Premenstrual Syndrome and Premenstrual Dysphoric Disorder as Centrally Based Disorders
Abstract
:1. Introduction
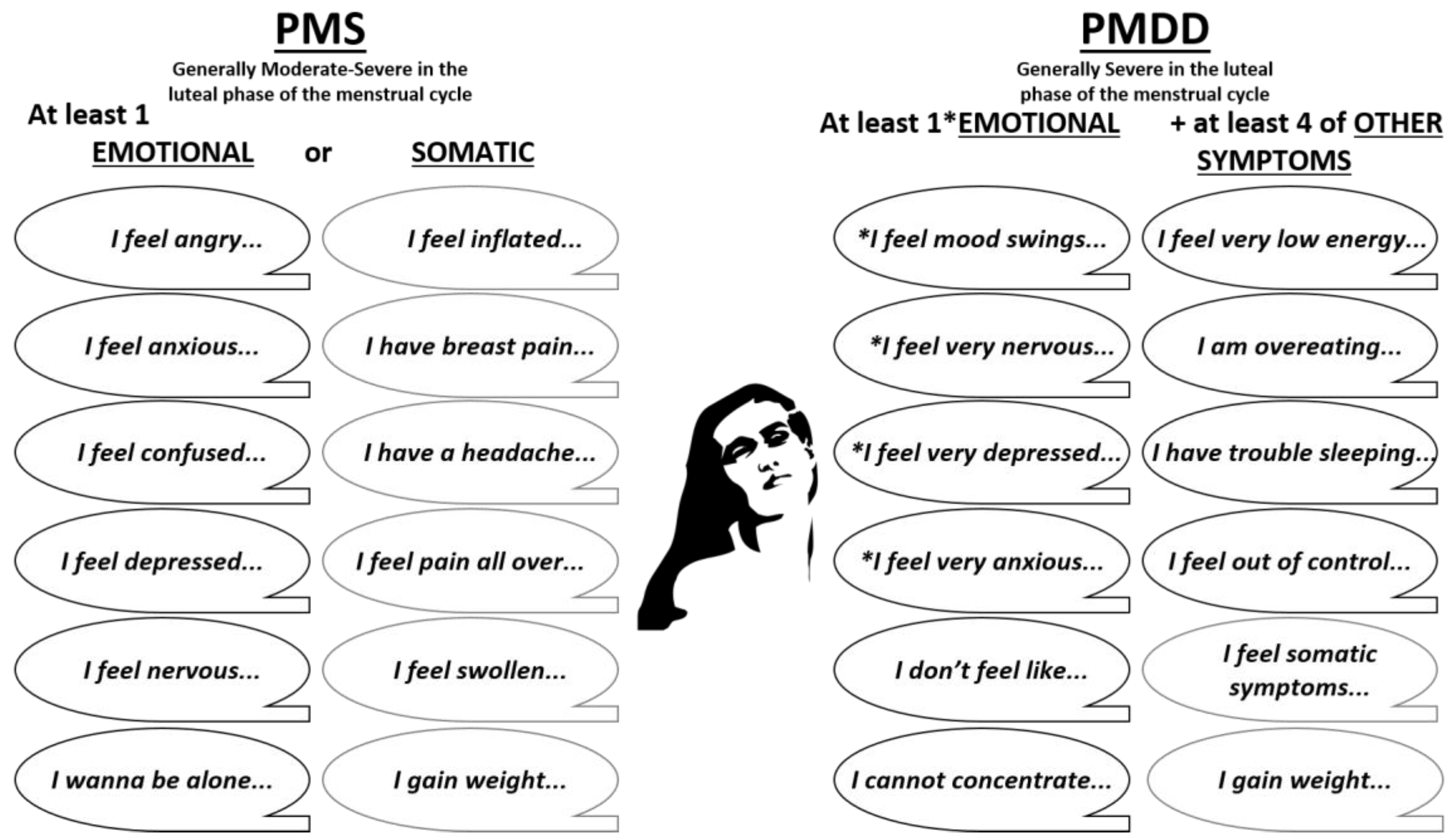
2. Definitions of Premenstrual Disorders
3. Epidemiology and Risks Factors of PMS/PMDD
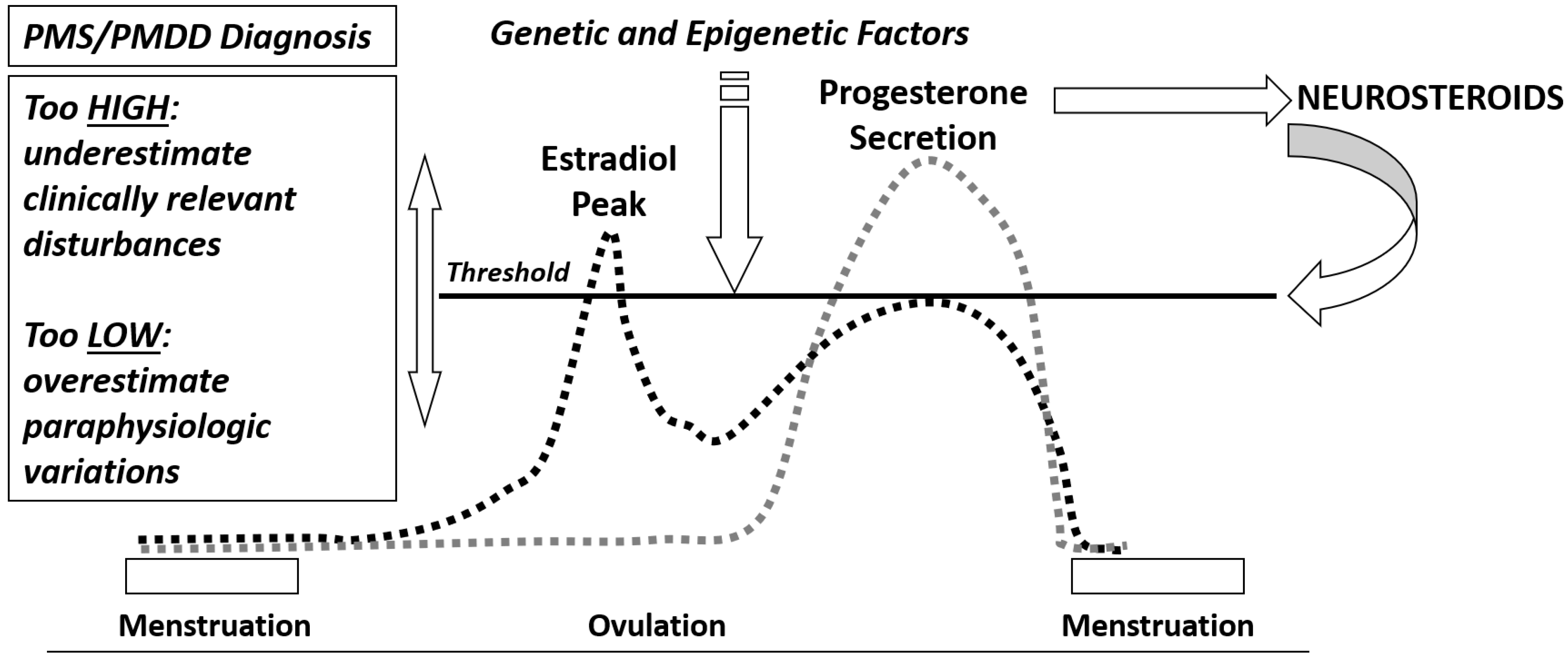
4. Neuroendocrine Aspects of PMS/PMDD
Estrogens and Progesterone
5. Allopregnanolone and GABAergic System
6. Opioid System
7. Serotoninergic System
8. Other Trends in Neuroinflammation
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Critchley, H.O.; Babayev, E.; Bulun, S.E.; Clark, S.; Garcia-Grau, I.; Gregersen, P.K.; Kilcoyne, A.; Kim, J.-Y.J.; Lavender, M.; Marsh, E.E.; et al. Menstruation: Science and society. Am. J. Obstet. Gynecol. 2020, 223, 624–664. [Google Scholar] [CrossRef] [PubMed]
- Roeder, H.J.; Leira, E.C. Effects of the Menstrual Cycle on Neurological Disorders. Curr. Neurol. Neurosci. Rep. 2021, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, J.V.; Guico-Pabia, C.J.; Taylor, H.S. Menstrual cycle-related exacerbation of disease. Am. J. Obstet. Gynecol. 2010, 202, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Barrington, D.J.; Robinson, H.J.; Wilson, E.; Hennegan, J. Experiences of menstruation in high income countries: A systematic review, qualitative evidence synthesis and comparison to low- and middle-income countries. PLoS ONE 2021, 16, e0255001. [Google Scholar] [CrossRef]
- Matteson, K.A.; Zaluski, K.M. Menstrual Health as a Part of Preventive Health Care. Obstet. Gynecol. Clin. N. Am. 2019, 46, 441–453. [Google Scholar] [CrossRef]
- Tassorelli, C.; Greco, R.; Allena, M.; Terreno, E.; Nappi, R.E. Transdermal Hormonal Therapy in Perimenstrual Migraine: Why, When and How? Curr. Pain Headache Rep. 2012, 16, 467–473. [Google Scholar] [CrossRef]
- Shulman, L.P. Gynecological Management of Premenstrual Symptoms. Curr. Pain Headache Rep. 2010, 14, 367–375. [Google Scholar] [CrossRef]
- Ismaili, E.; Consensus Group of the International Society for Premenstrual Disorders; Walsh, S.; O’Brien, P.M.S.; Bäckström, T.; Brown, C.; Dennerstein, L.; Eriksson, E.; Freeman, E.W.; Ismail, K.M.K.; et al. Fourth consensus of the International Society for Premenstrual Disorders (ISPMD): Auditable standards for diagnosis and management of premenstrual disorder. Arch. Women’s Ment. Health 2016, 19, 953–958. [Google Scholar] [CrossRef]
- Yonkers, K.A.; O’Brien, P.S.; Eriksson, E. Premenstrual syndrome. Lancet 2008, 371, 1200–1210. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual, 4th ed.; American College of Obstetricians and Gynecologists: Washington, DC, USA, 2014; pp. 607–613. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American College of Obstetricians and Gynecologists: Washington, DC, USA, 2013. [Google Scholar]
- Hofmeister, S.; Bodden, S. Premenstrual Syndrome and Premenstrual Dysphoric Disorder. Am. Fam. Physician 2016, 94, 236–240. [Google Scholar]
- Stute, P.; Bodmer, C.; Ehlert, U.; Eltbogen, R.; Ging, A.; Streuli, I.; Von Wolff, M. Interdisciplinary consensus on management of premenstrual disorders in Switzerland. Gynecol. Endocrinol. 2017, 33, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Epperson, C.N.; Steiner, M.; Hartlage, S.A.; Eriksson, E.; Schmidt, P.J.; Jones, I.; Yonkers, K.A. Premenstrual Dysphoric Disorder: Evidence for a New Category for DSM-5. Am. J. Psychiatry 2012, 169, 465–475. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 11th ed.; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Studd, J.; Nappi, R.E. Reproductive depression. Gynecol. Endocrinol. 2012, 28, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Studd, J. Severe premenstrual syndrome and bipolar disorder: A tragic confusion. Menopause Int. 2012, 18, 82–86. [Google Scholar] [CrossRef]
- Slyepchenko, A.; Minuzzi, L.; Frey, B.N. Comorbid Premenstrual Dysphoric Disorder and Bipolar Disorder: A Review. Front. Psychiatry 2021, 12, 719241. [Google Scholar] [CrossRef]
- Hall, E.; Steiner, M. Psychiatric Symptoms and Disorders Associated with Reproductive Cyclicity in Women: Advances in Screening Tools. Women’s Health 2015, 11, 399–415. [Google Scholar] [CrossRef] [Green Version]
- Steiner, M.; MacDougall, M.; Brown, E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch. Women’s Ment. Health 2003, 6, 203–209. [Google Scholar] [CrossRef]
- Mortola, J.F.; Girton, L.; Beck, L.; Yen, S.S. Diagnosis of premenstrual syndrome by a simple, prospective, and reliable instrument: The calendar of premenstrual experiences. Obstet. Gynecol. 1990, 76, 302–307. [Google Scholar] [CrossRef]
- Sattar, K. Epidemiology of Premenstrual Syndrome, A Systematic Review and Meta-Analysis Study. J. Clin. Diagn. Res. 2014, 8, 106–109. [Google Scholar] [CrossRef]
- Yonkers, K.A.; Simoni, M.K. Premenstrual disorders. Am. J. Obstet. Gynecol. 2018, 218, 68–74. [Google Scholar] [CrossRef]
- Pilver, C.E.; Kasl, S.; Desai, R.; Levy, B.R. Health advantage for black women: Patterns in pre-menstrual dysphoric disorder. Psychol. Med. 2011, 41, 1741–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapkin, A.J.; Winer, S.A. Premenstrual syndrome and premenstrual dysphoric disorder: Quality of life and burden of illness. Expert Rev. Pharm. Outcomes Res. 2009, 9, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Gao, D.; Sun, H.; Cheng, X.; An, L.; Qiao, M. Trends in Research Related to Premenstrual Syndrome and Premenstrual Dysphoric Disorder From 1945 to 2018: A Bibliometric Analysis. Front. Public Health 2021, 9, 596128. [Google Scholar] [CrossRef]
- Potter, J.; Bouyer, J.; Trussell, J.; Moreau, C. Premenstrual Syndrome Prevalence and Fluctuation over Time: Results from a French Population-Based Survey. J. Women’s Health 2009, 18, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sander, B.; Gordon, J.L. Premenstrual Mood Symptoms in the Perimenopause. Curr. Psychiatry Rep. 2021, 23, 73. [Google Scholar] [CrossRef]
- de Wit, A.E.; de Vries, Y.A.; de Boer, M.K.; Scheper, C.; Fokkema, A.; Janssen, C.A.; Giltay, E.J.; Schoevers, R.A. Efficacy of combined oral contraceptives for depressive symptoms and overall symptomatology in premenstrual syndrome: Pairwise and network meta-analysis of randomized trials. Am. J. Obstet. Gynecol. 2021, 225, 624–633. [Google Scholar] [CrossRef]
- Choi, S.H.; Hamidovic, A. Association Between Smoking and Premenstrual Syndrome: A Meta-Analysis. Front. Psychiatry 2020, 11, 575526. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R.; Hankinson, S.E.; Willett, W.C.; Johnson, S.R.; Manson, J.E. Adiposity and the Development of Premenstrual Syndrome. J. Women’s Health 2010, 19, 1955–1962. [Google Scholar] [CrossRef] [Green Version]
- Fernández, M.D.M.; Saulyte, J.; Inskip, H.; Takkouche, B. Premenstrual syndrome and alcohol consumption: A systematic review and meta-analysis. BMJ Open 2018, 8, e019490. [Google Scholar] [CrossRef] [Green Version]
- Pearce, E.; Jolly, K.; Jones, L.; Matthewman, G.; Zanganeh, M.; Daley, A. Exercise for premenstrual syndrome: A systematic review and meta-analysis of randomised controlled trials. BJGP Open 2020, 4, 25. [Google Scholar] [CrossRef]
- Perkonigg, A.; Yonkers, K.A.; Pfister, H.; Lieb, R.; Wittchen, H.-U. Risk Factors for Premenstrual Dysphoric Disorder in a Community Sample of Young Women: The role of traumatic events and posttraumatic stress disorder. J. Clin. Psychiatry 2004, 65, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Pessoa, A.R.; Madeira, N.; Macedo, A.; Pereira, A.T. Association between premenstrual dysphoric disorder and perinatal depression: A systematic review. Arch. Women’s Ment. Health 2021, 25, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Osborn, E.; Brooks, J.; O’Brien, P.M.S.; Wittkowski, A. Suicidality in women with Premenstrual Dysphoric Disorder: A systematic literature review. Arch. Women’s Ment. Health 2021, 24, 173–184. [Google Scholar] [CrossRef]
- Nobles, C.J.; Thomas, J.J.; Valentine, S.E.; Gerber, M.; Ba, A.S.V.; Marques, L. Association of premenstrual syndrome and premenstrual dysphoric disorder with bulimia nervosa and binge-eating disorder in a nationally representative epidemiological sample. Int. J. Eat. Disord. 2016, 49, 641–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nappi, R.E.; Nappi, G. Neuroendocrine aspects of migraine in women. Gynecol. Endocrinol. 2012, 28, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Amital, D.; Herskovitz, C.; Fostick, L.; Silberman, A.; Doron, Y.; Zohar, J.; Itsekson, A.; Zolti, M.; Rubinow, A.; Amital, H. The Premenstrual Syndrome and Fibromyalgia—Similarities and Common Features. Clin. Rev. Allergy Immunol. 2010, 38, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Graziottin, A. The shorter, the better: A review of the evidence for a shorter contraceptive hormone-free interval. Eur. J. Contracept. Reprod. Health Care 2016, 21, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. Estrogen Makes the Brain a Sex Organ. J. Clin. Psychiatry 1997, 58, 421–422. [Google Scholar] [CrossRef] [Green Version]
- Backstrom, T.; Sanders, D.; Leask, R.; Davidson, D.; Warner, P.; Bancroft, J. Mood, Sexuality, Hormones, and the Menstrual Cycle. II. Hormone Levels and Their Relationship to the Premenstrual Syndrome. Psychosom. Med. 1983, 45, 503–507. [Google Scholar] [CrossRef]
- Soares, C.N.; Zitek, B. Reproductive hormone sensitivity and risk for depression across the female life cycle: A continuum of vulnerability? J. Psychiatry Neurosci. 2008, 33, 331–343. [Google Scholar]
- Wise, D.D.; Felker, A.; Stahl, S.M. Tailoring treatment of depression for women across the reproductive lifecycle: The importance of pregnancy, vasomotor symptoms, and other estrogen-related events in psychopharmacology. CNS Spectr. 2008, 13, 647–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genazzani, A.; Gastaldi, M.; Bidzinska, B.; Mercuri, N.; Nappi, R.; Segre, A.; Petraglia, F. The brain as a target organ of gonadal steroids. Psychoneuroendocrinology 1992, 17, 385–390. [Google Scholar] [CrossRef]
- Bernardi, F.; Pluchino, N.; Stomati, M.; Pieri, M.; Genazzani, A.R. CNS: Sex Steroids and SERMs. Ann. N. Y. Acad. Sci. 2003, 997, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Giatti, S.; Diviccaro, S.; Serafini, M.M.; Caruso, D.; Garcia-Segura, L.M.; Viviani, B.; Melcangi, R.C. Sex differences in steroid levels and steroidogenesis in the nervous system: Physiopathological role. Front. Neuroendocr. 2019, 56, 100804. [Google Scholar] [CrossRef] [PubMed]
- Schweizer-Schubert, S.; Gordon, J.L.; Eisenlohr-Moul, T.A.; Meltzer-Brody, S.; Schmalenberger, K.M.; Slopien, R.; Zietlow, A.-L.; Ehlert, U.; Ditzen, B. Steroid Hormone Sensitivity in Reproductive Mood Disorders: On the Role of the GABAA Receptor Complex and Stress During Hormonal Transitions. Front. Med. 2021, 7, 479646. [Google Scholar] [CrossRef]
- McEvoy, K.; Osborne, L.; Nanavati, J.; Payne, J.L. Reproductive Affective Disorders: A Review of the Genetic Evidence for Premenstrual Dysphoric Disorder and Postpartum Depression. Curr. Psychiatry Rep. 2017, 19, 94. [Google Scholar] [CrossRef]
- Schmidt, P.J.; Nieman, L.K.; Danaceau, M.A.; Adams, L.F.; Rubinow, D.R. Differential Behavioral Effects of Gonadal Steroids in Women with and in Those without Premenstrual Syndrome. N. Engl. J. Med. 1998, 338, 209–216. [Google Scholar] [CrossRef]
- Studd, J.W. A guide to the treatment of depression in women by estrogens. Climacteric 2011, 14, 637–642. [Google Scholar] [CrossRef]
- Bixo, M.; Johansson, M.; Timby, E.; Michalski, L.; Bäckström, T. Effects of GABA active steroids in the female brain with a focus on the premenstrual dysphoric disorder. J. Neuroendocr. 2018, 30, e12553. [Google Scholar] [CrossRef]
- Yen, J.-Y.; Lin, H.-C.; Liu, T.-L.; Long, C.-Y.; Ko, C.-H. Early- and Late-Luteal-Phase Estrogen and Progesterone Levels of Women with Premenstrual Dysphoric Disorder. Int. J. Environ. Res. Public Health 2019, 16, 4352. [Google Scholar] [CrossRef] [Green Version]
- Bäckström, T.; Andreen, L.; Birzniece, V.; Björn, I.; Johansson, I.-M.; Nordenstam-Haghjo, M.; Nyberg, S.; Poromaa, I.S.; Wahlström, G.; Wang, M.; et al. The Role of Hormones and Hormonal Treatments in Premenstrual Syndrome. CNS Drugs 2003, 17, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Oinonen, K.A.; Mazmanian, D. To what extent do oral contraceptives influence mood and affect? J. Affect. Disord. 2002, 70, 229–240. [Google Scholar] [CrossRef]
- Schmidt, P.J.; Rubinow, D.R. Sex Hormones and Mood in the Perimenopause. Ann. N. Y. Acad. Sci. 2009, 1179, 70–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, A.F.; Mortola, J.F.; Wood, S.H.; Yen, S.S. Persistence of premenstrual syndrome during low-dose administration of the pro-gesterone antagonist RU 486. Obstet. Gynecol. 1994, 84, 1001–1005. [Google Scholar]
- Schmidt, P.J.; Nieman, L.K.; Grover, G.N.; Muller, K.L.; Merriam, G.R.; Rubinow, D.R. Lack of Effect of Induced Menses on Symptoms in Women with Premenstrual Syndrome. N. Engl. J. Med. 1991, 324, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Chodankar, R.R. 90 YEARS OF PROGESTERONE: Selective progesterone receptor modulators in gynaecological therapies. J. Mol. Endocrinol. 2020, 65, T15–T33. [Google Scholar] [CrossRef] [PubMed]
- Comasco, E.; Kallner, H.K.; Bixo, M.; Hirschberg, A.L.; Nyback, S.; de Grauw, H.; Epperson, C.N.; Sundström-Poromaa, I. Ulipristal Acetate for Treatment of Premenstrual Dysphoric Disorder: A Proof-of-Concept Randomized Controlled Trial. Am. J. Psychiatry 2021, 178, 256–265. [Google Scholar] [CrossRef]
- Baller, E.B.; Wei, S.-M.; Kohn, P.D.; Rubinow, D.R.; Alarcón, G.; Schmidt, P.J.; Berman, K.F. Abnormalities of Dorsolateral Prefrontal Function in Women with Premenstrual Dysphoric Disorder: A Multimodal Neuroimaging Study. Am. J. Psychiatry 2013, 170, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.-M.; Baller, E.B.; Martinez, P.E.; Goff, A.C.; Li, H.J.; Kohn, P.D.; Kippenhan, J.S.; Soldin, S.J.; Rubinow, D.R.; Goldman, D.; et al. Subgenual cingulate resting regional cerebral blood flow in premenstrual dysphoric disorder: Differential regulation by ovarian steroids and preliminary evidence for an association with expression of ESC/E(Z) complex genes. Transl. Psychiatry 2021, 11, 206. [Google Scholar] [CrossRef]
- Kaltsouni, E.; Fisher, P.M.; Dubol, M.; Hustad, S.; Lanzenberger, R.; Frokjaer, V.G.; Wikström, J.; Comasco, E.; Sundström-Poromaa, I. Brain reactivity during aggressive response in women with premenstrual dysphoric disorder treated with a selective progesterone receptor modulator. Neuropsychopharmacology 2021, 46, 1460–1467. [Google Scholar] [CrossRef]
- Wyatt, K.M.; Dimmock, P.W.; O’Brien, P.S.; Ismail, K.M.; Jones, P.W. The effectiveness of GnRHa with and without ‘add-back’ therapy in treating premenstrual syndrome: A meta analysis. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Segebladh, B.; Borgström, A.; Nyberg, S.; Bixo, M.; Sundström-Poromaa, I. Evaluation of different add-back estradiol and progesterone treatments to gonadotropin-releasing hormone agonist treatment in patients with premenstrual dysphoric disorder. Am. J. Obstet. Gynecol. 2009, 201, 139.e1–139.e8. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, R.; Holsboer, F. Neuroactive steroids: Mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999, 22, 410–416. [Google Scholar] [CrossRef]
- Zorumski, C.F.; Paul, S.M.; Covey, D.F.; Mennerick, S. Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiol. Stress 2019, 11, 100196. [Google Scholar] [CrossRef]
- Rapkin, A.J.; Morgan, M.; Goldman, L.; Brann, D.W.; Simone, D.; Mahesh, V.B. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet. Gynecol. 1997, 90, 709–714. [Google Scholar] [CrossRef]
- Monteleone, P.; Luisi, S.; Tonetti, A.; Bernardi, F.; Genazzani, A.D.; Luisi, M.; Petraglia, F.; Genazzani, A.R. Allopregnanolone concen-trations and premenstrual syndrome. Eur. J. Endocrinol. 2000, 142, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, I.; Luisi, S.; Quirici, B.; Monteleone, P.; Bernardi, F.; Liut, M.; Casarosa, E.; Palumbo, M.; Petraglia, F.; Genazzani, A.R. Adrenal response to adrenocorticotropic hormone stimulation in patients with premenstrual syndrome. Gynecol. Endocrinol. 2004, 18, 79–87. [Google Scholar] [CrossRef]
- Hantsoo, L.; Epperson, C.N. Allopregnanolone in premenstrual dysphoric disorder (PMDD): Evidence for dysregulated sensitivity to GABA-A receptor modulating neuroactive steroids across the menstrual cycle. Neurobiol. Stress 2020, 12, 100213. [Google Scholar] [CrossRef]
- Smith, S.S.; Gong, Q.H.; Li, X.; Moran, M.H.; Bitran, D.; Frye, C.A.; Hsu, F.-C. Withdrawal from 3α-OH-5α-Pregnan-20-One Using a Pseudopregnancy Model Alters the Kinetics of Hippocampal GABAA-Gated Current and Increases the GABAAReceptor α4 Subunit in Association with Increased Anxiety. J. Neurosci. 1998, 18, 5275–5284. [Google Scholar] [CrossRef] [Green Version]
- Andreen, L.; Sundström-Poromaa, I.; Bixo, M.; Nyberg, S.; Bäckström, T. Allopregnanolone concentration and mood—a bimodal association in postmenopausal women treated with oral progesterone. Psychopharmacology 2006, 187, 209–221. [Google Scholar] [CrossRef]
- Martinez, P.E.; Rubinow, D.R.; Nieman, L.K.; Koziol, D.E.; Morrow, A.L.; Schiller, C.E.; Cintron, D.; Thompson, K.D.; Khine, K.K.; Schmidt, P.J. 5α-Reductase Inhibition Prevents the Luteal Phase Increase in Plasma Allopregnanolone Levels and Mitigates Symptoms in Women with Premenstrual Dysphoric Disorder. Neuropsychopharmacology 2016, 41, 1093–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andréen, L.; Nyberg, S.; Turkmen, S.; van Wingen, G.; Fernandez, G.; Bäckström, T. Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators. Psychoneuroendocrinology 2009, 34, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Porcu, P.; Serra, M.; Concas, A. The brain as a target of hormonal contraceptives: Evidence from animal studies. Front. Neuroendocr. 2019, 55, 100799. [Google Scholar] [CrossRef] [PubMed]
- Rapkin, A.J.; Morgan, M.; Sogliano, C.; Biggio, G.; Concas, A. Decreased neuroactive steroids induced by combined oral contraceptive pills are not associated with mood changes. Fertil. Steril. 2006, 85, 1371–1378. [Google Scholar] [CrossRef]
- Lopez, L.M.; A Kaptein, A.; Helmerhorst, F.M. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst. Rev. 2012, 15, CD006586. [Google Scholar] [CrossRef]
- Paoletti, A.M.; Lello, S.; Fratta, S.; Orrù, M.; Ranuzzi, F.; Sogliano, C.; Concas, A.; Biggio, G.; Melis, G.B. Psychological effect of the oral contraceptive formulation containing 3 mg of drospirenone plus 30 μg of ethinyl estradiol. Fertil. Steril. 2004, 81, 645–651. [Google Scholar] [CrossRef]
- Bengtsdotter, H.; Lundin, C.; Danielsson, K.G.; Bixo, M.; Baumgart, J.; Marions, L.; Brynhildsen, J.; Malmborg, A.; Lindh, I.; Poromaa, I.S. Ongoing or previous mental disorders predispose to adverse mood reporting during combined oral contraceptive use. Eur. J. Contracept. Reprod. Health Care 2018, 23, 45–51. [Google Scholar] [CrossRef]
- Fruzzetti, F.; Fidecicchi, T. Hormonal Contraception and Depression: Updated Evidence and Implications in Clinical Practice. Clin. Drug Investig. 2020, 40, 1097–1106. [Google Scholar] [CrossRef]
- Rapkin, A.J.; Korotkaya, Y.; Taylor, K.C. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): Current perspectives. Open Access J. Contracept. 2019, 10, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Coffee, A.L.; Kuehl, T.J.; Willis, S.; Sulak, P.J. Oral contraceptives and premenstrual symptoms: Comparison of a 21/7 and extended regimen. Am. J. Obstet. Gynecol. 2006, 195, 1311–1319. [Google Scholar] [CrossRef]
- Maguire, J.; Stell, B.; Rafizadeh, M.; Mody, I. Ovarian cycle–linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat. Neurosci. 2005, 8, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Bäckström, T.; Das, R.; Bixo, M. Positive GABA A receptor modulating steroids and their antagonists: Implications for clinical treatments. J. Neuroendocr. 2021, 34, e13013. [Google Scholar] [CrossRef] [PubMed]
- Bäckström, T.; Ekberg, K.; Hirschberg, A.L.; Bixo, M.; Epperson, C.N.; Briggs, P.; Panay, N.; O’Brien, S. A randomized, double-blind study on efficacy and safety of sepranolone in premenstrual dysphoric disorder. Psychoneuroendocrinology 2021, 133, 105426. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Martignoni, E.; Petraglia, F.; Sanees, M.G.; Nappi, G.; Genazzani, A.R. Premenstrual fall of plasma β-endorphin in patients with premenstrual syndrome. Fertil. Steril. 1987, 47, 570–573. [Google Scholar] [CrossRef]
- Tassorelli, C.; Sandrini, G.; Cecchini, A.P.; Nappi, R.E.; Sances, G.; Martignoni, E. Changes in Nociceptive Flexion Reflex Threshold Across the Menstrual Cycle in Healthy Women. Psychosom. Med. 2002, 64, 621–626. [Google Scholar] [CrossRef]
- Rabin, D.S.; Schmidt, P.J.; Campbell, G.; Gold, P.W.; Jensvold, M.; Rubinow, D.R.; Chrousos, G.P. Hypothalamic-Pituitary-Adrenal Function in Patients with the Premenstrual Syndrome. J. Clin. Endocrinol. Metab. 1990, 71, 1158–1162. [Google Scholar] [CrossRef]
- Facchinetti, F.; Fioroni, L.; Martignoni, E.; Sances, G.; Costa, A.; Genazzani, A.R. Changes of opioid modulation of the hypothalamo-pituitary-adrenal axis in patients with severe premenstrual syndrome. Psychosom. Med. 1994, 56, 418–422. [Google Scholar] [CrossRef]
- Facchinetti, F.; Genazzani, A.D.; Martignoni, E.; Fioroni, L.; Nappi, G.; Genazzani, A.R. Neuroendocrine changes in luteal function in patients with premenstrual syndrome. J. Clin. Endocrinol. Metab. 1993, 76, 1123–1127. [Google Scholar] [CrossRef]
- Halbreich, U.; Tworek, H. Altered Serotonergic Activity in Women with Dysphoric Premenstrual Syndromes. Int. J. Psychiatry Med. 1993, 23, 1–27. [Google Scholar] [CrossRef]
- Barth, C.; Villringer, A.; Sacher, J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci. 2015, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Rapkin, A.J.; Akopians, A.L. Pathophysiology of premenstrual syndrome and premenstrual dysphoric disorder. Menopause Int. 2012, 18, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Griffin, L.D.; Mellon, S.H. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc. Natl. Acad. Sci. USA 1999, 96, 13512–13517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marjoribanks, J.; Brown, J.; O’Brien, P.M.S.; Wyatt, K. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst. Rev. 2013, 2013, CD001396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- di Scalea, T.L.; Pearlstein, T. Premenstrual Dysphoric Disorder. Med. Clin. N. Am. 2019, 103, 613–628. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R. Chronic Inflammation and Premenstrual Syndrome: A Missing Link Found? J. Women’s Health 2016, 25, 857–858. [Google Scholar] [CrossRef]
- Jabbour, H.N.; Kelly, R.W.; Fraser, H.M.; Critchley, H.O.D. Endocrine Regulation of Menstruation. Endocr. Rev. 2006, 27, 17–46. [Google Scholar] [CrossRef] [Green Version]
- Roomruangwong, C.; Sirivichayakul, S.; Carvalho, A.F.; Maes, M. The uterine-chemokine-brain axis: Menstrual cycle-associated symptoms (MCAS) are in part mediated by CCL2, CCL5, CCL11, CXCL8 and CXCL10. J. Affect. Disord. 2020, 269, 85–93. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R.; Ronnenberg, A.G.; Houghton, S.; Nobles, C.; Zagarins, S.E.; Takashima-Uebelhoer, B.B.; Faraj, J.L.; Whitcomb, B.W. Association of inflammation markers with menstrual symptom severity and premenstrual syndrome in young women. Hum. Reprod. 2014, 29, 1987–1994. [Google Scholar] [CrossRef] [Green Version]
- Gold, E.B.; Wells, C.; Rasor, M.O. The Association of Inflammation with Premenstrual Symptoms. J. Women’s Health 2016, 25, 865–874. [Google Scholar] [CrossRef]
- Granda, D.; Szmidt, M.; Kaluza, J. Is Premenstrual Syndrome Associated with Inflammation, Oxidative Stress and Antioxidant Status? A Systematic Review of Case–Control and Cross-Sectional Studies. Antioxidants 2021, 10, 604. [Google Scholar] [CrossRef]
- Cubeddu, A.; Bucci, F.; Giannini, A.; Russo, M.; Daino, D.; Russo, N.; Merlini, S.; Pluchino, N.; Valentino, V.; Casarosa, E.; et al. Brain-derived neurotrophic factor plasma variation during the different phases of the menstrual cycle in women with premenstrual syndrome. Psychoneuroendocrinology 2011, 36, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Bannister, E. There is increasing evidence to suggest that brain inflammation could play a key role in the aetiology of psychiatric illness. Could inflammation be a cause of the premenstrual syndromes PMS and PMDD? Post Reprod. Health 2019, 25, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Roomruangwong, C.; Carvalho, A.F.; Geffard, M.; Maes, M. The menstrual cycle may not be limited to the endometrium but also may impact gut permeability. Acta Neuropsychiatr. 2019, 31, 294–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappi, R.E.; Cucinella, L.; Bosoni, D.; Righi, A.; Battista, F.; Molinaro, P.; Stincardini, G.; Piccinino, M.; Rossini, R.; Tiranini, L. Premenstrual Syndrome and Premenstrual Dysphoric Disorder as Centrally Based Disorders. Endocrines 2022, 3, 127-138. https://doi.org/10.3390/endocrines3010012
Nappi RE, Cucinella L, Bosoni D, Righi A, Battista F, Molinaro P, Stincardini G, Piccinino M, Rossini R, Tiranini L. Premenstrual Syndrome and Premenstrual Dysphoric Disorder as Centrally Based Disorders. Endocrines. 2022; 3(1):127-138. https://doi.org/10.3390/endocrines3010012
Chicago/Turabian StyleNappi, Rossella E., Laura Cucinella, David Bosoni, Alessandra Righi, Federica Battista, Pietro Molinaro, Giulia Stincardini, Manuela Piccinino, Roberta Rossini, and Lara Tiranini. 2022. "Premenstrual Syndrome and Premenstrual Dysphoric Disorder as Centrally Based Disorders" Endocrines 3, no. 1: 127-138. https://doi.org/10.3390/endocrines3010012
APA StyleNappi, R. E., Cucinella, L., Bosoni, D., Righi, A., Battista, F., Molinaro, P., Stincardini, G., Piccinino, M., Rossini, R., & Tiranini, L. (2022). Premenstrual Syndrome and Premenstrual Dysphoric Disorder as Centrally Based Disorders. Endocrines, 3(1), 127-138. https://doi.org/10.3390/endocrines3010012






