Insulin Resistance and Glucose Metabolism during Infection
Abstract
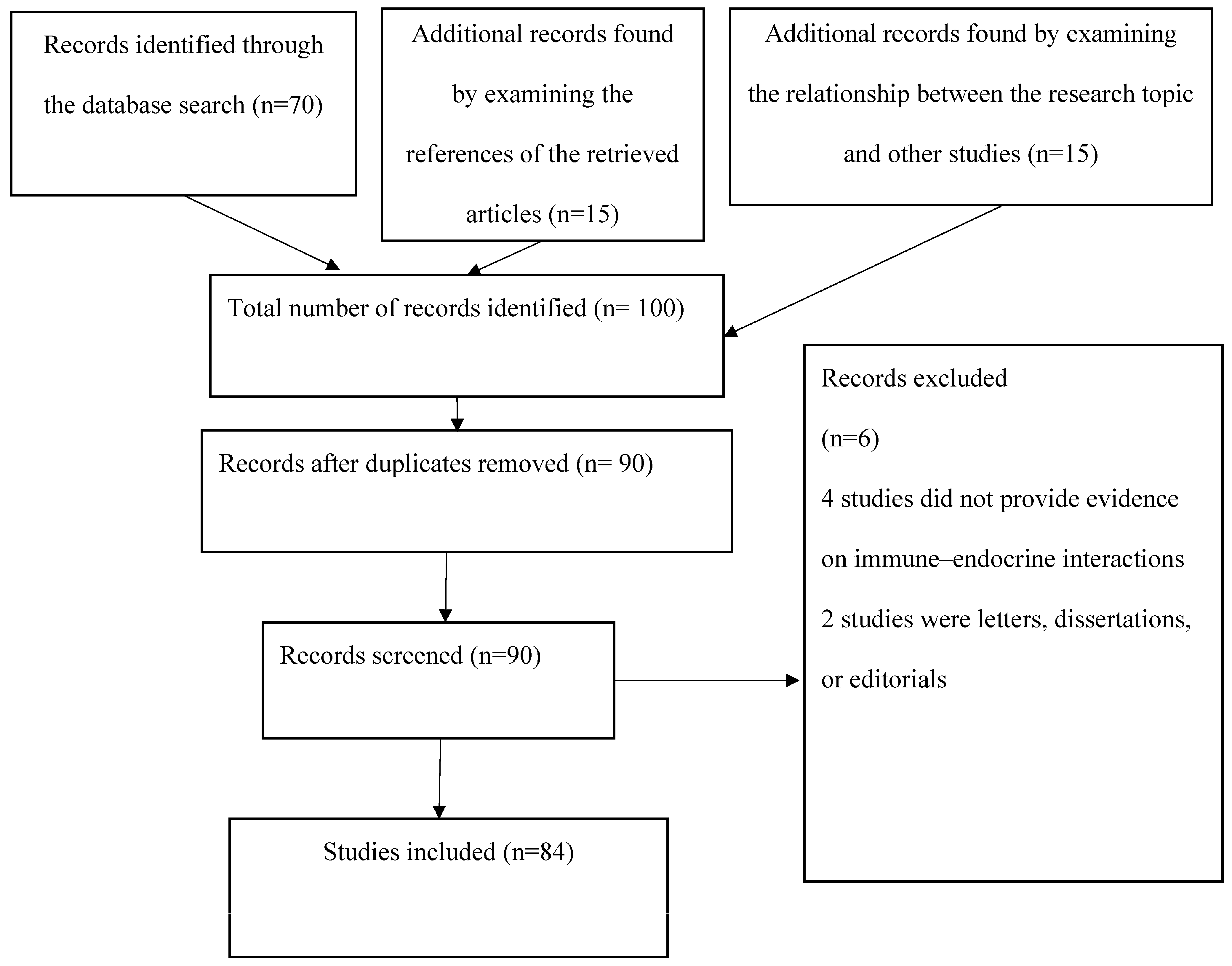
:1. Introduction
2. Methods
2.1. Endocrine System and Immunity
2.2. Differences in Immune–Endocrine Interactions during Viral, Bacterial, and Fungal Infections
3. Results
3.1. Euglycemic Hyperinsulinemia
3.2. Anorexia, Infection, and Blood Glucose Levels
3.3. Stress Hyperglycemia and Infections
3.4. Diabetes and Infections
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wensveen, F.M.; Sestan, M.; Wensveen, T.; Polic, B. Beauty and the beast’ in infection: How immune-endocrine interactions regulate systemic metabolism in the context of infection. Eur. J. Immunol. 2019, 49, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Knight, J. Endocrine system I: Overview of the endocrine system and hormones. Nurs. Times 2021, 117, 38–42. [Google Scholar]
- Buliman, A.; Tataranu, L.G.; Paun, D.L.; Mirica, A.; Dumitrache, C. Cushing’s disease: A multidisciplinary overview of the clinical features, diagnosis, and treatment. J. Med. Life 2016, 9, 12–18. [Google Scholar] [PubMed]
- Gavrieli, A.; Mantzoros, C.S. Novel molecules regulating energy homeostasis: Physiology and regulation by macronutrient intake and weight loss. Endocrinol. Metab. 2016, 31, 361–372. [Google Scholar] [CrossRef]
- Lee, W.Y. Articles in Endocrinology and Metabolism in 2016. Endocrinol. Metab. 2017, 32, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H. Endocrine risk factors for cognitive impairment. Endocrinol. Metab. 2016, 31, 185–192. [Google Scholar] [CrossRef]
- Foster, S.R.; Hauser, A.S.; Vedel, L.; Strachan, R.T.; Huang, X.P.; Gavin, A.C.; Shah, S.D.; Nayak, A.P.; Haugaard-Kedström, L.M.; Penn, R.B.; et al. Discovery of human signaling systems: Pairing peptides to G protein-coupled receptors. Cell 2019, 179, 895–908. [Google Scholar] [CrossRef]
- Roh, E.; Kim, M.S. Brain regulation of energy metabolism. Endocrinol. Metab. 2016, 31, 519–524. [Google Scholar] [CrossRef]
- Milling, S. Beyond cytokines: Influences of the endocrine system on human immune homeostasis. Immunology 2021, 163, 113–114. Available online: https://onlinelibrary.wiley.com/doi/10.1111/imm.13347?af=R (accessed on 1 January 2023). [CrossRef]
- Singh, A.T.; Mc Causland, F.R. Osmolality and blood pressure stability during haemodialysis. Semin. Dial. 2017, 30, 509–517. [Google Scholar] [CrossRef]
- Tsoli, M.; Boutzios, G.; Kaltsas, G. Immune system effects on the endocrine system. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279139/ (accessed on 1 January 2023).
- Straub, R.H.; Cutolo, M.; Buttgereit, F.; Pongratz, G. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J. Intern. Med. 2010, 267, 543–560. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Persani, L.; Cangiano, B.; Bonomi, M. The diagnosis and management of central hypothyroidism in 2018. Endocr. Connect. 2019, 8, R44–R54. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.Z. Interaction between neuroendocrinology and immunology: Hypothalamic-Pituitary-Thyroid Axis in immunoendocrinology. Open J. Endocr. Metab. Dis. 2021, 11, 63–69. [Google Scholar] [CrossRef]
- Rankin, L.; Artis, D. Beyond host defence: Emerging functions of the immune system in regulating complex tissue physiology. Cell 2018, 173, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Webber, T.; Ronacher, K.; Conradie-Smit, M.; Kleynhans, L. Interplay Between the Immune and Endocrine Systems in the Lung: Implications for TB Susceptibility. Front. Immunol. 2022, 13, 829355. [Google Scholar] [CrossRef]
- Silva, A.R.; Gonçalves-de-Albuquerque, C.F.; Pérez, A.R.; de Frias Carvalho, V. Immune-endocrine interactions related to a high risk of infections in chronic metabolic diseases: The role of PPAR gamma. Eur. J. Pharmacol. 2019, 854, 272–281. [Google Scholar] [CrossRef]
- Bansal, R.; Gubbi, S.; Muniyappa, R. Metabolic syndrome and COVID 19: Endocrine-immune-vascular interactions shapes clinical course. Endocrinology 2020, 161, bqaa112. [Google Scholar] [CrossRef]
- Muthusami, S.; Vidya, B.; Shankar, E.M.; Vadivelu, J.; Ramachandran, I.; Stanley, J.A.; Selvamurugan, N. The functional significance of endocrine-immune interactions in health and disease. Curr. Protein Pept. Sci. 2020, 21, 52–65. [Google Scholar] [CrossRef]
- Wensveen, F.M.; Šestan, M.; Wensveen, T.T.; Polić, B. Blood glucose regulation in context of infection. Vitam. Horm. 2021, 117, 253–318. [Google Scholar]
- Rowe, R.K.; Griesbach, G.S. Immune-endocrine interactions in the pathophysiology of sleep-wake disturbances following traumatic brain injury: A narrative review. Brain Res. Bull. 2022, 185, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Manley, K.; Han, W.; Zelin, G.; Lawrence, D.A. Crosstalk between the immune, endocrine, and nervous systems in immunotoxicology. Curr. Opin. Toxicol. 2018, 10, 37–45. [Google Scholar] [CrossRef]
- Zefferino, R.; Di Gioia, S.; Conese, M. Molecular links between endocrine, nervous and immune system during chronic stress. Brain Behav. 2021, 11, e01960. [Google Scholar] [CrossRef]
- Peters, L.; Posgai, A.; Brusko, T.M. Islet-immune interactions in type 1 diabetes: The nexus of beta cell destruction. Clin. Exp. Immunol. 2019, 198, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Davis, P.J.; Lin, H.Y.; Gionfra, F.; Percario, Z.A.; Affabris, E.; Pedersen, J.Z.; Marchese, C.; Trivedi, P.; Anastasiadou, E.; et al. Thyroid hormones interaction with immune response, inflammation and non-thyroidal illness syndrome. Front. Cell Dev. Biol. 2021, 8, 614030. [Google Scholar] [CrossRef]
- Demeneix, B. Endocrine Disruptors: From Scientific Evidence to Human Health Protection; EPRS: European Parliamentary Research Service: Brussels, Belgium, 2019. [Google Scholar]
- Klecha, A.J.; Arcos, M.L.; Frick, L.; Genaro, A.M.; Cremaschi, G. Immune-endocrine interactions in autoimmune thyroid diseases. Neuroimmunomodulation 2008, 15, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, M.D.; Pellizas, C.G. Thyroid hormone action on innate immunity. Front. Endocrinol. 2019, 10, 350. [Google Scholar] [CrossRef]
- Ruiz-Argüelles, A.; García-Carrasco, M. Thyroid dysfunction and the immune system. In Handbook of Systemic Autoimmune Diseases; Elsevier: Amsterdam, The Netherlands, 2008; Volume 9, pp. 75–80. [Google Scholar]
- Zhang, Z.; Reponen, T.; Hershey, G.K. Fungal Exposure and Asthma: IgE and Non-IgE-Mediated Mechanisms. Curr. Allergy Asthma Rep. 2016, 16, 86. [Google Scholar] [CrossRef]
- Sestan, M.; Marinovic, S.; Kavazovic, I.; Cekinovic, D.; Wueest, S.; Wensveen, T.; Brizic, I. Virus-induced interferon-gamma causes insulin resistance in skeletal muscle and derails glycaemic control in obesity. Immunity 2018, 49, 164–177.e166. [Google Scholar] [CrossRef]
- Gupta, S.S.; Wang, J.; Chen, M. Metabolic reprogramming in CD8+ T cells during acute viral infections. Front. Immunol. 2020, 11, 1013. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.A.; Kahan, S.M.; Zajac, A.J. Anti-viral CD8 T cells and the cytokines that they love. Virology 2013, 435, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heredero, G.; Gomez de las Heras, M.M.; Gabandé-Rodríguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, M. Adiponectin: A versatile player of innate immunity. J. Mol. Cell Biol. 2016, 8, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.R. Dynamic interactions between the immune system and the neuroendocrine system in health and disease. Front. Endocrinol. 2021, 12, 278. [Google Scholar] [CrossRef]
- Roep, B.O.; Thomaidou, S.; van Tienhoven, R.; Zaldumbide, A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat. Rev. Endocrinol. 2021, 17, 150–161. [Google Scholar] [CrossRef]
- Polito, R.; Di Meo, I.; Barbieri, M.; Daniele, A.; Paolisso, G.; Rizzo, M.R. Adiponectin role in neurodegenerative diseases: Focus on nutrition review. Int. J. Mol. Sci. 2020, 21, 9255. [Google Scholar] [CrossRef] [PubMed]
- Bliddal, S.; Nielsen, C.H.; Feldt-Rasmussen, U. Recent advances in understanding autoimmune thyroid disease: The tallest tree in the forest of polyautoimmunity. F1000Research 2017, 6, 1776. [Google Scholar] [CrossRef]
- Smith, B.L. Adaptation as a dynamic construct for studying stress resilience and susceptibility. Brain Behav. Immun. 2019, 81, 18–19. [Google Scholar] [CrossRef]
- Schiller, M.; Ben-Shaanan, T.L.; Rolls, A. Neuronal regulation of immunity: Why, how and where? Nature Rev. Immun. 2021, 21, 20–36. [Google Scholar] [CrossRef]
- Liu, Y.; Vu, V.; Sweeney, G. Examining the potential of developing and implementing use of adiponectin-targeted therapeutics for metabolic and cardiovascular diseases. Front. Endocrinol. 2019, 10, 842. [Google Scholar] [CrossRef] [PubMed]
- Bird, L. Getting enough energy for immunity. Nat. Rev. Immunol. 2019, 19, 269. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.P.; Deane, A.M. Dysglycemia and glucose control during sepsis. Clin. Chest Med. 2016, 37, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, C.; Gong, Y.; Fang, F.; Tian, H.; Li, J.; Cheng, X. Assessment of insulin resistance in subjects with normal glucose tolerance, hyperinsulinemia with normal blood glucose tolerance, impaired glucose tolerance, and newly diagnosed type 2 diabetes (Prediabetes Insulin Resistance Research). J. Diabetes Res. 2016, 2016, 9270768. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Patterson, S.P.; Speck, M.; Ehses, J.A.; Levings, M.K. Insulin inhibits IL-10-mediated regulatory T cell function: Implications for obesity. J. Immunol. 2014, 192, 623–629. [Google Scholar]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Le Zhan Yanxiang Guo, J.; White, E.; et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef]
- Ferrannini, E.; Mark, M.; Mayoux, E. CV protection in the EMPA-REGOUTCOME Trial: A “thrifty substrate” hypothesis. Diabetes Care 2016, 39, 1108–1114. [Google Scholar] [CrossRef]
- Benarroch, E. Brain glucose transporters: Implications for neurologic disease. Neurology 2014, 82, 1374–1379. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signalling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Højlund, K. Metabolism and insulin signalling in common metabolic disorders and inherited insulin resistance. Dan. Med. J. 2014, 61, B4890. [Google Scholar] [PubMed]
- Perrin, A.J.; Pariante, C.M. Endocrine and immune effects of non-convulsive neurostimulation in depression: A systematic review. Brain Behav. Immun. 2020, 87, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Tzanavari, T.; Giannogonas, P.; Karalis, K.P. TNF-alpha and obesity. Curr. Dir. Autoimmun. 2010, 11, 145–156. [Google Scholar] [PubMed]
- Rudd, C.E.; Taylor, A.; Schneider, H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev. 2009, 229, 12–26. [Google Scholar] [CrossRef]
- Dror, E.; Dalmas, E.; Meier, D.T.; Wueest, S.; Thévenet, J.; Thienel, C.; Timper, K.; Nordmann, T.M.; Traub, S.; Schulze, F.; et al. Postprandial macrophage-derived IL-1beta stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat. Immunol. 2017, 18, 283–292. [Google Scholar] [CrossRef]
- Sanchez, K.K.; Chen, G.Y.; Schieber, A.M.; Redford, S.E.; Shokhirev, M.N.; Leblanc, M.; Lee, Y.M.; Ayres, J.S. Cooperative metabolic adaptations in the host can favour asymptomatic infection and select for attenuated virulence in an enteric pathogen. Cell 2018, 175, 146–158.e115. [Google Scholar] [CrossRef]
- Paulsen, Ø.; Laird, B.; Aass, N.; Lea, T.; Fayers, P.; Kaasa, S.; Klepstad, P. The relationship between pro-inflammatory cytokines and pain, appetite and fatigue in patients with advanced cancer. PLoS ONE 2017, 12, e0177620. [Google Scholar] [CrossRef]
- Zenz, G.; Jačan, A.; Reichmann, F.; Farzi, A.; Holzer, P. Intermittent fasting exacerbates the acute immune and behavioral sickness response to the viral mimic poly (I:C) in mice. Front. Neurosci. 2019, 13, 359. [Google Scholar] [CrossRef]
- Balmer, M.L.; Ma, E.H.; Bantug, G.R.; Grählert, J.; Pfister, S.; Glatter, T.; Jauch, A.; Dimeloe, S.; Slack, E.; Dehio, P.; et al. Memory CD8(+) T cells require increased concentrations of acetate induced by stress for optimal function. Immunity 2016, 44, 1312–1324. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef] [PubMed]
- Tucey, T.M.; Verma, J.; Harrison, P.F.; Snelgrove, S.L.; Lo, T.L.; Scherer, A.K.; Barugahare, A.A.; Powell, D.R.; Wheeler, R.T.; Hickey, M.J.; et al. Glucose homeostasis is important for immune cell viability during candida challenge and host survival of systemic fungal infection. Cell Metab. 2018, 27, 988–1006.e7. [Google Scholar] [CrossRef] [PubMed]
- Okin, D.; Medzhitov, R. The effect of sustained inflammation on hepatic mevalonate pathway results in hyperglycaemia. Cell 2016, 165, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Sapra, A. Diabetes Mellitus; StatPearls Publishing: St. Petersburg, FL, USA, 2021; Available online: https://www.statpearls.com/ArticleLibrary/viewarticle/20429 (accessed on 1 January 2023).
- French, E.K.; Donihi, A.C.; Korytkowski, M.T. Diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome: Review of acute decompensated diabetes in adult patients. BMJ 2019, 365, l1114. [Google Scholar] [CrossRef]
- Rajaei, E.; Jalali, M.T.; Shahrabi, S.; Asnafi, A.A.; Pezeshki, S. HLAs in autoimmune diseases: Dependable diagnostic biomarkers? Curr. Rheumatol. Rev. 2019, 15, 269–276. [Google Scholar] [CrossRef]
- Chivese, T.; Norris, S.A.; Levitt, N.S. Progression to type 2 diabetes mellitus and associated risk factors after hyperglycemia first detected in pregnancy: A cross-sectional study in Cape Town, South Africa. PLoS Med. 2019, 16, e1002865. [Google Scholar] [CrossRef]
- Plummer, M.P.; Finnis, M.E.; Phillips, L.K.; Kar, P.; Bihari, S.; Biradar, V.; Moodie, S.; Horowitz, M.; Shaw, J.E.; Deane, A.M. Stress-induced hyperglycaemia and the subsequent risk of type 2 diabetes in survivors of critical illness. PLoS ONE 2016, 11, e0165923. [Google Scholar] [CrossRef]
- Abu-Ashour, W.; Twells, L.K.; Valcour, J.E.; Gamble, J.M. Diabetes and the occurrence of infection in primary care: A matched cohort study. BMC Infect. Dis. 2018, 18, 67. [Google Scholar] [CrossRef]
- Critchley, J.A.; Carey, I.M.; Harris, T.; DeWilde, S.; Hosking, F.J.; Cook, D.G. Glycaemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care 2018, 41, 2127–2135. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Infections and Autoimmunity- The Immune System and Vitamin D: A Systematic Review. Nutrients 2023, 15, 3842. [Google Scholar] [CrossRef]
- André, P.; Laugerette, F.; Féart, C. Metabolic endotoxemia: A potential underlying mechanism of the relationship between dietary fat intake and risk for cognitive impairments in humans? Nutrients 2019, 11, 1887. [Google Scholar] [CrossRef] [PubMed]
- Palacios, T.; Vitetta, L.; Coulson, S.; Madigan, C.D.; Lam, Y.Y.; Manuel, R.; Briskey, D.; Hendy, C.; Kim, J.N.; Ishoey, T.; et al. Targeting the intestinal microbiota to prevent type 2 diabetes and enhance the effect of metformin on glycaemia: A randomised controlled pilot study. Nutrients 2020, 12, 2041. [Google Scholar] [CrossRef] [PubMed]
- Al-Disi, D.; Ansari, M.G.; Sabico, S.; Wani, K.; Hussain, S.D.; Elshafie, M.M.; McTernan, P.; Al-Daghri, N.M. High glucose load and endotoxemia among overweight and obese Arab women with and without diabetes: An observational study. Medicine 2020, 99, e23211. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Reyes, J.; Escárcega-González, C.E.; Chavira-Suárez, E.; León-Buitimea, A.; Vázquez-León, P.; Morones-Ramírez, J.R.; Villalón, C.M.; Quintanar-Stephano, A.; Marichal-Cancino, B.A. Susceptibility for some infectious diseases in patients with diabetes: The key role of glycemia. Front. Public Health 2021, 9, 559595. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Yuan, K.; Zhou, L.; Wang, K.; Chen, H.N.; Lei, Y.; Lan, J. PRKAA/AMPK restricts HBV replication through the promotion of autophagic degradation. Autophagy 2016, 12, 1507–1520. [Google Scholar] [CrossRef] [PubMed]
- Yung, J.H.; Giacca, A. Role of c-Jun N-terminal kinase (JNK) in obesity and type 2 diabetes. Cells 2020, 9, 706. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C.; Tang, H.; Gao, S.; Sun, F.; Yang, Y.; Zhou, W.; Hu, Y.; Ke, C.; Wu, Y.; et al. CD2-associated protein contributes to Hepatitis C, Virus propagation and steatosis by disrupting insulin signalling. Hepatology 2018, 68, 1710–1725. [Google Scholar] [CrossRef]
- Esmailidehaj, M.; Kuchakzade, F.; Rezvani, M.E.; Farhadi, Z.; Esmaeili, H.; Azizian, H. 17β-Estradiol improves insulin signaling and insulin resistance in the aged female hearts: Role of inflammatory and anti-inflammatory cytokines. Life Sci. 2020, 253, 117673. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arneth, B. Insulin Resistance and Glucose Metabolism during Infection. Endocrines 2023, 4, 685-695. https://doi.org/10.3390/endocrines4040049
Arneth B. Insulin Resistance and Glucose Metabolism during Infection. Endocrines. 2023; 4(4):685-695. https://doi.org/10.3390/endocrines4040049
Chicago/Turabian StyleArneth, Borros. 2023. "Insulin Resistance and Glucose Metabolism during Infection" Endocrines 4, no. 4: 685-695. https://doi.org/10.3390/endocrines4040049
APA StyleArneth, B. (2023). Insulin Resistance and Glucose Metabolism during Infection. Endocrines, 4(4), 685-695. https://doi.org/10.3390/endocrines4040049






