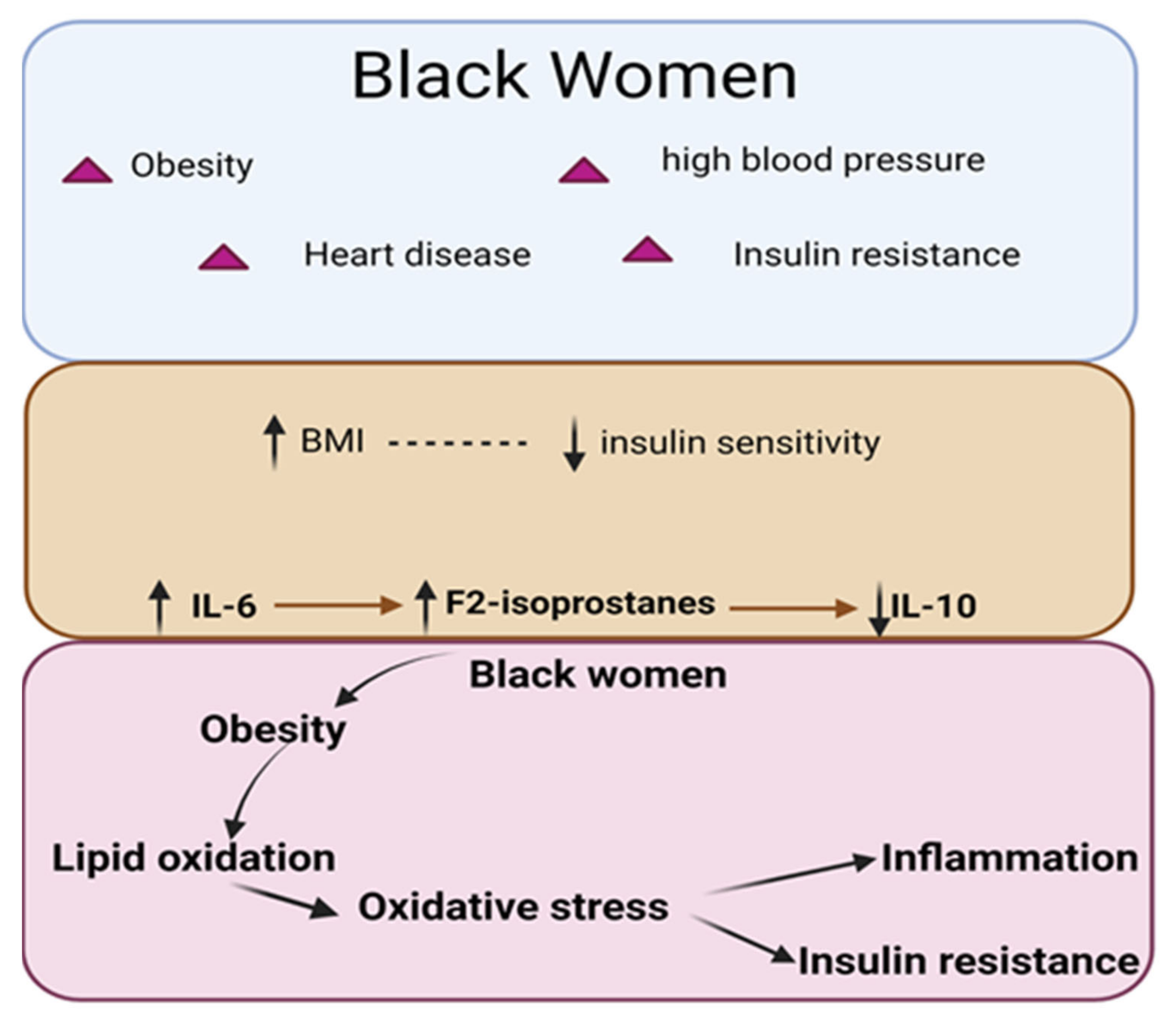
Obesity Is Associated with Increased F2-Isoprostanes and IL-6 in Black Women
Highlights
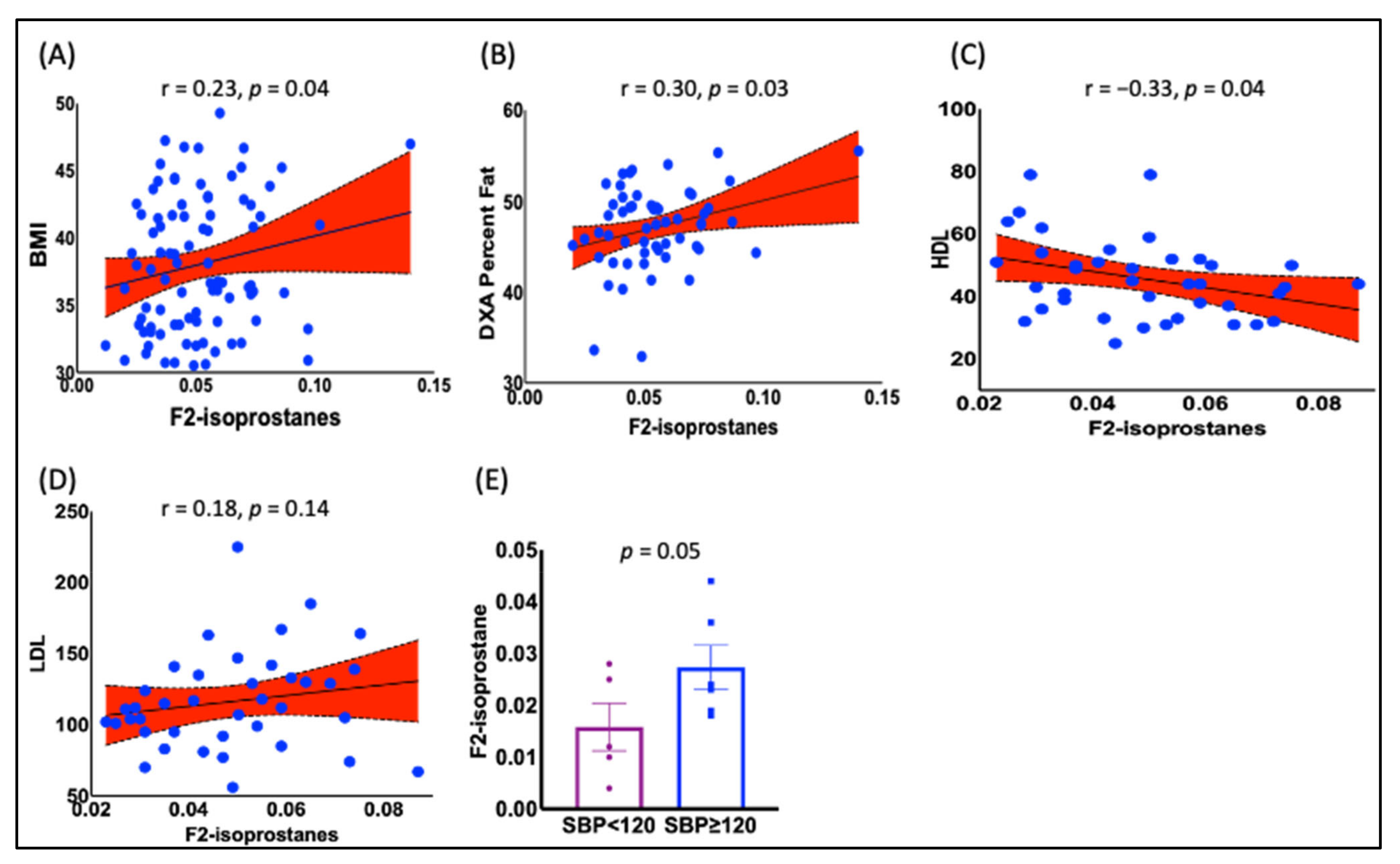
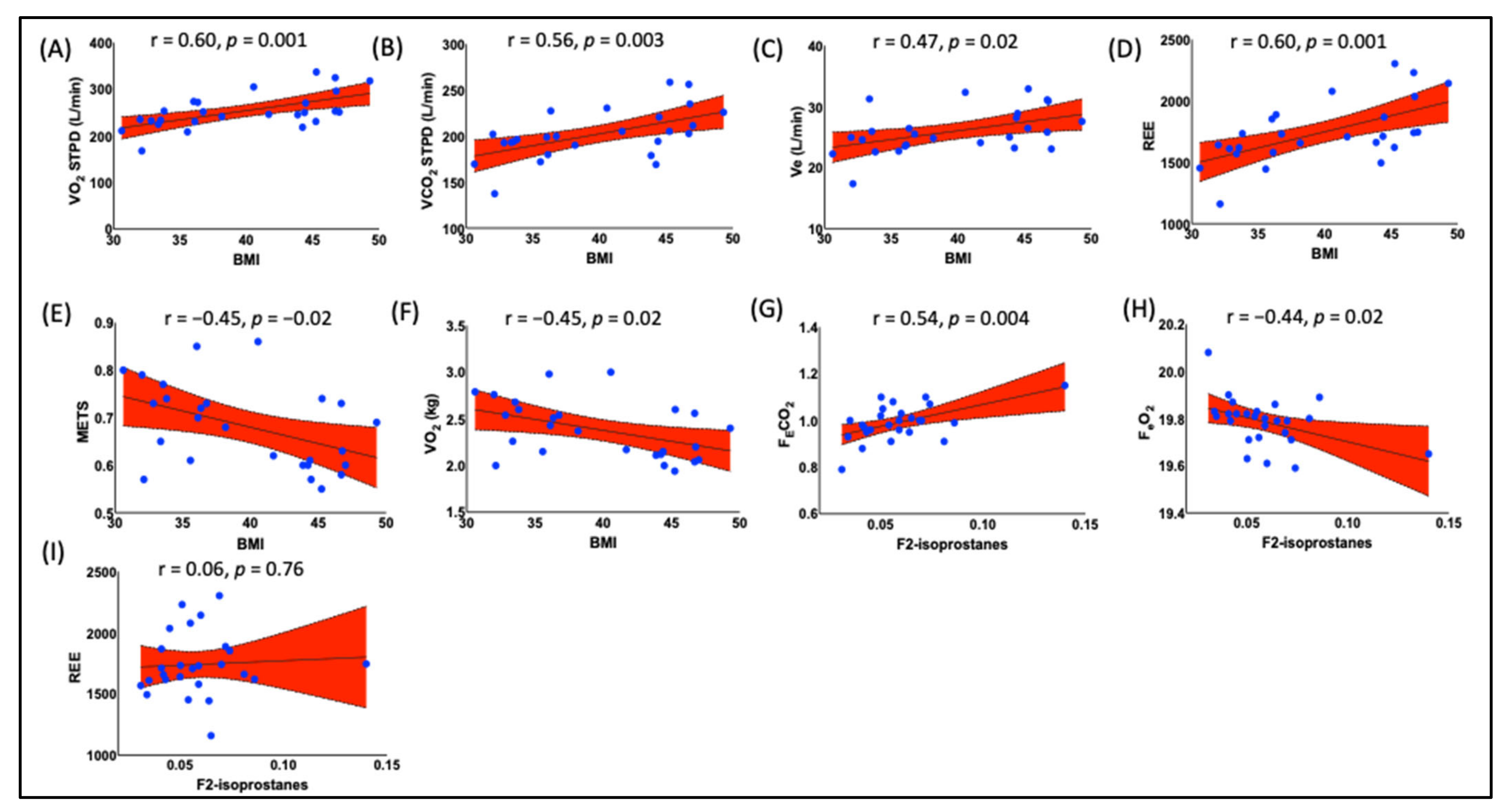
- Positive Correlation between BMI and F2-IsoPs: The study found that Body Mass Index (BMI) was positively correlated with the plasma F2-Isoprostanes (F2-IsoPs) levels in obese black women. This suggests that a higher BMI is associated with increased lipid peroxidation.
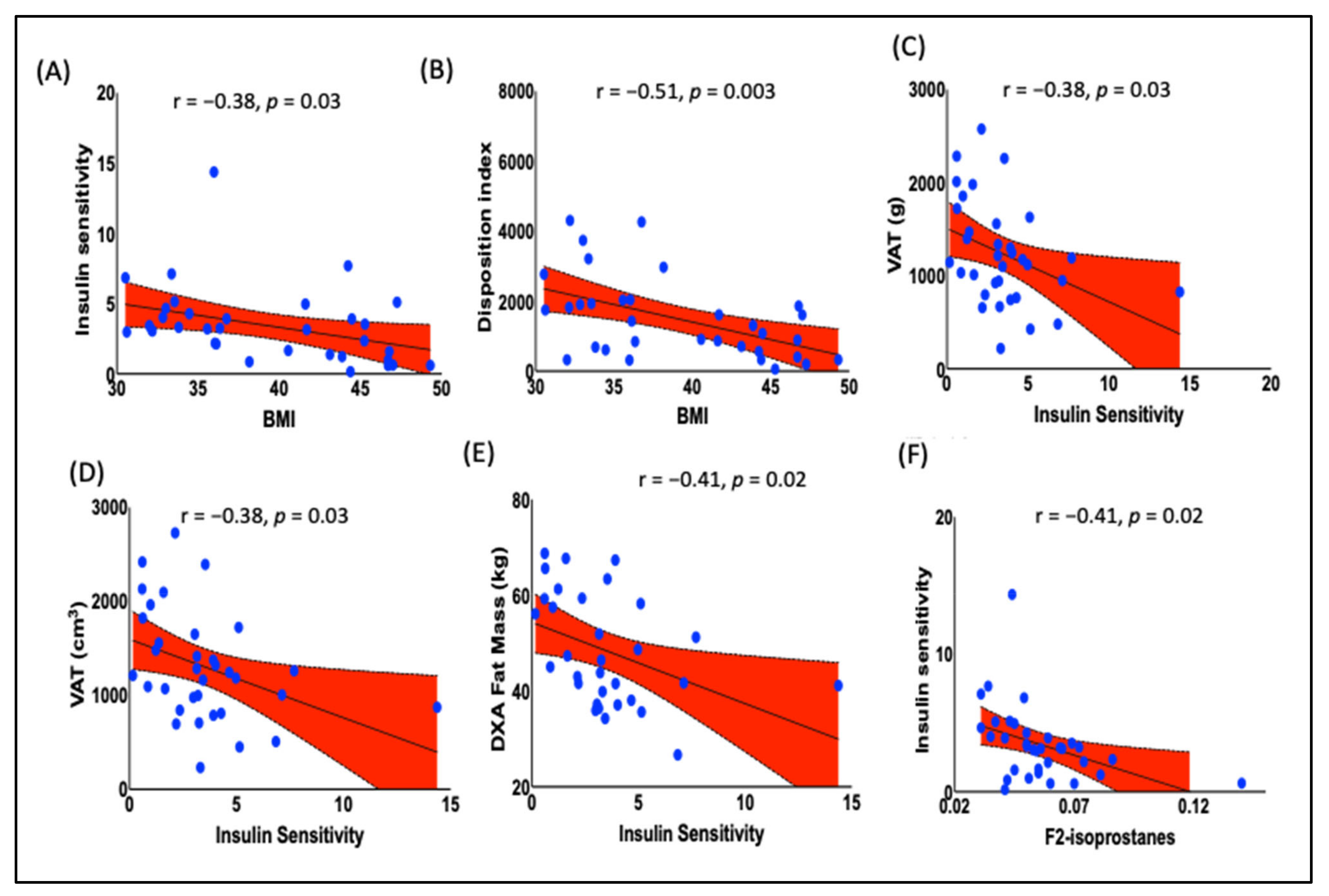
- Inverse Correlation between BMI and Insulin Sensitivity: BMI was inversely correlated with insulin sensitivity. This indicates that as BMI increases, the insulin sensitivity of the studied population decreases.
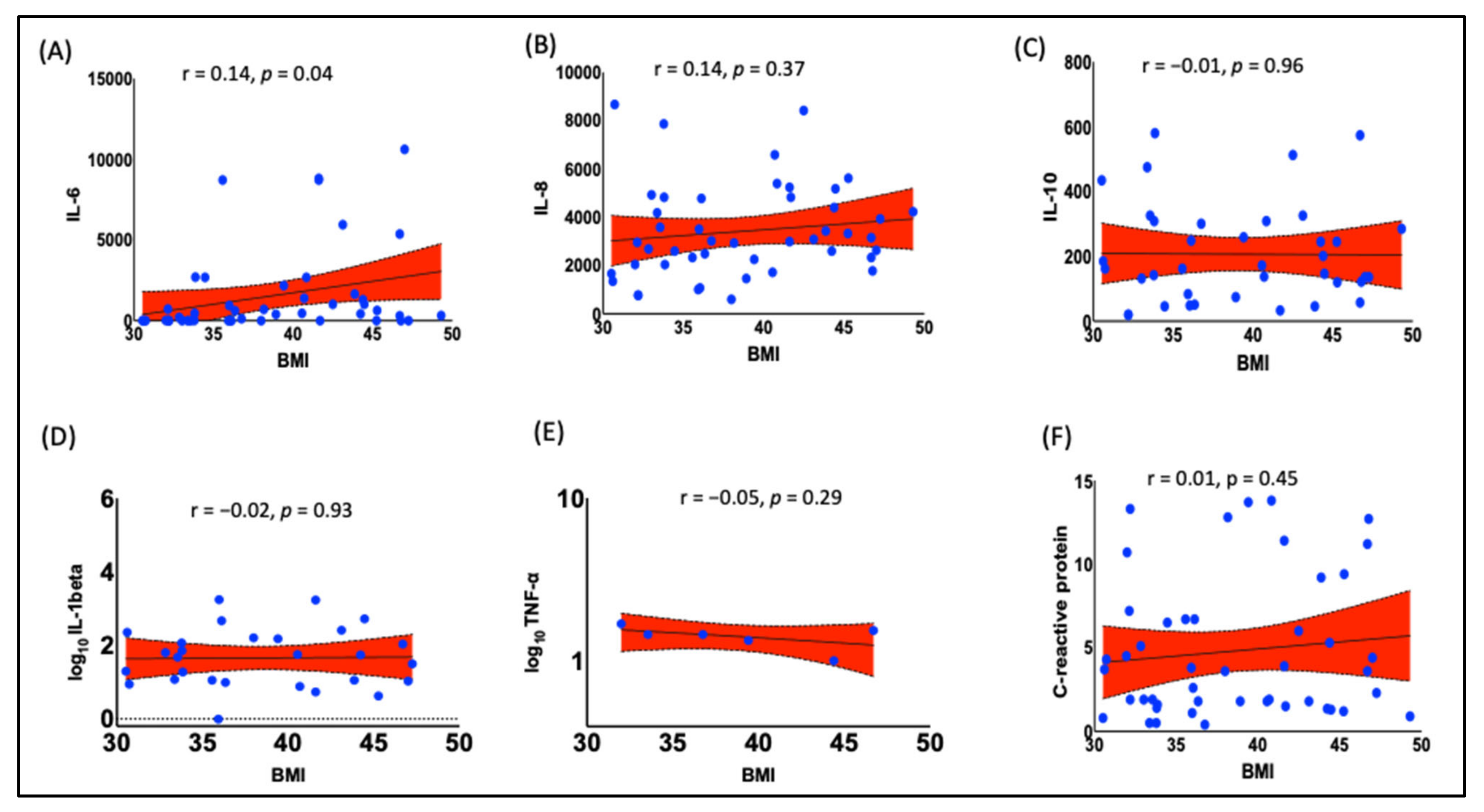
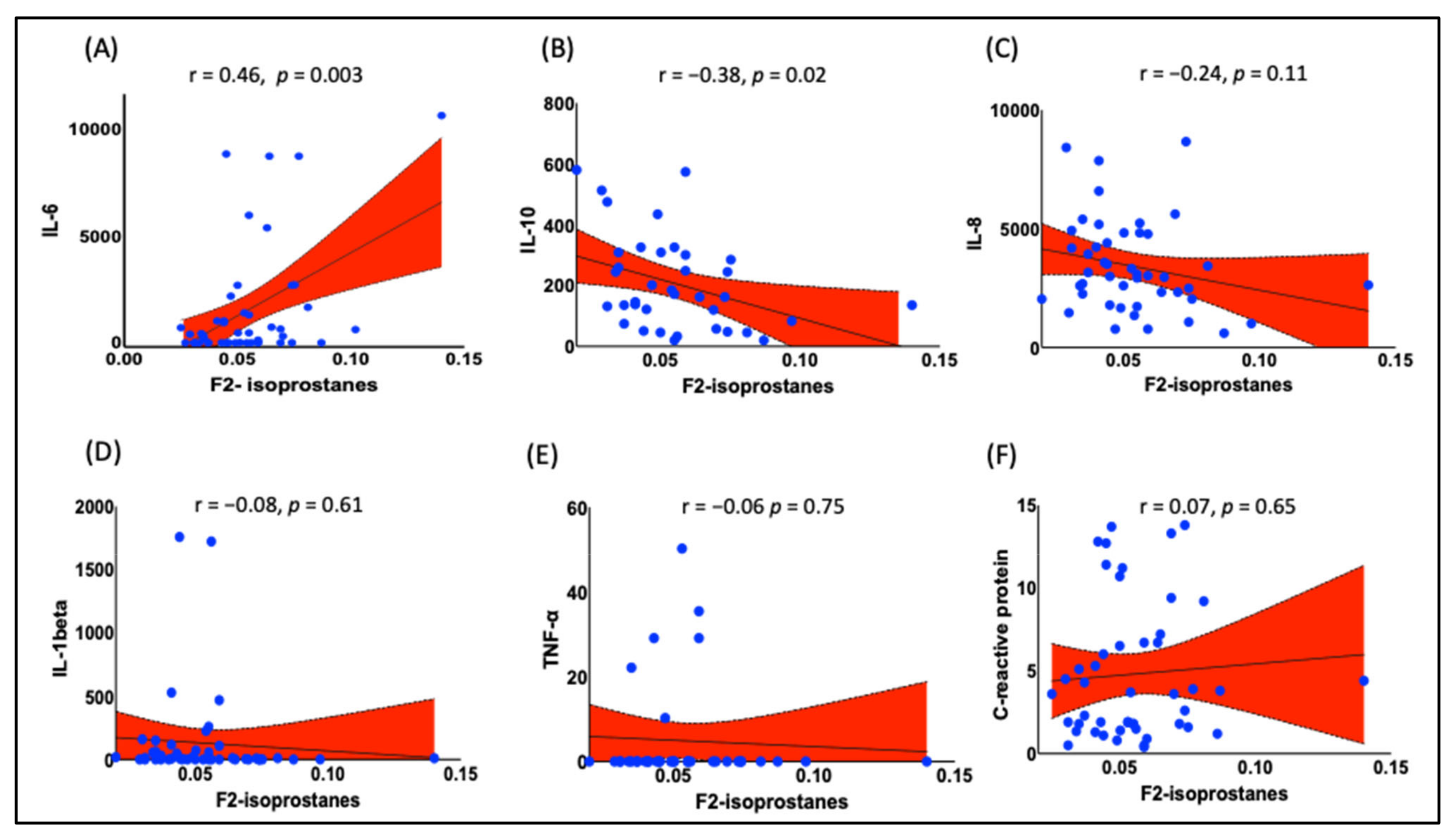
- Association of F2-IsoPs with Inflammatory Marker IL-6: F2-IsoPs were positively correlated with the levels of inflammatory cytokine IL-6. This indicates that higher levels of lipid peroxidation are associated with increased inflammation.
- Negative Correlation between F2-IsoPs and Anti-inflammatory Marker IL-10: F2-IsoPs were negatively correlated with the levels of anti-inflammatory cytokine IL-10. This suggests that higher lipid peroxidation is associated with reduced anti-inflammatory responses.
- Association of F2-IsoPs with Reduced Insulin Sensitivity: Higher plasma levels of F2-IsoPs were significantly associated with reduced insulin sensitivity. This finding indicates that lipid peroxidation may contribute to insulin resistance in obese black women.
Abstract
1. Introduction
2. Materials and Methods
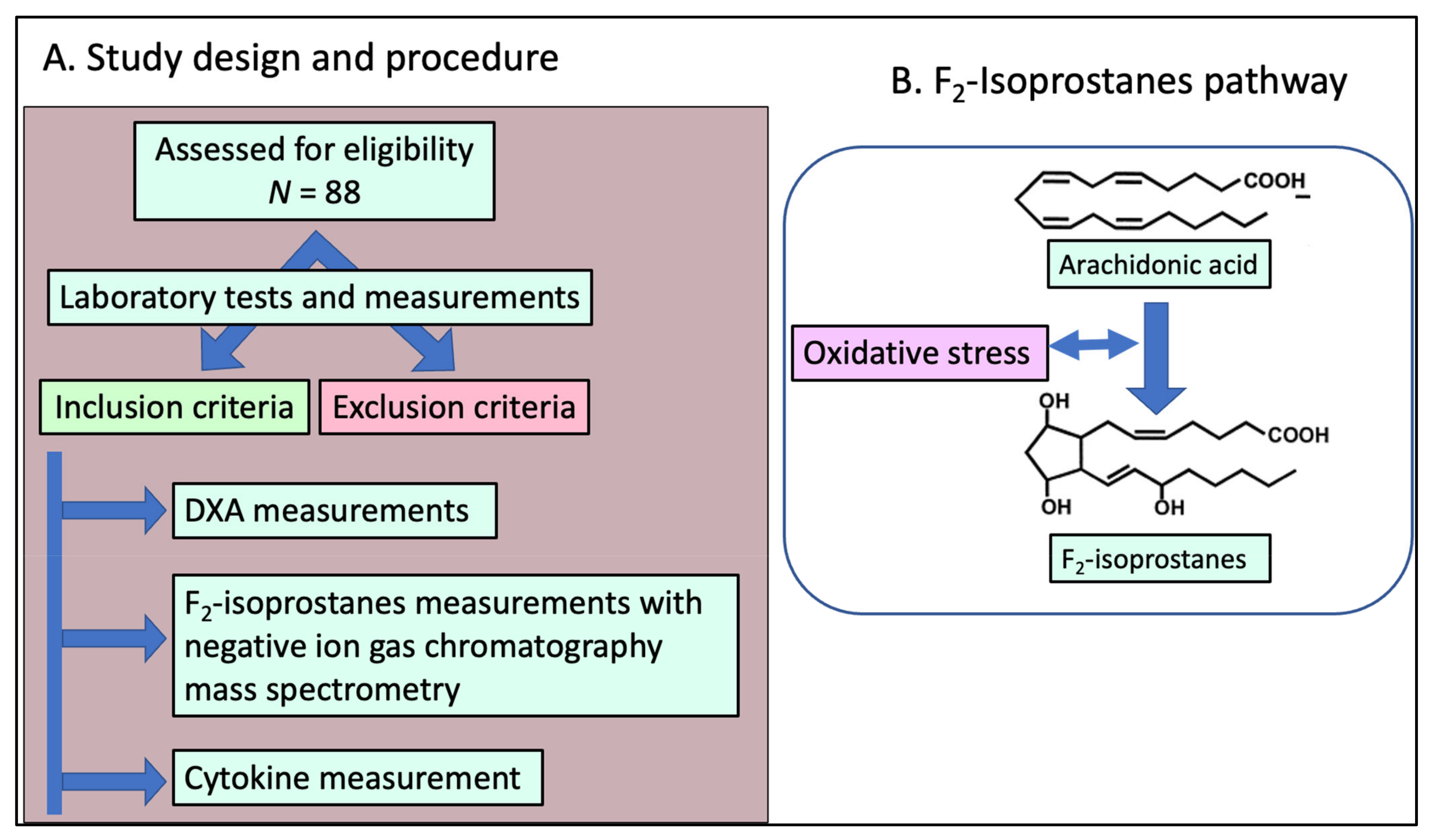
2.1. Study Design and Population Enrollment
2.2. Blood Sample Collection
2.3. Measurement of F2-IsoPs in Plasma and Peripheral Blood Mononuclear Cells (PBMCs)
2.4. Dual-Energy X-ray Absorptiometry
2.5. Resting Energy Expenditure (REE)
2.6. Cytokine Measurement
2.7. Statistics
3. Results
3.1. Obesity Is Associated with Increased Pro-Inflammatory Cytokine IL-6 in Black Women
3.2. The F2-IsoPs Are Associated with BMI, Lipoproteins, and Hypertension in Black Women
3.3. Plasma F2-IsoPs Are Associated with IL-6 and IL-10 in Obese Black Women
3.4. Prediabetic Indices Are Correlated with BMI
3.5. Obesity Is Correlated with Resting Energy Expenditure (REE) and Fractional Concentrations of Expired O2 and CO2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberto, C.A.; Swinburn, B.; Hawkes, C.; Huang, T.T.-K.; Costa, S.A.; Ashe, M.; Zwicker, L.; Cawley, J.H.; Brownell, K.D. Patchy progress on obesity prevention: Emerging examples, entrenched barriers, and new thinking. Lancet 2015, 385, 2400–2409. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer — Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.M.; Danaei, G.; Farzadfar, F.; Stevens, G.A.; Woodward, M.; Wormser, D.; Kaptoge, S.; Whitlock, G.; Qiao, Q.; Lewington, S.; et al. The Age-Specific Quantitative Effects of Metabolic Risk Factors on Cardiovascular Diseases and Diabetes: A Pooled Analysis. PLoS ONE 2013, 8, e65174. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration; Wormser, D.; Kaptoge, S.; Di Angelantonio, E.; Wood, A.M.; Pennells, L.; Thompson, A.; Sarwar, N.; Kizer, J.R.; Lawlor, D.A.; et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet 2011, 377, 1085–1095. [Google Scholar]
- Berrington de Gonzalez, A.; Hartge, P.; Cerhan, J.R.; Flint, A.J.; Hannan, L.; MacInnis, R.J.; Moore, S.C.; Tobias, G.S.; Anton-Culver, H.; Freeman, L.B.; et al. Body-mass index and mortality among 1.46 million white adults. N. Engl. J. Med. 2010, 363, 2211–2219. [Google Scholar] [CrossRef]
- Kumanyika, S. Ethnicity and Obesity Development in Children. Ann. N. Y. Acad. Sci. 1993, 699, 81–92. [Google Scholar] [CrossRef]
- Kumanyika, S.K. Special Issues Regarding Obesity in Minority Populations. Ann. Intern. Med. 1993, 119, 650–654. [Google Scholar] [CrossRef]
- Kumanyika, S. OBESITY IN BLACK WOMEN1. Epidemiol. Rev. 1987, 9, 31–50. [Google Scholar] [CrossRef]
- Bernstein, R.E. Human insulins and metabolic control. S. Afr. Med. J. 1983, 64, 605. [Google Scholar]
- Kumanyika, S.K.; Whitt-Glover, M.C.; Haire-Joshu, D. What works for obesity prevention and treatment in black Americans? Research directions. Obes. Rev. 2014, 15, 204–212. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014, 311, 806–814. [Google Scholar] [CrossRef]
- Flegal, K.M.; Carroll, M.D.; Kit, B.K.; Ogden, C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012, 307, 491–497. [Google Scholar] [CrossRef]
- Kong, A.; Tussing-Humphreys, L.M.; Odoms-Young, A.M.; Stolley, M.R.; Fitzgibbon, M.L. Systematic review of behavioural interventions with culturally adapted strategies to improve diet and weight outcomes in African American women. Obes. Rev. 2014, 15, 62–92. [Google Scholar] [CrossRef]
- Mensah, G.A.; Mokdad, A.H.; Ford, E.S.; Greenlund, K.J.; Croft, J.B. State of Disparities in Cardiovascular Health in the United States. Circulation 2005, 111, 1233–1241. [Google Scholar] [CrossRef]
- West, D.S.; Elaine Prewitt, T.; Bursac, Z.; Felix, H.C. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity 2008, 16, 1413–1420. [Google Scholar] [CrossRef]
- Tussing-Humphreys, L.M.; Fitzgibbon, M.L.; Kong, A.; Odoms-Young, A. Weight loss maintenance in African American women: A systematic review of the behavioral lifestyle intervention literature. J. Obes. 2013, 2013, 437369. [Google Scholar] [CrossRef]
- Wingo, B.C.; Carson, T.L.; Ard, J. Differences in weight loss and health outcomes among African Americans and whites in multicentre trials. Obes. Rev. 2014, 15 (Suppl. S4), 46–61. [Google Scholar] [CrossRef]
- Morales, K.H.; Kumanyika, S.K.; Fassbender, J.E.; Good, J.; Localio, A.R.; Wadden, T.A. Patterns of weight change in black Americans: Pooled analysis from three behavioral weight loss trials. Obesity 2014, 22, 2632–2640. [Google Scholar] [CrossRef]
- Kumanyika, S.K.; Gary, T.L.; Lancaster, K.J.; Samuel-Hodge, C.D.; Banks-Wallace, J.; Beech, B.M.; Hughes-Halbert, C.; Karanja, N.; Odoms-Young, A.M.; Prewitt, T.E.; et al. Achieving Healthy Weight in African-American Communities: Research Perspectives and Priorities. Obes. Res. 2005, 13, 2037–2047. [Google Scholar] [CrossRef]
- Kim, C.-S.; Park, H.-S.; Kawada, T.; Kim, J.-H.; Lim, D.; Hubbard, N.E.; Kwon, B.-S.; Erickson, K.L.; Yu, R. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int. J. Obes. 2006, 30, 1347–1355. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; KhazáAi, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Abe, I.; Gotoh, K.; Fukui, A.; Takanari, H.; Ishii, Y.; Ikebe, Y.; Kira, S.; Oniki, T.; Saito, S.; et al. Interleukin 10 Treatment Ameliorates High-Fat Diet–Induced Inflammatory Atrial Remodeling and Fibrillation. Circ. Arrhythmia Electrophysiol. 2018, 11, e006040. [Google Scholar] [CrossRef] [PubMed]
- Charles, B.A.; Doumatey, A.; Huang, H.; Zhou, J.; Chen, G.; Shriner, D.; Adeyemo, A.; Rotimi, C.N. The Roles of IL-6, IL-10, and IL-1RA in Obesity and Insulin Resistance in African-Americans. J. Clin. Endocrinol. Metab. 2011, 96, E2018–E2022. [Google Scholar] [CrossRef]
- McDonnell, W.J.; Koethe, J.R.; Mallal, S.A.; Pilkinton, M.A.; Kirabo, A.; Ameka, M.K.; Cottam, M.A.; Hasty, A.H.; Kennedy, A.J. High CD8 T-Cell Receptor Clonality and Altered CDR3 Properties Are Associated With Elevated Isolevuglandins in Adipose Tissue During Diet-Induced Obesity. Diabetes 2018, 67, 2361–2376. [Google Scholar] [CrossRef] [PubMed]
- Kirabo, A.; Fontana, V.; de Faria, A.P.; Loperena, R.; Galindo, C.L.; Wu, J.; Bikineyeva, A.T.; Dikalov, S.; Xiao, L.; Chen, W.; et al. DC isoketal-modified proteins activate T cells and promote hypertension. J. Clin. Investig. 2014, 124, 4642–4656. [Google Scholar] [CrossRef]
- Saleem, M.; Wang, X.; Pokkunuri, I.; Asghar, M. Superoxide via Sp3 mechanism increases renal renin activity, renal AT1 receptor function, and blood pressure in rats. Am. J. Physiol. Ren. Physiol. 2018, 315, F1478–F1483. [Google Scholar] [CrossRef]
- Pignatelli, P.; Menichelli, D.; Pastori, D.; Violi, F. Oxidative stress and cardiovascular disease: New insights. Kardiol. Polska 2018, 76, 713–722. [Google Scholar] [CrossRef]
- Saleem, M.; Pokkunuri, I.; Asghar, M. Superoxide increases angiotensin II AT 1 receptor function in human kidney-2 cells. FEBS Open Bio. 2016, 6, 1273–1284. [Google Scholar] [CrossRef]
- Zhang, H.; Davies, K.J.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free. Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef]
- Graille, M.; Wild, P.; Sauvain, J.-J.; Hemmendinger, M.; Canu, I.G.; Hopf, N. Urinary 8-isoprostane as a biomarker for oxidative stress. A systematic review and meta-analysis. Toxicol. Lett. 2020, 328, 19–27. [Google Scholar] [CrossRef]
- Bougoulia, M.; Triantos, A.; Koliakos, G. Plasma interleukin-6 levels, glutathione peroxidase and isoprostane in obese women before and after weight loss. Association with cardiovascular risk factors. Hormones 2006, 5, 192–199. [Google Scholar] [CrossRef]
- Helmersson, J.; Larsson, A.; Vessby, B.; Basu, S. Active smoking and a history of smoking are associated with enhanced prostaglandin F(2alpha), interleukin-6 and F2-isoprostane formation in elderly men. Atherosclerosis 2005, 181, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Tamm, M.; Bihl, M.; Eickelberg, O.; Stulz, P.; Perruchoud, A.P.; Roth, M. Hypoxia-Induced Interleukin-6 and Interleukin-8 Production Is Mediated by Platelet-Activating Factor and Platelet-Derived Growth Factor in Primary Human Lung Cells. Am. J. Respir. Cell Mol. Biol. 1998, 19, 653–661. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Kharitonov, S.A.; Resta, O.; Foschino-Barbaro, M.P.; Gramiccioni, E.; Barnes, P.J. Increased 8-Isoprostane and Interleukin-6 in Breath Condensate of Obstructive Sleep Apnea Patients. Chest 2002, 122, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Billings, F.T., IV; Pretorius, M.; Schildcrout, J.S.; Mercaldo, N.D.; Byrne, J.G.; Ikizler, T.A.; Brown, N.J. Obesity and oxidative stress predict AKI after cardiac surgery. J. Am. Soc. Nephrol. 2012, 23, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Pacini, G.; Bergman, R.N. MINMOD: A computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput. Methods Programs Biomed. 1986, 23, 113–122. [Google Scholar] [CrossRef]
- Milne, G.L.; Gao, B.; Terry, E.S.; Zackert, W.E.; Sanchez, S.C. Measurement of F2-isoprostanes and isofurans using gas chromatography-mass spectrometry. Free Radic. Biol. Med. 2013, 59, 36–44. [Google Scholar] [CrossRef]
- Milne, G.L.; Sanchez, S.C.; Musiek, E.S.; Morrow, J.D. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat. Protoc. 2007, 2, 221–226. [Google Scholar] [CrossRef]
- Parsa, D.; Aden, L.A.; Pitzer, A.; Ding, T.; Yu, C.; Diedrich, A.; Milne, G.L.; Kirabo, A.; Shibao, C.A. Enhanced parasympathetic cholinergic activity with galantamine inhibited lipid-induced oxidative stress in obese African Americans. Mol. Med. 2022, 28, 60. [Google Scholar] [CrossRef]
- Bi, X.; Seabolt, L.; Shibao, C.; Buchowski, M.; Kang, H.; Keil, C.D.; Tyree, R.; Silver, H.J. DXA-measured visceral adipose tissue predicts impaired glucose tolerance and metabolic syndrome in obese Caucasian and African-American women. Eur. J. Clin. Nutr. 2015, 69, 329–336. [Google Scholar] [CrossRef]
- Montesano, R.; Hall, J.; Hollstein, M.; Mironov, N.; Wild, C.P. Alkylation repair in human tissues. Basic Life Sci. 1990, 53, 437–452. [Google Scholar] [CrossRef]
- Shibao, C.; Gamboa, A.; Diedrich, A.; Ertl, A.C.; Chen, K.Y.; Byrne, D.W.; Farley, G.; Paranjape, S.Y.; Davis, S.N.; Biaggioni, I. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension 2007, 49, 27–33. [Google Scholar] [CrossRef]
- Nono Nankam, P.A.; Mendham, A.E.; De Smidt, M.F.; Keswell, D.; Olsson, T.; Bluher, M.; Goedecke, J.H. Changes in systemic and subcutaneous adipose tissue inflammation and oxidative stress in response to exercise training in obese black African women. J. Physiol. 2020, 598, 503–515. [Google Scholar] [CrossRef]
- Gower, B.A.; Hunter, G.R.; Chandler-Laney, P.C.; Alvarez, J.A.; Bush, N.C. Glucose metabolism and diet predict changes in adiposity and fat distribution in weight-reduced women. Obesity 2010, 18, 1532–1537. [Google Scholar] [CrossRef]
- Gower, B.A.; Fowler, L.A. Obesity in African-Americans: The role of physiology. J. Intern. Med. 2020, 288, 295–304. [Google Scholar] [CrossRef]
- Gower, B.A.; Alvarez, J.A.; Bush, N.C.; Hunter, G.R. Insulin sensitivity affects propensity to obesity in an ethnic-specific manner: Results from two controlled weight loss intervention studies. Nutr. Metab. 2013, 10, 3. [Google Scholar] [CrossRef]
- Montuschi, P.; Barnes, P.J.; Roberts, L.J., II. Isoprostanes: Markers and mediators of oxidative stress. FASEB J. 2004, 18, 1791–1800. [Google Scholar] [CrossRef]
- Davi, G.; Falco, A.; Patrono, C. Lipid peroxidation in diabetes mellitus. Antioxid. Redox Signal. 2005, 7, 256–268. [Google Scholar] [CrossRef]
- Davi, G.; Falco, A.; Patrono, C. Determinants of F2-isoprostane biosynthesis and inhibition in man. Chem. Phys. Lipids 2004, 128, 149–163. [Google Scholar] [CrossRef]
- Il’yasova, D.; Morrow, J.D.; Wagenknecht, L.E. Urinary F2-isoprostanes are not associated with increased risk of type 2 diabetes. Obes. Res. 2005, 13, 1638–1644. [Google Scholar] [CrossRef]
- Laight, D.W.; Desai, K.M.; Gopaul, N.K.; Anggard, E.E.; Carrier, M.J. F2-isoprostane evidence of oxidant stress in the insulin resistant, obese Zucker rat: Effects of vitamin E. Eur. J. Pharmacol. 1999, 377, 89–92. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Annuzzi, G.; Vari, R.; Filesi, C.; Giacco, R.; Scazzocchio, B.; Santangelo, C.; Giovannini, C.; Rivellese, A.A.; Masella, R. Predominant role of obesity/insulin resistance in oxidative stress development. Eur. J. Clin. Investig. 2012, 42, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Il’yasova, D.; Wang, F.; Spasojevic, I.; Base, K.; D’Agostino, R.B., Jr.; Wagenknecht, L.E. Racial differences in urinary F2-isoprostane levels and the cross-sectional association with BMI. Obesity 2012, 20, 2147–2150. [Google Scholar] [CrossRef] [PubMed]
- Annor, F.; Goodman, M.; Thyagarajan, B.; Okosun, I.; Doumatey, A.; Gower, B.A.; Il’yasova, D. African Ancestry Gradient Is Associated with Lower Systemic F(2)-Isoprostane Levels. Oxid. Med. Cell Longev. 2017, 2017, 8319176. [Google Scholar] [CrossRef]
- Il’yasova, D.; Wong, B.J.; Waterstone, A.; Kinev, A.; Okosun, I.S. Systemic F(2)-Isoprostane Levels in Predisposition to Obesity and Type 2 Diabetes: Emphasis on Racial Differences. Divers. Equal. Health Care 2017, 14, 91–101. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Keaney, J.F., Jr.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J.; et al. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 434–439. [Google Scholar] [CrossRef]
- Olusi, S.O. Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1159–1164. [Google Scholar] [CrossRef]
- Davi, G.; Ciabattoni, G.; Consoli, A.; Mezzetti, A.; Falco, A.; Santarone, S.; Pennese, E.; Vitacolonna, E.; Bucciarelli, T.; Costantini, F.; et al. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: Effects of improved metabolic control and vitamin E supplementation. Circulation 1999, 99, 224–229. [Google Scholar] [CrossRef]
- Gopaul, N.K.; Anggard, E.E.; Mallet, A.I.; Betteridge, D.J.; Wolff, S.P.; Nourooz-Zadeh, J. Plasma 8-epi-PGF2 alpha levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS Lett. 1995, 368, 225–229. [Google Scholar] [CrossRef]
- Kanaya, A.M.; Wassel, C.L.; Stoddard, P.J.; Harris, T.B.; Cummings, S.R.; Kritchevsky, S.B.; Goodpaster, B.H.; Green, C.; Satterfield, S.; Gross, M.D. F2-isoprostanes and adiposity in older adults. Obesity 2011, 19, 861–867. [Google Scholar] [CrossRef]
- Wagenknecht, L.E.; Mayer, E.J.; Rewers, M.; Haffner, S.; Selby, J.; Borok, G.M.; Henkin, L.; Howard, G.; Savage, P.J.; Saad, M.F.; et al. The insulin resistance atherosclerosis study (IRAS) objectives, design, and recruitment results. Ann. Epidemiol. 1995, 5, 464–472. [Google Scholar] [CrossRef]
- Leibel, R.L.; Rosenbaum, M.; Hirsch, J. Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med. 1995, 332, 621–628. [Google Scholar] [CrossRef]
- Weyer, C.; Pratley, R.E.; Salbe, A.D.; Bogardus, C.; Ravussin, E.; Tataranni, P.A. Energy expenditure, fat oxidation, and body weight regulation: A study of metabolic adaptation to long-term weight change. J. Clin. Endocrinol. Metab. 2000, 85, 1087–1094. [Google Scholar] [CrossRef]
- Heilbronn, L.; Smith, S.R.; Ravussin, E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int. J. Obes. Relat. Metab. Disord. 2004, 28 (Suppl. S4), S12–S21. [Google Scholar] [CrossRef]
- Crescenzo, R.; Bianco, F.; Mazzoli, A.; Giacco, A.; Liverini, G.; Iossa, S. Mitochondrial efficiency and insulin resistance. Front. Physiol. 2014, 5, 512. [Google Scholar] [CrossRef]
- Zurlo, F.; Lillioja, S.; Esposito-Del Puente, A.; Nyomba, B.L.; Raz, I.; Saad, M.F.; Swinburn, B.A.; Knowler, W.C.; Bogardus, C.; Ravussin, E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: Study of 24-h RQ. Am. J. Physiol. 1990, 259, E650–E657. [Google Scholar] [CrossRef]
- Ravussin, E.; Lillioja, S.; Knowler, W.C.; Christin, L.; Freymond, D.; Abbott, W.G.; Boyce, V.; Howard, B.V.; Bogardus, C. Reduced rate of energy expenditure as a risk factor for body-weight gain. N. Engl. J. Med. 1988, 318, 467–472. [Google Scholar] [CrossRef]
- Coe, C.L.; Love, G.D.; Karasawa, M.; Kawakami, N.; Kitayama, S.; Markus, H.R.; Tracy, R.P.; Ryff, C.D. Population differences in proinflammatory biology: Japanese have healthier profiles than Americans. Brain Behav. Immun. 2011, 25, 494–502. [Google Scholar] [CrossRef]
- Pollitt, R.A.; Kaufman, J.S.; Rose, K.M.; Diez-Roux, A.V.; Zeng, D.; Heiss, G. Cumulative life course and adult socioeconomic status and markers of inflammation in adulthood. J. Epidemiol. Community Health 2008, 62, 484–491. [Google Scholar] [CrossRef]
- Petersen, K.L.; Marsland, A.L.; Flory, J.; Votruba-Drzal, E.; Muldoon, M.F.; Manuck, S.B. Community socioeconomic status is associated with circulating interleukin-6 and C-reactive protein. Psychosom. Med. 2008, 70, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Koster, A.; Bosma, H.; Penninx, B.W.; Newman, A.B.; Harris, T.B.; van Eijk, J.T.; Kempen, G.I.; Simonsick, E.M.; Johnson, K.C.; Rooks, R.N.; et al. Association of inflammatory markers with socioeconomic status. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Slopen, N.; Lewis, T.T.; Gruenewald, T.L.; Mujahid, M.S.; Ryff, C.D.; Albert, M.A.; Williams, D.R. Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosom. Med. 2010, 72, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, N.; Diez-Roux, A.V.; Shea, S.; Cushman, M.; Ni, H.; Seeman, T. Socioeconomic position, race/ethnicity, and inflammation in the multi-ethnic study of atherosclerosis. Circulation 2007, 116, 2383–2390. [Google Scholar] [CrossRef]
- Gruenewald, T.L.; Cohen, S.; Matthews, K.A.; Tracy, R.; Seeman, T.E. Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Soc. Sci. Med. 2009, 69, 451–459. [Google Scholar] [CrossRef]
- Yamashita, A.; Soga, Y.; Iwamoto, Y.; Yoshizawa, S.; Iwata, H.; Kokeguchi, S.; Takashiba, S.; Nishimura, F. Macrophage-adipocyte interaction: Marked interleukin-6 production by lipopolysaccharide. Obesity 2007, 15, 2549–2552. [Google Scholar] [CrossRef]
- Mohamed-Ali, V.; Goodrick, S.; Rawesh, A.; Katz, D.R.; Miles, J.M.; Yudkin, J.S.; Klein, S.; Coppack, S.W. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J. Clin. Endocrinol. Metab. 1997, 82, 4196–4200. [Google Scholar] [CrossRef]
- Pasarica, M.; Rood, J.; Ravussin, E.; Schwarz, J.M.; Smith, S.R.; Redman, L.M. Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J. Clin. Endocrinol. Metab. 2010, 95, 4052–4055. [Google Scholar] [CrossRef]
- Pasarica, M.; Sereda, O.R.; Redman, L.M.; Albarado, D.C.; Hymel, D.T.; Roan, L.E.; Rood, J.C.; Burk, D.H.; Smith, S.R. Reduced adipose tissue oxygenation in human obesity: Evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009, 58, 718–725. [Google Scholar] [CrossRef]
| Variable | N | |
|---|---|---|
| Age | 87 | 42.61 ± 10.02 |
| Height (cm) | 83 | 162.9 ± 5.2 |
| Weight (kg) | 83 | 102 ± 15 |
| BMI, kg/m2 | 83 | 38.0 ± 5.0 |
| Baseline SBP, mmHg | 77 | 124 ± 14 |
| Baseline DBP, mmHg | 77 | 76.2 ± 9.9 |
| Heart rate, bpm | 80 | 68.31 ± 10.26 |
| Sodium | 87 | 139.0 ± 2.4 |
| Potassium | 87 | 3.93 ± 0.36 |
| Insulin, mU/ml | 50 | 15.0 ± 8.7 |
| Fasting insulin (microU/L) | 33 | 8.4 ± 7.3 |
| Fasting glucose (nmol/L) | 42 | 97 ± 10 |
| Fat-free mass, kg | 56 | 52.8 ± 6 |
| Percent fat mass | 56 | 47.1 ± 4.5 |
| HDL, mg/dL | 72 | 46.5 ± 10.9 |
| LDL mg/dL | 72 | 110.3 ± 32.9 |
| Triglycerides, mg/dL | 81 | 89.3 ± 49.2 |
| Total cholesterol, mg/dL | 72 | 175.4 ± 35.8 |
| Waist size, cm | 81 | 109.2 ± 11.5 |
| Creatinine, mg/dL | 88 | 0.8 ± 0.1 |
| BUN, mg/dL | 87 | 0.81 ± 0.11 |
| Isoprostanes, ng/mL | 88 | 0.050 ± 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, M.; Kastner, P.D.; Mehr, P.; Milne, G.L.; Ishimwe, J.A.; Park, J.H.; Shibao, C.A.; Kirabo, A. Obesity Is Associated with Increased F2-Isoprostanes and IL-6 in Black Women. Endocrines 2023, 4, 38-54. https://doi.org/10.3390/endocrines4010003
Saleem M, Kastner PD, Mehr P, Milne GL, Ishimwe JA, Park JH, Shibao CA, Kirabo A. Obesity Is Associated with Increased F2-Isoprostanes and IL-6 in Black Women. Endocrines. 2023; 4(1):38-54. https://doi.org/10.3390/endocrines4010003
Chicago/Turabian StyleSaleem, Mohammad, Paul D. Kastner, Pouya Mehr, Ginger L. Milne, Jeanne A. Ishimwe, Jennifer H. Park, Cyndya A. Shibao, and Annet Kirabo. 2023. "Obesity Is Associated with Increased F2-Isoprostanes and IL-6 in Black Women" Endocrines 4, no. 1: 38-54. https://doi.org/10.3390/endocrines4010003
APA StyleSaleem, M., Kastner, P. D., Mehr, P., Milne, G. L., Ishimwe, J. A., Park, J. H., Shibao, C. A., & Kirabo, A. (2023). Obesity Is Associated with Increased F2-Isoprostanes and IL-6 in Black Women. Endocrines, 4(1), 38-54. https://doi.org/10.3390/endocrines4010003







