; Tafuri, D.; Mazzeo, F.; Meccariello, R. Energy Homeostasis and Kisspeptin System, Roles of Exercise and Outcomes with a Focus on Male Reproductive Health. Endocrines 2025, 6, 43. https://doi.org/10.3390/endocrines6030043
Ruggiero M, Vicidomini A, Tafuri D, Mazzeo F, Meccariello R. Energy Homeostasis and Kisspeptin System, Roles of Exercise and Outcomes with a Focus on Male Reproductive Health. Endocrines. 2025; 6(3):43. https://doi.org/10.3390/endocrines6030043
Chicago/Turabian StyleRuggiero, Mario, Antonella Vicidomini, Domenico Tafuri, Filomena Mazzeo, and Rosaria Meccariello. 2025. "Energy Homeostasis and Kisspeptin System, Roles of Exercise and Outcomes with a Focus on Male Reproductive Health" Endocrines 6, no. 3: 43. https://doi.org/10.3390/endocrines6030043
APA StyleRuggiero, M., Vicidomini, A., Tafuri, D., Mazzeo, F., & Meccariello, R. (2025). Energy Homeostasis and Kisspeptin System, Roles of Exercise and Outcomes with a Focus on Male Reproductive Health. Endocrines, 6(3), 43. https://doi.org/10.3390/endocrines6030043










