Abstract
Background: Serum anti-Müllerian hormone (AMH) levels and antral follicle count are key in evaluating ovarian reserve (OR) for fertility. The performance of the Siemens Healthineers AMH assay was assessed on the ADVIA Centaur® System. Methods: Analytical characteristics, clinical performance, and method comparison studies were performed in a prospective cohort of 532 women at fertility clinics. Serum AMH levels were determined using ADVIA Centaur, Beckman Access®, and Roche Elecsys® assays. Results: The limit of quantitation for the ADVIA Centaur AMH assay was 0.030 ng/mL. Repeatability was ≤2.9% CV, within-lab repeatability was ≤3.2% CV, and reproducibility was ≤4.4% CV. Results using serum or lithium heparin sample types were equivalent. Diagnostic sensitivity across assays ranged from 77.3% to 90.2% and specificity ranged from 51.0 to 71.0%; corresponding positive and negative predictive values ranged from 66.6% to 74.3% and 74.2% to 83.0%, respectively. Receiver operating characteristic analyses demonstrated that the assays have a high probability for discriminating between diminished–normal and high OR. ADVIA and Beckman assays agreed according to ADVIA = 1.00 × Beckman + 0.014 ng/mL, τ = 0.909, while a more modest correlation of ADVIA = 1.41 × Roche − 0.024 ng/mL, τ = 0.777 was observed with Roche assay. Conclusions: The ADVIA Centaur assay demonstrates acceptable analytical characteristics and clinical performance comparable to the Roche AMH assay and is essentially interchangeable with the Beckman AMH assay for reliable OR assessment.
1. Introduction
Ovarian reserve (OR) is a term that represents the combined potential both in the quantity and quality of a woman’s remaining oocytes relative to age. The concept is crucial for predicting a woman’s reproductive potential as well as guiding appropriate fertility treatment and management in reproductive medicine [1]. Several methods exist to evaluate OR, including antral follicle count (AFC) and blood-based biomarkers such as anti-Müllerian hormone (AMH) levels [2].
AMH is a glycoprotein hormone secreted by granulosa cells that surround the oocytes of growing follicles. Although the primary function of AMH is to inhibit the development of the Müllerian ducts in the male fetus during development by preventing the formation of female reproductive organs, in adult females, AMH plays a key role in regulating follicular maturation and ovarian function.
The use of AMH for assessing OR has gained significant attention due to its potential to be a reliable and non-invasive marker. Unlike other traditional methods such as the measurement of follicle-stimulating hormone (FSH) and AFC, which are more prone to either cycle or inter-operator variability, AMH is relatively stable and an easily measurable biomarker [3].
The rationale behind using AMH for OR assessment lies in its close correlation with the number of antral follicles, the small fluid-filled structures that contain immature oocytes within the ovaries [4,5]. Since AMH is produced by the granulosa cells of these developing follicles, its blood levels serve as a surrogate for quantitatively assessing the overall ovarian follicle pool and provide valuable insight into a woman’s remaining egg supply. In addition, AMH levels demonstrate limited variability during the menstrual cycle, making it an attractive tool for assessing OR at any point in the menstrual cycle [6]. Finally, AMH levels are consistent over time, potentially providing an indication of the long-term decline in OR associated with aging. By providing valuable quantitative insights into a woman’s remaining egg supply, AMH measurement, along with other clinical findings, plays a crucial role in personalized fertility treatment strategies, enabling better patient counseling for family planning.
Although AMH assays are commercially available by other manufacturers, Siemens developed the ADVIA Centaur® AMH assay to complement the reproductive testing menu at many institutions globally. It is a sandwich immunoassay using direct acridinium ester-based chemiluminometric technology. Two mouse monoclonal anti-AMH antibodies are used in the assay, which is expected to detect total AMH. One antibody in the Lite Reagent is labeled with acridinium ester. The other antibody is a biotinylated antibody coupled to streptavidin-coated magnetic particles in the solid phase. A direct relationship exists between the amount of AMH present in the patient sample and the amount of relative light units detected by the system. Dose concentration results (ng/mL) are calculated based on a two-point calibration from a pre-defined master curve.
The objective of this study was to characterize the analytical and clinical performance of the ADVIA Centaur AMH assay in the assessment of OR and compare it to other commercially available AMH assays.
2. Materials and Methods
2.1. Clinical Study Design
This was a prospective multicenter clinical trial conducted at the following 11 fertility clinics in the United States: Bloom Reproductive Institute, Arizona; Utah Fertility Clinic, Utah; Fertility Treatment Center, Arizona; Center for Assisted Reproduction, Texas; Reproductive Associates of Delaware, Delaware; Houston Fertility Clinic, Texas; Center for Reproductive Medicine, Florida; Reproductive Endocrinology Associates of Charlotte, North Carolina; Fertility and IVF Center of Miami, Florida; Shady Grove Fertility Center, Maryland; and Women’s Medical Research Group, Florida. Testing of clinical samples was performed at the University of Maryland School of Medicine, Baltimore, Maryland.
The study enrolled female subjects 22 to 45 years of age who presented for evaluation of OR and who met the inclusion/exclusion criteria listed in Table 1. Such criteria were used to establish a study population representative of the AMH assay intended use population in clinical practice, i.e., women of reproductive age seeking an OR assessment before starting fertility therapy. Women with factors known to impact the correlation of AMH and AFC were excluded [7,8,9,10,11,12].

Table 1.
Inclusion and exclusion criteria for clinical study.
Each enrolled subject underwent a transvaginal ultrasound (TVUS) to measure AFC between days 2 and 4 of their menstrual cycle. A maximum of 20 mL of blood samples from each female subject were collected in two serum separator tubes (SST) between days 2 and 4 of the menstrual cycle. No lithium heparin (Li-heparin) plasma was collected during this clinical sample collection. Serum tubes were allowed to clot at room temperature for 30 min and then centrifuged at 1000 to 1300× g for 10 to 15 min and transferred into cryovial tubes. Specimens were then frozen and maintained at −70 °C at the collection sites within 4 h of collection. Subsequently, samples were shipped on dry ice to the central lab, where they were thawed, pooled, and pipetted into 500 µL aliquots in screw top vials and then stored at ≤−20 °C until analysis.
2.2. Ethical Approval
Each site obtained Institutional Review Board (IRB) approval prior to the start of the prospective enrollment, and all enrolled subjects signed patient informed consent forms (ICF).
2.3. Analytical Performance of AMH Assay
Analytical sensitivity studies to determine the limit of blank (LoB), limit of detection (LoD), and limit of quantitation (LoQ) were performed consistent with the Clinical and Laboratory Standards Institute (CLSI) EP17-A2. For LoB, six human female serum samples prescreened for undetectable levels of AMH were assayed across five days, resulting in 240 measurements per reagent lot. LoB was calculated non-parametrically as the 95th percentile of all measurements for each reagent lot. LoD and LoQ were established from 880 measurements per reagent lot of 11 low-level human female samples (0.008–0.166 ng/mL) using three reagent lots and two ADVIA Centaur XP instruments. A precision profile curve was generated per reagent lot to calculate LoD = LoB + 1.645 × (within-laboratory standard deviation) and LoQ as the AMH concentration on the curve at 20% within-laboratory coefficient of variation (CV) (Table S1). When the estimated LoB, LoD, LoQ values were lower than the design requirement goal for the assay, a conservative value was set and claimed for the assay.
Repeatability, within-lab precision, and reproducibility of the ADVIA Centaur AMH assay were evaluated according to CLSI EP05-A3 using eight human serum pools (consisting of sera from at least 3 subjects) and three levels (low: ~1 ng/mL, medium: ~5 ng/mL, and high: ~14 ng/mL) of ADVIA Centaur AMH quality controls (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA). In total, 480 replicates were produced for each sample over 240 runs and were analyzed using a validated statistical Microsoft Excel software application for performance evaluations.
Linearity testing consistent with CLSI EP06-A involved diluting a high-AMH concentration human serum pool (approximately 26 ng/mL) with a low serum pool derived from a total of 5 female subjects with an AMH level corresponding to the limit of detection of the assay. Twelve samples were prepared across the assay range and tested in replicates of five with mean values used for linear regression analysis via a validated Excel application. The assay was linear if the percentage bias for all levels tested was within 10% of the predicted values by the regression model.
Method comparison studies were completed in accordance with CLSI EP09C-ED3 using 120 frozen female serum samples acquired from Precision for Medicine (Carlsbad, CA, USA), Access Biologicals (Vista, CA, USA), and Discovery Life Sciences (Newtown, PA, USA) and covering the measuring interval (0.080 ng/mL to 22.0 ng/mL). Samples were tested using three assays: ADVIA Centaur AMH, Access® 2 AMH (Beckman Coulter, Brea, CA, USA), performed internally at Siemens site, and Elecsys® AMH (Cobas system e 601, Roche Diagnostics, Indianapolis, IN, USA) performed externally at University of Maryland. Results were analyzed by Passing–Bablok regression.
Specimen equivalence was evaluated consistent with CLSI EP09C-ED3 using frozen matched sample sets from female donors drawn in SST, Li-heparin glass tubes, and no anticoagulant/no gel barrier tubes (Access Biologicals, Vista, CA, USA). Samples were tested using the ADVIA Centaur AMH assay. AMH results from SST and Li-heparin tubes to those from no anticoagulant/no gel barrier tubes were compared via weighted Deming regression.
The interference testing study was completed in accordance with CLSI documents EP07-ED3 and EP37-ED1, using substances listed in Table S2, up to the indicated concentration. Testing was conducted using paired difference analysis at two AMH concentrations, approximately 1 ng/mL and 7 ng/mL, obtained by pooling 6 patient samples for each level. It was presumed that no exogenous interferents were present, but levels of cholesterol, hemoglobin, total bilirubin, conjugated bilirubin, total protein, and IgG were quantified. Additional interferent was added to achieve target concentrations. Samples were split into “control” (no interferent, with vehicle only) and “test” (with interferent) groups for each substance tested. Percent dose bias was calculated as follows: 100 × [(mean of test sample − mean of control sample)/mean of control sample]. For substances exceeding the maximum allowable bias of 10%, a dose–response titration was conducted to identify the highest concentration that met the bias criteria.
2.4. Clinical Sensitivity and Specificity of the ADVIA Centaur AMH Assay
Clinical sensitivity and specificity were determined using study subject specimens collected prospectively from the intended use population as outlined in Section 2.1. Serum AMH concentrations were correlated to AFC according to CLSI EP12-A2. AFC results were determined by TVUS for each subject, performed between day 2 and day 4 of the menstrual cycle, and included follicles 2–10 mm in diameter. AFC data were divided into two groups: >15 (high OR) or ≤15 (normal to diminished OR). AFC results were compared to AMH results using the previously established OR cutoff value of 1.77 ng/mL [5]. The AMH assay result was “positive” if the AMH concentration was >1.77 ng/mL and “negative” if the AMH concentration was ≤1.77 ng/mL.
2.5. Statistical Analysis
Statistical analysis was performed using SAS statistical software version 9.4. From each instrument test result, the estimated sensitivity, specificity, and the two-sided 95% Wilson’s score confidence intervals (CI) of each parameter were calculated. The positive percent value (PPV), negative percent value (NPV), and two-sided 95% CI were also calculated.
An analysis of the two-sided 95% CIs for the differences over the three assays for sensitivity and specificity was computed using the method recommended by Newcombe.
Receiver operating characteristic (ROC) curve analysis was performed for each method to calculate the area under the curve (AUC) and compare the predictive accuracy of each diagnostic test, knowing that AUC > 0.9, high accuracy test; AUC > 0.7 to ≤0.9, moderate accuracy test; 0.5 < AUC ≤ 0.7, low accuracy test [13].
Passing–Bablok regression analysis was performed between the methods. Bland–Altman plots and average bias were computed to evaluate the quantitative value difference between the methods.
3. Results
3.1. Analytical Performance
Detection parameters: LoB, LoD, and LoQ values were determined to be (and claimed) 0.003 (0.010) ng/mL, 0.009 (0.020) ng/mL, and 0.018 (0.043) ng/mL, respectively (Table S1).
Analytical Precision: For all levels tested, repeatability CVs were ≤2.9%. Within-lab CVs varied from 2.4% to 3.2%, and total imprecision CVs ranged from 2.5% to 4.4% (Table 2).

Table 2.
Serum samples repeatability, within-lab precision, and reproducibility.
Linearity: The assay showed linearity from 0.018 to 26.3 ng/mL with an average bias deviation from linearity of 1.3% over the range, confirming the assay analytical measuring range from 0.043 ng/mL to 24.0 ng/mL (Figure S1).
Method Comparison: The ADVIA Centaur assay correlated strongly with Beckman and Roche devices, with Pearson coefficients between 0.989 and 0.994 and Passing–Bablok regression slopes from 1.04 to 1.07. Mean biases were 1.6% for Beckman and 5.3% for Roche (Figure 1).
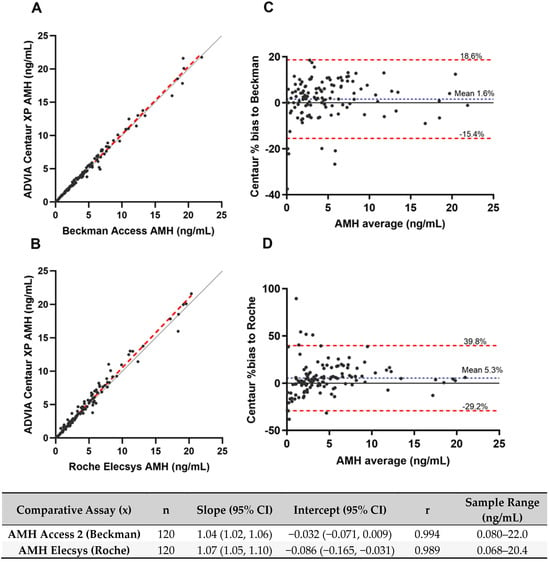
Figure 1.
Comparison of AMH quantitative values from commercially acquired samples between the ADVIA Centaur, Beckman Access, and Roche Elecsys assays. (A,B) Passing–Bablok regression analysis. Red dashed lines represent regression lines, and solid gray lines represent identity lines. Regression equations slope and intercept are reported in the bottom table. (C,D) Bland–Altman plots. Blue dotted lines represent mean bias. Red dashed lines indicate the limits of agreement, defined as the mean difference ±1.96 times the standard deviation of the differences.
Specimen Equivalence: AMH concentrations were comparable whether processed as serum (with or without a gel separator) or as Li-heparin plasma, with regression analyses showing slopes of 1.00 and 1.08, respectively and correlation coefficients of 0.997. Mean biases were 0.04% for serum with gel barrier samples and 8.5% for Li-heparin plasma samples (Figure S2).
Interferences Testing: No assay interference was observed for substances in Table S2 at specified serum concentrations.
3.2. Clinical Performance
Demographics: A total of 578 eligible women met all inclusion and exclusion criteria during prospective subject enrollment. Out of 578 samples, 532 had sufficient volume to conduct analyses presented in this study. Demographic data for these samples are shown in Table 3. Of the 532 subjects, 47.9% (n = 255) were classified as having normal to diminished OR (AFC ≤ 15), and 52.1% (n = 277) were classified as having high OR (AFC > 15). Most subjects were White (404 of 532) and not Hispanic or Latino (429 of 532). Black or African American subjects’ percentage aligned with current estimates in the total U.S. population (approximately 13.4%). Participants with AFC ≤15 had a mean age of 36.1 years and a mean BMI of 27.05 kg/m2; participants with AFC >15 had a mean age of 32.9 years and a mean BMI of 26.74 kg/m2.

Table 3.
Patients’ demographics.
Clinical Performance: This study utilized an AMH cutoff of 1.77 ng/mL and an AFC cutoff of 15. Subjects were classified as having high OR if the AFC was greater than 15. Table 4 shows that the estimated sensitivity and specificity of the ADVIA Centaur AMH assay were 90.2% and 51.8%, respectively, with a PPV of 67.0% and NPV of 83.0%. For the Beckman Access AMH assay, sensitivity was 89.9% and specificity was 51.0%, with a PPV and an NPV of 66.6% and 82.3%, respectively. Sensitivity was 77.3% and specificity was 71.0% for the Roche Elecsys AMH assay, with a PPV of 74.3% and NPV of 74.2%.

Table 4.
Diagnostic concordance between each instrument tested for AMH assay and AFC.
Notes at the bottom of Table 4 show for ADVIA Centaur and Beckman Access that the CI for the difference includes zero. This indicates that no statistical difference in sensitivity and specificity was observed between the ADVIA Centaur and Beckman Access AMH assays within this clinical study sample. In contrast, similar analysis between ADVIA Centaur and Roche Elecsys or Beckman Access and Roche Elecsys demonstrated a statistical difference between the AMH methods.
An additional analysis was performed to evaluate the impact of subject age on assay performance given that a woman’s OR decreases with age (Table S3). Subjects were sorted into two groups: women < 35 years (n = 264) of age and women ≥ 35 years of age (n = 268). In this study, the prevalence of AFC > 15 was 37.7% in women ≥ 35 years of age and 66.7% in women <35 years of age. Differences in clinical performance were observed between subjects <35 and ≥35 years of age. Across the three AMH methods tested, the number of subjects with a result >1.77 ng/mL and classified as having high OR was higher in subjects <35 years of age (ranging from 72.8% to 77.3% of subjects) compared to 57.1% to 69.2% of subjects ≥35 years of age, and the number of subjects with a result ≤1.77 ng/mL classified as having normal/diminished OR was higher in subjects ≥35 years of age (ranging from 83.2% to 89.7% of subjects ≥35 years of age compared to 56.6% to 65.9% of subjects <35 years of age).
Figure 2 shows the ROC curves comparing the ADVIA Centaur, Beckman Access, and Roche Elecsys AMH assays for the discrimination of OR. AUC ranged from 0.815 for ADVIA Centaur and Beckman Access to 0.825 for Roche Elecsys (Figure 2B), and AUC value comparison indicated no statistical difference between the methods with p value > 0.05 for each calculated chi-square. Youden’s index (J) was calculated, and the highest J was used to determine the optimal cutoff, which was estimated at 3.18 ng/mL (sensitivity 69.3%, specificity 77.6%, and J = 0.46961) for ADVIA Centaur, 2.94 ng/mL (sensitivity 74.0%, specificity 74.5%, and J = 0.48517) for Beckman Access, and 2.09 ng/mL (sensitivity 69.7%, specificity 79.6%, and J = 0.49283) for Roche Elecsys. All three optimal cutoffs differ from each of the three manufacturer-reported cutoffs of 1.77 ng/mL.
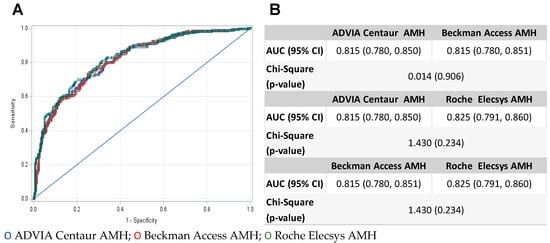
Figure 2.
(A) ROC curves comparing Centaur ADVIA (blue circle), Beckman Access (red circle), and Roche Elecsys (green circle) AMH assays for the discrimination of ovarian reserve. (B) Statistical comparison of area under the curve (AUC) values from ROC curve analysis.
The AMH values obtained across the three instruments from each clinical sample (n = 532) were compared. A strong correlation was observed between the ADVIA Centaur and the Beckman Access with a high Kendall’s tau correlation coefficient of 0.909 (Figure 3), while a moderate correlation was observed with Roche Elecsys (τ = 0.777). Scatter plots and fit lines were created using Passing–Bablok regression method and shown in Figure 3. The Passing–Bablok linear regression fit slope value for these methods ranged from 1.00 to 1.41 (95% CI of the slope ranging from 0.98 to 1.48). The Bland–Altman plots shown in Figure 3 demonstrate a mean bias of -0.3% for ADVIA Centaur vs. Beckman Access and 62.1% for ADVIA Centaur vs. Roche Elecsys. The 2233% bias and 2000% bias observed for the same sample in Figure 3E,F represent an absolute difference of 0.67 ng/mL (Roche Elecsys AMH 0.030 ng/mL and ADVIA Centaur AMH 0.70 ng/mL) and 0.64 ng/mL (Roche Elecsys AMH 0.030 ng/mL and Beckman Access AMH 0.63 ng/mL). AMH values for this sample obtained with all methods are well below the cutoff, and the observed difference would not be expected to impact clinical interpretation. Comparison of AMH values between Beckman Access and Roche Elecsys also demonstrated a mean bias of 67.4%, suggesting that the Roche Elecsys AMH assay is the outlier in our data set.
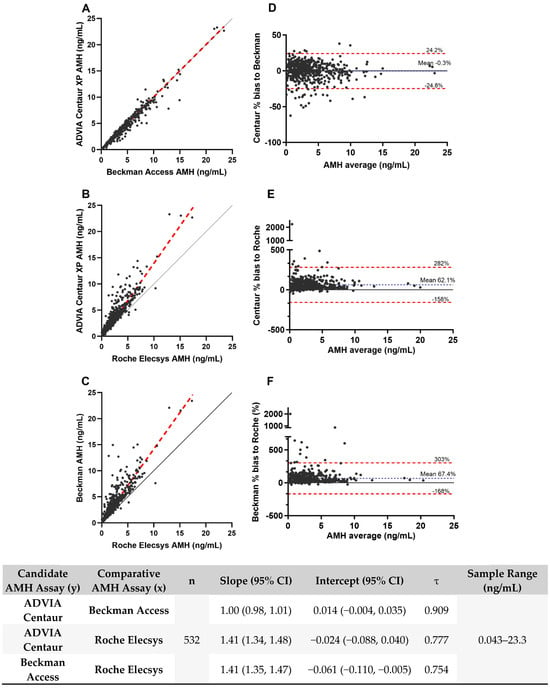
Figure 3.
Comparison of quantitative AMH values from prospective clinical samples between the ADVIA Centaur, Beckman Access, and Roche Elecsys assays. (A–C) Passing–Bablok regression analysis. Red dashed lines represent regression lines, and solid gray lines represent identity lines. Regression equations slope and intercept are reported in the bottom table. (D–F) Bland–Altman plots. Blue dotted lines represent mean bias. Red dashed lines indicate the limits of agreement, defined as the mean difference ± 1.96 times the standard deviation of the differences.
4. Discussion
The objective of this study was to characterize the analytical and clinical performance of the ADVIA Centaur AMH assay. Strong analytical and clinical performance of the new ADVIA Centaur AMH assay was observed, demonstrating equivalent performance to the commercialized Beckman Access AMH assay and confirming its suitability for routine clinical use.
Results of the precision studies show that the total %CV precision of the ADVIA Centaur (3.3% CV at 0.955 ng/mL AMH and 2.9% CV at 4.75 ng/mL AMH) is comparable to the commercially available Beckman Access assay, in the range of 1 to 5 ng/mL AMH concentration (3.2% at 1.0 ng/mL AMH and 2.8% CV at 5.04 ng/mL) [14].
The specimen equivalence results show that Li-heparin plasma samples met equivalency requirements per regression analysis but exhibit a consistent positive bias across the assay range compared to serum samples (Figure S2D). This bias is not clinically significant (total allowable error 30%) but suggests serum samples may be preferable. Notably, serum samples are already favored for AMH measurement in clinical laboratories due to their known superior stability [15].
The clinical sensitivity and specificity for the ADVIA Centaur AMH assay (90.5% sensitivity and 52.0% specificity) are comparable to those reported for the Beckman Access AMH assay (88.8% sensitivity and 59.0% specificity) [14]. Of note, the data set used for the ADVIA Centaur sensitivity and specificity calculations in this study (N = 532) and the ADVIA Centaur Instructions for Use (IFU) [16] (N = 533) have 501 individual samples in common. The reported sensitivity and specificity for the Roche Elecsys AMH assay at 88.3% and 68.3%, respectively [17], are slightly different than the ones observed in this study, but parameters such as study group inclusion/exclusion criteria and demographics may contribute to these differences. The clinical performance of the AMH assays stratified by age (Table S3) confirms the impact of the well-established women’s age factor and supports the decline of ovarian reserve with age [18].
The AMH cutoff used to evaluate OR can impact assay sensitivity and specificity. The optimal cutoff estimated by Youden’s index was approximately 3 ng/mL for both the ADVIA Centaur and Beckman Access AMH assays and 2 ng/mL for the Roche Elecsys AMH assay. Although the 1.77 ng/mL cutoff differs from the optimal and does not provide a balance between sensitivity and specificity for the ADVIA Centaur assay, it was selected to optimize sensitivity as caution is needed in interpretation of AMH test results to guide decision making prior to starting fertility therapy. The 1.77 ng/mL cutoff provides a high probability (>90%) of ruling-out subjects with high OR that would not need to undergo fertility therapy. The trade-off is that with a lower specificity, decision making for subjects with borderline results will be mainly guided by additional clinical factors and lab tests.
Both the ADVIA Centaur and Roche AMH assays are standardized against the Beckman Coulter AMH assay; however, substantial biases were observed when comparing ADVIA Centaur versus Roche Elecsys and Beckman Access versus Roche Elecsys. The average bias (5.3%) between the ADVIA Centaur and Roche Elecsys AMH assays was within acceptable requirement bias in the method comparison study performed as part of assay verification. However, the individual sample bias results shown in Figure 1D indicated a higher level of imprecision, with results that contribute to −30% to 40% observed bias to Roche as compared to −15% to 19% observed bias to Beckman (Figure 1C). We suspect that these observations might be linked to preanalytical factors. The sample testing using the Beckman assay was conducted in-house under well-controlled conditions for sample handling. However, the samples for the Roche assay were shipped and tested externally. Consequently, we cannot rule out the possibility of insufficient or inconsistent thawing or mixing during the handling process, which could lead to increased variability in the AMH measurements. Nevertheless, average biases >60% were observed against Roche in the method comparison study performed using the clinical samples. The samples used for verification studies were commercially acquired, and thus potential differences between these samples and samples collected as part of the prospective clinical study are unknown. Importantly, the verification study method comparison results were generated in 2020, prior to Roche’s AMH assay reformulation, while the method comparison study using the prospective clinical samples was performed in 2023 using the reformulated Roche AMH assay. Additional studies are needed to better understand if the reformulation of the Roche AMH assay has contributed to the observed difference. Of note, all AMH assay testing in our study was performed on samples that underwent two freeze/thaw cycles as described in the Methods section but occurred 18 months apart for the Roche AMH assay as compared to ADVIA and Beckman assays testing. While pointing out these methodological differences, we believe that sample handling and storage are not expected to have significantly contributed to the observed results. Previously published studies indicate that AMH is highly stable during extended freezer storage time and repeated freeze–thaw conditions in frozen serum samples [15]. In addition, method differences, including underestimation of AMH results with the Roche Elecsys, have been previously observed in head-to-head comparison studies between Beckman Access and Roche Elecsys AMH assays as well as other commercially available assays [19,20]. These quantitative differences emphasize the standardization challenges associated with AMH assays and the ongoing efforts to standardize these assays that have been well described within the literature [21,22]. Of interest, Xu and colleagues have developed an online tool for converting results between Roche Elecsys and Beckman Access AMH assays [23]. Whether their proposed formula may be extended to ADVIA Centaur remains to be verified.
The observed between-method bias in AMH measurements may be influenced by other factors such as proprietary epitope-specific antibody (Ab) designs, unknown isoform detection, assay matrix effects, and analytical interferents. For example, the use of biotinylated capture Ab in a preformed solid phase format for the ADVIA Centaur AMH assay allowed less than 10% bias with biotin levels up to 3500 ng/mL (0.350 mg/dL) [16]. For the Beckman Access AMH assay, none of the Abs used are biotinylated Ab, and no interference is described at the highest biotin concentration tested of 179 ng/mL [14]. In contrast, prior to assay reformulation, Roche Elecsys AMH assay claimed no biotin interference up to 30 ng/mL, which was extended to 1200 ng/mL by incorporating a scavenger antibody [17]. Still, samples with biotin above this level could experience negative bias in the Roche Elecsys AMH assay.
These results highlight the need to establish specific reference limits for individual AMH assays until an international reference standard is established and AMH assays are re-standardized against it. Additionally, clinical practice guidelines and standards [1,3] describe that the OR assessment should be made in conjunction with other clinical markers (age, follicle-stimulating hormone level, AFC, and ovarian volume) to better evaluate a woman’s fertility potential and guide clinical decision making in fertility practice. Overall, this study demonstrates that the performance of the most recent fully automated AMH assay (ADVIA Centaur) is comparable to other commercially available automated AMH assays and can be reliably incorporated into the overall assessment of OR.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/endocrines5040037/s1, Figure S1: Linearity evaluation for AMH assay on the ADVIA Centaur; Figure S2: Comparison of tube type specimen for the ADVIA Centaur AMH assay; Table S1: Summary of analytical sensitivity evaluation; Table S2: Interferences testing; Table S3: Clinical Performance of the AMH assay on each instrument tested stratified by age.
Author Contributions
Conceptualization, J.B., K.F., C.B., R.S., M.B., J.E.O., E.Z. and R.H.C.; methodology, J.B., K.F., C.B., R.S., M.B., J.E.O., E.Z. and R.C; software, C.B. and R.S.; validation, J.B., K.F., C.B. and R.S.; formal analysis, J.B., K.F., C.B. and R.S.; investigation, J.B., K.F., M.B., J.E.O., E.Z. and R.H.C.; data curation, J.B., K.F., C.B. and R.S.; writing—original draft preparation, J.B., K.F., C.B., R.S. and R.H.C.; writing—review and editing, J.B., K.F., C.B., R.S., M.B., J.E.O., E.Z. and R.H.C.; supervision, J.B., K.F., M.B., J.E.O., E.Z. and R.H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA.
Institutional Review Board Statement
Each site obtained approval by their local Institutional Review Board (IRB) prior to the start of prospective enrollment. Principal investigator IRB approvals for study protocol: Siemens Healthcare Diagnostics—CA-Centaur-201905.PRO, ADVIA Centaur AMH Ovarian Reserve Specimen Collection Protocol (Pro00043105). Protocol approved by Advarra: 6 May 2020. See Appendix A.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author.
Acknowledgments
The authors would like to thank Russell Foulk, Ronald Feinberg, Randall Loy, and Jack Crain, for supervision of subject enrollment at their respective institution; Jeanne Rhea-McManus and Geraldine Arrode-Bruses for writing support; and Edward De Vol for statistical support.
Conflicts of Interest
Robert H. Christenson has consulting agreements with Siemens Healthineers, Beckman Coulter Inc, Roche Diagnostics, QuidelOrtho, and Becton Dickinson. J. Bogdanovic, K. Freeman, C. Brown, and R. Singleton are employees of Siemens Healthineers. No potential conflicts of interest are reported by M. Behera, J. Obrien, and E. Zbella.
Appendix A. Institutional Review Board Site Approvals (Date and Code)

| Site | Code | Approval Date |
|---|---|---|
| Fertility and IVF Center of Miami | SSU00128953 | 14 August 2020 |
| Bloom Reproductive Institute | SSU00129719 | 25 August 2020 |
| Fertility Treatment Center | SSU00125180 | 2 July 2020 |
| Reproductive Endocrinology Associates of Charlotte | SSU00129791 | 25 August 2020 |
| Center for Assisted Reproduction | SSU00125251 | 2 July 2020 |
| Reproductive Associated of Delaware | SSU00128589 | 13 August 2020 |
| Utah Fertility Clinic | SSU00129326 | 19 August 2020 |
| Women’s Medical Research Group | SSU00125192 | 2 July 2020 |
| Center for Reproductive Medicine | SSU00132960 | 15 October 2020 |
| Shady Grove Fertility Center | SSU00126730 | 16 July 2020 |
| Houston Fertility Clinic | SSU00125481 | 9 July 2020 |
References
- Infertility Workup for the Women’s Health Specialist: ACOG Committee Opinion, Number 781. Obstet. Gynecol. 2019, 133, e377–e384. [CrossRef] [PubMed]
- Moolhuijsen, L.M.; Visser, J.A. Anti-Müllerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J. Clin. Endocrinol. Metab. 2020, 105, 3361–3373. [Google Scholar] [CrossRef] [PubMed]
- Penzias, A.; Azziz, R.; Bendikson, K.; Falcone, T.; Hansen, K.; Hill, M.; Hurd, W.; Jindal, S.; Kalra, S.; Mersereau, J.; et al. Testing and interpreting measures of ovarian reserve: A committee opinion. Fertil. Steril. 2020, 114, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Baker, V.L.; Gracia, C.; Glassner, M.J.; Schnell, V.L.; Doody, K.; Coddington, C.C.; Shin, S.S.; Marshall, L.A.; Alper, M.M.; Morales, A.J.; et al. Multicenter evaluation of the Access AMH antimüllerian hormone assay for the prediction of antral follicle count and poor ovarian response to controlled ovarian stimulation. Fertil. Steril. 2018, 110, 506–513.e3. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.H.; Reuter, L.M.; Baker, V.L.; Craig, L.B.; Sakkas, D.; Surrey, E.; Doody, K.J.; Jungheim, E.S.; Bayrak, A.B.; Hund, M.; et al. A multicentre evaluation of the Elecsys® anti-Müllerian hormone immunoassay for prediction of antral follicle count. Reprod. BioMedicine Online 2019, 38, 845–852. [Google Scholar] [CrossRef] [PubMed]
- van Disseldorp, J.; Lambalk, C.B.; Kwee, J.; Looman, C.W.N.; Eijkemans, M.J.C.; Fauser, B.C.; Broekmans, F.J. Comparison of inter-and intra-cycle variability of anti-Müllerian hormone and antral follicle counts. Hum. Reprod. 2010, 25, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Dumanski, S.M.; Ahmed, S.B. Fertility and reproductive care in chronic kidney disease. J. Nephrol. 2019, 32, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kelley, A.S.; Badon, S.E.; Lanham, M.S.; Fisseha, S.; Moravek, M.B. Body mass index restrictions in fertility treatment: A national survey of OB/GYN subspecialists. J. Assist. Reprod. Genet. 2019, 36, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Karvonen-Gutierrez, C.; Kong, S.; Arends, V.; Steffes, M.; McConnell, D.S.; Randolph, J.F.; Harlow, S.D. Antimüllerian hormone among women with and without type 1 diabetes: The Epidemiology of Diabetes Interventions and Complications Study and the Michigan Bone Health and Metabolism Study. Fertil. Steril. 2016, 106, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, A.L.; Kazemi, M.; Lujan, M.E. Impact of obesity on anti-mullerian hormone (Amh) levels in women of reproductive age. J. Clin. Med. 2021, 10, 3192. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.M.; Lee, C.H.; Baerwald, A. Interrelationships among reproductive hormones and antral follicle count in human menstrual cycles. Endocr. Connect. 2016, 5, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Lin, C.; Zhang, M.; Lv, F.; Zhu, X.; Han, X.; Cai, X.; Ji, L. Assessment of ovarian reserve in patients with type 1 diabetes: A systematic review and meta-analysis. Endocrine 2022, 77, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Akobeng, A.K. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. Int. J. Paediatr. 2007, 96, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Access®. Anti-Müllerian Hormone (AMH) Assay Instructions for Use; B15676_Rev C; Beckman: Marseille, France, 2019. [Google Scholar]
- Vrzáková, R.; Šimánek, V.; Topolčan, O.; Vurm, V.; Slouka, D.; Kučera, R. The Stability of the Anti-Müllerian Hormone in Serum and Plasma Samples under Various Preanalytical Conditions. Diagnostics 2023, 13, 1501. [Google Scholar] [CrossRef] [PubMed]
- ADVIA Centaur®. Anti-Müllerian Hormone (AMH) Assay Instructions for Use; 10998436_EN Rev.01; Siemens Diagnostics Inc.: Tarrytown, NY, USA, 2023. [Google Scholar]
- Cobas®. Elecsys Anti-Müllerian Hormone (AMH) Assay Instructions for Use; 08819378501_Rev 02; Roche: Mannheim, Germany, 2022. [Google Scholar]
- Committee opinion no. 589. Female age-related fertility decline. Obstet. Gynecol. 2014, 123, 719–721. [Google Scholar]
- Nelson, S.M.; Pastuszek, E.; Kloss, G.; Malinowska, I.; Liss, J.; Lukaszuk, A.; Plociennik, L.; Lukaszuk, K. Two new automated, compared with two enzyme-linked immunosorbent, antimüllerian hormone assays. Fertil. Steril. 2015, 104, 1016–1021e6. [Google Scholar] [CrossRef] [PubMed]
- Punchoo, R.; Bhoora, S. Variation in the Measurement of Anti-Müllerian Hormone–What Are the Laboratory Issues? Front. Endocrinol. 2021, 12, 719029. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.; Hockley, J.; Rigsby, P.; Burns, C. Establishment of a WHO Reference Reagent for anti-Mullerian hormone. Reprod. Biol. Endocrinol. 2020, 18, 86. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.R.; Robertson, D.M.; Burns, C.; Ledger, W.L. Challenges in Measuring AMH in the Clinical Setting. Front. Endocrinol. 2021, 12, 691432. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Feng, G.; Ma, C.; Han, Y.; Zhou, J.; Song, J.; Su, Y.; Zhong, Q.; Chen, F.; Cui, L.; et al. AMH converter: An online tool for converting results between the different anti-Müllerian hormone assays of Roche Elecsys®, Beckman Access, and Kangrun. PeerJ 2023, 11, e15301. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).