Estro-Progestins and Pain Relief in Endometriosis
Abstract
:1. Introduction
2. Methods
3. Endometriosis-Related Pain
4. Medical Management: An Overview
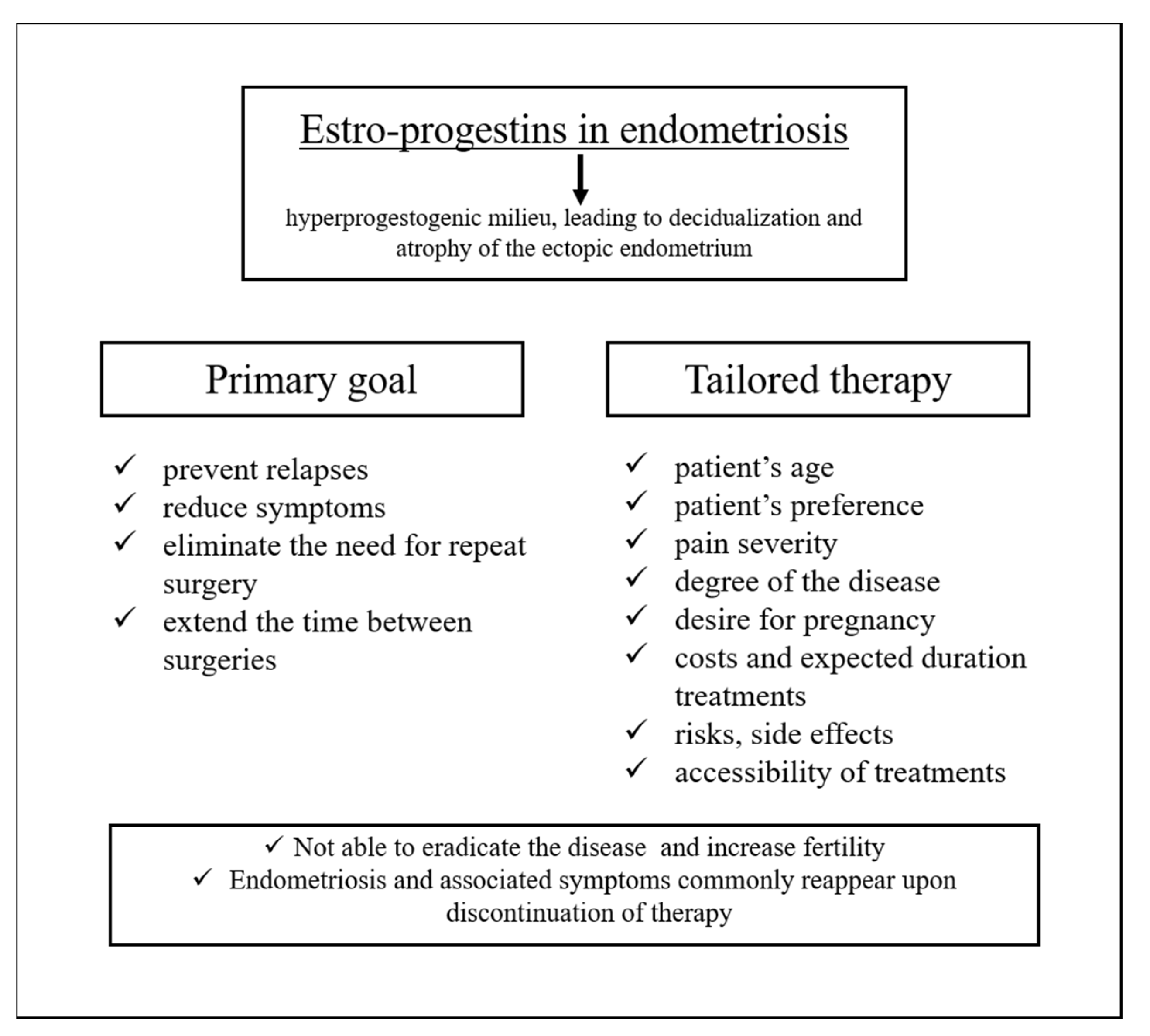
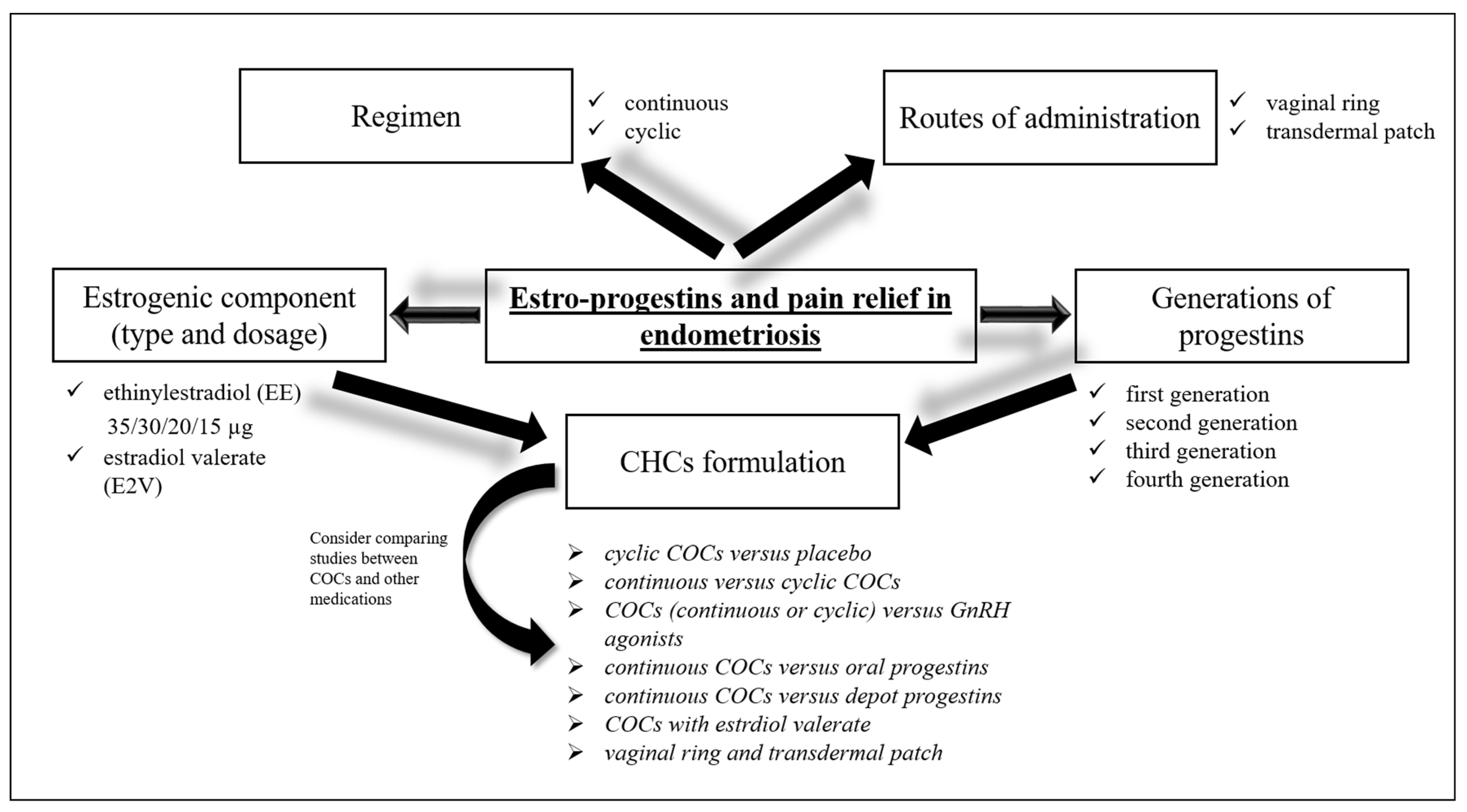
5. Estro-Progestins and Endometriosis
5.1. Cyclic COCs vs. Placebo
5.2. Continuous vs. Cyclic COCs
5.3. COCs (Continuous or Cyclic) vs. GnRH Agonists and Antagonists
5.4. Continuous COCs vs. Oral Progestins
5.5. Continuous COCs vs. Depot Progestins
5.6. COCs with Estrdiol Valerate
5.7. Vaginal Ring and Transdermal Patch
6. Estro-Progestins and Endometrioma
7. Estro-Progestins and Deep Infiltrating Endometriosis
8. Discussion
9. Conclusions
Funding
Conflicts of Interest
References
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Practice bulletin no. 114: Management of endometriosis. Obstet. Gynecol. 2010, 116, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Treatment of pelvic pain associated with endometriosis: A committee opinion. Fertil. Steril. 2014, 101, 927–935. [CrossRef] [PubMed]
- Bafort, C.; Beebeejaun, Y.; Tomassetti, C.; Bosteels, J.; Duffy, J.M. Laparoscopic surgery for endometriosis. Cochrane Database Syst. Rev. 2020, 23, CD011031. [Google Scholar] [CrossRef]
- Falcone, T.; Shakiba, K.; Bena, J.F.; McGill, K.M.; Minger, J. Surgical treatment of endometriosis: A 7-year follow-up on the requirement for further surgery. Obstet. Gynecol. 2008, 111, 1285–1292. [Google Scholar] [CrossRef] [Green Version]
- Leone Roberti Maggiore, U.; Gupta, J.K.; Ferrero, S. Treatment of endometrioma for improving fertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 81–85. [Google Scholar] [CrossRef]
- Ferrero, S.; Alessandri, F.; Racca, A.; Leone Roberti Maggiore, U. Treatment of pain associated with deep endometriosis: Alternatives and evidence. Fertil. Steril. 2015, 104, 771–792. [Google Scholar] [CrossRef] [Green Version]
- Tafi, E.; Leone Roberti Maggiore, U.; Alessandri, F.; Bogliolo, S.; Gardella, B.; Vellone, V.G.; Grillo, F.; Mastracci, L.; Ferrero, S. Advances in pharmacotherapy for treating endometriosis. Expert Opin. Pharmacother. 2015, 16, 2465–2483. [Google Scholar] [CrossRef]
- Ferrero, S.; Evangelisti, G.; Barra, F. Current and emerging treatment options for endometriosis. Expert Opin. Pharmacother. 2018, 19, 1109–1125. [Google Scholar] [CrossRef]
- Barra, F.; Scala, C.; Mais, V.; Guerriero, S.; Ferrero, S. Investigational drugs for the treatment of endometriosis, an update on recent developments. Expert Opin. Investig. Drugs 2018, 27, 445–458. [Google Scholar] [CrossRef]
- Nezhat, C.; Vang, N.; Tanaka, P.P.; Nezhat, C. Optimal Management of Endometriosis and Pain. Obstet. Gynecol. 2019, 134, 834–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asally, R.; Markham, R.; Manconi, F. The Expression and Cellular Localisation of Neurotrophin and Neural Guidance Molecules in Peritoneal Ectopic Lesions. Mol. Neurobiol. 2019, 56, 4013–4022. [Google Scholar] [CrossRef] [PubMed]
- Anaf, V.; Simon, P.; El Nakadi, I.; Fayt, I.; Buxant, F.; Simonart, T.; Peny, M.O.; Noel, J.C. Relationship between endometriotic foci and nerves in rectovaginal endometriotic nodules. Hum. Reprod. 2000, 15, 1744–1750. [Google Scholar] [CrossRef]
- Zager, E.L.; Pfeifer, S.M.; Brown, M.J.; Torosian, M.H.; Hackney, D.B. Catamenial mononeuropathy and radiculopathy: A treatable neuropathic disorder. J. Neurosurg. 1998, 88, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Morotti, M.; Vincent, K.; Becker, C.M. Mechanisms of pain in endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 20, 8–13. [Google Scholar] [CrossRef]
- Dunselman, G.A.J.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef]
- Falcone, T.; Flyckt-Rebecca, R. Clinical management of endometriosis. Obstet. Gynecol. 2018, 131, 557–571. [Google Scholar] [CrossRef] [Green Version]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Prim. 2018, 4, 9. [Google Scholar] [CrossRef]
- Harada, T.; Momoeda, M.; Taketani, Y.; Hoshiai, H.; Terakawa, N. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: A placebo-controlled, double-blind, randomized trial. Fertil. Steril. 2008, 90, 1583–1588. [Google Scholar] [CrossRef]
- Vercellini, P.; Pietropaolo, G.; De Giorgi, O.; Pasin, R.; Chiodini, A.; Crosignani, P.G. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil. Steril. 2005, 84, 1375–1387. [Google Scholar] [CrossRef]
- Murji, A.; Biberoğlu, K.; Leng, J.; Mueller, M.D.; Römer, T.; Vignali, M.; Yarmolinskaya, M. Use of dienogest in endometriosis: A narrative literature review and expert commentary. Curr. Med. Res. Opin. 2020, 36, 895–907. [Google Scholar] [CrossRef] [Green Version]
- Casper, R.F. Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil. Steril. 2017, 107, 533–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou-Setta, A.M.; Houston, B.; Al-Inany, H.G.; Farquhar, C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst. Rev. 2013, 31, CD005072. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Pan, A.; Hart, R.J. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst. Rev. 2010, 8, CD008475. [Google Scholar] [CrossRef]
- Farmer, J.E.; Prentice, A.; Breeze, A.; Ahmad, G.; Duffy, J.M.; Watson, A.; Pick, A. Gonadotrophin-releasing hormone analogues for endometriosis: Bone mineral density. Cochrane Database Syst. Rev. 2003, 2003, CD001297. [Google Scholar] [CrossRef]
- Bedaiwy, M.A.; Allaire, C.; Alfaraj, S. Long-term medical management of endometriosis with dienogest and with a gonadotropin-releasing hormone agonist and add-back hormone therapy. Fertil. Steril. 2017, 107, 537–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, H.S.; Giudice, L.C.; Lessey, B.A.; Abrao, M.S.; Kotarski, J.; Archer, D.F.; Diamond, M.P.; Surrey, E.; Johnson, N.P.; Watts, N.B.; et al. Treatment of Endometriosis-Associated Pain with Elagolix, an Oral GnRH Antagonist. N. Engl. J. Med. 2017, 377, 28–40. [Google Scholar] [CrossRef]
- Bedaiwy, M.A.; Alfaraj, S.; Yong, P.; Casper, R. New developments in the medical treatment of endometriosis. Fertil. Steril. 2017, 107, 555–565. [Google Scholar] [CrossRef] [Green Version]
- Grandi, G.; Barra, F.; Ferrero, S.; Sileo, F.G.; Bertucci, E.; Napolitano, A.; Facchinetti, F. Hormonal contraception in women with endometriosis: A systematic review. Eur. J. Contracept. Reprod. Health Care 2019, 24, 61–70. [Google Scholar] [CrossRef]
- Bourdel, N.; Alves, J.; Pickering, G.; Ramilo, I.; Roman, H.; Canis, M. Systematic review of endometriosis pain assessment: How to choose a scale? Hum. Reprod. Update 2015, 21, 136–152. [Google Scholar] [CrossRef]
- Harada, T.; Kosaka, S.; Elliesen, J.; Yasuda, M.; Ito, M.; Momoeda, M. Ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen for the management of endometriosis-associated pelvic pain: A randomized controlled trial. Fertil. Steril. 2017, 108, 798–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, S.; Iraci, M.; Cianci, S.; Fava, V.; Casella, E.; Cianci, A. Comparative, open-label prospective study on the quality of life and sexual function of women affected by endometriosis-associated pelvic pain on 2 mg dienogest/30 µg ethinyl estradiol continuous or 21/7 regimen oral contraceptive. J. Endocrinol. Investig. 2016, 39, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Guzick, D.S.; Huang, L.S.; Broadman, B.A.; Nealon, M.; Hornstein, M.D. Randomized trial of leuprolide versus continuous oral contraceptives in the treatment of endometriosis-associated pelvic pain. Fertil. Steril. 2011, 95, 1568–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vercellini, P.; Trespidi, L.; Colombo, A.; Vendola, N.; Marchini, M.; Crosignani, P.G. A gonadotropin-releasing hormone agonist versus a low-dose oral contraceptive for pelvic pain associated with endometriosis. Fertil. Steril. 1993, 60, 75–79. [Google Scholar] [CrossRef]
- Zupi, E.; Marconi, D.; Sbracia, M.; Zullo, F.; De Vivo, B.; Exacustos, C.; Sorrenti, G. Add-back therapy in the treatment of endometriosis-associated pain. Fertil. Steril. 2004, 82, 1303–1308. [Google Scholar] [CrossRef]
- Parazzini, F.; Di Cintio, E.; Chatenoud, L.; Moroni, S.; Ardovino, I.; Struzziero, E.; Falsetti, L.; Bianchi, A.; Bracco, G.; Pellegrini, A.; et al. Estroprogestin vs. gonadotrophin agonists plus estroprogestin in the treatment of endometriosis-related pelvic pain: A randomized trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 8, 11–14. [Google Scholar] [CrossRef]
- Di Francesco, A.; Pizzigallo, D. Use of micronized palmitoylethanolamide and trans-polydatin in chronic pelvic pain associated with endometriosis. An open-label study. G. Ital. di Ostet. e Ginecol. 2014, 53, 125–134. [Google Scholar] [CrossRef]
- Granese, R.; Perino, A.; Calagna, G.; Saitta, S.; De Franciscis, P.; Colacurci, N.; Triolo, O.; Cucinella, G. Gonadotrophin-releasing hormone analogue or dienogest plus estradiol valerate to prevent pain recurrence after laparoscopic surgery for endometriosis: A multi-center randomized trial. Acta Obstet. Gynecol. Scand. 2015, 94, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Fedele, L.; Bianchi, S.; Montefusco, S.; Frontino, G.; Carmignani, L. A gonadotropin-releasing hormone agonist versus a continuous oral contraceptive pill in the treatment of bladder endometriosis. Fertil. Steril. 2008, 90, 183–184. [Google Scholar] [CrossRef]
- Vercellini, P.; De Giorgi, O.; Mosconi, P.; Stellato, G.; Vicentini, S.; Crosignani, P.G. Cyproterone acetate versus a continuous monophasic oral contraceptive in the treatment of recurrent pelvic pain after conservative surgery for symptomatic endometriosis. Fertil. Steril. 2002, 77, 52–61. [Google Scholar] [CrossRef]
- Razzi, S.; Luisi, S.; Ferretti, C.; Calonaci, F.; Gabbanini, M.; Mazzini, M.; Petraglia, F. Use of a progestogen only preparation containing desogestrel in the treatment of recurrent pelvic pain after conservative surgery for endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 135, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Morotti, M.; Remorgida, V.; Venturini, P.L.; Ferrero, S. Progestogen-only contraceptive pill compared with combined oral contraceptive in the treatment of pain symptoms caused by endometriosis in patients with migraine without aura. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Piacenti, I.; Viscardi, M.F.; Masciullo, L.; Sangiuliano, C.; Scaramuzzino, S.; Piccioni, M.G.; Muzii, L.; Benedetti Panici, P.; Porpora, M.G. Dienogest versus continuous oral levonorgestrel/EE in patients with endometriosis: What’s the best choice? Gynecol. Endocrinol. 2021, 37, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Cheewadhanaraks, S.; Choksuchat, C.; Dhanaworavibul, K.; Liabsuetrakul, T. Postoperative depot medroxyprogesterone acetate versus continuous oral contraceptive pills in the treatment of endometriosis-associated pain: A randomized comparative trial. Gynecol. Obstet. Investig. 2012, 74, 151–156. [Google Scholar] [CrossRef]
- Grandi, G.; Xholli, A.; Napolitano, A.; Palma, F.; Cagnacci, A. Pelvic pain and quality of life of women with endometriosis during quadriphasic estradiol valerate/dienogest oral contraceptive: A patient-preference prospective 24-week pilot study. Reprod. Sci. 2015, 22, 626–632. [Google Scholar] [CrossRef] [Green Version]
- Morelli, M.; Sacchinelli, A.; Venturella, R.; Mocciaro, R.; Zullo, F. Postoperative administration of dienogest plus estradiol valerate versus levonorgestrel-releasing intrauterine device for prevention of pain relapse and disease recurrence in endometriosis patients. J. Obstet. Gynaecol. Res. 2013, 39, 985–990. [Google Scholar] [CrossRef]
- Vercellini, P.; Barbara, G.; Somigliana, E.; Bianchi, S.; Abbiati, A.; Fedele, L. Comparison of contraceptive ring and patch for the treatment of symptomatic endometriosis. Fertil. Steril. 2010, 93, 2150–2161. [Google Scholar] [CrossRef]
- Maggiore, U.L.R.; Remorgida, V.; Scala, C.; Tafi, E.; Venturini, P.L.; Ferrero, S. Desogestrel-only contraceptive pill versus sequential contraceptive vaginal ring in the treatment of rectovaginal endometriosis infiltrating the rectum: A prospective open-label comparative study. Acta Obstet. Gynecol. Scand. 2014, 93, 239–247. [Google Scholar] [CrossRef]
- Koga, K.; Takamura, M.; Fujii, T.; Osuga, Y. Prevention of the recurrence of symptom and lesions after conservative surgery for endometriosis. Fertil. Steril. 2015, 104, 793–801. [Google Scholar] [CrossRef]
- NICE. Endometriosis: Diagnosis and management; NICE guideline: London, UK, 2017; ISBN 978-1-4731-2661-9. [Google Scholar]
- Taniguchi, F.; Enatsu, A.; Ota, I.; Toda, T.; Arata, K.; Harada, T. Effects of low dose oral contraceptive pill containing drospirenone/ethinylestradiol in patients with endometrioma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 191, 116–120. [Google Scholar] [CrossRef]
- Vercellini, P.; Somigliana, E.; Daguati, R.; Vigano, P.; Meroni, F.; Crosignani, P.G. Postoperative oral contraceptive exposure and risk of endometrioma recurrence. Am. J. Obstet. Gynecol. 2008, 198, 504.e1–504.e5. [Google Scholar] [CrossRef] [PubMed]
- Muzii, L.; Maneschi, F.; Marana, R.; Porpora, M.G.; Zupi, E.; Bellati, F.; Angioli, R.; Benedetti Panici, P. Oral Estroprogestins after Laparoscopic Surgery to Excise Endometriomas: Continuous or Cyclic Administration? Results of a Multicenter Randomized Study. J. Minim. Invasive Gynecol. 2011, 18, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Takamura, M.; Koga, K.; Osuga, Y.; Takemura, Y.; Hamasaki, K.; Hirota, Y.; Yoshino, O.; Taketani, Y. Post-operative oral contraceptive use reduces the risk of ovarian endometrioma recurrence after laparoscopic excision. Hum. Reprod. 2009, 24, 3042–3048. [Google Scholar] [CrossRef] [Green Version]
- Seracchioli, R.; Mabrouk, M.; Frascà, C.; Manuzzi, L.; Montanari, G.; Keramyda, A.; Venturoli, S. Long-term cyclic and continuous oral contraceptive therapy and endometrioma recurrence: A randomized controlled trial. Fertil. Steril. 2010, 93, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; De Matteis, S.; Somigliana, E.; Buggio, L.; Frattaruolo, M.P.; Fedele, L. Long-term adjuvant therapy for the prevention of postoperative endometrioma recurrence: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2013, 92, 8–16. [Google Scholar] [CrossRef]
- Muzii, L.; Di Tucci, C.; Achilli, C.; Di Donato, V.; Musella, A.; Palaia, I.; Panici, P.B. Continuous versus cyclic oral contraceptives after laparoscopic excision of ovarian endometriomas: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2016, 214, 203–211. [Google Scholar] [CrossRef]
- Wattanayingcharoenchai, R.; Rattanasiri, S.; Charakorn, C.; Attia, J.; Thakkinstian, A. Postoperative hormonal treatment for prevention of endometrioma recurrence after ovarian cystectomy: A systematic review and network meta-analysis. BJOG An Int. J. Obstet. Gynaecol. 2021, 128, 25–35. [Google Scholar] [CrossRef]
- Tarjanne, S.; Ng, C.H.M.; Manconi, F.; Arola, J.; Mentula, M.; Maneck, B.; Fraser, I.S.; Heikinheimo, O. Use of hormonal therapy is associated with reduced nerve fiber density in deep infiltrating, rectovaginal endometriosis. Acta Obstet. Gynecol. Scand. 2015, 94, 693–700. [Google Scholar] [CrossRef]
- Mabrouk, M.; Frascà, C.; Geraci, E.; Montanari, G.; Ferrini, G.; Raimondo, D.; Alvisi, S.; Paradisi, R.; Villa, G.; Seracchioli, R. Combined Oral Contraceptive Therapy in Women with Posterior Deep Infiltrating Endometriosis. J. Minim. Invasive Gynecol. 2011, 18, 470–474. [Google Scholar] [CrossRef]
- Ferrero, S.; Leone Roberti Maggiore, U.; Scala, C.; Di Luca, M.; Venturini, P.L.; Remorgida, V. Changes in the size of rectovaginal endometriotic nodules infiltrating the rectum during hormonal therapies. Arch. Gynecol. Obstet. 2013, 287, 447–453. [Google Scholar] [CrossRef]
- Ferrari, S.; Persico, P.; Di Puppo, F.; Vigano, P.; Tandoi, I.; Garavaglia, E.; Giardina, P.; Mezzi, G.; Candiani, M. Continuous low-dose oral contraceptive in the treatment of colorectal endometriosis evaluated by rectal endoscopic ultrasonography. Acta Obstet. Gynecol. Scand. 2012, 91, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.T.; Schlaff, W.; Gordon, K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: A systematic review of the evidence. Fertil. Steril. 2018, 110, 137–152.e1. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S. Endometriosis: Modern management of an ancient disease. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 1–2. [Google Scholar] [CrossRef]
- Endrikat, J.; Parke, S.; Trummer, D.; Serrani, M.; Duijkers, I.; Klipping, C. Pituitary, ovarian and additional contraceptive effects of an estradiol-based combined oral contraceptive: Results of a randomized, open-label study. Contraception 2013, 87, 227–234. [Google Scholar] [CrossRef]
- Vandever, M.A.; Kuehl, T.J.; Sulak, P.J.; Witt, I.; Coffee, A.; Wincek, T.J.; Reape, K.Z. Evaluation of pituitary-ovarian axis suppression with three oral contraceptive regimens. Contraception 2008, 77, 162–170. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, B.; Mueller, M.; Montgomery, G. Progesterone Resistance in Endometriosis: An Acquired Property? Trends Endocrinol. Metab. 2018, 29, 535–548. [Google Scholar] [CrossRef]
- Vercellini, P. Are combined hormonal contraceptives the neglected treatment for symptomatic endometriosis? Fertil. Steril. 2018, 110, 61–62. [Google Scholar] [CrossRef]
- Martone, S.; Troìa, L.; Marcolongo, P.; Luisi, S. Role of medical treatment of endometriosis. Minerva Obstet. Gynecol. 2021, 73, 304–316. [Google Scholar] [CrossRef]
| Hormonal Formulation | Study Reference | Patients Selection | Duration | Interventions | Main Outcomes |
|---|---|---|---|---|---|
| Cyclic COCs vs. placebo | Harada et al. [19] | 100 symptomatic endometriosis (diagnosed by surgery or imaging) | 4 months | EE 35 µg plus norethisterone 1 mg or placebo | In COCs users: -Three-fold greater reduction of dysmenorrhea VAS scores. -No clinically significant reduction in non-menstrual pain. |
| Harada et al. [31] | 312 symptomatic endometriosis (diagnosed by surgery or imaging) | 52 weeks | Extended flexible regimen with EE 20 µg plus DRSP 3 mg versus placebo versus DNG 2 mg | -Flexible extended regimen significantly reduced severe pelvic pain compared with placebo (mean difference in pain score −26.3 mm using a 100-mm VAS). -In the dienogest group the pain score decreased even more (decrease of 50.0 mm). | |
| Continuous vs. cyclic COCs | Caruso et al. [32] | 63 versus 33 patients with endometriosis-associated pelvic pain | 6 months | Continuous versus a 21-day cyclic regimen of EE 30 µg plus DNG 2 mg | Continuous regimen reported greater and faster reduction of endometriosis-associated pelvic pain and significant improvement of sexual activity and QoL than cyclical regimen. |
| COCs (continuous or cyclic) vs. GnRH agonists | Guzick et al. [33] | 47 patients with endometriosis-associated pelvic pain. | 48 weeks | Continuous EE 35 µg plus norethindrone 1 mg versus add-back norethindrone acetate 5mg and intramuscular injection of placebo or depot LA 11,25 mg every 12 weeks | Significant improvement in pain scores from baseline in both treatment groups and no significant difference in the extent of pain relief. |
| Vercellini et al. [34] | 57 patients with surgical diagnosis of endometriosis and pelvic pain | 6 months | cyclic EE 20/30 µg and DSG 0.15 mg versus goserelin 3.6 mg in a 28-day subcutaneous depot formulation | -Significant reduction in deep dyspareunia in both groups, with goserelin being superior to COCs. -Significant improvement in dysmenorrhea and non-menstrual pain with no difference between groups. | |
| Zupi et al. [35] | 133 patients with pelvic pain recurrence after surgery | 12 months | group 1: LA alone, group 2: LA plus add-back therapy (transdermal E2 and oral norethindrone), group 3: cyclic EE 30 µg plus GSD 0.75 mg | -Groups 1 and 2 showed greater pain improvement compared to group 3. -Add-back therapy showed a reduced rate of adverse effects, good pain control, and better QoL than the other two treatments. | |
| Parazzini et al. [36] | 47 versus 55 patients with laparoscopically confirmed endometriosis and pelvic pain | 12 months | EE 30 µg plus gestroden 0.75 mg versus 4 months of tryptorelin 3.75 mg every 28 days followed by 8 months of COC | No significant differences between groups in pain relief. | |
| Di Francesco and Pizzigallo [37] | 30 patients with chronic pelvic pain associated to endometriosis | 6 months | Palmitoylethanolamide + trans-polydatin versus LA versus cyclic EE 30 µg plus DRSP 3 mg. | Dysmenorrhea, chronic pelvic pain, and dyspareunia intensity significantly decreased over time in all three groups, irrespective of the treatment applied. | |
| Granese et al. [38] | 78 patients who underwent laparoscopic surgery for endometriosis combined with chronic pelvic pain | 9 months | multiphasic pill with E2V 2 mg plus DNG versus LA 3.75 mg monthly | -Similar endometriosis relpase rate and VAS score. -Substantial improvement in QoL and health satisfaction with both treatments in all women with higher scores than preoperative values. | |
| Fedele et al. [39] | 10 patients with bladder endometriosis. | 6 months | continuous COC treatment versus GnRH agonist | Both regimens resulted in regression of the bladder lesions, with slightly better results with GnRH agonist than with COC. | |
| COCs (continuous or cyclic) vs. oral progestins | Vercellini et al. [40] | 90 patients with pain relapse after conservative surgery | 6 months | Continuous monophasic EE 20 µg plus DSG 0.15 mg versus CPA 12.5 mg | -Similar improvement in non-menstrual pelvic pain, dysmenorrhea, dyspareunia, QoL, psychological profile and sexual satisfaction from both treatments. -Slightly higher satisfaction in CPA users. -Dysmenorrhea improved much more significantly than nonmenstrual pain with both treatments. |
| Vercellini et al. [20] | 90 patients with symptomatic rectovaginal endometriosis after surgery | 12 months | Continuous EE 10 µg plus CPA 3 mg versus NETA 2.5 mg | -Dysmenorrhea, deep dyspareunia, nonmenstrual pelvic pain, and dyschezia scores were substantially reduced without major between-group differences -Slightly higher satisfaction in NETA users | |
| Razzi et al. [41] | 40 patients with recurrent pelvic pain after conservative surgery | 6 months. | EE 20 µg plus DSG 0.15 mg versus DSG 75 mcg | -Significant improvement of pelvic pain and dysmenorrhea in both groups. -More frequently breakthrough bleeding in POP group. | |
| Morotti et al. [42] | 144 patients with symptomatic rectovaginal endometriosis and migraine without aura | 6 months | Cyclic EE 20 µg plus DSG 0.15 mg versus continuous DSG 75 mcg | -Similar decrease in chronic pelvic pain and dyspareunia for both treatments. -POP is better tolerated than COC and it seems to ameliorate migraine attacks | |
| Continuous COCs vs. long-acting progestins | Cheewadhanaraks et al. [43] | 84 patients with symptomatic endometriosis after conservative surgery | 24 weeks | EE 30 µg plus GSD 0.075 mg versus intramuscular DMPA 150 mg every 12 weeks | -No differences in treatment satisfaction and withdrawal rates between the two groups -Significantly higher VAS score per week for dysmenorrhea in COC group. |
| Morelli et al. [38] | 92 patients undergoing surgery for endometriosis | 24 months | Multiphasic pill with E2V 2 mg plus DNG versus 52 mg LNG-IUS | -Statistically greater reduction in Ca125 levels and VAS scores in COC group. -Slightly lower recurrence rate in COC group. -Significantly higher patient satisfaction in LNG-IUS group. | |
| Continuous COCs vs. NSAID | Grandi et al. [44] | 34 patients with menstrual pain and endometriosis | 24 weeks | Multiphasic pill with E2V 2 mg plus DNG versus ketoprofen 200 mg tablets | Significantly greater reduction in menstrual and intermenstrual pain and improvement of QoL during E2V/DNG treatment than NSAID therapy. |
| Inclusion Criteria | Study Reference | Number of Patients | Duration | Interventions | Main Outcomes |
|---|---|---|---|---|---|
| Ovarian endometrioma, without recent medical or surgical treatment | Taniguchi et al. [51] | 49 | 6 months | Cyclic EE 20 µg plus DRSP 3 mg compared with pretreatment | -Maximum diameter and volume of ovarian endometriomas significantly decreased after 3 and 6 cycles. -VAS scores of dysmenorrhea were reduced after 1, 3 and 6 cycles. |
| Harada et al. [19] | 100 | 4 months | EE 35 µg plus norethisterone 1 mg or placebo | Endometriomas significantly reduced their volume only in the COCs group. | |
| Laparoscopic excision of ovarian endometriomas | Vercelli et al. [52] | 277 | 36 months | EE 20 µg plus DSG 0.15 mg versus non-users | Postoperative risk of endometrioma recurrence was 6% in users compared with 49% in the never users. |
| Muzii et al. [53] | 57 | 6 months | Continuous versus cyclic EE 20 µg plus DSG 0.15 mg | -Endometrioma recurrence rate was 4% in the cyclical regimen group, compared with 0% in the continuous group. -Improvements in pain scores in both groups with no significant differences. -More adverse effects and significantly higher treatment discontinuation rate in the continuous group. | |
| Takamura et al. [54] | 87 | 24 months | Cyclic, 35 µg plus norethisterone 1 mg versus non-users | Endometrioma recurrence rate was 2.9% in users compared with 35.8% in the never used or discontinued. | |
| Seracchioli et al. [55] | 217 | 24 months | Continuous versus cyclic EE 20 µg plus GSD 0.075 mg or no therapy. | -Lower endometrioma recurrence rate in continuous and cyclic regimen groups (14.7% and 8.2%) than non-users (29%). -Shorter recurrence-free time in non-users. -The mean increase in endometrioma diameter every 6 months was significantly reduced in COCs-users. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troìa, L.; Luisi, S. Estro-Progestins and Pain Relief in Endometriosis. Endocrines 2022, 3, 349-366. https://doi.org/10.3390/endocrines3020028
Troìa L, Luisi S. Estro-Progestins and Pain Relief in Endometriosis. Endocrines. 2022; 3(2):349-366. https://doi.org/10.3390/endocrines3020028
Chicago/Turabian StyleTroìa, Libera, and Stefano Luisi. 2022. "Estro-Progestins and Pain Relief in Endometriosis" Endocrines 3, no. 2: 349-366. https://doi.org/10.3390/endocrines3020028
APA StyleTroìa, L., & Luisi, S. (2022). Estro-Progestins and Pain Relief in Endometriosis. Endocrines, 3(2), 349-366. https://doi.org/10.3390/endocrines3020028





