Abstract
Introduction: During the formation of neural circuits, the developing brain demonstrates extraordinary plasticity, heavily influenced by hormones. These chemical messengers interact with specific receptors to regulate vital physiological functions. The thyroid gland plays a pivotal role in maintaining hormonal balance and guiding brain development. However, emerging threats like endocrine-disrupting chemicals (EDCs) can interfere with this intricate system. EDCs are exogenous substances that can mimic, enhance, or block the actions of endogenous hormones, disrupting hormonal signaling in the brain at various developmental stages. Exposure can impair cognitive function and behavior due to disruptions in thyroid function. Studies indicate that mixtures of EDCs negatively impact brain development, leading to lower IQ and behavioral problems. Reducing EDC exposure through regulations and public awareness is crucial, and further research is needed to elucidate their mechanisms. Conclusions: Protecting vulnerable populations, such as pregnant women and children, is essential through prompt regulatory measures.
1. Introduction
The human body is an intricate communication network, with hormones acting as critical chemical messengers that interact with specific receptors in target cells [1]. Hormones are produced by specialized glands, tissues, and even individual cells, and coordinate and regulate several functions, impacting growth, development, differentiation, energy balance, and reproduction [2]. Central to this intricate hormonal system are the thyroid gland and the mammalian brain, which play pivotal roles in ensuring the proper balance and function of these critical processes [3,4]. However, the integrity of these systems is increasingly threatened by endocrine-disrupting chemicals (EDCs), which can interfere with hormonal balance and brain function. Imbalances in thyroid hormone levels can lead to severe neurological disorders, emphasizing the importance of studying their role in brain development and function [5,6,7].
The term “endocrine-disrupting chemicals” was coined at the 1991 Wingspread Conference in Racine, Wisconsin. This scientific meeting highlighted that various man-made and natural substances released into the environment have the potential to disrupt the endocrine system’s normal function in animals, including humans [8]. This article will focus on the intricate relationship between the brain and the thyroid hormones, highlighting the impact of EDC exposure on their function.
The production and release of manufactured chemicals into the environment has grown exponentially since the 1940s. Experimental and epidemiological research data show that exposure to specific synthetic chemicals at environmentally relevant concentrations during critical developmental windows, such as the fetal or neonatal periods, leads to a range of abnormalities in both vertebrate and invertebrate species [9,10]. These disruptions can manifest as morphological anomalies, altered biochemical processes, physiological dysfunction, and behavioral changes [11].
This phenomenon was first predicted by Rachel Carson in her book Silent Spring, which raised concerns about the potential health consequences of ECDs. Carson’s work led to the United States’s subsequent banning of the agricultural and industrial use of dichloro-diphenyl-trichloroethane (DDT) in 1973 and polychlorinated biphenyls (PCBs) in 1977. Regulatory agencies worldwide, recognizing the risks posed by EDCs, have since implemented measures to minimize their environmental impact, including public awareness campaigns [11,12].
Initially, it was believed that EDCs primarily interacted with cells through hormone receptors, such as estrogen and thyroid receptors. This interaction could result in various effects, including acting as agonists, partial agonists, or antagonists of the natural hormone effects [13,14]. However, over time, a far more intricate picture has emerged, with EDCs employing a much broader range of disrupting mechanisms than previously recognized, including (a) altered receptor expression and signal transduction, (b) post-translational modifications and/or ion flux, (c) disrupted transport across cell membranes, (d) compromised distribution or circulating plasma protein transporters, (e) altered hormone metabolism or clearance, (f) altering the fates of hormone-producing or hormone-responsive cells, and (g) dysregulated epigenetic modifications [15].
Considering the intricate interplay of hormones across various tissues, we must acknowledge that EDCs will have similarly complex effects, although not perfectly mimicking those of natural hormones. Therefore, a clear definition of an EDC is essential, as it determines the evidence needed for classification and shapes how we evaluate exposure risks. In agreement, in 2012, the Endocrine Society stated that EDCs are defined as “an exogenous chemical, or mixture of chemicals, which can interfere with any aspect of hormone action” [16,17].
For example, microcystin-LR (MC-LR) is a cyanobacterial toxin that has been shown to disrupt the hypothalamic–pituitary–thyroid (HPT) axis in both female rats and zebrafish. Additionally, MC-LR has been observed to damage the morphology of thyroid follicular cells, leading to endocrine imbalances. While TSH levels increased in females, T3 and T4 levels remained unchanged, suggesting a compensatory response [18,19].
1.1. Thyroid Hormone Action on the Brain
The central nervous system (CNS) is particularly vulnerable during development, relying on the actions of thyroid hormones (THs) for appropriate growth and function [20]. The consequences of EDCs on thyroid function might decrease circulating TH levels, mimicking a deficiency of these hormones [21]. Others might disrupt the hormones’ ability to bind to receptors in brain cells, blocking cell communication even when hormone levels are normal. [22,23]. The complexity of EDCs’ actions makes it challenging to identify the exact mechanisms by which these chemicals disrupt brain development and/or alter adult physiological maintenance.
1.1.1. Thyroid Hormone Regulation and Negative Feedback Loop
It is worth noting that the brain regulates thyroid function through the hypothalamus–pituitary–thyroid axis. The hypothalamus releases thyrotropin-releasing hormone (TRH), which stimulates the pituitary gland to secrete thyroid-stimulating hormone (TSH). TSH then prompts the thyroid gland to produce THs, especially T3 and T4. These hormones exert negative effects on TRH and TSH production and secretion through negative feedback loops that ensure the balance of TH levels in the body [24,25].
1.1.2. Thyroid Hormone Transport and Metabolism in the Brain
Moreover, the CNS is a crucial target for THs because these hormones play a vital role in brain maturation by influencing developmental processes, differentiation, metabolism, myelination, cytoskeletal stabilization, and neuronal and glial signaling [26,27,28,29]. Interestingly, the blood–brain barrier and glial cells act as critical regulators of TH signaling in the brain [30]. They achieve this through the widespread expression of monocarboxylate transporter 8 (MCT8) and organic anion transporter polypeptide 1C1 (OATP1C1), which facilitate the selective transport of THs through their membranes [31,32]. Glial cells, astrocytes, and tanycytes regulate the amount of T3 that reaches neurons by expressing the type 2 deiodinase (D2) enzyme that catalyzes the conversion of pro-hormone T4 to the active hormone T3. Accordingly, the inactivation of glial cell selective D2 in mice leads to a hypothyroidism phenotype [33,34]. T3 is subsequently metabolized to 3,5-diiodothyronine (T2) by type 3 deiodinase (D3), which is highly expressed in neurons. In many cases, tight control of these pathways by T3 is achieved with coordinated reciprocal changes in D2-mediated thyroid hormone activation and D3-mediated thyroid hormone inactivation [35].
New evidence suggests that T3 directly enters neurons and is selectively taken up by specific compartments within the cell (clathrin-dependent, MCT8- and D3-containing endosomal/NDL). It is shielded from degradation during transport to the nucleus because it enters directly from the surrounding space. These experiments explain the previously puzzling observation of high nuclear T3 levels in the brain despite significant D3 activity. They also suggest how L-T3 therapy for hypothyroidism can bypass D3-mediated breakdown and effectively reach the nucleus to restore TH signaling [36].
1.1.3. Nuclear Thyroid Hormone Receptors
Within the nucleus, T3 interacts with highly expressed receptors in the brain and has a significantly stronger binding affinity (around 10 times higher) to nuclear TH receptors (THRs) compared to T4. The four THR subtypes (TRα1, TRβ1, TRβ2, and TRβ3), which bind both T3 and DNA, are encoded by two different genes—THRA and THRB [37,38].
This makes T3 the primary driver of T3′s influence on brain gene expression [39]. The developmental time points for the expression of THRs in humans, rats, and mice are similar. These species exhibit a critical period of THR expression during early development, particularly in the brain.
Both THR mRNA subtypes, THRA1 and THRB2, seem to be expressed overall and overlap throughout the mouse brain, although the hippocampus, amygdala, and hypothalamus show some regional variations. In the cerebellum, THRA1 expression is concentrated in the granular layer, while THRB1 expression is primarily localized in the Purkinje cell layer [40].
The expression of the T3-binding receptor in the rat brain occurs earlier than in other tissues. Receptor activity can be measured in the whole brain as early as embryonic day 14 (E14), and messenger RNA for the receptor is detectable in the neural tube by E11.5 and, in specific regions of the diencephalon and ventral rhombencephalon, by E12.5. Consequently, restricted populations of cells may be targets of thyroid hormones during these early developmental stages [37].
Single-cell RNA sequencing has recently shed light on thyroid hormone receptor (THR) targets in various neuronal subtypes. Studies of T3 responses in the adult cortex and the hypothalamus of mice expressing the TRα1R384C variant suggest that diverse neuronal populations exhibit distinct sets of THR target genes [41,42].
THRA and THRB are expressed in the adult rat brain with distinct and overlapping patterns. Ercan-Fang and colleagues determined that Thrα1 constitutes approximately 70–80% of the total receptors present in the adult rat cerebrum and cerebellum [43].
Given the prevalence of TRα1 in the adult brain, it is probable that most of the effects of thyroid hormone in this region are mediated through this receptor subtype. While specific quantitative data for the developing brain are limited, mRNA expression patterns suggest that TRα1 is also the dominant receptor in humans and rodents, particularly during the fetal period [37].
In humans, maternal T4 enters the embryonic cavities early in development, with T4 concentrations reaching biologically relevant levels during the first trimester. T3, generated from T4 by D2 deiodinase, increases in the cerebral cortex until mid-gestation, reaching adult-like levels despite low T3 in fetal fluids. D2 and D3 deiodinases regulate T3 bioavailability, ensuring cell-specific and timely thyroid hormone action. Nuclear receptor bound T3 is detected in the cerebral cortex early in the first trimester, supporting the critical role of THs in early brain development [44].
1.1.4. Genomic and Non-Genomic Actions of Thyroid Hormones
TH signaling involves a complex interplay between coactivators and corepressors that influence nuclear receptors. Inside the cell, THRs are in specific DNA regions known as thyroid hormone-responsive elements (TREs), found in the regulatory region of T3 target genes [45,46]. Notably, THR binding to DNA as a heterodimer with RXR is crucial for genes positively regulated by TH. Additionally, the unliganded receptor can repress positively regulated genes, and specific functions vary between THRA and THRB isoforms. The interaction of the TH/nuclear receptor complex with TREs leads to the activation or inhibition of the transcription of specific genes and the synthesis of the proteins that they encode [47,48,49,50].
THs are traditionally known for their nuclear actions regulating gene expression and have emerged as versatile modulators of cellular processes through non-genomic mechanisms. These rapid (seconds to minutes) effects involve interactions with proteins expressed outside the nucleus, including cell membrane proteins like integrin αvβ3, cytoplasmic proteins, and truncated isoforms of the nuclear TH receptor THRA, such as p30 TRα1 [51].
1.1.5. The Impact of Thyroid Hormone Imbalances on Brain Development and Function
This intricate signaling network orchestrates a diverse array of cellular functions, including nutrient transport regulation, cellular metabolism, apoptosis regulation, cell proliferation, and angiogenesis. TH, particularly T4, stimulates the proliferation of cancer and endothelial cells, contributing to tumor growth and angiogenesis. Intriguingly, the T4 prohormone, while primarily serving as a precursor to the genomically active T3, exhibits a distinct and potentially more prominent role in cancer cell function compared to T3 [52,53,54].
Interestingly, data from the ventricular zone of the newborn rat brain has not only demonstrated that TH action targets multiple cell junction components, suggesting potential involvement of both genomic and nongenomic TH signaling pathways, but also pointed toward a possible effect of hypothyroidism on the integrity of the blood–brain and blood–cerebrospinal fluid barriers [55].
In adult rats, hypothyroidism decreases the differentiation, survival, and neurogenesis of hippocampal progenitor cells, which can contribute to cognitive and behavioral deficits. These effects can be reversed by TH replacement [56,57,58]. Current research with brain organoids that were derived from healthy individuals and compared with thyroid gene mutations (congenital hypothyroidism and resistance to thyroid hormone) showed how THs influence the early development of the human cerebral cortex [59]. These findings offer valuable insights into the developmental differences observed in children born to mothers with varying degrees of gestational hypothyroidism, mild-to-moderate gestational iodine deficiency (GID), or hypothyroidism developed early in life.
In the brain, gestational iodine deficiency processing disorder (GIDPD), even in mild forms, can impair hippocampal development and myelination, affecting neuroprocessing speed, impulse control, response inhibition, and working memory. This can contribute to learning disorders like dyslexia, language delays, ASD, and ADHD [60,61].
Studies in rodents have shown that GIDPD is associated with delayed axonal growth and reduced myelin basic protein (MBP), essential for myelination. The hippocampus plays a key role in memory formation and recall. Impaired hippocampal function and myelination can affect memory retrieval and neurotransmission [61].
While severe GIDPD can result in cretinism, milder forms have significant cognitive and psychosocial consequences. Preventative iodine programs are crucial to address both severe and mild GID. A prospective iodine supplementation intervention targeting pregnant and breastfeeding women at risk of mild iodine deficiency, with longitudinal follow-up of their offspring, is essential to establish appropriate supplementation guidelines [60,61].
Hypothyroidism alterations can affect various brain regions and pathways, including the cerebral cortex, hippocampus, cerebellum, interhemispheric and corticospinal tracts, and associative nuclei. It is noteworthy that the formation of new neurons, a process known as neurogenesis, continues throughout adulthood in most vertebrates, including humans, and depends on TH action [62,63].
Finally, THs are considered neuroprotective hormones: In vitro models using human neuroblastoma cell lines have shown that treatment with T3 under hypoxic conditions increases the expression of hypoxia-inducible factor 2α (HIF-2α), a protein that promotes cell survival and neurogenesis [64]. TH treatment on rat brain-derived endothelial cells has suggested that these hormones promote cell expansion and the formation of tube-like structures, characteristics of angiogenesis. These effects are mediated by the upregulation of two important factors involved in blood vessel formation: vascular endothelial growth factor A (VEGF-A) and basic fibroblast growth factor (FGF-2) [65,66].
Several studies have investigated the potential link between exposure to EDCs and changes in TH levels. These hormonal changes may lead to alterations in neuronal proliferation, migration, process outgrowth, synaptic development, and myelin formation in specific brain regions, ultimately affecting functions like cognition and locomotion, or behavior like anxiety and depression [67,68,69].
1.2. Thyroid Hormones and Endocrine-Disrupting Chemicals: A Risky Mix for Brain Development and Function
The intricate relationship between the brain and THs underscores the importance of maintaining endocrine health. Therefore, EDCs pose a significant threat to this balance, with potentially far-reaching consequences for brain function and overall health (Figure 1).
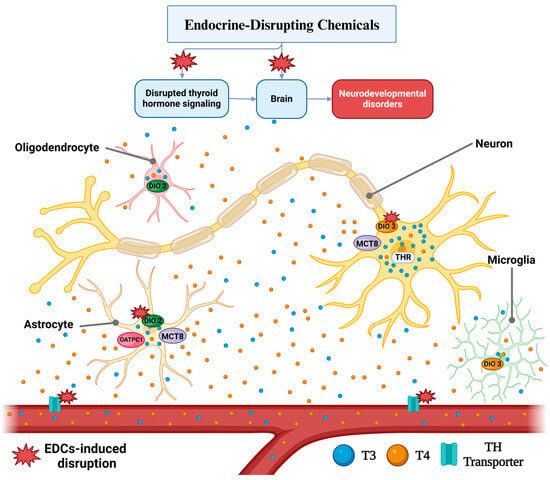
Figure 1.
Thyroid Hormone Action and Deleterious effects of EDC Exposure on The Brain through TH signaling dysregulation. Studies have reported that exposure to EDCs, especially during critical phases of development, disrupts the expression and function of proteins involved in the metabolism, transport, and action of THs in various cells of the central nervous system (CNS). The outcomes of this dysregulation are compromised neurodevelopment and altered cognitive functions, both in children and adults. Created with BioRender.com, accessed on 23 October 2024.
1.2.1. The Complexity of EDC Mixtures
EDC Exposure and Thyroid Health
These contaminants are found in numerous everyday products and pollutants, including contaminated water, air pollution, pesticides and agricultural chemicals, flame retardants, cleaning supplies, personal care products, food additives and packaging materials, paints and solvents, and medical devices [16]. EDCs interfere with THs in several ways, affecting their production, transport, breakdown, and overall effectiveness. Reducing exposure to these harmful chemicals and understanding their mechanisms can help mitigate their impact, safeguarding both thyroid and brain health [70].
Prenatal human exposure to EDCs and disrupted TH action has been associated with lower IQ scores, adverse neurodevelopment, and impaired brain function in children and into adulthood [71,72,73]. Animal studies have already demonstrated that chronic low-dose exposure to EDCs can also interfere with brain development [74,75,76].
Specific EDCs and Their Effects
Over 1000 pesticides have been confirmed to be EDCs or classified as potential EDCs. Among these, several non-persistent pesticides, including organophosphates (OPs), dithiocarbamates, and pyrethroids, are suspected thyroid disrupters [77]. Experimental studies suggest they may interfere with the hypothalamic–pituitary–thyroid axis at various levels, leading to hypothyroid and hyperthyroid phenotypes [78].
- CHAMACOS
The CHAMACOS study, initiated in 1999, was a longitudinal investigation examining the health of children born to pregnant women in California’s Salinas Valley farmworker communities. This study focused on the effects of early life, childhood, adolescent, and adult exposure to EDCs. Findings from this study suggested that exposure to organophosphate pesticides (OPs), dichloro-diphenyl-trichloroethane (DDT), bisphenol A (BPA), and polychlorinated biphenyls (PCBs) can interfere with thyroid function and brain development and function [79,80,81,82,83].
- Organophosphate Pesticides (OPs)
The link between childhood exposure to OPs and brain function in adolescence was investigated in a study that measured levels of dialkylphosphate (DAP) metabolites, a biomarker of OP exposure, in pregnant mothers (at 13 and 26 weeks) and their children (at 6 months and 1, 2, 3, and 5 years old). Later, at age 18, the children underwent functional near-infrared spectroscopy (fNIRS) while performing tasks related to executive function and semantic language. Interestingly, a secondary analysis focusing on male adolescents revealed that those with higher childhood OP pesticide exposure, as indicated by DAP levels, exhibited altered brain connectivity patterns. This finding suggests that these altered brain functions in males may partially explain the working memory deficits associated with childhood OP exposure [84,85,86].
- Polychlorinated Biphenyls (PCBs)
Several behavioral tests suggest negative effects on children’s cognitive function from both occupational and environmental PCB exposure. PCB levels in maternal milk were linked to slower mental development in German children at 30 and 42 months [87]. Accordingly, studies showed a link between prenatal PCB exposure and intellectual impairment decline in young children. A study showed that children born to parents exposed to PCB-contaminated cooking rice oil in Taiwan scored lower on developmental assessments compared to controls [88]. Furthermore, prenatal PCB and dioxin exposure in pregnant women was associated with decreased cognitive scores in Dutch children at 42 months [89]. Additionally, Vreugdenhil et al. identified subtle alterations in cognitive function in 6.5-year-old children, suggesting perinatal PCB exposure may have had a lasting impact [90]. Studies of the Great Lakes region linked prenatal PCB exposure in mothers who consumed contaminated fish to lower IQ scores in their children at 4 and 11 years old [91,92]. Studies have also linked perinatal PCB exposure to an increased risk of behaviors resembling attention-deficit hyperactivity disorder (ADHD) in children aged 7 to 11 [93]. In addition, higher PCB concentrations in children’s placentas correlated with a decrease in their IQ at 11 years old and were associated with lower scores on developmental assessments in 4-year-old Spanish children [94,95,96].
Evidence has linked PCB exposure to depression through the disruption of the dopamine and thyroid systems. Studies have identified a negative association between low-level PCB exposure (LPCB) and free thyroxine (fT4) levels, which correlated with increased levels of the main dopamine metabolite (HVA) and depressive symptoms [97].
PCB exposure has also been related to HPT dysfunctions in animal models. Indeed, PCBs lowered T4 in rats and harmed developing brains by mimicking a hypothyroid state [98,99]. These results are coherent with studies that demonstrated reduced hearing and cerebellar damage in animals exposed to PCB mixtures [100]. Additionally, both PCBs and T4 deficiency have been associated with increased levels of glial fibrillary acidic protein (GFAP) and linked to behavioral issues in children, along with a smaller corpus callosum size, which can affect cerebellum development and motor function [101]. However, inconsistencies still exist, as PCB exposure does not always fully mimic hypothyroidism. Some PCBs might even mimic T4 effects, as evidenced by their ability to bind to T4 receptors and promote nerve cell development in experimental models [98,99].
- Polybrominated Diphenyl Ethers (PBDEs)
Polybrominated diphenyl ethers (PBDEs) have also been linked to thyroid disruption and neurobehavioral deficits. Despite the prohibitions on certain PBDE congeners in Europe and some U.S. states, they remain prevalent due to their long half-lives, particularly in areas where regulations require their use as flame retardants [102]. Investigations of the behavioral effects of PBDE exposure in humans are limited but have associated it with cognitive impairment. Similarly, higher BDE47 levels have been associated with increased attention-deficit hyperactivity disorder (ADHD) symptoms and lower TH levels in 4-year-old children [103]. Other studies have explored these associations with TH levels and suggest distinct mechanisms [104,105]. Interestingly, these effects appear to be independent of maternal TH levels, indicating the need for further research into other aspects of TH action.
- Bisphenol A (BPA)
BPA, a common EDC found in plastics, presents challenges in definitively linking prenatal exposure to children’s cognitive function. Establishing a link between human exposure and BPA disruption of TH action poses a challenge due to BPA’s short half-life, which makes it difficult to reliably measure exposure during pregnancy. Additionally, dietary patterns can influence observed results, given that BPA exposure is linked to food consumption [106].
BPA concentrations have been detected directly in brain tissue. For example, a study conducted at the University Hospital of Antwerp in 2002 found BPA levels of 0.91 ng/g in brain samples taken during the autopsy of eleven patients [107]. Similarly, in an experimental study involving nulliparous Fischer female rats, oral administration of 100 mg/kg of BPA resulted in the significant accumulation of the chemical in various brain regions after 48 h. The concentrations were measured at 0.540 μg/g in plasma, 0.745 μg/g in the pituitary gland, 0.180 μg/g in the hypothalamus, 0.103 μg/g in the brain stem, 0.102 μg/g in the cerebellum, 0.097 μg/g in the frontal cortex, 0.181 μg/g in the hippocampus, and 0.220 μg/g in the caudate nucleus [108].
BPA primarily exerts its effects by binding to estrogen receptors (ERs), including ERα and ERβ, as well as the G protein-coupled receptor GPR30 [109]. Due to its chemical structure, BPA can act as both an agonist and antagonist depending on the receptor and context, thereby influencing receptor-dependent signaling pathways. In addition to its interactions with estrogen receptors, BPA is also capable of binding to other receptors, such as the androgen receptor (AR), estrogen-related receptor gamma (ERRγ), thyroid hormone receptor (THR), and glucocorticoid receptor (GR) [110].
These interactions enable BPA to disrupt multiple endocrine pathways, contributing to its broad range of biological effects [111,112]. Epidemiological studies have extensively documented the association between prenatal exposure to BPA and the risk of developing neurodevelopmental disorders in humans [112,113]. For example, longitudinal cohort studies have shown that prenatal exposure to BPA is linked to an increased risk of ADHD, autism spectrum disorder (ASD), and anxiety in children [114].
Human biomonitoring studies have shown that exposure to BPA and its analogs is linked to health issues such as reproductive and neurodevelopmental changes, obesity, and metabolic disorders. These studies have identified and prioritized BPA-related biomarkers, including brain-derived neurotrophic factor (BDNF) and kisspeptin (KiSS), which are associated with neurodevelopmental and reproductive effects [115,116]
Recent studies in pluripotent stem cell-derived organoid systems established a forebrain organoid model that recapitulates early human cortical development. Exposing these organoids to BPA led to pronounced disruptions in cortical development, characterized by reduced progenitor cell proliferation, premature neuronal differentiation, accelerated progenitor depletion, and aberrant cortical layer organization. These effects were attenuated by T3 supplementation, suggesting an antagonistic interaction between BPA and thyroid hormone signaling pathways [117].
Mechanisms of BPA Action
Plastic use is also associated with contamination from the leakage of plastic components into the environment. Regarding externalizing behaviors such as aggression and hyperactivity, findings are mixed, with some studies indicating associations in girls with higher prenatal BPA exposure [106]. Interestingly, the thyroid–brain–BPA mechanisms of disruption are shown in animal models to be acting as an antagonist to the THRs, particularly the beta isoform (THRβ). This antagonism has been observed in both laboratory studies (in vitro) and animal studies (in vivo) [118]. BPA’s main effects appear to include interfering with the negative feedback loop of THs in the pituitary gland and acting as a THR antagonist in vitro. These effects were associated with increased serum thyroxine levels and alterations in RC3/neurogranin expression in the developing rat brain. [119,120].
Although animal studies provide valuable insights, it is essential to consider the limitations and potential differences when applying findings to human populations. Extrapolating results from animal studies to humans requires careful consideration of species-specific differences in physiology, metabolism, and behavior.
1.3. Factors Influencing EDCs’ Effects
While TH disruption has been considered a risk factor for neurodevelopmental impairments, it is likely that other factors are also involved: EDCs can directly affect the brain, interacting with other hormone receptors and signaling pathways [76]. Additionally, the combined exposure to EDCs and other environmental factors, such as air pollution or nutritional deficiencies, can exacerbate their negative impacts on neurodevelopment [121,122,123]. Genetic variations and epigenetic mechanisms can also influence susceptibility to these harmful effects [124]. Therefore, a comprehensive understanding of the epidemiological effects of low IQ requires considering the complex interplay between thyroid hormone disruption, EDCs, environmental factors, genetics, and epigenetics.
1.4. Challenges and Future Directions in Studying EDCs’ Impact on the Brain
Research on EDCs has expanded in recent decades. The impact of EDCs on cognitive function suggests that even a slight decrease in IQ can have a significant ripple effect at the population level. For example, research indicates that a one-point loss in IQ corresponds to a 2% reduction in lifetime economic productivity [125]. To study these mechanisms, various strategies have been employed to understand the endocrine-disrupting potential of chemicals on living organisms.
There is a growing recognition of the need for studies using mixtures of EDCs, which offer a more realistic approach to assessing health risks. In fact, most studies focus on exposure to individual EDCs. This approach neglects the complex reality of pregnant women’s exposure to mixtures of multiple contaminants [126]. This gap in knowledge hinders our understanding of real-world health risks and impedes the development of effective mitigation strategies. Unlike the controlled settings of most EDC research, real life exposes individuals to a constant mix of EDCs from diverse sources like food, water, air, and consumer products. This simultaneous, lifelong exposure to multiple compounds can have additive, amplifying, or even antagonistic effects on the body compared to the effects of individual EDCs [127].
1.4.1. Disruption of TH Signaling by Chemical Mixtures
Two recent studies investigated the potential impact of a mixture of EDCs on early brain development. Researchers exposed tadpoles, a model organism with conserved TH signaling to individuals, to a mixture of 15 common chemicals found in human amniotic fluid (containing phenols, phthalates, pesticides, and heavy metals, as well as perfluorinated, polychlorinated, and polybrominated compounds). The exposure to this chemical mixture altered TH signaling, gene expression, and brain structure in the tadpoles. Interestingly, while some aspects, like behavior alterations, suggested an overall anti-thyroid effect, others appeared to mimic T3 activity. This complexity might be explained by the mixture’s influence on peripheral deiodination mechanisms during a critical developmental stage. Notably, tadpoles were exposed when their thyroid glands were just beginning to synthesize THs. This highlights the potential significance of EDCs on these peripheral pathways in early development, occurring even before the thyroid gland is fully functional. Finally, these findings have raised concerns about the potential adverse effects of everyday exposure to chemical mixtures on fetal brain development in humans [128,129].
1.4.2. Future Directions
Despite the significant amount of data generated in recent years on EDCs, new approaches and methodologies continue to emerge, enhancing our understanding of their harmful effects on human health. However, several key aspects still require further refinement and improvement, such as the investigation of long-term effects of low-dose EDC exposure. In this context, many studies have focused on high-dose exposures, and it is well known that real-world exposure is often related to low concentrations and chronic exposure to these contaminants. Longitudinal studies are also needed to assess the long-term health consequences of low-dose EDC exposure.
Another aspect that needs to be further explored is the role of epigenetics in EDC-induced brain alterations. Indeed, epigenetic modifications, such as DNA methylation and histone acetylation, can alter gene expression without changing the underlying DNA sequence. Therefore, research is needed to investigate how EDCs may influence epigenetic mechanisms and contribute to long-term changes in the brain.
Finally, it is crucial to understand and explore the potential interactions between EDCs and other environmental factors. EDCs may interact with other environmental factors, such as air pollution, heavy metals, or nutritional deficiencies, that may exacerbate their negative effects on brain health. Then, studies should account for the combined effects of multiple exposures to reflect real-life scenarios and improve our understanding of their impacts more accurately.
2. Conclusions
EDCs pose a significant threat to brain development and function by interfering with TH signaling. Exposure to EDCs, particularly during critical developmental periods, can disrupt the production, transport, and action of THs in the CNS. Researchers must select relevant EDC combinations, determine appropriate doses, and develop methods to assess the complex interplay between these compounds, including synergistic and antagonistic effects. Despite these challenges, studies being conducted with mixtures of EDCs have demonstrated their negative effects on brain development and function, along with thyroid function dysregulation. This disruption can lead to a range of adverse outcomes, including lower IQ scores, impaired cognitive function, and behavioral problems.
It is crucial to reduce exposure to EDCs through regulatory measures, public awareness campaigns, and the development of safer alternatives. Further research is needed to better understand the mechanisms by which EDCs interact with the thyroid hormone system and brain development, and to develop effective strategies for mitigating their harmful effects. While past research primarily focused on severe thyroid disorders like cognitive impairment and hypothyroidism, contemporary understanding emphasizes a broader spectrum of cognitive processing impairments and thyroid function levels. Our findings also support the importance of iodine supplementation for expectant mothers and the general population to mitigate the potential negative effects of EDCs on TH production.
Therefore, strategies regulating the production and use of known and potential new EDCs must be swiftly established to protect the populations most susceptible to the detrimental effects of this exposure, such as pregnant women, newborns, and children.
Author Contributions
N.A.D., B.F.P., R.G.d.S., V.G.R., R.d.S.B. and M.M.L.K.; writing—original draft preparation, M.I.C., R.M.M., C.S.-N. and G.G.; rewriting, review, and editing, C.S.-N. and G.G.; supervision, C.S.-N. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the São Paulo Research Foundation (FAPESP)—2022/03094-5 to B.P.F. and G.G.; 2021/02752-6 to G.G., C.S.-N, M.M.L.K and R.M.M.; 2022/10804-9 to M.M.L.K.; and 2016/18517-8 to C.S.-N; Brazilian National Council for Scientific and Technological Development (CNPq)—406643/2022-9 and 405825/2021-8 to G.G., C.S.-N., and M.I.C; 406997/2023-3 to C.S.-N.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jameson, J.L. Endocrinology: Adult & Pediatric, 7th ed.; Elsevier Saunders: Philadelpia, PA, USA, 2016. [Google Scholar]
- Kronenberg, H.M.; Melmed, S.; Polonsky, K.S.; Larsen, P.R. (Eds.) Williams Textbook of Endocrinology, 13th ed.; Elsevier: Phildelphia, PA, USA, 2015. [Google Scholar]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Flamant, F.; Koibuchi, N.; Bernal, J. Thyroid Hormone in Brain and Brain Cells. Front. Endocrinol. 2015, 6, 99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zoeller, T.R.; Dowling, A.L.; Herzig, C.T.; Iannacone, E.A.; Gauger, K.J.; Bansal, R. Thyroid hormone, brain development, and the environment. Environ. Health Perspect. 2002, 110 (Suppl. S3), 355–361. [Google Scholar] [CrossRef] [PubMed]
- Demeneix, B.A. Evidence for Prenatal Exposure to Thyroid Disruptors and Adverse Effects on Brain Development. Eur. Thyroid. J. 2019, 8, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Caporale, N.; Leemans, M.; Birgersson, L.; Germain, P.-L.; Cheroni, C.; Borbély, G.; Engdahl, E.; Lindh, C.; Bressan, R.B.; Cavallo, F.; et al. From cohorts to molecules: Adverse impacts of endocrine disrupting mixtures. Science 2022, 375, abe8244. [Google Scholar] [CrossRef]
- Markey, C.M.; Rubin, B.S.; Soto, A.M.; Sonnenschein, C. Endocrine disruptors: From Wingspread to environmental developmental biology. J. Steroid Biochem. Mol. Biol. 2002, 83, 235–244. [Google Scholar] [CrossRef]
- McLachlan, J.A. Environmental signaling what embryos and evolution teach us about endocrine disrupting chemicals. Endocr. Rev. 2001, 22, 319–341. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Stephens, A.E.; Jepson, P.D.; Jobling, S.; Johnson, A.C.; Matthiessen, P.; Sumpter, J.P.; Tyler, C.R.; McLean, A.R. A restatement of the natural science evidence base on the effects of endocrine disrupting chemicals on wildlife. Proc. Biol. Sci. 2019, 286, 20182416. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Kassotis, C.D.; Vandenberg, L.N.; Demeneix, B.A.; Porta, M.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Economic, regulatory, and policy implications. Lancet Diabetes Endocrinol. 2020, 8, 719–730. [Google Scholar] [CrossRef]
- Opitz, R.; Hartmann, S.; Blank, T.; Braunbeck, T.; Lutz, I.; Kloas, W. Evaluation of Histological and Molecular Endpoints for Enhanced Detection of Thyroid System Disruption in Xenopus laevis Tadpoles. Toxicol. Sci. 2006, 90, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.M.; Maffini, M.V.; Schaeberle, C.M.; Sonnenschein, C. Strengths and weaknesses of in vitro assays for estrogenic and androgenic activity. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 15–33. [Google Scholar] [CrossRef] [PubMed]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2019, 16, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-Disrupting Chemicals and Public Health Protection: A Statement of Principles from The Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef] [PubMed]
- Modica, R.; Benevento, E.; Colao, A. Endocrine-disrupting chemicals (EDCs) and cancer: New perspectives on an old relationship. J. Endocrinol. Investig. 2022, 46, 667–677. [Google Scholar] [CrossRef]
- Chen, L.; Shi, T.; Wang, Y.-T.; He, J.; Zhao, X.; Wang, Y.-K.; Giesy, J.P.; Chen, F.; Chen, Y.; Tuo, X.; et al. Effects of acute exposure to microcystins on hypothalamic-pituitary-adrenal (HPA), -gonad (HPG) and -thyroid (HPT) axes of female rats. Sci. Total Environ. 2021, 778, 145196. [Google Scholar] [CrossRef]
- Shi, T.; Xu, L.-L.; Chen, L.; He, J.; Wang, Y.-K.; Chen, F.; Chen, Y.; Giesy, J.P.; Wang, Y.-T.; Wu, Q.-H.; et al. Acute exposure to microcystins affects hypothalamic-pituitary axes of male rats. Environ. Pollut. 2022, 318, 120843. [Google Scholar] [CrossRef]
- Mughal, B.B.; Fini, J.-B.; Demeneix, B.A. Thyroid-disrupting chemicals and brain development: An update. Endocr. Connect. 2018, 7, R160–R186. [Google Scholar] [CrossRef]
- Oliveira, K.J.; Chiamolera, M.I.; Giannocco, G.; Pazos-Moura, C.C.; Ortiga-Carvalho, T.M. Thyroid function disruptors: From nature to chemicals. J. Mol. Endocrinol. 2019, 62, R1–R19. [Google Scholar] [CrossRef]
- Gilbert, M.E.; O’Shaughnessy, K.L.; Axelstad, M. Regulation of Thyroid-disrupting Chemicals to Protect the Developing Brain. Endocrinology 2020, 161, bqaa106. [Google Scholar] [CrossRef]
- Seralini, G.-E.; Jungers, G. Endocrine disruptors also function as nervous disruptors and can be renamed endocrine and nervous disruptors (ENDs). Toxicol. Rep. 2021, 8, 1538–1557. [Google Scholar] [CrossRef] [PubMed]
- Ortiga-Carvalho, T.M.; Chiamolera, M.I.; Pazos-Moura, C.C.; Wondisford, F.E. Hypothalamus-Pituitary-Thyroid Axis. Compr. Physiol. 2016, 6, 1387–1428. [Google Scholar] [PubMed]
- Batistuzzo, A.; Salas-Lucia, F.; Gereben, B.; Ribeiro, M.O.; Bianco, A.C. Sustained Pituitary T3 Production Explains the T4-mediated TSH Feedback Mechanism. Endocrinology 2023, 164, bqad155. [Google Scholar] [CrossRef]
- Manzano, J.; Bernal, J.; Morte, B. Influence of thyroid hormones on maturation of rat cerebellar astrocytes. Int. J. Dev. Neurosci. 2007, 25, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.M.; El-Gareib, A.; El-Bakry, A.; El-Tawab, S.A.; Ahmed, R. Thyroid hormones states and brain development interactions. Int. J. Dev. Neurosci. 2007, 26, 147–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Teng, W.; Shan, Z.; Yu, X.; Gao, Y.; Wang, S.; Fan, C.; Wang, H.; Zhang, H. The Effect of Maternal Subclinical Hypothyroidism During Pregnancy on Brain Development in Rat Offspring. Thyroid 2010, 20, 909–915. [Google Scholar] [CrossRef]
- Bernal, J.; Morte, B.; Diez, D. Thyroid hormone regulators in human cerebral cortex development. J. Endocrinol. 2022, 255, R27–R36. [Google Scholar] [CrossRef]
- Vancamp, P.; Darras, V.M. From zebrafish to human: A comparative approach to elucidate the role of the thyroid hormone transporter MCT8 during brain development. Gen. Comp. Endocrinol. 2018, 265, 219–229. [Google Scholar] [CrossRef]
- Bernal, J.; Guadaño-Ferraz, A.; Morte, B. Thyroid hormone transporters—Functions and clinical implications. Nat. Rev. Endocrinol. 2015, 11, 406–417. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Montero-Pedrazuela, A.; Guadaño-Ferraz, A.; Rausell, E. Thyroid Hormone Transporters MCT8 and OATP1C1 Are Expressed in Pyramidal Neurons and Interneurons in the Adult Motor Cortex of Human and Macaque Brain. Int. J. Mol. Sci. 2023, 24, 3207. [Google Scholar] [CrossRef]
- Morte, B.; Bernal, J. Thyroid hormone action: Astrocyte-neuron communication. Front. Endocrinol. 2014, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Bocco, B.M.L.C.; Werneck-De-Castro, J.P.; Oliveira, K.C.; Fernandes, G.W.; Fonseca, T.L.; Nascimento, B.P.P.; McAninch, E.A.; Ricci, E.; Kvárta-Papp, Z.; Fekete, C.; et al. Type 2 Deiodinase Disruption in Astrocytes Results in Anxiety-Depressive-Like Behavior in Male Mice. Endocrinology 2016, 157, 3682–3695. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; da Conceição, R.R. The Deiodinase Trio and Thyroid Hormone Signaling. Methods Mol. Biol. 2018, 1801, 67–83. [Google Scholar] [PubMed]
- Salas-Lucia, F.; Fekete, C.; Sinkó, R.; Egri, P.; Rada, K.; Ruska, Y.; Gereben, B.; Bianco, A.C. Axonal T3 uptake and transport can trigger thyroid hormone signaling in the brain. eLife 2023, 12, e82683. [Google Scholar] [CrossRef]
- Bernal, J. Thyroid hormone receptors in brain development and function. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 249–259. [Google Scholar] [CrossRef]
- Flamant, F.; Gauthier, K.; Richard, S. Genetic Investigation of Thyroid Hormone Receptor Function in the Developing and Adult Brain. Curr. Top. Dev. Biol. 2017, 125, 303–335. [Google Scholar]
- Vella, K.R.; Hollenberg, A.N. The actions of thyroid hormone signaling in the nucleus. Mol. Cell. Endocrinol. 2017, 458, 127–135. [Google Scholar] [CrossRef]
- Gil-Ibañez, P.; Morte, B.; Bernal, J. Role of Thyroid Hormone Receptor Subtypes α and β on Gene Expression in the Cerebral Cortex and Striatum of Postnatal Mice. Endocrinology 2013, 154, 1940–1947. [Google Scholar] [CrossRef]
- Sreenivasan, V.K.A.; Dore, R.; Resch, J.; Maier, J.; Dietrich, C.; Henck, J.; Balachandran, S.; Mittag, J.; Spielmann, M. Single-cell RNA-based phenotyping reveals a pivotal role of thyroid hormone receptor alpha for hypothalamic development. Development 2023, 150, dev201228. [Google Scholar] [CrossRef]
- Flamant, F.; Richard, S. Thyroid Hormone Receptors Function in GABAergic Neurons During Development and in Adults. Endocrinology 2024, 165, bqae101. [Google Scholar] [CrossRef]
- Ercan-Fang, S.; Schwartz, H.L.; Oppenheimer, J.H. Isoform-specific 3,5,3′-triiodothyronine receptor binding capacity and mes-senger ribonucleic acid content in rat adenohypophysis: Effect of thyroidal state and comparison with extrapituitary tissues. Endocrinology 1996, 137, 3228–3233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Escobar, G.M.; Obregón, M.J.; del Rey, F.E. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract. Res. Clin. Endocrinol. Metab. 2004, 18, 225–248. [Google Scholar] [CrossRef] [PubMed]
- Gagne, R.; Green, J.R.; Dong, H.; Wade, M.G.; Yauk, C.L. Identification of thyroid hormone receptor binding sites in developing mouse cerebellum. BMC Genom. 2013, 14, 341. [Google Scholar] [CrossRef] [PubMed]
- Suen, C.; Yen, P.; Chin, W. In vitro transcriptional studies of the roles of the thyroid hormone (T3) response elements and minimal promoters in T3-stimulated gene transcription. J. Biol. Chem. 1994, 269, 1314–1322. [Google Scholar] [CrossRef]
- Brent, G.A.; Moore, D.D.; Larsen, R.P. Thyroid Hormone Regulation of Gene Expression. Annu. Rev. Physiol. 1991, 53, 17–35. [Google Scholar] [CrossRef]
- Epstein, F.H.; Brent, G.A. The Molecular Basis of Thyroid Hormone Action. N. Engl. J. Med. 1994, 331, 847–853. [Google Scholar] [CrossRef]
- Brent, G.A. A Historical Reflection on Scientific Advances in Understanding Thyroid Hormone Action. Thyroid 2023, 33, 1140–1149. [Google Scholar] [CrossRef]
- Sinha, R.A.; Yen, P.M. Metabolic Messengers: Thyroid Hormones. Nat. Metab. 2024, 6, 639–650. [Google Scholar] [CrossRef]
- Davis, P.J.; Leonard, J.L.; Lin, H.Y.; Leinung, M.; Mousa, S.A. Molecular Basis of Nongenomic Actions of Thyroid Hormone. Vitam. Horm. 2018, 106, 67–96. [Google Scholar]
- Luidens, M.K.; Mousa, S.A.; Davis, F.B.; Lin, H.-Y.; Davis, P.J. Thyroid hormone and angiogenesis. Vasc. Pharmacol. 2009, 52, 142–145. [Google Scholar] [CrossRef]
- Taylor, E.; Heyland, A. Evolution of thyroid hormone signaling in animals: Non-genomic and genomic modes of action. Mol. Cell. Endocrinol. 2017, 459, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-C.S.H.; Ko, P.-J.; Pan, Y.-S.; Lin, H.-Y.; Whang-Peng, J.; Davis, P.J.; Wang, K. Role of thyroid hormone-integrin αvβ3-signal and therapeutic strategies in colorectal cancers. J. Biomed. Sci. 2021, 28, 24. [Google Scholar] [CrossRef] [PubMed]
- O’shaughnessy, K.L.; McMichael, B.D.; Sasser, A.L.; Bell, K.S.; Riutta, C.; Ford, J.L.; Stoker, T.E.; Grindstaff, R.D.; Pandiri, A.R.; Gilbert, M.E. Thyroid hormone action controls multiple components of cell junctions at the ventricular zone in the newborn rat brain. Front. Endocrinol. 2023, 14, 1090081. [Google Scholar] [CrossRef] [PubMed]
- Desouza, L.A.; Ladiwala, U.; Daniel, S.M.; Agashe, S.; Vaidya, R.A.; Vaidya, V.A. Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol. Cell. Neurosci. 2005, 29, 414–426. [Google Scholar] [CrossRef]
- Tan, Z.S.; Vasan, R.S. Thyroid function and Alzheimer’s disease. J. Alzheimers Dis. 2009, 16, 503–507. [Google Scholar] [CrossRef]
- Jo, S.; Fonseca, T.L.; Bocco, B.M.L.C.; Fernandes, G.W.; McAninch, E.A.; Bolin, A.P.; Da Conceição, R.R.; Werneck-De-Castro, J.P.; Ignacio, D.L.; Egri, P.; et al. Type 2 deiodinase polymorphism causes ER stress and hypothyroidism in the brain. J. Clin. Investig. 2018, 129, 230–245. [Google Scholar] [CrossRef]
- de Souza, J.S.; Carromeu, C.; Torres, L.B.; Araujo, B.H.; Cugola, F.R.; Maciel, R.M.; Muotri, A.R.; Giannocco, G. IGF1 neuronal response in the absence of MECP2 is dependent on TRalpha 3. Hum. Mol. Genet. 2017, 26, 270–281. [Google Scholar] [CrossRef]
- Hegedüs, L.; Bianco, A.C.; Jonklaas, J.; Pearce, S.H.; Weetman, A.P.; Perros, P. Primary hypothyroidism and quality of life. Nat. Rev. Endocrinol. 2022, 18, 230–242. [Google Scholar] [CrossRef]
- Hay, I.; Hynes, K.L.; Burgess, J.R. Mild-to-Moderate Gestational Iodine Deficiency Processing Disorder. Nutrients 2019, 11, 1974. [Google Scholar] [CrossRef]
- Hynes, K.L.; Otahal, P.; Hay, I.; Burgess, J.R. Mild Iodine Deficiency During Pregnancy Is Associated with Reduced Educational Outcomes in the Offspring: 9-Year Follow-up of the Gestational Iodine Cohort. J. Clin. Endocrinol. Metab. 2013, 98, 1954–1962. [Google Scholar] [CrossRef]
- Salas-Lucia, F. Mapping Thyroid Hormone Action in the Human Brain. Thyroid 2024, 34, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Donangelo, I.; Abe, K.; Scremin, O.; Ke, S.; Li, F.; Milanesi, A.; Liu, Y.-Y.; Brent, G.A. Thyroid hormone treatment activates protective pathways in both in vivo and in vitro models of neuronal injury. Mol. Cell. Endocrinol. 2017, 452, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cooper-Kuhn, C.M.; Nannmark, U.; Blomgren, K.; Kuhn, H.G. Stimulatory effects of thyroid hormone on brain an-giogenesis in vivo and in vitro. J. Cereb. Blood Flow Metab. 2010, 30, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, L.; Lapi, D.; Del Seppia, C. Factors and Mechanisms of Thyroid Hormone Activity in the Brain: Possible Role in Recovery and Protection. Biomolecules 2024, 14, 198. [Google Scholar] [CrossRef]
- Schantz, S.L.; Widholm, J.J. Cognitive effects of endocrine-disrupting chemicals in animals. Environ. Health Perspect. 2001, 109, 1197–1206. [Google Scholar] [CrossRef]
- Venero, C.; Guadaño-Ferraz, A.; Herrero, A.I.; Nordström, K.; Manzano, J.; de Escobar, G.M.; Bernal, J.; Vennström, B. Anxiety, memory impairment, and locomotor dysfunction caused by a mutant thyroid hormone receptor alpha1 can be ameliorated by T3 treatment. Genes Dev. 2005, 19, 2152–2163. [Google Scholar] [CrossRef]
- Bathla, M.; Singh, M.; Relan, P. Prevalence of anxiety and depressive symptoms among patients with hypothyroidism. Indian J. Endocrinol. Metab. 2016, 20, 468–474. [Google Scholar] [CrossRef]
- Pearce, E.N. Endocrine Disruptors and Thyroid Health. Endocr. Pract. 2023, 30, 172–176. [Google Scholar] [CrossRef]
- Bouchard, M.F.; Chevrier, J.; Harley, K.G.; Kogut, K.; Vedar, M.; Calderon, N.; Trujillo, C.; Johnson, C.; Bradman, A.; Barr, D.B.; et al. Prenatal Exposure to Organophosphate Pesticides and IQ in 7-Year-Old Children. Environ. Health Perspect. 2011, 119, 1189–1195. [Google Scholar] [CrossRef]
- Bloom, M.S.; Jansing, R.L.; Kannan, K.; Rej, R.; Fitzgerald, E.F. Thyroid hormones are associated with exposure to persistent organic pollutants in aging residents of upper Hudson River communities. Int. J. Hyg. Environ. Health 2013, 217, 473–482. [Google Scholar] [CrossRef]
- Sagiv, S.K.; Harris, M.H.; Gunier, R.B.; Kogut, K.R.; Harley, K.G.; Deardorff, J.; Bradman, A.; Holland, N.; Eskenazi, B. Prenatal Organo-phosphate Pesticide Exposure and Traits Related to Autism Spectrum Disorders in a Population Living in Proximity to Ag-riculture. Environ. Health Perspect. 2018, 126, 047012. [Google Scholar]
- Mastorakos, G.; Karoutsou, E.I.; Mizamtsidi, M.; Creatsas, G. The menace of endocrine disruptors on thyroid hormone physiology and their impact on intrauterine development. Endocrine 2007, 31, 219–237. [Google Scholar] [CrossRef]
- Kortenkamp, A. Low dose mixture effects of endocrine disrupters: Implications for risk assessment and epidemiology. Int. J. Androl. 2008, 31, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Salazar, P.; Villaseca, P.; Cisternas, P.; Inestrosa, N.C. Neurodevelopmental impact of the offspring by thyroid hormone sys-tem-disrupting environmental chemicals during pregnancy. Environ. Res. 2021, 200, 111345. [Google Scholar] [CrossRef] [PubMed]
- Schug, T.T.; Johnson, A.F.; Birnbaum, L.S.; Colborn, T.; Guillette, L.J., Jr.; Crews, D.P.; Collins, T.; Soto, A.M.; Vom Saal, F.S.; McLachlan, J.A.; et al. Minireview: Endocrine Disruptors: Past Lessons and Future Directions. Mol. Endocrinol. 2016, 30, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Campos, É.; Freire, C. Exposure to non-persistent pesticides and thyroid function: A systematic review of epidemiological evidence. Int. J. Hyg. Environ. Health 2016, 219, 481–497. [Google Scholar] [CrossRef]
- Eskenazi, B.; Bradman, A.; Castorina, R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ. Health Perspect. 1999, 107, 409–419. [Google Scholar] [CrossRef]
- Chevrier, J.; Eskenazi, B.; Bradman, A.; Fenster, L.; Barr, D.B. Associations between Prenatal Exposure to Polychlorinated Biphenyls and Neonatal Thyroid-Stimulating Hormone Levels in a Mexican-American Population, Salinas Valley, California. Environ. Health Perspect. 2007, 115, 1490–1496. [Google Scholar] [CrossRef]
- Eskenazi, B.; Rosas, L.G.; Marks, A.R.; Bradman, A.; Harley, K.; Holland, N.; Johnson, C.; Fenster, L.; Barr, D.B. Pesticide Toxicity and the Developing Brain. Basic Clin. Pharmacol. Toxicol. 2008, 102, 228–236. [Google Scholar] [CrossRef]
- Chevrier, J.; Gunier, R.B.; Bradman, A.; Holland, N.T.; Calafat, A.M.; Eskenazi, B.; Harley, K.G. Maternal Urinary Bisphenol A during Pregnancy and Maternal and Neonatal Thyroid Function in the CHAMACOS Study. Environ. Health Perspect. 2013, 121, 138–144. [Google Scholar] [CrossRef]
- Sagiv, S.K.; Mora, A.M.; Rauch, S.; Kogut, K.R.; Hyland, C.; Gunier, R.B.; Bradman, A.; Deardorff, J.; Eskenazi, B. Prenatal and Childhood Exposure to Organophosphate Pesticides and Behavior Problems in Adolescents and Young Adults in the CHAMACOS Study. Environ. Health Perspect. 2023, 131, 67008. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, S.K.; Rauch, S.; Kogut, K.R.; Hyland, C.; Gunier, R.B.; Mora, A.M.; Bradman, A.; Deardorff, J.; Eskenazi, B. Prenatal exposure to organophosphate pesticides and risk-taking behaviors in early adulthood. Environ. Health 2022, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, R.; Ma, Q.; Baker, J.M.; Rauch, S.; Gunier, R.B.; Mora, A.M.; Kogut, K.; Bradman, A.; Eskenazi, B.; et al. Childhood exposure to organophosphate pesticides: Functional connectivity and working memory in adolescents. NeuroToxicology 2024, 103, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Binter, A.-C.; Mora, A.M.; Baker, J.M.; Bruno, J.L.; Kogut, K.; Rauch, S.; Reiss, A.L.; Eskenazi, B.; Sagiv, S.K. Exposure to DDT and DDE and functional neuroimaging in adolescents from the CHAMACOS cohort. Environ. Res. 2022, 212, 113461. [Google Scholar] [CrossRef]
- Walkowiak, J.; Wiener, J.A.; Fastabend, A.; Heinzow, B.; Krämer, U.; Schmidt, E.; Steingrüber, H.J.; Wundram, S.; Winneke, G. Envi-ronmental exposure to polychlorinated biphenyls and quality of the home environment: Effects on psychodevelopment in early childhood. Lancet 2001, 358, 1602–1607. [Google Scholar] [CrossRef]
- Guo, Y.L.; Lambert, G.H.; Hsu, C.-C.; Hsu, M.M.L. Yucheng: Health effects of prenatal exposure to polychlorinated biphenyls and dibenzofurans. Int. Arch. Occup. Environ. Health 2004, 77, 153–158. [Google Scholar] [CrossRef]
- Patandin, S.; Lanting, C.I.; Mulder, P.G.; Boersma, E.R.; Sauer, P.J.; Weisglas-Kuperus, N. Effects of environmental exposure to poly-chlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. J. Pediatr. 1999, 134, 33–41. [Google Scholar] [CrossRef]
- Vreugdenhil, H.J.; Lanting, C.I.; Mulder, P.G.; Boersma, E.; Weisglas-Kuperus, N. Effects of prenatal PCB and dioxin background exposure on cognitive and motor abilities in Dutch children at school age. J. Pediatr. 2002, 140, 48–56. [Google Scholar] [CrossRef]
- Jacobson, J.L.; Jacobson, S.W.; Humphrey, H.E. Effects of in utero exposure to polychlorinated biphenyls and related contaminants on cognitive functioning in young children. J. Pediatr. 1990, 116, 38–45. [Google Scholar] [CrossRef]
- Jacobson, J.L.; Jacobson, S.W. Evidence for PCBs as neurodevelopmental toxicants in humans. Neurotoxicology 1997, 18, 415–424. [Google Scholar]
- Sagiv, S.K.; Thurston, S.W.; Bellinger, D.C.; Tolbert, P.E.; Altshul, L.M.; Korrick, S.A. Prenatal Organochlorine Exposure and Behaviors Associated with Attention Deficit Hyperactivity Disorder in School-Aged Children. Am. J. Epidemiol. 2010, 171, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.W.; Lonky, E.; Reihman, J.; Pagano, J.; Gump, B.B.; Darvill, T. The relationship between prenatal PCB exposure and intel-ligence (IQ) in 9-year-old children. Environ. Health Perspect. 2008, 116, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Forns, J.; Torrent, M.; Garcia-Esteban, R.; Grellier, J.; Gascon, M.; Julvez, J.; Guxens, M.; Grimalt, J.O.; Sunyer, J. Prenatal exposure to polychlorinated biphenyls and child neuropsychological development in 4-year-olds: An analysis per congener and specific cognitive domain. Sci. Total Environ. 2012, 432, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Pinson, A.; Bourguignon, J.P.; Parent, A.S. Exposure to endocrine disrupting chemicals and neurodevelopmental alterations. Andrology 2016, 4, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Gaum, P.M.; Gube, M.; Esser, A.; Schettgen, T.; Quinete, N.; Bertram, J.; Putschögl, F.M.; Kraus, T.; Lang, J. Depressive Symptoms After PCB Exposure: Hypotheses for Underlying Pathomechanisms via the Thyroid and Dopamine System. Int. J. Environ. Res. Public Health 2019, 16, 950. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Dowling, A.L.; Vas, A.A. Developmental exposure to polychlorinated biphenyls exerts thyroid hormone-like effects on the expression of RC3/neurogranin and myelin basic protein messenger ribonucleic acids in the developing rat brain. Endocrinology 2000, 141, 181–189. [Google Scholar] [CrossRef]
- Bansal, R.; You, S.-H.; Herzig, C.T.; Zoeller, R.T. Maternal thyroid hormone increases HES expression in the fetal rat brain: An effect mimicked by exposure to a mixture of polychlorinated biphenyls (PCBs). Dev. Brain Res. 2005, 156, 13–22. [Google Scholar] [CrossRef]
- Goldey, E.S.; Crofton, K.M. Thyroxine Replacement Attenuates Hypothyroxinemia, Hearing Loss, and Motor Deficits Following Developmental Exposure to Aroclor 1254 in Rats. Toxicol. Sci. 1998, 45, 94–105. [Google Scholar] [CrossRef]
- Nguon, K.; Baxter, M.G.; Sajdel-Sulkowska, E.M. Perinatal exposure to polychlorinated biphenyls differentially affects cerebellar development and motor functions in male and female rat neonates. Cerebellum 2005, 4, 112–122. [Google Scholar] [CrossRef]
- Costa, L.G.; de Laat, R.; Tagliaferri, S.; Pellacani, C. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol. Lett. 2014, 230, 282–294. [Google Scholar] [CrossRef]
- Gascon, M.; Vrijheid, M.; Martínez, D.; Forns, J.; Grimalt, J.O.; Torrent, M.; Sunyer, J. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age. Environ. Int. 2011, 37, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Chevrier, J.; Rauch, S.A.; Kogut, K.; Harley, K.G.; Johnson, C.; Trujillo, C.; Sjödin, A.; Bradman, A. In Utero and Childhood Polybrominated Diphenyl Ether (PBDE) Exposures and Neurodevelopment in the CHAMACOS Study. Environ. Health Perspect. 2013, 121, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, S.K.; Kogut, K.; Gaspar, F.W.; Gunier, R.B.; Harley, K.G.; Parra, K.; Villaseñor, D.; Bradman, A.; Holland, N.; Eskenazi, B. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9–12 years of age. Neurotoxicology Teratol. 2015, 52, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Roen, E.L.; Wang, Y.; Calafat, A.M.; Wang, S.; Margolis, A.; Herbstman, J.; Hoepner, L.A.; Rauh, V.; Perera, F.P. Bisphenol A exposure and behavioral problems among inner city children at 7–9 years of age. Environ. Res. 2015, 142, 739–745. [Google Scholar] [CrossRef]
- Geens, T.; Neels, H.; Covaci, A. Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere 2012, 87, 796–802. [Google Scholar] [CrossRef]
- Kim, C.; Sapienza, P.; Ross, I.; Johnson, W.; Luu, H.; Hutter, J. Distribution of bisphenol A in the neuroendocrine organs of female rats. Toxicol. Ind. Health 2004, 20, 41–50. [Google Scholar] [CrossRef]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef]
- Prasanth, G.K.; Divya, L.M.; Sadasivan, C. Bisphenol—A can bind to human glucocorticoid receptor as an agonist: An in silico study. J. Appl. Toxicol. 2010, 30, 769–774. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Bansal, R.; Parris, C. Bisphenol-A, an Environmental Contaminant that Acts as a Thyroid Hormone Receptor Antagonist in Vitro, Increases Serum Thyroxine, and Alters RC3/Neurogranin Expression in the Developing Rat Brain. Endocrinology 2005, 146, 607–612. [Google Scholar] [CrossRef]
- Costa, H.E.; Cairrao, E. Effect of bisphenol A on the neurological system: A review update. Arch. Toxicol. 2023, 98, 1–73. [Google Scholar] [CrossRef]
- Nesan, D.; Sewell, L.C.; Kurrasch, D.M. Opening the black box of endocrine disruption of brain development: Lessons from the characterization of Bisphenol, A. Horm. Behav. 2018, 101, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Myridakis, A.; Chalkiadaki, G.; Fotou, M.; Kogevinas, M.; Chatzi, L.; Stephanou, E.G. Exposure of Preschool-Age Greek Children (RHEA Cohort) to Bisphenol A, Parabens, Phthalates, and Organophosphates. Environ. Sci. Technol. 2015, 50, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Mustieles, V.; D’Cruz, S.C.; Couderq, S.; Rodríguez-Carrillo, A.; Fini, J.-B.; Hofer, T.; Steffensen, I.-L.; Dirven, H.; Barouki, R.; Olea, N.; et al. Bisphenol A and its analogues: A comprehensive review to identify and prioritize effect biomarkers for human biomonitoring. Environ. Int. 2020, 144, 105811. [Google Scholar] [CrossRef] [PubMed]
- Howdeshell, K.L.; Beverly, B.E.J.; Blain, R.B.; Goldstone, A.E.; Hartman, P.A.; Lemeris, C.R.; Newbold, R.R.; Rooney, A.A.; Bucher, J.R. Evaluating endocrine disrupting chemicals: A perspective on the novel assessments in CLARITY-BPA. Birth Defects Res. 2023, 115, 1345–1397. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, D.; Cai, C.; Zhou, M.; Dai, P.; Lai, Q.; Zhang, L.; Fan, Y.; Gao, Z. Modeling early human cortical development and evaluating neurotoxicity with a forebrain organoid system. Environ. Pollut. 2023, 337, 122624. [Google Scholar] [CrossRef]
- Moriyama, K.; Tagami, T.; Akamizu, T.; Usui, T.; Saijo, M.; Kanamoto, N.; Hataya, Y.; Shimatsu, A.; Kuzuya, H.; Nakao, K. Thyroid Hormone Action Is Disrupted by Bisphenol A as an Antagonist. J. Clin. Endocrinol. Metab. 2002, 87, 5185–5190. [Google Scholar] [CrossRef]
- Zoeller, R.T. Environmental chemicals as thyroid hormone analogues: New studies indicate that thyroid hormone receptors are targets of industrial chemicals? Mol. Cell. Endocrinol. 2005, 242, 10–15. [Google Scholar] [CrossRef]
- Gentilcore, D.; Porreca, I.; Rizzo, F.; Ganbaatar, E.; Carchia, E.; Mallardo, M.; De Felice, M.; Ambrosino, C. Bisphenol A interferes with thyroid specific gene expression. Toxicology 2013, 304, 21–31. [Google Scholar] [CrossRef]
- Kusters, M.S.W.; Essers, E.; Muetzel, R.; Ambrós, A.; Tiemeier, H.; Guxens, M. Air pollution exposure during pregnancy and childhood, cognitive function, and emotional and behavioral problems in adolescents. Environ Res. 2022, 214, 113891. [Google Scholar] [CrossRef]
- Ambròs, A.; Fernández-Barrés, S.; Pérez-Crespo, L.; Guxens, M.; Arija, V. Maternal exposure to air pollution during pregnancy and child’s cognitive, language, and motor function: ECLIPSES study. Environ Res. 2022, 212, 113501. [Google Scholar]
- Simeone, G.; Bergamini, M.; Verga, M.C.; Cuomo, B.; D’antonio, G.; Iacono, I.D.; Di Mauro, D.; Di Mauro, F.; Di Mauro, G.; Leonardi, L.; et al. Do Vegetarian Diets Provide Adequate Nutrient Intake during Complementary Feeding? A Systematic Review. Nutrients 2022, 14, 3591. [Google Scholar] [CrossRef] [PubMed]
- Lindner, S.; Lucchini, R.; Broberg, K. Genetics and Epigenetics of Manganese Toxicity. Curr. Environ. Health Rep. 2022, 9, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Liu, Y. Reducing the Staggering Costs of Environmental Disease in Children, Estimated at $76.6 Billion in 2008. Health Aff. 2011, 30, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.J.; Zota, A.R.; Schwartz, J.M. Environmental Chemicals in Pregnant Women in the United States: NHANES 2003–2004. Environ. Health Perspect. 2011, 119, 878–885. [Google Scholar] [CrossRef]
- Duh-Leong, C.; Maffini, M.V.; Kassotis, C.D.; Vandenberg, L.N.; Trasande, L. The regulation of endocrine-disrupting chemicals to minimize their impact on health. Nat. Rev. Endocrinol. 2023, 19, 600–614. [Google Scholar] [CrossRef]
- Fini, J.-B.; Mughal, B.B.; Le Mével, S.; Leemans, M.; Lettmann, M.; Spirhanzlova, P.; Affaticati, P.; Jenett, A.; Demeneix, B.A. Human amniotic fluid contaminants alter thyroid hormone signalling and early brain development in Xenopus embryos. Sci. Rep. 2017, 7, srep43786. [Google Scholar] [CrossRef]
- Leemans, M.; Spirhanzlova, P.; Couderq, S.; Le Mével, S.; Grimaldi, A.; Duvernois-Berthet, E.; Demeneix, B.; Fini, J.-B. A Mixture of Chemicals Found in Human Amniotic Fluid Disrupts Brain Gene Expression and Behavior in Xenopus laevis. Int. J. Mol. Sci. 2023, 24, 2588. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).