Abstract
Dysmetabolic iron overload syndrome (DIOS) corresponds to the increase in iron stores associated with components of metabolic syndrome (MtS) and in the absence of an identifiable cause of iron excess. The objective of this work was to review the main aspects of DIOS. PUBMED and EMBASE were consulted, and PRISMA guidelines were followed. DIOS is usually asymptomatic and can be diagnosed by investigating MtS and steatosis. About 50% of the patients present altered hepatic biochemical tests (increased levels of γ-glutamyl transpeptidase itself or associated with increased levels of alanine aminotransferase). The liver may present parenchymal and mesenchymal iron overload, but the excess of iron is commonly mild. Steatosis or steatohepatitis is observed in half of the patients. Fibrosis is observed in about 15% of patients. Hyperferritinemia may damage the myocardium, liver, and several other tissues, increasing morbidity and mortality. Furthermore, DIOS is closely related to oxidative stress, which is closely associated with several pathological conditions such as inflammatory diseases, hypertension, diabetes, heart failure, and cancer. DIOS is becoming a relevant finding in the general population and can be associated with high morbidity/mortality. For these reasons, investigation of this condition could be an additional requirement for the early prevention of cardiovascular diseases.
1. Introduction
Cardiovascular diseases (CVD) are responsible for most causes of death worldwide and are strongly associated with metabolic syndrome (MtS), which comprises risk factors related to high morbidity. The primary underlying mechanism related to the onset and maintenance of CVD is atherosclerosis [1,2,3,4,5,6].
In addition to MtS, authors have described the occurrence of another condition, the dysmetabolic iron overload syndrome (DIOS) corresponds to the increase in the body iron stores, associated with components of MtS, and in the absence of identifiable cause of iron excess [7,8,9,10,11,12,13] (Figure 1). Most DIOS patients also possess nonalcoholic fatty liver disease (NAFLD), a condition that is best defined as metabolic associated fat liver disease (MAFLD) [14]. Although MAFLD, which is also a result of insulin resistance and MtS, is usually but not invariably followed by expanded body iron stores, iron depletion can attenuate steatosis in MAFLD [10,15,16,17]. The histological patterns found in DIOS associates both mesenchymal and parenchymal areas. The iron deposition on the hepatic reticuloendothelial system cells is implicated with increased hepatic apoptosis in MAFLD patients, additionally to higher percentages of advanced hepatic fibrosis, higher portal inflammation, and augmented hepatocellular ballooning [18,19,20,21,22,23]. Figure 1 summarizes DIOS definition visually.
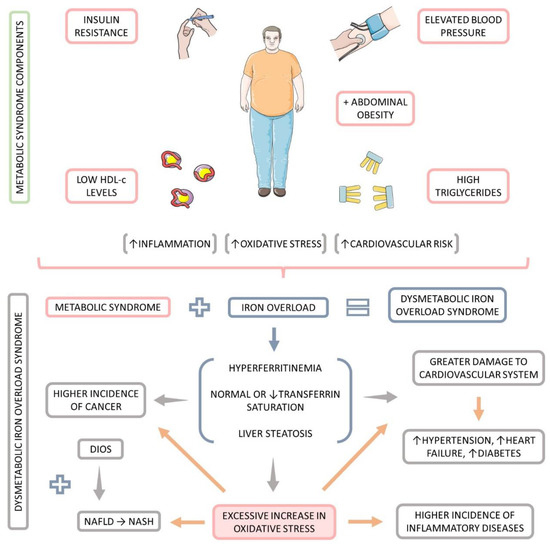
Figure 1.
Definition of dysmetabolic iron overload syndrome (DIOS). ↑: increase, ↓: decrease.
Iron is a fundamental element for the maintenance of homeostasis. It is essential to electron transport, oxygen transport, DNA synthesis, and several other actions, but it may also be toxic. Disruption in iron metabolism as observed in patients with DIOS may be multifactorial. Diets rich in iron associated with genetic factors may trigger the overload condition, related to crosstalk observed between liver and visceral adipose tissue. Macrophages seem to be especially important in the homeostasis of systemic iron levels, and ferroportin is the iron exporter and plays a role in mediating the exit of iron from macrophages to circulation [24,25,26,27].
DIOS is closely related to oxidative stress (OS), and this condition is closely associated with several pathological conditions such as inflammatory diseases, hypertension, diabetes, heart failure, and cancer [28,29,30,31].
The presence of MtS factors, hyperferritinemia, and altered transferrin saturation are disorders that represent a serious global problem. Both MAFLD and its aggressive form, nonalcoholic steatohepatitis (NASH), are linked with higher morbidity and mortality. The incidence of NASH is estimated to increase by more than 50% in the next ten years. Moreover, MAFLD incidence nowadays may vary from up to one-quarter of the world population and may be higher than 60% in diabetic patients and about 90% in patients who underwent bariatric surgery [10,32,33,34,35,36,37,38].
As DIOS has increased in incidence and due to its clinical importance, the aim of this study is to review the main aspects of this condition and perform a review of the existing studies with human beings.
2. Materials and Methods
2.1. Information Sources
We searched the electronic databases PubMed (Medline) and EMBASE. The keywords and MeSH (Medical Subject Headings) terms were dysmetabolic iron overload syndrome or DIOS or hyperferritinemia and insulin resistance or metabolic syndrome or obesity or dyslipidemia or NAFLD or NASH or cardiovascular diseases. The search included articles published until November 2022.
2.2. Study Selection
Only studies in English and with humans were included. Two independent reviewers (S.M.B. and L.F.L.) consulted the databases, and according to titles and abstracts, we independently retrieved relevant studies. Full-text articles were retrieved to support decision-making. Discordance between the two reviewers were evaluated by the other two reviewers (M.D.B. and R.d.A.G.).
2.3. Inclusion Criteria
We included the articles that investigated the presence of DIOS related to MtS, obesity, insulin resistance/diabetes, and oxidative stress.
2.4. Exclusion Criteria
Studies not in English, abstracts, poster presentations, reviews, and clinical guidelines were excluded from our search.
3. Results
Fifteen studies involving humans (totaling 1227 individuals) were included. Selection criteria included observational studies (cross-sectional, cohorts, and case-control) and interventional studies (quasi-experimental studies, randomized trials, open-label, and double-blind clinical trials). There was complete agreement among the reviewers regarding the selection of the studies. The results of this search are shown in Table 1.

Table 1.
Studies that investigated dysmetabolic iron overload syndrome in humans.
4. Discussion
The results of the revision are included in Table 1 and discussed at the end of this section. In addition, we have searched the literature for the survey of key points related to DIOS that are briefly commented below.
4.1. Ferritin and Hyperferritinemia
Iron is a crucial element for all living systems, essential for oxygen transport in hemoglobin and cellular energy production and serving as a cofactor or catalyst in several enzymatic processes. The iron-containing protein ferritin reflects the homeostasis of human iron storage and iron delivery, which is crucial for the maintenance of the anteriorly mentioned biological processes [54,55,56,57]. Figure 2 summarizes the iron metabolism in the human body.
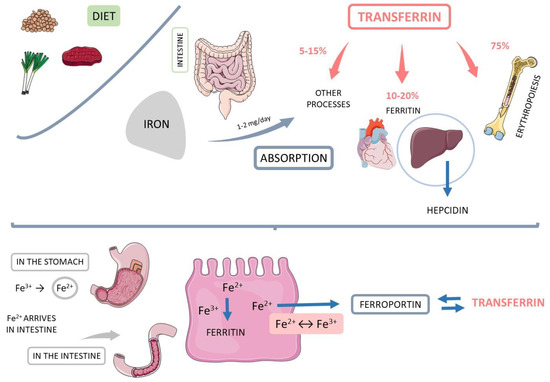
Figure 2.
Iron metabolism. Hb: hemoglobin.
Hyperferritinemia is a condition where excessively high levels of ferritin are observed, indirectly indicating iron overload, which damages the myocardium, the liver, and several other tissues, increasing morbidity and mortality (Figure 3). Nevertheless, more than 90% of hyperferritinemia cases are derived from four major causes: inflammatory conditions, cytolysis, alcoholism, and MtS. Another critical cause that should be seen separately is genetic hemochromatosis. The differentiation of these conditions can be performed with laboratory tests such as hemogram, transferrin saturation, liver function tests, creatinine phosphokinase (CPK), C reactive protein, glycemia, total cholesterol, and triglycerides. Immune and autoimmune affections can also possibly cause hyperferritinemia [54,58,59,60,61,62].
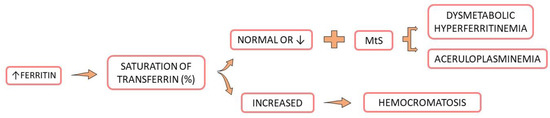
Figure 3.
Consequences of hyperferritinemia. Hyperferritinemia and normal or reduced saturation of transferrin and metabolic syndrome risk factors result in dysmetabolic hyperferritinemia. If the saturation of transferrin is elevated, the condition is probably hemochromatosis. MtS: metabolic syndrome, ↓: decrease.
Ferritin seems to play an essential role in angiogenesis, cells proliferation, and immunosuppression. Additionally, it can also be considered as an inflammatory acute-phase protein. Some studies suggested an association between high ferritin levels and chronic diseases, such as CVD and cancer. In CVD, ferritin can lead to a dual pro-inflammatory effect because high levels can represent an acute phase similar to C reactive protein. Low levels can also precipitate inflammation and, thus, increase the pro-inflammatory cytokines. These conditions can be related to CVD progression [54,59,63,64,65]. Hyperferritinemia has also been associated with NAFLD/MAFLD and with polycystic ovary syndrome [8,16,66,67].
4.2. Hepcidin
Hepcidin is a peptide hormone released by liver hepatocytes involved in regulating ferroportin expression, which is the major iron export protein presented in cells. Hepcidin can bind to ferroportin, leading to its internalization and degradation, which only reduces iron export from different vias. This process inhibits cellular iron exportation from macrophages. Hepcidin also inhibits iron uptake in the gut that, together with inhibition of recycling iron from macrophages, decreases iron levels in plasma. The axis hepcidin-ferroportin has a central regulatory role in iron homeostasis. In hereditary hemochromatosis, both the expression and function of hepcidin are disturbed and lead to increase in ferroportin due to the low circulating hepcidin levels resulting in augmentation of iron absorption in the gut and pathological deposition of this element in tissues [29,44,68,69,70].
Stimulation of hepcidin in the liver reduces ferroportin levels, resulting in the inhibition of cellular iron mobilization to the plasma. Acute inflammatory and infectious conditions may also increase hepcidin expression. Induction of hepcidin resulting from inflammatory processes possibly interferes with iron metabolism in acute or chronic inflammation disorders. In case of infections, hepcidin induction is associated with sequestration of intracellular iron, depriving microorganisms of this important factor. In chronic inflammation, hepcidin expression is increased principally due to interleukin 6 (IL-6) and Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) expressions [69,71,72].
A disrupted low synthesis of hepcidin has been associated with NAFLD and could help the iron uptake and increase predisposition for DIOS. Furthermore, both obesity and diabetes are related to augmenting hepcidin release. Marmur et al. [44], showed that in NAFLD subjects, hepcidin levels in serum and liver correlate to body iron stores. These authors found no association with body mass index (BMI), lipid parameters, the degree of steatohepatitis, or C reactive protein. In patients with DIOS and NAFLD, serum hepcidin levels are close to those observed in other hepatic diseases with iron overload, except for hereditary hemochromatosis. Authors postulate that an adequate hepcidin production in NAFLD in comparison to iron stores and the accumulation of iron in DIOS cannot be explained by a deficiency of hepcidin, differently from what is observed in hereditary hemochromatosis.
In animal models, the expression of hepcidin is inhibited in insulin resistance conditions. After administration of glucose, levels of hepcidin are elevated as well as in overweight and patients with NAFLD. The levels of hepcidin are also increased in patients with DIOS when comparing to obese patients with regular levels of ferritin. Elevated levels of hepcidin in DIOS may suggest hepcidin resistance [48].
4.3. Metabolic Syndrome
MtS is a cluster of conditions associated with the development of CVD. These factors may include insulin resistance, low levels of HDL-c, high levels of triglycerides, hypertension, altered values for waist circumference (WC), and obesity. These factors are associated with a complex dysregulation of iron homeostasis. Ferritin concentrations in serum increase with the number of risk factors of the MtS, especially insulin resistance, visceral fat mass, BMI, and hypertension. For these reasons, high ferritin levels have been related to a higher risk for the development of DM2 [5,46,60,73,74,75,76,77,78].
Furthermore, in subjects with morbid obesity, ferritin is strongly associated with IR and waist circumference. This may indicate that serum ferritin levels are closely related to important insulin resistance in overweight or obesity patients independent from other risk factors of the MtS. Particularly, serum ferritin concentrations can indicate severe liver insulin resistance and a higher risk for progression of relevant clinical hard endpoints such as cardiovascular death [79,80,81].
Serum ferritin levels are moderately elevated in MtS, but serum iron and transferrin saturation are usually normal, although transferrin saturation can be increased in up to 35% of cases. It is estimated that a third of MtS patients suffer from hyperferritinemia, although with normal transferrin saturation levels. The levels of hepcidin in serum can also be elevated in MtS [82,83].
An observational study with 1391 subjects (616 men and 775 women) showed that patients with MtS present significantly higher serum levels of ferritin and hepcidin than subjects without the syndrome. Iron regulatory feedback is preserved in MtS, and hepcidin tends to progressively augment in response to the augmented iron stores [84].
Risk factors of the MtS can also be related to inflammatory processes. Regardless of its cause, acute or chronic inflammation can augment ferritin levels in serum. Transferrin saturation may decrease or remain normal depending on DIOS parameters in this condition. In inflammatory processes, several cytokines are released, and especially IL-6 has a particular role in stimulating the production of ferritin and hepcidin. When there is an increase in hepcidin levels, iron sequestration in macrophages and enterocytes results in ferritin synthesis [26,58,80,83].
4.4. Oxidative Stress
OS in the cells is related to a significant imbalance of a plethora of biological signaling pathways. Reactive oxygen species (ROS) release usually happens via the reduction of molecular oxygen or due to oxidation of water. In balanced conditions, mitochondria produce ROS in consequence of aerobic respiration. Three to five percent of the oxygen is converted to ROS during this process. These molecular events require endogen enzymes such as superoxide dismutase, catalase, and glutathione peroxidase. When the ROS production surpasses the cell’s antioxidant capacity, a disrupted condition (OS) starts and causes cellular macromolecules damages such as nucleic acids, lipids, and proteins may occur. Increased levels of ROS can also accelerate cell apoptosis and necrosis due to the activation of poly (adenosine diphosphate ribose) polymerase related to the development of several pathological conditions. On the other hand, ROS are needed in the heart under basal conditions for regulating myocyte growth, maintaining vascular smooth muscle tone, and other critical cellular responses. OS is also related to the development of incapacitant and degenerative conditions, such as neurological and cancer [85,86,87,88,89,90,91,92].
OS is the main reason why iron and IR damage the liver tissue in both animals and humans. The consequences are related to damage to DNA, lipids, and protein, glutathione depletion, energy loss, increased release of pro-inflammatory cytokines, fibrogenesis, steatosis, and cell death. Iron may also induce liver injury due to its role in the up-regulation of cholesterol production, stress induction in the endoplasmic reticulum, and activation of macrophages and stellate cells [8,93,94]. Furthermore, iron is also associated with an increase in the release of inflammatory cytokines, associated with fibrosis, steatosis, and hepatocellular carcinoma [8,92,95,96]. Figure 4 shows the correlation between iron overload and OS leading to increased hepcidin levels.
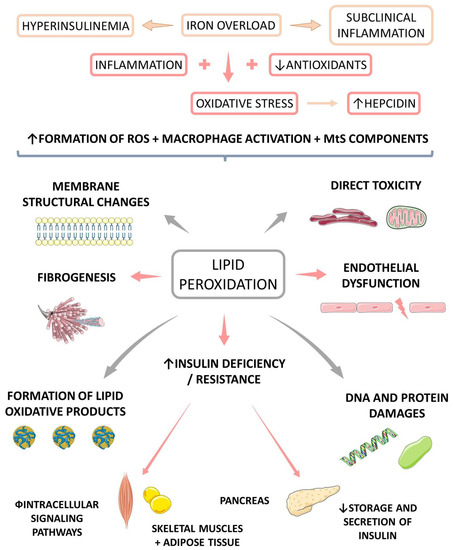
Figure 4.
Iron overload increases oxidative stress and is related to the increase in hepcidin levels. Furthermore, oxidative stress is associated with lipidic peroxidation that interferes in insulin secretion and may lead to insulin resistance (IR), diabetes (DM), fibrogenesis, and endothelial dysfunction. Iron overload is also associated with hyperinsulinemia and subclinical inflammation. ROS: reactive oxygen species, MtS: metabolic syndrome, ↑: increase, ↓: decrease.
The hepatic steatosis seems to result in an environment of the augmented OS, as well as IR, and necrotic signalization in obese patients leading to hepatic damage [92,97]. DM2 and fluctuations in glycemia may be related to several micro and macrovascular complications that may result from OS and inflammatory processes leading to endothelial dysfunction and aberrant angiogenic capacity. Furthermore, atherosclerosis is a chronic process that affects large and medium-sized arteries and is also a consequence of OS [93,98,99,100].
Iron is also now related to a new non-apoptotic form of cells-death, called ferroptosis. This phenomenon is caused mainly by redox imbalance and can occur through two mechanisms: transporter-dependent (extrinsic) and enzyme-dependent (intrinsic). In resume, when a cell produces more oxidants than antioxidants, an abnormal presence of ROS. In iron-overload syndrome, the production of ROS and lipid peroxidation are all events mediated by iron ions. Extrinsic ferroptosis is caused mainly by elevation of iron uptake in consequence of decreased cysteine or glutamine uptake. The intrinsic form is caused principally by inhibition of glutathione peroxidase 4 (GPX4). All these forms lead in final analyses to the accumulation of ROS and this accumulation to oxidative damage. Ferroptosis is finished if oxidation reaches the cell membrane. Although ferroptosis is a relatively new concept, it is implicated in various human diseases, such as neurodegenerative diseases, CVD, infectious, and cancer [57,101,102].
4.5. Dysmetabolic Iron Overload Syndrome
DIOS was defined in the 1990s and shows a close relationship between hepatic iron overload and features of MtS in subjects without apparent cause of iron overload. It is more frequent than genetic hemochromatosis, and most patients are middle-aged males [8,12,13,22,39,41,62,103]. Now that MtS is increasing worldwide, the presence of DIOS follows this tendency. Figure 5 shows some consequences of iron overload in β-cell, muscle, and adipocyte.
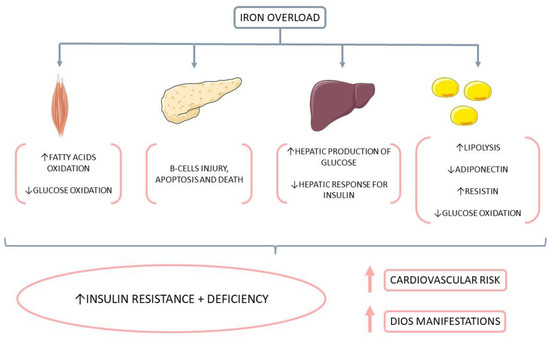
Figure 5.
Consequences of iron overload in β-cell, muscle, and adipocyte. Iron overload is related to a decrease in insulin release in β-cells. In muscle, it is associated with decreased glucose disposal and an increase in the oxidation of free fat acids (FFA). In adipocytes, there is a reduction in glucose uptake. This scenario contributes to insulin resistance (IR), diabetes mellitus (DM), and cardiovascular diseases (CVD). ↑: increase, ↓: decrease.
DIOS is usually asymptomatic and can be diagnosed in the investigation of MtS and steatosis. Ferritin levels in the serum are increased (up to 1000–1200 ng/mL), but iron levels and transferrin saturation are usually normal. About 50% of the patients present altered hepatic biochemical tests (increased levels of γ-glutamyl transpeptidase itself or associated with increased levels of alanine aminotransferase). The liver may present parenchymal and mesenchymal iron overload, but the excess of iron is commonly mild. Steatosis (defined by the accumulation of triglycerides and fatty acids in the liver) or steatohepatitis was observed in half of the patients. Fibrosis or cirrhosis are observed in about 15% of patients [16,39,43,104,105].
The mechanisms of occurrence of an iron overload in DIOS are not entirely understood. Impairment in hepcidin synthesis and an imbalanced regulation of iron export are involved. The modification of metabolism of iron observed in patients with DIOS may result from a complex process triggered by the excess of iron in the diet together with environmental and genetic factors associated with a crosstalk between the hepatic tissue and visceral adipose tissue [11,12,22,43,48,52,103].
In a rat model of NAFLD, Fujiwara et al. [13] evaluated the role of iron overload. The animals were treated with a high-fat and high-fructose diet leading to hepatic steatosis, dyslipidemia and increase in the body weight. Furthermore, they observed increased hepatic inflammation represented by iron deposition in sinusoidal macrophages/Kupffer cells, together with nuclear translocation of nuclear factor-κB (NF κB) and increase of pro-inflammatory cytokines (such as tumor necrosis factor-α (TNF-α), interferon-γ (IFN γ), and interleukin (IL)-1β) due to the upregulation of TH1/M1.
Studies with dietary iron loading animals showed increased resistin expression and reduced leptin levels. Increased resistin and decreased leptin levels contribute respectively to IR and increased appetite. These two conditions together increase the chances of developing obesity and DM. The metabolism of adipose tissue results in increased resistin, TNF-α, IFN-γ, interleukins (IL-6, IL-8, and IL-12), free fat acids (FFA), and a decrease in leptin, and IL-10 levels. This secretory pattern leads to an increase in the inflammatory activity in obese patients (low-grade inflammation) [106,107] showed that leptin directly interferes with iron metabolism. These authors showed that leptin levels were lower in leptin-deficient mice and higher in leptin receptor-deficient when compared with control. These results of this study suggest that the activation of the leptin receptor has effects on hepcidin expression. Authors concluded that this inappropriate signaling of this hormone could nearly link obesity, MtS, CVD, autoimmunity, and cancer. In this sense, iron-mediated OS could also be contributing to this unfavorable scenario.
The iron metabolism is commonly imbalanced in obese patients in both cellular and tissue levels. Moreover, adipose tissue plays an essential role in iron regulation. The presence of obesity may modify macrophage iron content in visceral adipose tissue in the liver and adipocytes. Furthermore, iron reduces the expression of adiponectin that is related to improving IR. The level of this adipokine is reduced in MtS subjects and negatively correlated with serum ferritin levels [8,83,108,109,110].
Higher ferritin levels are associated with obesity and overweight children and may be influenced by a liver injury that, associated with hypertrophied adipocytes and low-grade inflammation, follow the clinical course of overweight and obesity to modify the circulating markers of iron status. Still, the decreased absorption of iron observed in obesity can be the consequence of higher hepcidin levels that are also triggered by inflammatory processes. On the other hand, the levels of serum transferrin and serum iron decreases during inflammation [83,111,112,113].
Modifications in iron metabolism (ferritin or serum iron) are also associated with altered lipid concentration in adults and children [114]. Gonzalez-Dominguez et al. and Zhu et al. [73,83] showed that dyslipidemic children and adolescents presented lower levels of serum iron and increased ferritin levels in the obese.
Figure 6 summarizes the effects of increased visceral fat accumulation and oxidative stress in the pathogenesis of diabetes and cardiovascular diseases.
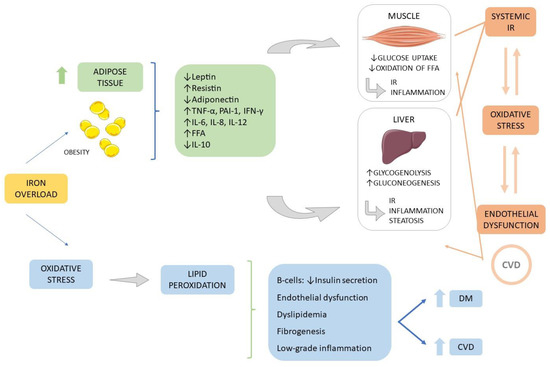
Figure 6.
Obesity and oxidative stress in the pathogenesis of Diabetes mellitus and cardiovascular diseases. Increased abdominal adipose tissue is related to the release of pro-inflammatory cytokines (such as resistin, TNF-α, IFN-γ, IL-6, and IL-8), an increase of free fatty acids, reduction in adiponectin release and reduction of the anti-inflammatory IL-10). This scenario leads to an imbalance in the metabolism of glucose in the liver and muscles resulting in insulin resistance and the installation of an inflammatory scenario related to the development of DM and CVD. Associated with this scenario is also installed oxidative stress, which in turn is also associated with DM and CVD. CVD: cardiovascular disease; DM: diabetes mellitus; FFA: free fat acid; PAI-1: inhibitor of plasminogen activator; INF-γ: interferon-γ; IL: interleukin; IR: insulin resistance; TNFα: tumor necrosis factor-α, ↑: increase, ↓: decrease.
Dongiovanni et al. [109] showed that an iron-enriched diet supplementation augmented hepatic iron and serum hepcidin in mice. An increment of 40% in fasting glucose is related to insulin resistance and increased triglycerides. Supplemented animals showed decreased visceral adipose tissue mass plus the accumulation of iron in adipocytes. These animals also showed reduced insulin signaling in the visceral adipose tissue and up-regulation of iron-responsive genes and adipokines. These conditions led to insulin resistance, down-regulation of lipoprotein lipase, and hyperresistinemia. Furthermore, acute administration of hepcidin down-regulated lipoprotein lipase.
In Table 1 it is shown fifteen studies that investigated the presence of DIOS in humans. These studies are commented below.
Lahaye et al. [39] conducted a case-control study with 60 MtS, DIOS, or healthy participants to evaluate if MtS or DIOS alters macrophage profiles. The results showed no differences between the expression of inflammatory genes, but there were differences between the expression of genes related to iron metabolism between individuals with MtS and DIOS. Although iron metabolism was altered in these patients, there was an up regulatory activity of transferrin receptor 1 (TFRC) expression, which is important in limiting iron toxicity in patients affected by DIOS. A possible bias to this study is the small number of included patients.
Vaquero et al. [40] conducted a cross-sectional study with 50 overweight participants to evaluate if there was a relationship between iron status and biomarkers for insulin resistance. Although the results showed that in obese/overweight individuals, the iron transport and the iron storage are altered, these occurred without iron overload or deficiency. Although this study presented sample calculation, the sample size was small.
In a prospective study, Castiella et al. [41] evaluated 276 participants with hyperferritinemia (135 with MtS) and found no significant differences in liver iron concentrations between individuals with hyperferritinemia affected or not by MtS. This study presented a large sample size, which can be a strength.
Lobbes et al. [42] showed the use of proanthocyanidin does not interfere in iron absorption both in DIOS or in hemochromatosis (HH) patients. Since this is a cross-over trial, the authors did not show the characteristics of the subjects included in the study.
In a cross-sectional study, Rauber et al. [43] found that hepcidin can be assessed to investigate ferritin increases among MtS patients. However, the included individuals were not evaluated for iron overload-deposit in hepatocytes, limiting the study’s generalizability. Nevertheless, these patients were classified for hemochromatosis C282Y and H63D genes mutations.
Marmur et al. [44] found that hepcidin levels are higher in patients with liver disease, both DIOS and NAFLD. In this study, the authors indicated 84 individuals in the Methods section but then presented 85 patients distributed according to the ferritin level. Although they used a sample of 84 patients in the study, the number of members in each group was small (n = 5–22).
Stechemesser et al. [46] showed that patients with MtS and high levels of ferritin present higher values for glycemia and HBA1c than patients without hyperferritinemia. The individuals in the control group had lower BMI and waist-to-hip ratio than the other groups, although at a similar age.
Rametta [48] also verified that DIOS patients present higher levels of hepcidin and even higher transferrin saturation (these patients show an imbalance in hepcidin’s ability to control iron absorption, suggesting hepcidin resistance). The control group followed in this study consisted of members with differences concerning patient groups regarding gender (more women), age (younger), and nutritional status (BMI and lower waist circumference). Furthermore, there is a reduced number of individuals in each group (n = 10–18) and less sensitive techniques in the analysis of some study variables, such as iron absorption and serum hepcidin dosage.
Ruivard et al. [115] showed that ferritin levels are reduced in patients submitted to venesection, but they did not observe an improvement of the metabolic and hepatic characteristics after this intervention. Jézéquel et al. [49] also studied patients submitted to venesection and noted that transferrin levels were lower in DIOS. This study also included a small number of patients (n = 24), one half of whom presented with DIOS, and the other was the control group. Furthermore, the authors used statistical methodology to calculate the sample; however, at the end of the article, they point out the need for studies with a larger number of individuals. [103] also investigated the effects of the venesection and found that individuals with high ferritin levels are at risk of iron overload recurrence. These authors indicated a limitation of the study that many participants have a genetic mutation, which may interfere with iron reaccumulation.
Dongiovanni et al. [50] found that the levels of hepatic TfR-1 mRNA were upregulated in subjects with fatty liver and DIOS. In this study, the authors included a small number of control patients (n = 10) compared to sick individuals (n = 46). The design of the study presents differences in several important variables, including gender (more men than women), age (older), and BMI (lowest). However, they did not apply statistical tests to infer the magnitude of such differences. Limitations were also observed in the study by [51], who used a control group with healthy individuals significantly younger than the group with the disease; in addition, the authors did not present or analyze any other variable to characterize similarities or differences between the groups, such as BMI or waist circumference. In this, the authors showed that hepcidin levels are reduced after iron depletion. A populational study (Nicola Martinelli et al., 2012) showed that hepcidin levels tend to be as high as the iron stores, despite using a small number of patients.
Ruivard et al. [52] also developed a study to compare intestinal absorption of iron in men with or without DIOS and concluded that intestinal iron absorption was lower in DIOS compared to both overweight and lean controls. However, this study did not control the dietary intake of iron and its absorptive facilitators or inhibitors. For this reason, bias should be considered in this research.
We can observe from the studies shown in Table 1 that there are significant limitations to consider. The main ones can be the small sample number in various studies; male prevalence, which hinders the perception of the disease in women; the presence of genetic mutations, which may interfere with the evaluated results; no investigation of daily iron intake; limitations of laboratory techniques for assessing serum or urine hepcidin levels.
Management of DIOS may involve the treatment of overweight, hyperlipidemia, hypertension, and insulin resistance/diabetes. Although managing these risk factors is essential, it is not sufficient to normalize body iron stores that usually excess mildly and average 100 μmol/g [116,117,118]. Modifications in lifestyle and iron intake are also in the first line of therapeutic intervention in patients with DIOS [8].
4.6. Future Directions
An increase in the hepatic and body iron stores and the presence of MtS components characterizes DIOS. Although increasing evidence suggests an overlap between NAFLD with iron overload and, therefore, DIOS, the pathogenic pathways involved in DIOS occurrence are still not completely understood. Due to these reasons, soon, not only animal models sharing similarities with human patients with DIOS must be designed, but also non-invasive methods for iron overload evaluation in dysmetabolic human patients must be improved. Furthermore, there is a need to investigate how iron overload overlaps with NAFLD and causes dysmetabolic changes in the body’s homeostasis [13,62]. Since a mild to moderate excess of iron in the liver can aggravate the risk of NAFLD progression to NASH, it is also necessary to unravel the mechanistic events by which pro-inflammatory cytokines, ROS, OS, and lipid peroxidation can be related to iron overload toxicity [22].
The pathways of how DIOS affects other diseases, such as inflammatory and immunomodulated diseases, must also be a field of study. Authors highlighted that in DIOS, macrophages might present impaired polarization toward the M2 alternative phenotype, which is considered an adaptative role of the up-regulation of the TFRC in DIOS macrophages that may limit iron toxicity during the dysmetabolic process [39]. However, these events can affect the occurrence and progression of diseases other than DIOS, like atherosclerosis, arthritis, cardiometabolic affections, and other degenerative conditions. The relationship between iron status and obesity is another field that must be highlighted [40], principally because individuals with augmented visceral adiposity and waist circumference do not necessarily have increased body mass index.
5. Conclusions
DIOS is becoming a relevant finding in the general population and can be associated with altered function of adipose tissue, immunological cells, inflammation, oxidative stress, insulin resistance, diabetes, cardiovascular diseases, and cardiovascular risk factors. In this context, the need to investigate serum ferritin levels and transferrin saturation in clinical practice in patients with obesity, MtS, MAFLD, and CVD becomes imperative.
Although the findings of DIOS are still heterogeneous, iron overload degrades cell functions and survival, principally due to ferroptosis. Mostly in MAFLD and NASH, excessive iron deposition impairs the liver’s function and leads to organ failure due to toxicity. Because of these reasons, DIOS investigation should be everyday practice in clinical care.
Author Contributions
Conceptualization, S.M.B., L.F.L., R.J.T., U.A.P.F., C.G.M., R.d.A.G., A.M.G.M.B. and M.D.B.; methodology, S.M.B., L.F.L. and M.D.B.; software, S.M.B., R.d.A.G., A.M.G.M.B. and M.D.B.; validation, S.M.B., L.F.L., R.J.T., U.A.P.F., C.G.M., R.d.A.G., A.M.G.M.B. and M.D.B., S.M.B., L.F.L., R.J.T., U.A.P.F., C.G.M., R.d.A.G., A.M.G.M.B. and M.D.B.; formal analysis, S.M.B., R.J.T., U.A.P.F., C.G.M. and M.D.B.; investigation, S.M.B., L.F.L., R.J.T., U.A.P.F., C.G.M., R.d.A.G., A.M.G.M.B. and M.D.B.; resources, S.M.B., L.F.L., R.J.T., U.A.P.F., C.G.M., R.d.A.G., A.M.G.M.B. and M.D.B.; data curation, S.M.B. and M.D.B.; writing—original draft preparation, S.M.B., L.F.L., R.J.T., U.A.P.F., A.M.G.M.B. and M.D.B.; writing—review and editing, S.M.B. and M.D.B.; supervision, S.M.B. and M.D.B.; project administration, S.M.B. and M.D.B.; funding acquisition, S.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors declare attribution to Smart Servier (https://smart.servier.com/ (accessed on 12 November 2022)) due to the provision of some scientific images that were used in this article under an attribution license of public copyrights https://creativecommons.org/licenses/by/3.0/ (accessed on 12 November 2022) and under disclaimer of warranties. All Smart Servier’s images were not changed in the writing of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barbalho, S.M.; Tofano, R.J.; Chagas, E.F.B.; Detregiachi, C.R.P.; Goulart, A.R.; Flato, U.A.P. Benchside to the bedside of frailty and cardiovascular aging: Main shared cellular and molecular mechanisms. Exp. Gerontol. 2021, 148, 111302. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Badimon, L.; Montecucco, F.; Lüscher, T.F.; Libby, P.; Camici, G.G. Inflammation, Aging, and Cardiovascular Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 79, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Aboonabi, A.; Meyer, R.R.; Singh, I. The association between metabolic syndrome components and the development of atherosclerosis. J. Hum. Hypertens. 2019, 33, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Saedi, S.; Watson, S.E.; Young, J.L.; Tan, Y.; Wintergerst, K.A.; Cai, L. Does maternal low-dose cadmium exposure increase the risk of offspring to develop metabolic syndrome and/or type 2 diabetes? Life Sci. 2023, 9, 121385. [Google Scholar] [CrossRef] [PubMed]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes/Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- Sinatora, R.V.; Chagas, E.F.B.; Mattera, F.O.P.; Mellem, L.J.; Santos, A.; Pereira, L.P.; Aranão, A.L.C.; Guiguer, E.L.; Araújo, A.C.; Haber, J.; et al. Relationship of Inflammatory Markers and Metabolic Syndrome in Postmenopausal Women. Metabolites 2022, 12, 73. [Google Scholar] [CrossRef]
- Dos Santos Vieira, D.A.; Hermes Sales, C.; Galvão Cesar, C.L.; Marchioni, D.M.; Fisberg, R.M. Influence of Haem, Non-Haem, and Total Iron Intake on Metabolic Syndrome and Its Components: A Population-Based Study. Nutrients 2018, 10, 314. [Google Scholar] [CrossRef]
- Li, H.; Lin, L.; Xia, Y.L.; Xie, Y.; Yang, X. Research progress on the role of ferroptosis in cardiovascular disease. Front Cardiovasc Med. 2022, 22, 1077332. [Google Scholar] [CrossRef]
- Lainé, F.; Reymann, J.M.; Morel, F.; Langouët, S.; Perrin, M.; Guillygomarc’h, A.; Brissot, P.; Turmel, V.; Mouchel, C.; Pape, D.; et al. Effects of phlebotomy therapy on cytochrome P450 2e1 activity and oxidative stress markers in dysmetabolic iron overload syndrome: A randomized trial. Aliment. Pharmacol. Ther. 2006, 24, 1207–1213. [Google Scholar] [CrossRef]
- Qiu, R.; Alikhanyan, K.; Volk, N.; Marques, O.; Mertens, C.; Agarvas, A.R.; Singh, S.; Pepperkok, R.; Altamura, S.; Muckenthaler, M.U. Repression of the iron exporter ferroportin may contribute to hepatocyte iron overload in individuals with type 2 diabetes. Mol. Metab. 2022, 66, 101644. [Google Scholar] [CrossRef]
- Sachinidis, A.; Doumas, M.; Imprialos, K.; Stavropoulos, K.; Katsimardou, A.; Athyros, V.G. Dysmetabolic Iron Overload in Metabolic Syndrome. Curr. Pharm. Des. 2020, 26, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Castiella, A.; Urreta, I.; Zapata, E.; de Juan, M.; Alústiza, J.M.; Emparanza, J.I. Dysmetabolic iron overload syndrome and its relationship with HFE gene mutations and with liver steatosis. Dig. Liver Dis. 2020, 52, 683–685. [Google Scholar] [CrossRef]
- Fujiwara, S.; Izawa, T.; Mori, M.; Atarashi, M.; Yamate, J.; Kuwamura, M. Dietary iron overload enhances Western diet induced hepatic inflammation and alters lipid metabolism in rats sharing similarity with human DIOS. Sci. Rep. 2022, 12, 21414. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Sánchez, N.; Bugianesi, E.; Gish, R.G.; Lammert, F.; Tilg, H.; Nguyen, M.H.; Sarin, S.K.; Fabrellas, N.; Zelber-Sagi, S.; Fan, J.G.; et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol. Hepatol. 2022, 7, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Gattermann, N.; Muckenthaler, M.U.; Kulozik, A.E.; Metzgeroth, G.; Hastka, J. The Evaluation of Iron Deficiency and Iron Overload. Dtsch. Arztebl. Int. 2021, 118, 847–856. [Google Scholar] [CrossRef]
- Moris, W.; Verhaegh, P.; Jonkers, D.; Deursen, C.V.; Koek, G. Hyperferritinemia in Nonalcoholic Fatty Liver Disease: Iron Accumulation or Inflammation? Semin. Liver Dis. 2019, 39, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.H.G.; Ross, D.G.F.; Jaskowski, L.-A.; Burke, L.J.; Britton, L.J.; Musgrave, N.; Briskey, D.; Rishi, G.; Bridle, K.R.; Subramaniam, V.N. Iron depletion attenuates steatosis in a mouse model of non-alcoholic fatty liver disease: Role of iron-dependent pathways. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166142. [Google Scholar] [CrossRef] [PubMed]
- Deugnier, Y.; Turlin, B. Iron and hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2001, 16, 491–494. [Google Scholar] [CrossRef]
- Tam, E.; Sung, H.K.; Lam, N.H.; You, S.; Cho, S.; Ahmed, S.M.; Abdul-Sater, A.A.; Sweeney, G. Role of Mitochondrial Iron Overload in Mediating Cell Death in H9c2 Cells. Cells 2022, 28, 118. [Google Scholar] [CrossRef]
- Maliken, B.D.; Nelson, J.E.; Klintworth, H.M.; Beauchamp, M.; Yeh, M.M.; Kowdley, K.V. Hepatic reticuloendothelial system cell iron deposition is associated with increased apoptosis in nonalcoholic fatty liver disease. Hepatology 2013, 57, 1806–1813. [Google Scholar] [CrossRef]
- Nelson, J.E.; Wilson, L.; Brunt, E.M.; Yeh, M.M.; Kleiner, D.E.; Unalp-Arida, A.; Kowdley, K.V. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology 2011, 53, 448–457. [Google Scholar] [CrossRef]
- Fernandez, M.; Lokan, J.; Leung, C.; Grigg, A. A critical evaluation of the role of iron overload in fatty liver disease. J. Gastroenterol. Hepatol. 2022, 37, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Ameka, M.; Hasty, A.H. Paying the Iron Price: Liver Iron Homeostasis and Metabolic Disease. Compr. Physiol. 2022, 12, 3641–3663. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Golabi, P.; Paik, J.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): A systematic review. Hepatology. 2023. [Google Scholar] [CrossRef]
- Camaschella, C.; Nai, A.; Silvestri, L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Kang, R.; Tang, D. Iron Metabolism in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226. [Google Scholar] [CrossRef]
- Xiang, Y.; Fan, X.; Zhao, M.; Guo, Q.; Guo, S. CKIP-1 alleviates oxygen-glucose deprivation/reoxygenation-induced apoptosis and oxidative stress in cultured hippocampal neurons by downregulating Keap1 and activating Nrf2/ARE signaling. Eur. J. Pharmacol. 2019, 848, 140–149. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, W.; Yan, X.; Huang, T.; Yang, A. Overexpression of Hepcidin Alleviates Steatohepatitis and Fibrosis in a Diet-induced Nonalcoholic Steatohepatitis. J. Clin. Transl. Hepatol. 2022, 10, 577–588. [Google Scholar] [CrossRef]
- Kim, C.H.; Leitch, H.A. Iron overload-induced oxidative stress in myelodysplastic syndromes and its cellular sequelae. Crit. Rev. Oncol./Hematol. 2021, 163, 103367. [Google Scholar] [CrossRef]
- Gordan, R.; Fefelova, N.; Gwathmey, J.K.; Xie, L.-H. Iron Overload, Oxidative Stress and Calcium Mishandling in Cardiomyocytes: Role of the Mitochondrial Permeability Transition Pore. Antioxidants 2020, 9, 758. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Lazo, M.; Hernaez, R.; Eberhardt, M.S.; Bonekamp, S.; Kamel, I.; Guallar, E.; Koteish, A.; Brancati, F.L.; Clark, J.M. Prevalence of nonalcoholic fatty liver disease in the United States: The Third National Health and Nutrition Examination Survey, 1988-1994. Am. J. Epidemiol. 2013, 178, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Murali, A.R.; Gupta, A.; Brown, K. Systematic review and meta-analysis to determine the impact of iron depletion in dysmetabolic iron overload syndrome and non-alcoholic fatty liver disease. Hepatol. Res. 2018, 48, E30–E41. [Google Scholar] [CrossRef]
- Satiya, J.; Snyder, H.S.; Singh, S.P.; Satapathy, S.K. Narrative review of current and emerging pharmacological therapies for nonalcoholic steatohepatitis. Transl. Gastroenterol. Hepatol. 2021, 6, 60. [Google Scholar] [CrossRef]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef]
- Carpi, R.Z.; Barbalho, S.M.; Sloan, K.P.; Laurindo, L.F.; Gonzaga, H.F.; Grippa, P.C.; Zutin, T.L.M.; Girio, R.J.S.; Repetti, C.S.F.; Detregiachi, C.R.P.; et al. The Effects of Probiotics, Prebiotics and Synbiotics in Non-Alcoholic Fat Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH): A Systematic Review. Int. J. Mol. Sci. 2022, 23, 8805. [Google Scholar] [CrossRef]
- Chen, H.; Zhan, Y.; Zhang, J.; Cheng, S.; Zhou, Y.; Chen, L.; Zeng, Z. The Global, Regional, and National Burden and Trends of NAFLD in 204 Countries and Territories: An Analysis From Global Burden of Disease 2019. JMIR Public Health Surveill. 2022, 8, e34809. [Google Scholar] [CrossRef]
- Lahaye, C.; Gladine, C.; Pereira, B.; Berger, J.; Chinetti-Gbaguidi, G.; Lainé, F.; Mazur, A.; Ruivard, M. Does iron overload in metabolic syndrome affect macrophage profile? A case control study. J. Trace Elem. Med. Biol. 2021, 67, 126786. [Google Scholar] [CrossRef]
- Vaquero, M.P.; Martínez-Maqueda, D.; Gallego-Narbón, A.; Zapatera, B.; Pérez-Jiménez, J. Relationship between iron status markers and insulin resistance: An exploratory study in subjects with excess body weight. PeerJ 2020, 8, e9528. [Google Scholar] [CrossRef]
- Castiella, A.; Urreta, I.; Zapata, E.; Zubiaurre, L.; Alústiza, J.M.; Otazua, P.; Salvador, E.; Letamendi, G.; Arrizabalaga, B.; Rincón, M.L.; et al. Liver iron concentration in dysmetabolic hyperferritinemia: Results from a prospective cohort of 276 patients. Ann. Hepatol. 2020, 19, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Lobbes, H.; Gladine, C.; Mazur, A.; Pereira, B.; Dualé, C.; Cardot, J.-M.; Ruivard, M. Effect of procyanidin on dietary iron absorption in hereditary hemochromatosis and in dysmetabolic iron overload syndrome: A crossover double-blind randomized controlled trial. Clin. Nutr. 2020, 39, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Rauber, M.R.; Pilger, D.A.; Cecconello, D.K.; Falcetta, F.S.; Marcondes, N.A.; Faulhaber, G.A.M. Hepcidin is a useful biomarker to evaluate hyperferritinemia associated with metabolic syndrome. Acad. Bras. Cienc. 2019, 91, e20180286. [Google Scholar] [CrossRef] [PubMed]
- Marmur, J.; Beshara, S.; Eggertsen, G.; Onelöv, L.; Albiin, N.; Danielsson, O.; Hultcrantz, R.; Stål, P. Hepcidin levels correlate to liver iron content, but not steatohepatitis, in non-alcoholic fatty liver disease. BMC Gastroenterol. 2018, 18, 78. [Google Scholar] [CrossRef] [PubMed]
- Lainé, F.; Ruivard, M.; Loustaud-Ratti, V.; Bonnet, F.; Calès, P.; Bardou-Jacquet, E.; Sacher-Huvelin, S.; Causse, X.; Beusnel, C.; Renault, A. Metabolic and hepatic effects of bloodletting in dysmetabolic iron overload syndrome: A randomized controlled study in 274 patients. Hepatology 2017, 65, 465–474. [Google Scholar] [CrossRef]
- Stechemesser, L.; Eder, S.K.; Wagner, A.; Patsch, W.; Feldman, A.; Strasser, M.; Auer, S.; Niederseer, D.; Huber-Schönauer, U.; Paulweber, B. Metabolomic profiling identifies potential pathways involved in the interaction of iron homeostasis with glucose metabolism. Mol. Metab. 2017, 6, 38–47. [Google Scholar] [CrossRef]
- Deugnier, Y.; Bardou-Jacquet, É.; Lainé, F. Dysmetabolic iron overload syndrome (DIOS). La Presse Méd. 2017, 46, e306–e311. [Google Scholar] [CrossRef]
- Rametta, R.; Dongiovanni, P.; Pelusi, S.; Francione, P.; Iuculano, F.; Borroni, V.; Fatta, E.; Castagna, A.; Girelli, D.; Fargion, S. Hepcidin resistance in dysmetabolic iron overload. Liver Int. 2016, 36, 1540–1548. [Google Scholar] [CrossRef]
- Jézéquel, C.; Lainé, F.; Laviolle, B.; Kiani, A.; Bardou-Jacquet, E.; Deugnier, Y. Both hepatic and body iron stores are increased in dysmetabolic iron overload syndrome. A case-control study. PLoS ONE 2015, 10, e0128530. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Lanti, C.; Gatti, S.; Rametta, R.; Recalcati, S.; Maggioni, M.; Fracanzani, A.L.; Riso, P.; Cairo, G.; Fargion, S.; et al. High fat diet subverts hepatocellular iron uptake determining dysmetabolic iron overload. PLoS ONE 2015, 10, e0116855. [Google Scholar] [CrossRef]
- Trombini, P.; Paolini, V.; Pelucchi, S.; Mariani, R.; Nemeth, E.; Ganz, T.; Piperno, A. Hepcidin response to acute iron intake and chronic iron loading in dysmetabolic iron overload syndrome. Liver Int. 2011, 31, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Ruivard, M.; Lainé, F.; Ganz, T.; Olbina, G.; Westerman, M.; Nemeth, E.; Rambeau, M.; Mazur, A.; Gerbaud, L.; Tournilhac, V. Iron absorption in dysmetabolic iron overload syndrome is decreased and correlates with increased plasma hepcidin. J. Hepatol. 2009, 50, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Chang, S.D.; Sreenivasan, G.M.; Tsang, P.W.; Broady, R.C.; Li, C.H.; Zypchen, L.N. Dysmetabolic hyperferritinemia is associated with normal transferrin saturation, mild hepatic iron overload, and elevated hepcidin. Ann. Hematol. 2011, 90, 139–143. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Biddulph, J.P.; Rafnsson, S.B.; Trivella, M.; Nihoyannopoulos, P.; Demakakos, P. The association of ferritin with cardiovascular and all-cause mortality in community-dwellers: The English longitudinal study of ageing. PLoS ONE 2017, 12, e0178994. [Google Scholar] [CrossRef] [PubMed]
- Shander, A.; Goodnough, L.T.; Javidroozi, M.; Auerbach, M.; Carson, J.; Ershler, W.B.; Ghiglione, M.; Glaspy, J.; Lew, I. Iron deficiency anemia—Bridging the knowledge and practice gap. Transfus. Med. Rev. 2014, 28, 156–166. [Google Scholar] [CrossRef]
- Takatoku, M. Japanese National Research Group on Idiopathic Bone Marrow Failure Syndromes. Retrospective nationwide survey of Japanese patients with transfusion-dependent MDS and aplastic anemia highlights the negative impact of iron overload on morbidity/mortality. Eur. J. Haematol. 2007, 78, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Plays, M.; Müller, S.; Rodriguez, R. Chemistry and biology of ferritin. Metallomics 2021, 13, mfab021. [Google Scholar] [CrossRef] [PubMed]
- Lorcerie, B.; Audia, S.; Samson, M.; Millière, A.; Falvo, N.; Leguy-Seguin, V.; Berthier, S.; Bonnotte, B. Diagnosis of hyperferritinemia in routine clinical practice. La Presse Méd. 2017, 46, e329–e338. [Google Scholar] [CrossRef] [PubMed]
- Sandnes, M.; Ulvik, R.J.; Vorland, M.; Reikvam, H. Hyperferritinemia—A Clinical Overview. J. Clin. Med. 2021, 10, 2008. [Google Scholar] [CrossRef] [PubMed]
- Tofano, R.J.; Pescinni-Salzedas, L.M.; Chagas, E.F.B.; Detregiachi, C.R.P.; Guiguer, E.L.; Araujo, A.C.; Bechara, M.D.; Rubira, C.J.; Barbalho, S.M. Association of Metabolic Syndrome and Hyperferritinemia in Patients at Cardiovascular Risk. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3239–3248. [Google Scholar] [CrossRef]
- Barreto, B.F.M.; Punaro, G.R.; Elias, M.C.; Parise, E.R. Is homeostasis model assessment for insulin resistance > 2.5 a distinguished criteria for metabolic dysfunction-associated fatty liver disease identification? Arq. Gastroenterol. 2022, 59, 402–407. [Google Scholar] [CrossRef]
- Branisso, P.P.F.; de Oliveira, C.; Filho, H.M.L.; Lima, F.R.; Santos, A.S.; Mancini, M.C.; de Melo, M.E.; Carrilho, F.J.; Rocha, M.S.; Clark, P.; et al. Non-invasive methods for iron overload evaluation in dysmetabolic patients. Ann. Hepatol. 2022, 27, 100707. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, J.; Wei, L.; He, B.; Wang, C.; Wang, B. Iron deficiency activates pro-inflammatory signaling in macrophages and foam cells via the p38 MAPK-NF-κB pathway. Int. J. Cardiol. 2011, 152, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.C.; Kang, J.H.; Shin, H.S. Relationship of cardiovascular risk factors and serum ferritin with C-reactive protein. Arch. Med. Res. 2007, 38, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Jang, H.; Gupta, B.; Jeong, J.H.; Ge, Y.; Yong, C.S.; Kim, J.O.; Bae, J.S.; Song, I.S.; Kim, I.S.; et al. Tie2-mediated vascular remodeling by ferritin-based protein C nanoparticles confers antitumor and anti-metastatic activities. J. Hematol. Oncol. 2020, 13, 123. [Google Scholar] [CrossRef]
- Adamska, A.; Łebkowska, A.; Krentowska, A.; Adamski, M.; Kowalska, I. The Association Between Serum Ferritin Concentration and Visceral Adiposity Estimated by Whole-Body DXA Scan in Women With Polycystic Ovary Syndrome. Front. Endocrinol. 2020, 10, 873. [Google Scholar] [CrossRef]
- Trasolini, R.; Cox, B.; Galts, C.; Yoshida, E.M.; Marquez, V. Elevated serum ferritin in non-alcoholic fatty liver disease is not predictive of fibrosis. Can. Liver J. 2022, 5, 152–159. [Google Scholar] [CrossRef]
- Kanamori, Y.; Murakami, M.; Sugiyama, M.; Hashimoto, O.; Matsui, T.; Funaba, M. Hepcidin and IL-1β. Vitam. Horm. 2019, 110, 143–156. [Google Scholar]
- Kowdley, K.V.; Gochanour, E.M.; Sundaram, V.; Shah, R.A.; Handa, P. Hepcidin Signaling in Health and Disease: Ironing Out the Details. Hepatol. Commun. 2021, 5, 723–735. [Google Scholar] [CrossRef]
- Zhou, W.; Qiu, K. The correlation between lncRNA NEAT1 and serum hepcidin in the peripheral blood of non-alcoholic fatty liver disease patients. Am. J. Transl. Res. 2022, 14, 2593–2599. [Google Scholar]
- Gozzelino, R.; Arosio, P. Iron homeostasis in health and disease. Int. J. Mol. Sci. 2016, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Meynard, D.; Babitt, J.L.; Lin, H.Y. The Journal of the American Society of Hematology. The liver: Conductor of systemic iron balance. Blood J. Am. Soc. Hematol. 2014, 123, 168–176. [Google Scholar]
- Zhu, Y.; He, B.; Xiao, Y.; Chen, Y. Iron metabolism and its association with dyslipidemia risk in children and adolescents: A cross-sectional study. Lipids Health Dis. 2019, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Datz, C.; Müller, E.; Aigner, E. Iron overload and non-alcoholic fatty liver disease. Minerva Endocrinol. 2016, 42, 173–183. [Google Scholar] [CrossRef]
- Iwasaki, T.; Nakajima, A.; Yoneda, M.; Yamada, Y.; Mukasa, K.; Fujita, K.; Fujisawa, N.; Wada, K.; Terauchi, Y. Serum ferritin is associated with visceral fat area and subcutaneous fat area. Diabetes Care 2005, 28, 2486–2491. [Google Scholar] [CrossRef] [PubMed]
- Piperno, A.; Trombini, P.; Gelosa, M.; Mauri, V.; Pecci, V.; Vergani, A.; Salvioni, A.; Mariani, R.; Mancia, G. Increased serum ferritin is common in men with essential hypertension. J. Hypertens. 2002, 20, 1513–1518. [Google Scholar] [CrossRef]
- Kao, T.-W.; Huang, C.-C. Recent Progress in Metabolic Syndrome Research and Therapeutics. Int. J. Mol. Sci. 2021, 22, 6862. [Google Scholar] [CrossRef]
- Lyu, J.; Lin, Q.; Fang, Z.; Xu, Z.; Liu, Z. Complex impacts of gallstone disease on metabolic syndrome and nonalcoholic fatty liver disease. Front. Endocrinol. 2022, 13, 1032557. [Google Scholar] [CrossRef]
- Ellervik, C.; Marott, J.L.; Tybjærg-Hansen, A.; Schnohr, P.; Nordestgaard, B.G. Total and cause-specific mortality by moderately and markedly increased ferritin concentrations: General population study and metaanalysis. Clin. Chem. 2014, 60, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Rametta, R.; Fracanzani, A.L.; Fargion, S.; Dongiovanni, P. Dysmetabolic Hyperferritinemia and Dysmetabolic Iron Overload Syndrome (DIOS): Two Related Conditions or Different Entities? Curr. Pharm. Des. 2020, 26, 1025–1035. [Google Scholar] [CrossRef]
- Li, N.; Liao, Y.; Huang, H.; Fu, S. Co-regulation of hepatic steatosis by ferritinophagy and unsaturated fatty acid supply. Hepatol. Commun. 2022, 6, 2640–2653. [Google Scholar] [CrossRef] [PubMed]
- Mendler, M.-H.; Turlin, B.; Moirand, R.; Jouanolle, A.-M.; Sapey, T.; Guyader, D.; le Gall, J.-Y.; Brissot, P.; David, V.; Deugnier, Y. Insulin resistance–associated hepatic iron overload. Gastroenterology 1999, 117, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, Á.; Visiedo-García, F.M.; Domínguez-Riscart, J.; González-Domínguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M. Iron Metabolism in Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 5529. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, N.; Traglia, M.; Campostrini, N.; Biino, G.; Corbella, M.; Sala, C.; Busti, F.; Masciullo, C.; Manna, D.; Previtali, S.; et al. Increased serum hepcidin levels in subjects with the metabolic syndrome: A population study. PLoS ONE 2012, 7, e48250. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants-An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- Singh, E.; Devasahayam, G. Neurodegeneration by oxidative stress: A review on prospective use of small molecules for neuroprotection. Mol. Biol. Rep. 2020, 47, 3133–3140. [Google Scholar] [CrossRef] [PubMed]
- García-Guede, Á.; Vera, O.; Ibáñez-de-Caceres, I. When Oxidative Stress Meets Epigenetics: Implications in Cancer Development. Antioxidants 2020, 9, 468. [Google Scholar] [CrossRef]
- Ghosh, R.; Alajbegovic, A.; Gomes, A.V. NSAIDs and cardiovascular diseases: Role of reactive oxygen species. Oxidative Med. Cell. Longev. 2015, 2015, 536962. [Google Scholar] [CrossRef]
- Brown, G.C.; Murphy, M.P.; Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial proton and electron leaks. Essays Biochem. 2010, 47, 53–67. [Google Scholar] [CrossRef]
- Chen, Q.; Vazquez, E.J.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Production of reactive oxygen species by mitochondria: Central role of complex III. J. Biol. Chem. 2003, 278, 36027–36031. [Google Scholar] [CrossRef]
- Watson, A.J.; Askew, J.N.; Benson, R.S. Poly (adenosine diphosphate ribose) polymerase inhibition prevents necrosis induced by H2O2 but not apoptosis. Gastroenterology 1995, 109, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Sonavane, M.; Smith, K.R.; Seiger, J.; Migaud, M.E.; Gassman, N.R. Dihydroxyacetone suppresses mTOR nutrient signaling and induces mitochondrial stress in liver cells. PLoS ONE 2022, 17, e0278516. [Google Scholar] [CrossRef]
- Maamoun, H.; Benameur, T.; Pintus, G.; Munusamy, S.; Agouni, A. Crosstalk between oxidative stress and endoplasmic reticulum (ER) stress in endothelial dysfunction and aberrant angiogenesis associated with diabetes: A focus on the protective roles of heme oxygenase (HO)-1. Front. Physiol. 2019, 10, 70. [Google Scholar] [CrossRef]
- Shin, G.C.; Lee, H.M.; Kim, N.; Yoo, S.K.; Park, H.S.; Choi, L.S.; Kim, K.P.; Lee, A.R.; Seo, S.U.; Kim, K.H. Paraoxonase-2 contributes to promoting lipid metabolism and mitochondrial function via autophagy activation. Sci. Rep. 2022, 12, 21483. [Google Scholar] [CrossRef]
- Britton, L.J.; Subramaniam, V.N.; Crawford, D.H. Iron and non-alcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 8112. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.A.; Hwang, Y.; Hwang, H.J.; Park, N. Hepatoprotective Effects of Radish (Raphanus sativus L.) on Acetaminophen-Induced Liver Damage via Inhibiting Oxidative Stress and Apoptosis. Nutrients 2022, 14, 5082. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 2015, 15, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, K.G.; Gómez-Fernandez, C.R.; Vazquez-Padron, R. A comprehensive review of oxidative stress as the underlying mechanism in atherosclerosis and the inefficiency of antioxidants to revert this process. Curr. Pharm. Des. 2018, 24, 4705–4710. [Google Scholar] [CrossRef]
- Du, R.; Wu, X.; Peng, K.; Lin, L.; Li, M.; Xu, Y.; Xu, M.; Chen, Y.; Li, D.; Lu, J. Serum apolipoprotein B is associated with increased risk of metabolic syndrome among middle-aged and elderly Chinese: A cross-sectional and prospective cohort study. J. Diabetes 2019, 11, 752–760. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Bueno Ottoboni, A.M.M.; Fiorini, A.M.R.; Guiguer, É.L.; Nicolau, C.C.T.; Goulart, R.d.A.; Flato, U.A.P. Grape juice or wine: Which is the best option? Crit. Rev. Food Sci. Nutr. 2020, 60, 3876–3889. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Zhang, M.W.; Li, X.T.; Zhang, Z.Z.; Liu, Y.; Song, J.W.; Liu, X.M.; Chen, Y.H.; Wang, N.; Guo, Y.; Liang, L.R.; et al. Elabela blunts doxorubicin-induced oxidative stress and ferroptosis in rat aortic adventitial fibroblasts by activating the KLF15/GPX4 signaling. Cell Stress Chaperones 2022, 1–13. [Google Scholar] [CrossRef]
- Bardou-Jacquet, E.; Lainé, F.; Morcet, J.; Perrin, M.; Guyader, D.; Deugnier, Y. Long-term course after initial iron removal of iron excess in patients with dysmetabolic iron overload syndrome. Eur. J. Gastroenterol. Hepatol. 2014, 26, 418–421. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Fracanzani, A.L.; Fargion, S.; Valenti, L. Iron in fatty liver and in the metabolic syndrome: A promising therapeutic target. J. Hepatol. 2011, 55, 920–932. [Google Scholar] [CrossRef]
- Turlin, B.; Mendler, M.H.; Moirand, R.; Guyader, D.; Guillygomarc’h, A.; Deugnier, Y. Histologic features of the liver in insulin resistance–associated iron overload: A study of 139 patients. Am. J. Clin. Pathol. 2001, 116, 263–270. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Novelle, M.G.; Catalán, V.; Ortega, F.; Moreno, M.; Gomez-Ambrosi, J.; Xifra, G.; Serrano, M.; Guerra, E.; Ricart, W. Insulin resistance modulates iron-related proteins in adipose tissue. Diabetes Care 2014, 37, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Kuragano, T.; Kimura, T.; Nanami, M.; Hasuike, Y.; Nakanishi, T. Interplay of adipocyte and hepatocyte: Leptin upregulates hepcidin. Biochem. Biophys. Res. Commun. 2018, 495, 1548–1554. [Google Scholar] [CrossRef]
- Orr, J.S.; Kennedy, A.; Anderson-Baucum, E.K.; Webb, C.D.; Fordahl, S.C.; Erikson, K.M.; Zhang, Y.; Etzerodt, A.; Moestrup, S.K.; Hasty, A.H. Obesity alters adipose tissue macrophage iron content and tissue iron distribution. Diabetes 2014, 63, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Ruscica, M.; Rametta, R.; Recalcati, S.; Steffani, L.; Gatti, S.; Girelli, D.; Cairo, G.; Magni, P.; Fargion, S. Dietary iron overload induces visceral adipose tissue insulin resistance. Am. J. Pathol. 2013, 182, 2254–2263. [Google Scholar] [CrossRef]
- Nazari, M.; Ho, K.W.; Langley, N.; Cha, K.M.; Kodsi, R.; Wang, M.; Laybutt, D.R.; Cheng, K.; Stokes, R.A.; Swarbrick, M.M.; et al. Iron chelation increases beige fat differentiation and metabolic activity, preventing and treating obesity. Sci. Rep. 2022, 12, 776. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Gerasimidis, K.; Edwards, C.A.; Shaikh, M.G. Role of gut microbiota in the aetiology of obesity: Proposed mechanisms and review of the literature. J. Obes. 2016, 2016, 7353642. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Xu, H.; Dong, G.; Huang, K.; Wu, W.; Ye, J.; Fu, J. Ferritin as a key risk factor for nonalcoholic fatty liver disease in children with obesity. J. Clin. Lab. Anal. 2020, 35, e23602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, X.; Shen, Y.; Fang, X.; Wang, Y.; Wang, F. Obesity and iron deficiency: A quantitative meta-analysis. Obes. Rev. 2015, 16, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.K.; Yeo, K.J.; Huang, P.H.; Chang, S.H.; Chang, C.K.; Lan, J.L.; Chen, D.Y. Increased Lipid Peroxidation May Be Linked to Ferritin Levels Elevation in Adult-Onset Still’s Disease. Biomedicines 2021, 9, 1508. [Google Scholar] [CrossRef]
- Ruivard, M.; Laine, F.; Deugnier, Y. Iron absorption in nonalcoholic steatohepatitis and dysmetabolic iron overload syndrome. Hepatology 2016, 63, 1737–1738. [Google Scholar] [CrossRef]
- Deugnier, Y.; Turlin, B. Pathology of hepatic iron overload. Semin. Liver Dis. 2011, 31, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Trombini, P.; Mariani, R.; Salvioni, A.; Coletti, S.; Bonfadini, S.; Paolini, V.; Pozzi, M.; Facchetti, R.; Bovo, G.; et al. Revaluation of clinical and histological criteria for diagnosis of dysmetabolic iron overload syndrome. World J. Gastroenterol. 2008, 14, 4745–4752. [Google Scholar] [CrossRef]
- Deugnier, Y.; Turlin, B. Pathology of hepatic iron overload. World J. Gastroenterol. 2007, 13, 4755–4760. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).