Abstract
According to current guidelines, growth hormone (GH) therapy is strongly recommended in children and adolescents with GH deficiency (GHD) in order to accelerate growth rate and attain normal adult height. The diagnosis of GHD requires demonstration of decreased GH secretion in stimulation tests, below the established threshold value. Currently, GHD in children is classified as secondary insulin-like growth factor-1 (IGF-1) deficiency. Most children diagnosed with isolated GHD present with normal GH secretion at the attainment of near-final height or even in mid-puberty. The most important clinical problems, related to the diagnosis of isolated GHD in children and to optimal duration of rhGH therapy include: arbitrary definition of subnormal GH peak in stimulation tests, disregarding factors influencing GH secretion, insufficient diagnostic accuracy and poor reproducibility of GH stimulation tests, discrepancies between spontaneous and stimulated GH secretion, clinical entity of neurosecretory dysfunction, discrepancies between IGF-1 concentrations and results of GH stimulation tests, significance of IGF-1 deficiency for the diagnosis of GHD, and a need for validation IGF-1 reference ranges. Many of these issues have remained unresolved for 25 years or even longer. It seems that finding solutions to them should optimize diagnostics and therapy of children with short stature.
1. Introduction
According to current guidelines, growth hormone (GH) therapy is strongly recommended in children and adolescents with GH deficiency (GHD) in order to accelerate growth rate and attain normal adult height [1]. The diagnosis of GHD is based on decreased GH secretion in stimulation tests, below the established threshold value. Along with the increase in availability of recombinant human GH (rhGH), less restrictive diagnostic criteria of GHD have been introduced, however, without compelling evidence for any of the proposed cut-offs of GH peak in stimulation tests. From early 2000s, rhGH therapy has also been approved in children born small for gestational age (SGA) with no catch-up growth during first years of life. In United States (US), children with idiopathic short stature (ISS), who meet the established auxological criteria, may also be treated with rhGH, conversely to European children with ISS who generally remain untreated. Moreover, rhGH therapy with no requirement to confirm GHD is recommended in other clinical situations, e.g., in girls with Turner syndrome, children with Prader–Willi syndrome, in short children with chronic kidney disease and—in some countries—in children with skeletal dysplasia. Effectiveness of rhGH therapy with respect to growth response is the best in children with GHD, especially if the deficit of GH is severe and caused by genetic defects or organic abnormalities in the hypothalamic–pituitary region.
In addition to promoting growth, GH also exerts numerous metabolic effects that are relevant throughout life. All the patients with persistent severe GHD, confirmed after completion of linear growth, should continue GH therapy in adulthood, even for a lifetime. It is recommended to re-evaluate GH secretion (e.g., to perform so-called retesting) in the majority of patients diagnosed in childhood with GHD, who completed growth-promoting therapy, apart from ones with deficiencies in at least three other pituitary hormones, with confirmed specific genetic mutations and/or major structural defects of hypothalamic–pituitary region [1]. The cut-off value of stimulated GH peak for the diagnosis of severe GHD in adults is much lower than that established for children. Therefore, only a minority of patients diagnosed with isolated, idiopathic GHD in childhood require continuation of rhGH therapy as adults, while a significant proportion of such patients exhibit completely normal GH secretion with respect to pediatric criteria after the therapy withdrawal. There is also the evidence that normal GH secretion may appear well before completion of linear growth, most likely already in mid-puberty [2].
The main peripheral mediator of most GH effects is insulin-like growth factor-1 (IGF-1). In current classifications, GHD in pediatric patients is considered synonymous with secondary IGF-1 deficiency (IGFD); however, overlapping between IGF-1 levels in children with mild GHD and with ISS should be taken into account [3,4]. In the Guidelines from 2016 [1], measurements of IGF-1 concentration were recommended for monitoring adherence to rhGH therapy and suggested for adjusting rhGH doses. However, this was a conditional recommendation only, as the authors have concluded there were no published data sufficient for recommending IGF-1-based dosing of rhGH. Nevertheless, in the paper published in 2020, Wit et al. [5] proposed the measurement of IGF-1 concentration as an important part of screening in children with short stature. Assessment of short-term IGF-1 response to exogenous rhGH administration in the so-called IGF-1 generation test was introduced for diagnosing GH insensitivity (GHI). Next, the same test was also been considered a marker of GH sensitivity [6,7]. Nevertheless, the significance of this test in diagnosing GHD and GHI has been questioned over the years by other authors [8,9,10]. The most important clinical problems, related to the diagnosis of isolated GHD in children and to the optimal duration of rhGH therapy in them, are listed in Table 1 and commented on sequentially.

Table 1.
Clinical problems related to the diagnosis of isolated GHD and to the optimal duration of rhGH therapy in children.
The experts from the Growth Hormone Research Society (GRS) Workshop, held in March 2019, divided the issues relating to the diagnosis of short stature into different categories. One of them included long-standing topics of controversy and debate, such as in whom and how to perform GH stimulation tests, how to optimize rhGH dosing, and how to identify and manage suboptimal responses to treatment. The second group of problems consisted of new research areas, mainly related to selection the children with short stature for genetic testing and to interpretation of genetic tests. The authors stressed the necessity of accurate and repeated auxological assessment of the patients, with special attention paid to dysmorphic features and body proportions, while laboratory tests should be individualized with respect to clinical settings. They repeated previous statements of GRS, for example, that the diagnosis of GHD should not be based solely on the results of laboratory tests. Taking into account the new standards of immunoassays for GH measurement, they suggested that the cut-offs for GHD should be reduced to 7.0 ng/mL (µg/L), as has occurred, e.g., in Australia, New Zealand, Canada, Japan, and some European countries. With respect to IGF-1 measurements, the need to use reliable reference data, adjusted for age, sex, and pubertal stage of patients, was underlined. Unfortunately, the use of sex steroid priming turned out to be a dividing issue between the participants of the meeting, and no clear consensus was worked out [11]. From a practical point of view, it was very interesting to see a recent paper by Binder et al. [12], in which pediatric endocrinologists from eight European countries and the US discussed the national rules of GHD diagnostics and clinical practice in this field. The differences were reported concerning GH cut-offs, preferred stimulation tests, and the use of priming. Ultimately, there was even no full agreement with regard to recommended pretest criteria of short stature and slow height velocity.
2. Problems Related to Direct Assessment of GH Secretion
The diagnosis of GHD seems certain in short children with hypothalamic–pituitary defects (congenital malformations, tumors, and/or irradiation) and deficiencies in other anterior pituitary hormones, while it is still a challenge in children in whom the only clinical disorder is short stature [1].
The main diagnostic tools for assessment of GH secretion are stimulation tests with pharmacological agents. Due to the pulsatile pattern of GH release from the pituitary gland, it is impossible to consider even very low GH concentration in single measurement as a reliable marker of GHD. Current guidelines indicate that the diagnosis of GHD requires decreased GH secretion in two GH provocative tests with different pharmacological stimuli to be demonstrated, while a waiver of such testing is possible only in selected patients with multiple pituitary hormone deficiency of a confirmed genetic or organic cause [1].
Some controversy about the credibility of GH stimulation tests was presented by Rosenfeld et al. in 1995 [13]. The most important doubts concerned: the non-physiological nature of these tests, the arbitrary definition of normal and subnormal GH response to stimulation, disregarding dependencies of GH secretion on the age and effect of sex steroids, and the unproved reproducibility of test results. The authors stated that GH stimulation tests could identify the patients with severe GHD but were of limited value to distinguish short normal children from those with partial GHD. Further reservations were related to the quality of assays used for measurement of GH concentration, as well as to the relatively high cost, inconvenience, and potential risk of such diagnostic procedures. Unfortunately, most of these issues still remain relevant today, after more than a quarter of a century. The most recent review, confirming the topicality of these problems, was published by Kamoun et al. in 2021 [14].
2.1. Arbitrarily Defined Subnormal GH Peak in Stimulation Tests
The problem of arbitrary definition of normal and subnormal GH response to stimulation was described by Rosenfeld in 1995 [13]. Initially the cut-off value of GH peak in provocative tests was relatively low and increased gradually with increasing availability of rhGH from 5.0 ng/mL by 7.0 ng/mL to 10.0 ng/mL (µg/L), however, with no evidence to support any of these thresholds. The authors of the Consensus guidelines, published in 2000 [15], stated that GH peak below 10.0 µg/L had been traditionally used to confirm the diagnosis of GHD; however, it should be verified (lowered) using next-generation tests based on monoclonal assays and rhGH reference preparations. In the same document, the existence of a continuum of GH secretion and overlap of GH peaks in healthy children and those with GHD were emphasized. This issue was widely discussed by Savage et al. [4] who stated that there is a continuum of GH secretion and GH sensitivity, which range from severe GHD to severe GHI.
In a study by Broeck et al. [16] on a large cohort of Dutch prepubertal children, no difference in rhGH therapy effectiveness was observed between children with GH peaks between 10.0 and 20.0 mU/L (i.e., between 5.0 and 10.0 µg/L) and ones with GH peaks over 20.0 mU/L (i.e., over 10.0 µg/L). In another study, comparing auxological parameters (height SDS at diagnosis and predicted adult height), the results of GH stimulation tests and IGF-1 concentrations in 540 children with short stature, the only difference between the patients diagnosed with partial GHD (i.e., with GH peak in two stimulation tests between 5.0 and 10.0 µg/L) and ones with ISS (i.e., with GH peak over 10.0 µg/L) was the one between GH peaks in stimulation tests. Differently, children diagnosed with severe GHD (i.e., with GH peak below 5.0 µg/L) had more pronounced deficit of height, lower IGF-1 levels, and worse prediction of adult height [17].
While GH stimulation tests are quite accurate in the diagnosis of severe GHD, larger discrepancies appear in the patients with less severe disorders of GH secretion. In this situation, the diagnosis of severe GHD, based on GH peak after pharmacological stimulation below 5.0 µg/L, is usually reliable, especially as deficiencies of other anterior pituitary hormones or abnormalities in the hypothalamic pituitary region are observed in a significant proportion of these patients. Conversely, the diagnosis of partial GHD, based on GH peak between 5.0 and 10.0 µg/L, is burdened with a definitely higher risk of error [18]. The information that a very low GH peak in stimulation tests is diagnostic for severe GHD, while the threshold for discriminating between children with partial GHD and ISS has not been established, is included in the recommendations of Grimberg et al. [1].
Although it seems obvious that different pharmacological agents should elicit different GH responses to stimulation, the diagnosis of GHD in children is still based on a single, arbitrarily established cut-off value for decreased GH secretion, which may vary by country, but not by the type of test, except for the use of somatoliberin (GHRH). In the previously cited Consensus Guidelines [15], using a limited number of provocative agents (clonidine, glucagon, insulin, arginine, and L-dopa) in well standardized protocols was recommended for the assessment of GH secretion.
In 2014, Wagner et al. [19] made an attempt to validate GH concentrations measured with different immunoassays and to establish new cut-off limits for GH peak in stimulation tests. The authors stated that these cut-offs should be different for particular assays, and on a lower level than 10.0 µg/L, for example 7.77 µg/L for IMMULITE 2000 (appropriate equations enabling the conversion of these values for various assays were listed in the cited manuscript). Otherwise, Murray et al. [20] described the difficulties in establishing the optimal cut-off point for GH peak, noting that using auxological criteria was hampered, as findings in other disorders could be similar to that in GHD.
In the current Guidelines of Grimberg et al. [1] relating to GH therapy in children and adolescents, another important issue concerning the lack of randomized controlled trials that correlated GH response to stimulation with the effect of rhGH on adult height is stressed. Moreover, the authors noted that the generally accepted threshold value diagnostic for GHD at the level of 10.0 µg/L was close to the median value of GH peak in stimulation tests documented in normally growing children. Thus, a strong recommendation was issued against relying on GH provocative tests results as the sole diagnostic criterion of GHD.
2.2. Disregarding Factors Influencing GH Secretion in Interpreting the Results of Stimulation Tests
It is generally accepted that GH stimulation tests require prior exclusion of hypothyroidism, as a condition that may impair GH secretion. It is also very important to recognize the influence of concomitant medications, particularly glucocorticoids and psychotropic drugs. Among the physiological factors, sex steroids and nutritional status have a significant impact on GH secretion [15].
One of first studies concerning dependency of GH secretion on age and pubertal stage, published in 1979 by Gourmelen et al. [21], documented the increase in GH release during puberty or after receiving sex hormones. Age dependency of GH secretion with particularly low values in early puberty, increasing gradually during mid-puberty up to late puberty, was confirmed almost 35 years ago by Mauras et al. [22] and a few years later by Marin et al. [23]. Relatively low spontaneous GH secretion and GH response to pharmacological stimulation in prepubertal children is the cause of difficulties in proper differentiating between patients with true GHD and ones with constitutional delay of growth and puberty. In the previously cited study of Marin et al. [23], positive effects of exogenous estrogen administration on GH secretion, similar to those observed during puberty, were also reported, which was the rationale for introducing the estrogen priming for a few days preceding GH stimulation tests. However, in the guidelines of the GH Research Society from 2000 [15], a problem of low GH levels in stimulation tests during the peripubertal period was stressed, but with a constatation that there was no consensus on the use of sex steroids as priming before these tests.
In 2016, Grimberg et al. [1] suggested sex steroid priming prior to GH stimulation tests in selected groups of prepubertal children (boys over 11 and girls over 10 years old) with a prognosis of adult height within the normal range. This procedure was aimed at avoiding unnecessary rhGH therapy in case of constitutional delay of growth and puberty, misdiagnosed as GHD. However, this was only a conditional recommendation.
The same year, Murray et al. [20] highlighted the existence of different approaches to priming and different schemes to carry it out. The main, and so far, valid doubts have concerned the question of whether priming reduced the rate of false positive results of GH stimulation tests, or whether it deprived children with transient prepubertal GHD from treatment. In that review, only one study, by Gonc et al. [24], was cited, which confirmed that untreated boys with subnormal GH peak in unprimed GH stimulation tests, but normal GH response after sex steroid priming, had no impaired final height.
Next year, Radetti et al. [25] proposed using pegvisomant as a new type of primer to enhance GH secretion, enabling to decrease the rate of false positive results of GH stimulation tests in children. As the study was performed on a relatively small group of patients, its promising results seem to require further validation.
Obesity and overweight have a well-documented negative influence on GH secretion. Stanley et al. [26] reported an inverse correlation between GH peak in stimulation tests (expressed as natural log) and body mass index (BMI) SD score (SDS). This association remained significant even after controlling for age, sex and pubertal status, and was confirmed for a wide range of BMI values, not only for obesity. Higher BMI values increased the likelihood of a diagnosis of GHD for different cut-off points for GH peak in stimulation tests (5.0, 7.0, and 10.0 µg/L). The authors drew the conclusion that BMI should be regarded while interpreting the results of GH stimulation tests, as higher values of BMI SDS—even within normal range—could be associated with overdiagnosing GHD.
Another approach to the same issue was proposed by Barrett et al. [27], who compared short prepubertal children with GH peaks in stimulation tests below or over 10.0 µg/L, who were either overweight/obese or normal-weight. The main findings in this study were: significantly lower GH peaks in overweight/obese children than in normal-weight ones, despite similar IGF-1 levels and growth rates in both groups; and the much higher likelihood of obtaining subnormal GH peak in case of overweight/obesity. In a Korean study on short children with short stature, a negative impact of BMI on GH peak in stimulation tests was also confirmed, especially for the test with clonidine; moreover, among children with GHD, better outcomes of rhGH therapy were observed in those with obesity than in non-obese ones [28]. Lenartsson et al. [29] reported similar clinical characteristics of children with GH peaks below or over 7.0 µg/L, except for body weight and BMI SDS.
Recently, a systematic review and meta-analysis, concerning the impact of BMI on the results of GH stimulation tests in children and adolescents was published [30]. As a summary of the meta-analysis, the authors proposed different cut-offs for GH peak in stimulation tests for children with normal weight, overweight, and obesity.
Despite the well documented fact that GH responses in stimulation tests are blunted in overweight and obese patients, this phenomenon is taken into account when interpreting these tests in adults but not yet in children [1].
2.3. Insufficient Diagnostic Accuracy and Poor Reproducibility of GH Stimulation Tests
There is no doubt that no single GH stimulation test has sufficient specificity to confirm isolated GHD. Performing at least two tests has been suggested as a way to increase their sensitivity and specificity when interpreted together, however, with rather weak evidence justifying such a procedure [13,18]. The principle of performing two different stimulation tests results from the relatively high rate of false positive results in a single test, i.e., decreased GH peak in an actually GH-sufficient child, which may be caused, e.g., by a spontaneous GH pulse occurring shortly before the test [31].
In 1995, Rosenfeld et al. [13] stated that performing GH provocative tests sequentially or with combined stimuli might be time-saving and increase cost-effectiveness, but there was no evidence that such approach could increase the sensitivity or specificity of these tests. It seems reasonable that for independent assessment of GH secretion in the two stimulation tests, these tests should be performed on separate days to allow time to restore the GH pituitary reserve, while the result of the second from the two consecutive tests may be affected by down-regulation of the hypothalamic-pituitary axis [32]. If it is known that GH peak in a stimulation test performed shortly after a spontaneous GH surge may be blunted [26], the more it applies to the result of the second of two stimulation tests performed consecutively on the same day. Webb and Dattani [18] suggested taking special care in interpreting the second of the successive tests and applying different cut-off criteria for each test. Although in this context, it may seem somewhat puzzling, according to the current Guidelines [1], there is still no evidence against performing two GH provocative tests sequentially in one day. The authors of these recommendations, however, have paid attention to a possibility that stimulation tests with combined agents might yield different results from the same tests performed separately.
Problems with poor reproducibility of GH stimulation tests in poorly growing children, reported in 1990 by Zadik et al. [33] and 5 years later by Hinz [34], have remained unresolved for over 30 years. In fact, the high incidence of false positive results of GH stimulation tests has been the main reason for performing two stimulation tests in order to avoid overdiagnosing GHD, and thereafter the overtreatment of non-GH-deficient children [20].
In fact, if the result of one GH stimulation test is normal, the abnormal result of the second test should be considered as a false-positive. Moreover, it should be borne in mind that obtaining a false positive result (e.g., decreased GH peak in a GH-sufficient child) in one test does not guarantee that the result of the second test is true. In the analysis using the theory of probability, including the results of two GH stimulation tests with different pharmacological agents, performed consecutively in a group of 780 children with short stature and excluding other disorders of pituitary gland, the frequency of decreased GH peak (below 10.0 µg/L) in both tests was as high as 40.8%, while the frequency of falsely positive results of both tests was estimated at 19.2% [35]. However, as the real incidence of GHD in children with short stature is undoubtedly much lower, these data indicate also that stimulation tests with the cut-off for GH peak on the level of 10.0 µg/L did not give reliable results in the studied group.
Twenty years ago, Loche et al. [36] proved the hypothesis that in prepubertal children with normal magnetic resonance (MR) of the hypothalamic–pituitary region, normalization of GH response to stimulation might occur much earlier than at the attainment of final height, even before entering puberty and within a few months from previous assessment. Thus, the authors suggested that patients with subnormal GH response in stimulation tests despite normal MR required follow-up and reevaluation before the diagnosis of GHD was established. Moreover, they noted that the observed early normalization of the results of the GH stimulation tests might be related either to real improvement of GH secretion or to the poor reproducibility of the GH stimulation tests, resulting in a high incidence of false subnormal results in these tests in normal subjects. In that study, serum IGF-1 concentrations did not correlate with the results of the GH stimulation tests both at the first evaluation and during retesting.
In order to explain the described phenomenon, our research group [37] decided to repeat GH stimulation tests and IGF-1 measurements in a group of patients with a wide range of GH responses to stimulation and with no other hormonal deficiencies, and no organic abnormalities in hypothalamic–pituitary region. We observed not only normalization of previously decreased GH secretion but also the opposite situation, i.e., lowering the previously normal GH secretion. Somewhat surprisingly, IGF-1 concentrations in both assessments correlated with each other more strongly than GH peaks in the stimulation tests repeated at the same two time points. These findings pointed to the poor reproducibility of GH stimulation tests rather than the true normalization (or deterioration) of GH secretion.
2.4. Discrepancies between Spontaneous and Stimulated GH Secretion
Despite the fact that—theoretically—the 24 h profile of GH secretion should be a more reliable procedure in diagnosing GHD than GH stimulation tests, there is also a strong recommendation against the assessment of spontaneous GH secretion in diagnosing GHD, due to the scarcity of data showing an advantage in this procedure, an overlap between the results obtained during frequent GH sampling in children with GHD (diagnosed according to standard criteria) and healthy controls, as well as the poor reproducibility of spontaneous GH secretion [1,38]. Thus, the results of GH stimulation tests are generally considered more reliable in diagnosing GHD than assessment of the nocturnal or 24 h profile of GH secretion [1].
Pediatric endocrinologists in Poland have relatively large experience in assessment of GH secretion after falling asleep. Obara-Moszyńska et al. [39] showed that sleep was a stronger stimulus for GH secretion than commonly used pharmacological agents (insulin, clonidine, L-dopa, and glucagon) in prepubertal children. Presenting the results of statistical analysis concerning the accuracy of standard pharmacological stimulation tests, the authors clearly declared that “it was assumed that pituitary insufficiency might be diagnosed by GH levels below 20 µIU/L (10.0 µg/L) in the GH surge test after sleep onset”, drawing from these results a far-reaching (albeit coinciding with the previously adopted assumption) conclusion that the test of GH secretion after falling asleep might be a screening procedure in diagnosing GHD. Unfortunately, the cited study only compared the results of different tests of GH secretion, with no follow-up including the effectiveness of GH therapy in the patients diagnosed with GHD according to different criteria, as well as the observation of spontaneous growth of untreated subjects labelled consistently with these criteria as GH-sufficient. It seems that such an approach may raise some reservations regarding the adopted methodology of the study, especially in relation to the introduction of a nationwide screening test, which allows, in practice, further diagnostics towards GHD in a short child to be waived. Furthermore, in Poland, the 3-h test of spontaneous GH secretion after falling asleep (5 time points, every 30 min from 60 to 180 min of sleep) has gained a range of an obligatory screening procedures for many years.
In order to ensure that the test of GH secretion after falling asleep meets the requirements for screening tests (mostly 95% sensitivity), a different approach was used. The accuracy of the result of the nocturnal test of GH secretion was assessed with respect to the results of two pharmacological GH stimulation tests interpreted together, according to current rules (considered a reference procedure). In a group of one thousand children with short stature, only 70.4% sensitivity and 61.2% specificity of the nocturnal test was proved using the same cut-off value of GH peak (10.0 µg/L) for all tests, while the 95% sensitivity of the nocturnal test required increasing the cut-off GH peak up to 19.0 µg/L, which, however, was associated with a reduction in its specificity to just below 25%, making the test under validation rather useless as a screening procedure [40].
In the context of these considerations, of particular importance seems to be the report by Rose et al. from the late 1980s [41], who stated that the 24 h profile of spontaneous GH secretion had better reproducibility than GH stimulation tests; however, it could not identify as much as 57% of the children diagnosed with GHD by stimulation tests.
Similarly, in a very recent study, Lennartsson et al. [29] reported significant differences between spontaneous and stimulated GH peaks, with higher frequencies of divergent results for higher cut-offs applied. In this study, a problem of refractoriness, described as decreased GH peaks in stimulation tests started within short time after a spontaneous GH peak, has also been raised. The duration of such a refractory period was estimated to be approximately 2 h. This phenomenon may partly explain both divergent results and poor reproducibility of the tests assessing spontaneous and stimulated GH secretion. The authors have suggested that the evaluation of spontaneous GH secretion just before stimulation tests could help to avoid overdiagnosing GHD.
2.5. Neurosecretory Dysfunction of GH Secretion as a Clinical Entity
In the Consensus Guidelines from 2000 [15], evaluation of spontaneous GH secretion was proposed for patients with a normal GH peak after pharmacological stimulation but with a decreased IGF-1 level, being suspected of having neurosecretory dysfunction (NSD) of GH secretion; however, with the comment that this diagnosis was uncommon, except for the patients with a history of cranial irradiation. The diagnosis of NSD is included in ESPE Classification of Pediatric Endocrine Disorders [3], but with the comment that spontaneous GH secretion is highly variable and may be very low in normally growing children. It seems worth mentioning that the effectiveness of GH therapy in children with NSD was documented in 1984 for the small group of patients in the study of Spiliotis et al. [42]. In 1995, Hintz [34] proposed to diagnose NSD in extremely short children (height below or equal to first centile for age) with slow growth rate, delayed bone age, and normal GH peak (over or equal to 10.0 ng/mL) on provocative testing but with a low 12 or 24 h GH secretory pattern, however, pointing to the lack of reproducibility of both GH provocative testing and 24 h GH sampling. Strong support for the idea of assessing spontaneous GH secretion in diagnosing GHD came from a study by Radetti et al. from 2003 [43], which indicated that rhGH administration in children with short stature but normal spontaneous GH secretion was not associated with a clinically significant increase in height SD score, irrespectively from normal or decreased GH peak in stimulation tests. The authors of the Guidelines published in 2016 [1], stated that this was the only study assessing the effectiveness of rhGH therapy in children with NSD with respect to adult height.
In 2003, Rogol et al. [44] published a study showing that in prepubertal children the differences between spontaneous GH secretion between the patients diagnosed with idiopathic GHD and with ISS were lower than the differences in GH peaks after stimulation, however, with more disordered patterns of spontaneous GH secretion in ISS. Additionally, there was no difference in IGF-1 levels between the groups diagnosed with GHD and ISS. Last but not the least was the observation that there was no significant difference between the increase in height SDS between the groups of children with idiopathic GHD and ISS, wherein height SDS increase correlated inversely with variables related to spontaneous GH secretion and with IGF-1 concentrations only in the subgroup of children with severe GHD but not in ones with partial GHD or ISS. The authors concluded that in children with severe deficit of height diagnosed with ISS, the problem might be related to less coordinated, and thus less effective, spontaneous GH secretion; however, such diagnostics seemed unnecessary as rhGH therapy effectiveness was similar in children with ISS and mild GHD. Unfortunately, in Europe, children with even very severe short stature, but normal results for GH stimulation tests are not qualified to receive rhGH therapy, unless they are born SGA or have other clinical conditions that entitle to treatment regardless of GH secretion.
Taking into account the scarcity of data supporting GH therapy in patients with NSD, as well as the difficulties in establishing normative data for spontaneous GH secretion (overnight GH secretory patterns compatible with diagnosis of GHD in normally growing children, overlapping of spontaneous GH secretion between normally growing children and ones diagnosed with GHD, and poor reproducibility of serial GH sampling under identical conditions on two separate occasions), the authors of the cited Guidelines [1] strongly recommended against the measurement of spontaneous GH secretion in diagnosing GHD.
Recently, NSD has been reconsidered as a disease associated with good GH sensitivity but decreased GH secretion [20,45]. Wit et al. [38] have summarized the results of the research on physiological GH secretion in children, pointing to its relationships not only with height SDS and height velocity, but also with nutritional status and pubertal stage, which presents as the major determinant in spontaneous GH secretion. The authors also paid attention to the relative scarcity of studies comparing 24 h GH secretion in children with short stature and healthy controls. Nevertheless, it has recently been suggested by Lennartsson et al. [29] that the assessment of the 12 or 24 h profile of GH secretion may reduce the qualification to rhGH therapy children with false positive results of GH stimulation tests by about 20%; however, the data concerning final height of treated and untreated children were not available, which the authors themselves considered a limitation of their study. On the other hand, children with decreased spontaneous GH secretion (i.e., ones with NSD) may benefit during rhGH therapy despite normal results of GH stimulation tests.
Wit et al. [38] described various strategies for dealing with such patients: a trial of rhGH therapy (permitted in Sweden and the US), abstaining from any treatment (as currently, e.g., in Poland), or the very interesting option of performing an IGF-1 generation test and starting rhGH therapy only if the IGF-1 response is good (permitted in Netherlands). Binder et al. [46], in a very recent study, have shown similar effectiveness of rhGH therapy with respect to the attained adult height in children with NSD and with idiopathic GHD. The results of this study correspond to the previously cited report of Rogol et al. [41].
3. Problems Related to Diagnosing GHD as Secondary IGF-1 Deficiency
Since GHD has been defined as a secondary IGF-1 deficiency, assessment of IGF-1 secretion has become a mandatory component of GHD diagnostics [3]. Other possible causes of low IGF-1, such as malabsorption syndromes, malnutrition, liver diseases, renal failure, or other severe chronic diseases, should be considered in diagnostic process. Similar to physiological GH release, IGF-1 secretion is also age-dependent, increasing particularly during puberty; however, serum concentration of IGF-1 is much more stable.
Recently, Wit et al. [5] proposed the assessment of IGF-1 concentration as a part of laboratory screening in children with growth failure with different cut-offs dependent on clinical pre-test likelihood of GHD. The authors stressed that the results of IGF-1 measurements should be expressed as SDS with respect to appropriate reference data for age and sex of the patients, and adjusted for their pubertal stage and nutritional status. It was also proposed that lower cut-offs should be applied for the patients with low and very low clinical likelihood of GHD, while higher for those in whom GH stimulation tests might be indicated regardless of IGF-1 concentration (e.g., for children after irradiation).
Very important seems to be the statement of Inoue-Lima et al. [47], who mentioned that in peripubertal children with short stature, the interpretation of IGF-1 concentrations with respect to chronological age might be inappropriate, while there was also no evidence to recommend interpretation of IGF-1 levels in relation to bone age or pubertal status. The authors showed that IGF-1 assessment with respect to chronological age could be useful as a screening test in diagnosing GHD, while IGF-1 assessment taking into account pubertal status had the best positive predictive power for this diagnosis in peripubertal children.
3.1. Discrepancies between IGF-1 Levels and the Results of GH Stimulation Tests
The lack of good correlation between GH secretory status and serum IGF-1 concentrations was described by Rosenfeld et al. [13], who presented the results of previous research on this issue. Interestingly, in one of studies cited in this article, published by Blum et al. [48], a significant correlation (r = 0.78) was reported between the calculated 24 h GH secretion rates and IGF-1 concentrations in healthy children.
It was quite clearly stated in the Consensus of the GH Research Society [15] that IGF-1 concentrations below the cut-off on the level of −2.0 SD for age and sex were strongly suggestive of GHD after exclusion of other causes of decreased IGF-1 secretion; nevertheless, normal IGF-1 values could also be found in children with GHD. The same statement is currently valid for adults, despite the fact that in children, GHD has been classified as a secondary IGF-1 deficiency [3,49]. Murray et al. [20] have stressed the difficulties related to overlap of IGF-1 concentrations in GH-deficient and healthy children, especially in younger age groups, decreased IGF-1 levels in children with malnutrition, hypothyroidism, renal failure, diabetes, and other chronic diseases, as well as an increase in IGF-1 secretion during puberty. From the clinical point of view, very important is the suggestion that in children with delayed puberty, IGF-1 concentrations should be interpreted in relation to their bone age and pubertal stage.
The authors of many studies have evaluated the diagnostic value of IGF-1 assessments in children with respect to GH peak in stimulation tests, taken as a reference procedure. Recently, Ibba et al. [50] established the best cut-off value of IGF-1 for the diagnosis of GHD on the level −1.5 SDS, however simultaneously documenting the poor accuracy of IGF-1 measurements in discriminating patients with confirmed or excluded GHD (that is with GH peak in stimulation tests below or over 7.0 µg/L, respectively). Moreover, in a very recent study of Iwayama et al. [51], a poor diagnostic accuracy of IGF-1 measurement as a screening test for GHD was found in Japanese children with short stature or decreased growth velocity where GH peak in stimulation tests was considered a reference test. Very similar were earlier observations concerning the assessment of IGF-1 concentrations as a screening procedure in diagnosing GHD in Polish children [52]. However, in other study, the same research group [37] reported the stability of IGF-1 concentrations despite divergent results of GH stimulation tests repeated in the same patients, which raised doubts as to whether the results of GH stimulation tests were actually more reliable than measurements of IGF-1 concentration.
A possible solution to these problems may be introduction of new diagnostic tests, such as the assessment of GH secretion in stimulation tests after priming with pegvisomant. It has been shown that GH peaks in this test correlated with IGF-1 levels [25]. Unfortunately, this study was performed on a relatively small group of children, and thus requires further validation.
In this context, there were very interesting data presented by Meazza et al. [53], who measured serum levels of α-klotho—a transmembrane protein whose circulating soluble form (s-klotho) concentration reflects GH secretory status. The authors reported no significant difference in both IGF-1 and s-klotho between the groups of short children diagnosed with GHD and ISS on the basis of the pegvisomant-primed insulin-tolerance test, however, with a good correlation between s-klotho and IGF-1 in both groups. They concluded that s-klotho could not be considered a reliable marker of GH secretion in children, as it reflected IGF-1 levels but not GH peaks. However, it seems that opposite interpretation of these results is justified, pointing to the agreement of different markers of GH secretory status with each other, with the exception of GH stimulation tests.
Regarding the evident discrepancies between the results of GH stimulation tests and IGF-1 concentrations, there seems to be no strong evidence to show that a subnormal GH peak is more reliable for the diagnosis of GHD than a decreased IGF-1 level. Otherwise, assuming IGF-1 concentration as the reference test, the accuracy of GH stimulation tests would be poor. Moreover, taking into account the lack of correlation between the results of different GH stimulation tests or even the same test repeated in the same patient in a short time interval, it may actually be difficult to find any test that correlates with the results of other tests that do not correlate with each other.
3.2. Importance of Direct Confirmation of Secondary IGF-1 Deficiency in the Diagnosis of GHD
Since it has become possible to use recombined human IGF-1 (rhIGF-1) in extremely short children diagnosed with GHI, the problem of proper differentiation between primary and secondary IGFD (i.e., between GHI and GHD) has become particularly important. Considering all the caveats about GH stimulation test, including their arbitrary cut-off points, insufficient sensitivity and specificity, poor reproducibility and discrepancy with spontaneous GH secretion, it would seem prudent to directly assess IGF-1 response to GH administration. For this purpose, IGF-1 generation tests could be used as a diagnostic tool. The test based on measurements of IGF-1 concentrations before and after short-term rhGH administration was initially accepted for diagnosing GHI, with the lack of IGF-1 increase diagnostic for this condition [54]. The use of IGF-1 generation tests (in a 10-day variant) for prediction of growth response to human GH was proposed 40 years ago by Rudman et al. [55], who confirmed the relationship between somatomedin C (former name of IGF-1) increase during the test and growth response to treatment in a small group of children with normal-variant short stature. Unfortunately, the poor reproducibility of the IGF-1 generation test was also reported, which became a significant limitation of its clinical utility [56].
Rosenfeld at al. [13] presented a statement that IGF-1 concentrations might reflect functional GH secretion better than direct assessment of GH peaks in stimulation tests. Documenting impaired GH secretion in patients with IGFD had significant value for the exclusion of GHI as a cause of growth retardation in such patients. The authors directly questioned GH stimulation tests as the “gold standard” in diagnosing GHD, concluding that available methods for assessment of GH secretion were neither convenient nor reliable. As, in turn, IGF-1 concentrations might be influenced by factors other than GH secretion, the need for clinical evaluation of children with growth retardation, including serial measurements of height and calculating height velocity, was emphasized in the cited paper. At the same time, the authors cautioned against the expansion of the diagnosis of GHD and increasing the market for commercial rhGH.
Currently in different countries, children with extremely short stature, severe IGFD and normal results of GH stimulation tests may be diagnosed with primary IGFD and treated with rhIGF-1, with no necessity of confirming GHI by performing an IGF-1 generation test even in if a causative genetic diagnosis has not been established. In Poland, documenting lacking or insufficient increase in IGF-1 in generation tests remains an obligatory procedure before qualifying children to receive therapy with rhIGF-1. Thus, children with confirmed GHI may be treated with rhIGF-1, while those with a good increase in IGF-1 concentration during the short-generation test are still diagnosed with ISS and should remain untreated if they are born appropriate for gestational age (AGA). In fact, in Poland, SGA but not AGA children with normal GH secretion may qualify for rhGH therapy, even if they fulfill the criteria suggesting GHI, with no requirement to rule out this condition. This situation results from the applicable regulations, but it is difficult to rationally explain to the patients and their parents.
Our study group has assumed that children with confirmed IGF-1 response to rhGH in a generation test (e.g., with excluded GHI) could have secondary IGFD, related either to the disorders of GH secretion, not matching the standard criteria of GHD, or to GH bioinactivity. Furthermore, these patients remained GH-sensitive. So, we compared the effectiveness of rhGH therapy in such patients (labelled as IGFD group) divided into SGA and AGA subgroups, and in children with isolated GHD, observed up to the attainment of near-FH. The increase in height SDS was similar in the defined IGFD group and in the GHD group. Interestingly, the therapy turned out to be the most effective in the IGFD-AGA subgroup, while the less effective in IGFD-SGA; however, the differences between all three groups was found to be insignificant [57]. It seems worth mentioning that IGFD-AGA was the only subgroup that did not fulfill the standard criteria of qualifying for growth promoting therapies, as these patients were neither GH-deficient nor GH-insensitive, nor SGA. Our observations supported the possibility of growth response to rhGH therapy in such children. Nevertheless, in some other countries, in which the IGF-1 generation test is not required (or even not recommended) for the diagnosis of GHI, children with normal GH peak and severe GHD who were born AGA are directly diagnosed with GHI and treated only with rhIGF-1. It should be stressed here that Grimberg et al. [1], in the recent Guidelines concerning GH and IGF-1 treatment in children and adolescents, strongly recommended a trial of rhGH therapy before the initiation of IGF-1 administration in children with unexplained IGFD. The authors have also stated that rhGH, if effective, should be preferable to IGF-1 even in patients with a partial GHI, for a number of reasons, including IGF-1 independent effects of GH on growth plates, half the number of injections, no risk of hypoglycemia, and simultaneous increase in IGFBP-3 with IGF-1, which is considered favorable regarding the risk of cancers.
Moreover, it has been shown that a significant increase in the IGF-1/IGFBP-3 molar ratio predicted a good response to rhGH therapy in children with IGFD but normal results of GH stimulation tests [58]. The usefulness of this parameter for monitoring and optimizing rhGH therapy in children with GHD has also been demonstrated [59]. Recently, the IGF-1/IGFBP-3 molar ratio has been considered a useful parameter for assessing safety of rhGH therapy [60].
Other important issue in this aspect is dissociation of IGF-1 from ternary complexes with IGFBP-3 and acid-labile subunit, regulated by the proteolytic activity of IGFBP-3. It has been documented that in SGA children with low IGF-1 levels, IGFBP-3 proteolytic activity increased to preserve IGF-1 bioavailability [61,62]. Studies from the last few years have also shown the important role of pregnancy-associated plasma protein-A (PAPP-A) as a regulatory mechanism for releasing IGF-1 (and other IGFs) from corresponding binding proteins [63] and possible links between IGF-1 bioavailability regulated by IGFBP-3 proteolysis and insulin sensitivity [64]. Unfortunately, a more detailed discussion regarding these undoubtedly interesting issues goes far beyond the assumptions of this review.
3.3. Re-Standardization of Assays and the Need for Validation of IGF-1 Reference Ranges
Interpretation of IGF-1 concentrations requires appropriate age- and sex-related reference data, which should be established for particular assays and—optimally, for particular laboratories. The latter approach may be difficult in practice as it requires obtaining blood samples from a large cohort of healthy children of different ages in each center. To overcome this problem, central measurement of IGF-1 levels has been proposed; however, such a solution entails the necessity to properly secure the samples for transport, and generates additional costs and delays getting results of the tests. In practice, it is possible to use the reference data published for the laboratory method used in a given center.
In our laboratory, IGF-1 and IGFBP-3 concentrations were measured with kits provided by IMMULITE and the reference data provided by Elmlinger et al. [65] were used, which were constructed with appropriate methodology, taking into account the fact that due to the log-normal distribution of IGF-1 concentrations, calculation of IGF-1 SDS requires the use of log-transformed values [66].
Unfortunately, in 2016, new re-standardized assays for IGF-1 measurement were introduced on IMMULITE systems. According to the information provided by Siemens Healthcare Diagnostics Inc., Tarrytown, NY 10591-5097 USA [67], the results obtained with previously used and re-standardized assays have correlated very well and a linear equation has been proposed to re-calculate values obtained with “old” and “new” assays. However, in the provided new reference data, the log-normal IGF-1 distribution has not been taken into account and several-year age ranges for children, instead of annual ones, have been introduced. Centile charts drawn with the use of these Pediatric Reference Ranges, provided in Table 5 of the cited Bulletin [67], illustrate problems with their use in clinical practice (Figure 1).
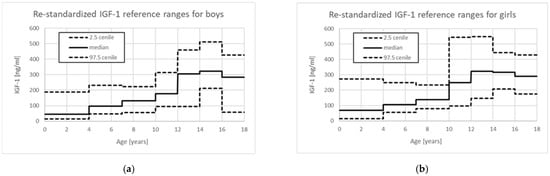
Figure 1.
Centile charts based on Pediatric reference ranges for re-standardized IGF-1 assays provided for IMMULITE 2000 Immunoassay System [67]: (a) for boys; (b) for girls.
In fact, there is no lower limit of IGF-1 in age group 0–3 years, as it is defined as <15 ng/mL, making it impossible to use this assay to confirm IGFD in the youngest children. For boys, the median value for age group 0 to 3 years (44 ng/mL) is even lower than 2.5 centile for age group 4 to 6 years (47 ng/mL), while the median for age group 12 to 13 years (305 ng/mL) is only slightly below the 97.5 centile for age group 10 to 11 years (315 ng/mL). Quite surprisingly, for girls, the value of 97.5 centile is higher in age group 0 to 3 years than in subsequent age groups up to 10 years, while the values of median and 2.5 centile in the same age groups increase with age; even more surprisingly, the median value for age group 10 to 11 years (248 ng/mL) is even higher than 97.5 centile for age group 7 to 9 years (233 ng/mL). Unfortunately, the clinicians usually interpret a single measurement (or sometimes serial measurements in the same patients) using the reference range provided by the laboratory for a given result and they may not be aware of how the reference ranges change for different age groups. The presented situation is only an example of the problems that clinicians may encounter when interpreting the results of IGF-1 concentration measurements.
4. Problems Related to the Duration of rhGH Therapy in Children with Idiopathic GHD
There is no doubt that rhGH therapy in a child diagnosed with GHD should be started as soon as possible. The main goals of treating short children with GHD are to improve their growth rate and to achieve normal adult height, and—in the case of severe GHD—to correct metabolic disorders. Response to rhGH therapy should be carefully monitored, especially in children diagnosed with moderate GHD despite normal IGF-1 concentrations and normal MR of hypothalamic–pituitary region [15]. This enables the identification of non-responders who might be misdiagnosed, e.g., due to falsely positive results of all GH stimulation tests performed on them. In the majority of patients treated with rhGH due to idiopathic GHD, secretion of GH appears normal after the discontinuation of growth-promoting treatment; however, it is unclear when exactly GH secretion would be normalized. Therefore, the optimal time for retesting still remains a matter of discussion. For the patients who completed linear growth, the diagnostic criteria of adult GHD should be applied during retesting after the growth-promoting therapy withdrawal in order to identify the patients with persistent severe GHD who may be candidates for even lifelong rhGH substitution.
4.1. Early Identification of Non-Responders
The most important parameter in monitoring the effectiveness of rhGH therapy in children with GHD is growth response to treatment, assessed on the basis of the increase in height and in growth rate. The authors of the Consensus Guidelines in 2000 [15] recommended starting rhGH therapy at the youngest possible age, before the onset of puberty. It has been considered necessary to document the pre-treatment growth rate of the patients as a benchmark for assessing its improvement during the therapy. The need for careful documentation of height velocity was stressed by Rosenfeld et al. [13], who also suggested that normal height velocity in an untreated child spoke strongly against the diagnosis of GHD. The patients who do not respond to treatment should be reevaluated very carefully in order to either modify the therapy (e.g., by increasing rhGH dose or just improving patient’s compliance) or its withdrawal if the diagnosis of GHD is excluded. Such management allows long-term but non-effective treatment to be avoided, which can also be inconvenient, expensive, and burdened with the possibility of side effects.
The first issue to resolve is a definition of poor-responder, as there are many suggested criteria, leading to classifying different proportions of children as poor responders [68]. The second problem is establishing the rules for dealing with such patients. Bang et al. [69] discussed the strategy for prevention of poor growth response to rhGH therapy, underlying age-related dependency of responses and the difficulties in assessment of pubertal children. The authors indicated that both height velocity and increase in height SDS were considered as endpoints in prediction models, while first-year response to treatment was a standard endpoint in clinical trials. The problems with identifying poor responders, based on first-year response to rhGH administration, have recently been widely discussed by Straetemans et al. [70,71], who stated that using different previously proposed criteria led to classifying comparable percentages of patients as poor responders; however, it turned out that different criteria were met by different patients. Unfortunately, the studies did not provide sufficient evidence on the advantage of any of the proposed criteria and—in general—have led to the conclusion that all the criteria based on first-year growth response “perform poorly as predictors of poor final height outcome” [70]. Unfortunately, extending the observation of growth response up to 2 years did not improve the prediction of poor adult height outcome after rhGH therapy [72].
The need to develop prediction models of expected growth response to treatment, enabling the estimation of the anticipated effects of rhGH therapy, was stressed at the end of the 20th century [73]. Such models determine the potential effectiveness of treatment and make the expectations of patients and their families more realistic. They can also be useful for early identification of non-responders. Introducing the use of prediction models into clinical practice should contribute to the personalized approach to rhGH therapy [74].
Mathematical models have been applied in prediction of GH therapy effectiveness in children since the end of 1990s. An overview of the prediction models developed so far has been presented by Smyczyńska U. et al. [75]. Unfortunately, most of these models had only a few-years prediction horizon, while adult height was a predicted variable in only two of them, provided by Carel et al. [76] and de Ridder et al. [77]. Ranke et al. developed multiple linear regression models of 1–4 years response to treatment [78] and of total pubertal growth [79]. Our contribution to the creation of prediction models has been the use of neural networks for this purpose [80]. Neural networks are classified as techniques of artificial intelligence, being—in fact—advanced computational statistical methods (also known as machine learning). Neural models do not require assuming any particular character of dependencies between explained variable (output) and explanatory ones (inputs), while such assumptions are necessary for linear models. A model of first-year response and a model of final height were created. The first one was based on pre-treatment auxological and hormonal data, in the second one, the inputs were the same variables and real first-year response to treatment. Conversely to the majority of previously published regression models created for children starting rhGH therapy before puberty, the neural models included both prepubertal and pubertal children. Nevertheless, they have been characterized by comparable accuracy and prediction error. The problem with all prediction models is the lack of tools that would allow them to be easily used in clinical practice, for example, in the form of user-friendly “calculators”.
Unfortunately, the management of poor-responders were not included in the recent Guidelines [1], and it still remains a matter of discussion [20,70,71].
4.2. Optimal Time for rhGH Therapy Withdrawal and Retsting of GH Secretion
According to the guidelines provided by the Consensus Workshop on adult GHD at Port Stephens in 1997 [81], retesting of GH and IGF-1 secretion after the attainment of final height should be undertaken by pediatric endocrinologists. Severe GHD in adults was defined in that document as GH peak in response to hypoglycemia below 3.0 µg/L (or decreased GH peak in alternative tests with appropriate cut-offs). Re-initiation of rhGH therapy should be considered in the patients fulfilling adult criteria of GHD and arranged in collaboration between pediatric and adult endocrinologists.
Grimberg et al. [1] have strongly recommended that rhGH therapy in pediatric doses should be withdrawn after slowing down the growth rate below 2.0–2.5 cm/year, while earlier discontinuation of treatment requires individual decisions. The moment of rhGH therapy cessation should be attainment of near-adult (near-final) height, characterized by a definitely slowing down growth rate and appropriately advanced bone age (14–15 years for girls and 16–17 years for boys). Another strong recommendation has concerned the need for reassessment of GH secretion after completion of growth promotion. Although measurement of IGF-1 concentration as an initial test in reevaluation of the somatotropic axis has only been a conditional recommendation, the performance of GH stimulation tests in the case of low IGF-1 has gained the status of a strong recommendation in the next paragraph of the same document [1], which seems somewhat inconsistent.
Furthermore, over the years, reports have been published indicating that normalization of GH secretion may occur in adolescence, much earlier than at reaching near-final height.
The phenomenon of decreased GH response to pharmacological stimulation in prepubertal children with decreased growth rate and normalization of GH secretion after the onset of puberty was described more than 40 years ago by Gourmelen et al. [21]. The authors speculated that their observation would suggest a transient and functional GHD before puberty and influence of sex hormones on GH secretion. This justified the need to reassess GH secretion in patients diagnosed with GHD as children. Similarly, Zucchini et al. [82] reported normal results of GH stimulation tests after the onset of puberty in the majority of children, diagnosed with isolated GHD in prepubertal period. Moreover, the authors documented that there was no significant difference in final height between the patients who suspended rhGH therapy due to normal GH secretion at retesting and those with confirmed GHD, who were treated up to completion of linear growth.
More recently, Penta et al. [83] confirmed permanent GHD in the transition period in only 16% of the patients diagnosed with isolated, idiopathic GHD in childhood, and suggested that the time of retesting should be anticipated in order to avoid overtreatment. Cavarzere et al. [2] performed retesting of GH secretion in mid-puberty in a group of children diagnosed previously with idiopathic GHD, and they discontinued rhGH therapy in over half of them with normal GH peaks at retesting, while restarting those with confirmed GHD. Interestingly, in this study, the only predictor of persistent and transient GHD was IGF-1 concentration (and IGF-1 SDS) after 1 year of rhGH therapy. Both groups of patients were observed up to the achievement of near-final height, and no difference between them was found in both height increase and final height. Thus, the authors concluded that GH secretion should be retested in mid-puberty. In line with these studies remain the results presented by Krukowska-Andrzejczyk et al. [84], who compared height SDS increase obtained during rhGH therapy in a group of patients diagnosed in childhood with partial GHD (i.e., with GH peaks in stimulation tests between 5.0 and 10.0 µg/L), which turned out to be transient in retesting, with this occurring spontaneously in a group of untreated children with ISS. Mean final height was normal in both groups, and there was no significant difference in the observed height gain between them. The authors also stated that there was a necessity to determine other criteria of qualifying children for rhGH therapy than only using the arbitrarily established cut-off value for GH peak in the stimulation test.
Poor reproducibility of the results of GH stimulation tests has been reconsidered as a possible cause of early normalization of GH secretion that might occur during mid-puberty [2] or even earlier [36,37]. Nevertheless, there is still no consensus regarding retesting of GH secretion earlier than at the attainment of near-final height.
Other explanation regarding the high incidence of transient GHD may be a physiologically lower GH response to pharmacological stimulation in the peripubertal and early pubertal periods of life rather than in late puberty, which is not taken into account while interpreting the results of the GH stimulation test. The possible solutions for this problem include establishing different cut-offs for GH peaks for particular stages of puberty (which seems to be very difficult, if not impossible) or performing sex steroid priming in selected groups of children. The latter approach has been suggested in the recent Guidelines of Grimberg et al. [1]. Thus, sex steroid priming preceding GH stimulation tests in prepubertal boys over 11 and girls over 10 years old seems to be an important procedure avoiding the overdiagnosing of GHD and subsequent unnecessary treatment. The use of priming should also limit the indications to early retesting of children qualified properly for rhGH therapy.
5. Concluding Remarks
According to Rosenfeld et al. [13], the question of who should be tested for GHD was even more important than the question how to test for GHD. This constatation seems to be also valid today, after 25 years. More recently, Ranke et al. [85] summarized 50 years of GH use in children with non-acquired GHD, treated in a single center, and observed the tendency to more stringent diagnostic criteria for GHD in recent years, including assessment of pre-treatment growth rate, sex steroid priming, and lowering cut-offs for GH stimulation tests, restricting diagnoses of GHD to more severe cases. Felício et al. [86] proposed the diagnosis of idiopathic GHD based on clinical, auxological, and radiological assessment, confirmed by GH peak in stimulation tests below 7.0 µg/L and IGF-1 SDS below −2.0. On the other hand, the proven effectiveness of rhGH therapy in children diagnosed with ISS has been in the background to approve this indication for treatment [87]; however, not in all countries.
It seems that finding solutions to the problems discussed in the present study may contribute to the optimization of diagnostics and therapy of children with short stature. Many of these issues have also been addressed in the review published by Henry in 2020 [88]. Moreover, an important part of that paper was devoted to ethical implications of diagnosing GHD in children. In trying to answer, why the cut-off value of GH peak in stimulation tests on the level of 10.0 µg/L has not been changed despite the evidence that it should be lower, the author stated that it might have been caused by difficulties in establishing normal values for GH secretion at a population level, but also by issues far from evidence-based medicine, such as a fear of reduction in revenue from therapy. He has also stated that, as current rules of GH testing carry a considerable risk of false positive results, stimulation tests should be performed only in the patients with a high clinical likelihood of GHD. This remark is in line with the previously discussed proposal of Wit et al. [5]. Moreover, Kamoun et al. [14] summed up the limitations of GH stimulation tests and stressed the need for developing new diagnostic modalities in future, while now improving the clinical application of GH stimulation tests by taking into account all factors that may influence their results. The authors pointed to the poor performance of provocative GH tests in diagnosing GHD, as well as at the limited progress in overcoming the existing problems over the years. Very recently, Rodari at al. [89] confirmed the rationale for decreasing the cut-off value of GH peak in stimulation tests, together with highlighting the need for reconsidering NSD as a rhGH-treatable disease related to hypothalamic derangement with a clinical presentation similar to that of isolated GHD.
According to recent proposals of GRS [11], personalized genetic or even epigenetic testing should become an important part of diagnostics of children with short stature. On the other hand, development of prediction algorithms for growth response to rhGH therapy could seem necessary to optimize treatment modalities and to identify non-responders as early as possible.
Although bioethical and economic issues have not been the subject of the present study, they are nevertheless also relevant to the proper management of patients. In this context, it is worth citing once more the recent paper of Henry [88], who presented the thesis that rhGH use in conditions other than “true” GHD may be considered as “cosmetic endocrinology”. This opinion may seem somewhat surprising, but it nevertheless requires some reflection. However, taking into account the limitations of the available diagnostic tests, it may be difficult to qualify for rhGH therapy all the patients who will benefit from this, and only these ones. It seems settled that the use of rhGH for growth promotion may not only be effective and justified not only in children with decreased GH peak in stimulation tests but also in some patients with normal results of these tests. On the other hand, the therapy duration in children diagnosed with isolated idiopathic GHD could be shortened, at least in some cases, with no harm to patients.
Funding
This research received no external funding.
Acknowledgments
I would like to thank the co-authors of cited papers from Medical University of Lodz, Poland and Polish Mother’s Memorial Hospital—Research Institute in Lodz, Poland.
Conflicts of Interest
The author declares no conflict of interest.
References
- Grimberg, A.; DiVall, S.A.; Polychronakos, C.; Allen, D.B.; Cohen, L.E.; Quintos, J.B.; Rossi, W.C.; Feudtner, C.; Murad, M.H. Guidelines for Growth Hormone and Insulin-Like Growth Factor-I Treatment in Children and Adolescents: Growth Hormone Deficiency, Idiopathic Short Stature, and Primary Insulin-Like Growth Factor-I Deficiency. Horm. Res. Paediatr. 2016, 86, 361–397. [Google Scholar] [CrossRef] [PubMed]
- Cavarzere, P.; Gaudino, R.; Sandri, M.; Ramaroli, D.A.; Pietrobelli, A.; Zaffanello, M.; Guzzo, A.; Salvagno, G.L.; Piacentini, G.; Antoniazzi, F. Growth hormone retesting during puberty: A cohort study. Eur. J. Endocrinol. 2020, 182, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Wit, J.-M.; Ranke, M.B.; Kelnar, C.J.H. The ESPE classification of paediatric endocrine diagnoses. Horm. Res. 2007, 68, 1–5. [Google Scholar]
- Savage, M.O.; Burren, C.P.; Rosenfeld, R.G. The continuum of growth hormone-IGF-I axis defects causing short stature: Diagnostic and therapeutic challenges. Clin. Endocrinol. 2010, 72, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Wit, J.M.; Bidlingmaier, M.; De Bruin, C. A Proposal for the Interpretation of Serum IGF-I Concentration as Part of Laboratory Screening in Children with Growth Failure. J. Clin. Res. Pediatr. Endocrinol. 2020, 12, 130–139. [Google Scholar] [CrossRef]
- Spiliotis, B.E.; Alexandrides, T.K.; Karystianos, C.; Vassilakos, P.; Zadik, Z.; Nikolakopoulou, N.M.; Nikiforidis, G.; Beratis, N.G. The insulin-like growth factor-I (IGF-I) generation test as an indicator of growth hormone status. Hormones 2009, 8, 117–128. [Google Scholar] [CrossRef]
- Buckway, C.K.; Rosenfeld, R.G. The IGF-I Generation Test Revisited: A Marker of GH Sensitivity. J. Clin. Endocrinol. Metab. 2001, 86, 5176–5183. [Google Scholar] [CrossRef]
- Cotterill, A.M.; Camacho-Hübner, C.; Duquesnoy, P.; Savage, M.O. Changes in serum IGF-I and IGFBP-3 concentrations during the IGF-I generation test performed prospectively in children with short stature. Clin. Endocrinol. 1998, 48, 719–724. [Google Scholar] [CrossRef]
- Coutant, R.; Dorr, H.-G.; Gleeson, H.; Argente, J. Diagnosis of endocrine disease: Limitations of the IGF1 generation test in children with short stature. Eur. J. Endocrinol. 2012, 166, 351–357. [Google Scholar] [CrossRef]
- Storr, H.L.; Chatterjee, S.; Metherell, L.A.; Foley, C.; Rosenfeld, R.G.; Backeljauw, P.F.; Dauber, A.; Savage, M.O.; Hwa, V. Nonclassical GH Insensitivity: Characterization of Mild Abnormalities of GH Action. Endocr. Rev. 2019, 40, 476–505. [Google Scholar] [CrossRef]
- Collett-Solberg, P.F.; Ambler, G.; Backeljauw, P.F.; Bidlingmaier, M.; Biller, B.M.K.; Boguszewski, M.C.S.; Cheung, P.T.; Choong, C.S.Y.; Cohen, L.E.; Cohen, P.; et al. Diagnosis, Genetics, and Therapy of Short Stature in Children: A Growth Hormone Research Society International Perspective. Horm. Res. Paediatr. 2019, 92, 1–14. [Google Scholar] [CrossRef]
- Binder, G.; Reinehr, T.; Ibáñez, L.; Thiele, S.; Linglart, A.; Woelfe, J.; Saenger, P.; Bettendorf, M.; Zachurzok, A.; Goglke, B.; et al. GHD Diagnostics in Europe and the US: An Audit of National Guidelines and Practice. Horm. Res. Paediatr. 2020, 92, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, R.G.; Albertsson-Wikland, K.; Cassorla, F.; Frasier, S.D.; Hasegawa, Y.; Hintz, R.L.; Lafranchi, S.; Lippe, B.; Loriaux, L.; Melmed, S.; et al. Diagnostic Controversy: The Diagnosis of Childhood Growth-Hormone Deficiency Revisited. J. Clin. Endocrinol. Metab. 1995, 80, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, C.; Hawkes, C.P.; Grimberg, A. Provocative growth hormone testing in children: How did we get here and where do we go now? J. Pediatr. Endocrinol. Metab. 2021, 34, 679–696. [Google Scholar] [CrossRef] [PubMed]
- Growth Hormone Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: Summary statement of the GH Research Society. J. Clin. Endocrinol. Metab. 2000, 85, 3990–3993. [Google Scholar] [CrossRef]
- Van den Broeck, J.; Arends, N.; Hokken-Koelega, A. Growth response to recombinant human growth hormone (GH) in children with idiopathic growth retardation by level of maximum GH peak during GH stimulation tests. Horm. Res. 2000, 53, 267–273. [Google Scholar] [CrossRef]
- Smyczyńska, J.; Lewiński, A.; Hilczer, M.; Stawerska, R.; Karasek, M. Partial growth hormone deficiency (GHD) in children has more similarities to idiopathic short stature than to severe GHD. Endokrynol. Pol. 2007, 58, 182–187. [Google Scholar]
- Webb, E.A.; Dattani, M.T. Diagnosis of growth hormone deficiency. Endocr. Dev. 2010, 18, 55–66. [Google Scholar] [CrossRef]
- Wagner, I.V.; Paetzold, C.; Gausche, R.; Vogel, M.; Koerner, A.; Thiery, J.; Arsene, C.G.; Henrion, A.; Guettler, B.; Keller, E.; et al. Clinical evidence-based cutoff limits for GH stimulation tests in children with a backup of results with reference to mass spectrometry. Eur. J. Endocrinol. 2014, 171, 389–397. [Google Scholar] [CrossRef]
- Murray, P.G.; Dattani, M.T.; Clayton, P.E. Controversies in the diagnosis and management of growth hormone deficiency in childhood and adolescence. Arch. Dis. Child. 2016, 101, 96–100. [Google Scholar] [CrossRef]
- Gourmelen, M.; Pham-Huu-Tsrung, M.T.; Girard, F. Transient partial hGH deficiency in prepubertal children with delay of growth. Pediatr. Res. 1979, 13, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Mauras, N.; Blizzard, R.M.; Link, K.; Johnson, M.L.; Rogol, A.D.; Veldhuis, J.D. Augmentation of growth hormone secretion during puberty: Evidence for a pulse amplitude-modulated phenomenon. J. Clin. Endocrinol. Metab. 1987, 64, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Marin, G.; Domené, H.M.; Barnes, K.M.; Blackwell, B.J.; Cassorla, F.G.; Cutler, G.B.J. The effects of estrogen priming and puberty on the growth hormone response to standardized treadmill exercise and arginine-insulin in normal girls and boys. J. Clin. Endocrinol. Metab. 1994, 79, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Gonc, E.N.; Kandemir, N.; Ozon, A.; Alikasifoglu, A. Final heights of boys with normal growth hormone responses to provocative tests following priming. J. Pediatr. Endocrinol. Metab. 2008, 21, 963–971. [Google Scholar] [CrossRef]
- Radetti, G.; Elsedfy, H.H.; Khalaf, R.; Meazza, C.; Pagani, S.; El Kholy, M.; Albertini, R.; De Stefano, A.M.; Navarra, A.; De Silvestri, A.; et al. Pegvisomant-primed growth hormone (GH) stimulation test is useful in identifying true GH deficient children. Hormones 2017, 16, 291–296. [Google Scholar] [CrossRef]
- Stanley, T.L.; Levitsky, L.L.; Grinspoon, S.K.; Misra, M. Effect of body mass index on peak growth hormone response to provocative testing in children with short stature. J. Clin. Endocrinol. Metab. 2009, 94, 4875–4881. [Google Scholar] [CrossRef]
- Barrett, J.; Maranda, L.; Nwosu, B.U. The relationship between subnormal peak-stimulated growth hormone levels and auxological characteristics in obese children. Front. Endocrinol. 2014, 5, 35. [Google Scholar] [CrossRef]
- Yang, A.; Cho, S.Y.; Kwak, M.J.; Kim, S.J.; Park, S.W.; Jin, D.K.; Lee, J.E. Impact of BMI on peak growth hormone responses to provocative tests and therapeutic outcome in children with growth hormone deficiency. Sci. Rep. 2019, 9, 16181. [Google Scholar] [CrossRef]
- Lennartsson, O.; Nilsson, O.; Lodefalk, M. Discordance Between Stimulated and Spontaneous Growth Hormone Levels in Short Children Is Dependent on Cut-Off Level and Partly Explained by Refractoriness. Front. Endocrinol. 2020, 11, 584906. [Google Scholar] [CrossRef]
- Abawi, O.; Augustijn, D.; Hoeks, S.E.; de Rijke, Y.B.; van den Akker, E.L.T. Impact of body mass index on growth hormone stimulation tests in children and adolescents: A systematic review and meta-analysis. Crit. Rev. Clin. Lab. Sci. 2021, 58, 576–595. [Google Scholar] [CrossRef]
- Kelnar, C.J.H. The evidence base for growth hormone effectiveness in children. Endocr. Dev. 2010, 18, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Hindmarsh, P.C.; Dunger, D.B. Growth hormone (GH) provocation tests and the response to GH treatment in GH deficiency. Arch. Dis. Child. 2004, 89, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Zadik, Z.; Chalew, S.A.; Gilula, Z.; Kowarski, A.A. Reproducibility of growth hormone testing procedures: A comparison between 24-hour integrated concentration and pharmacological stimulation. J. Clin. Endocrinol. Metab. 1990, 71, 1127–1130. [Google Scholar] [CrossRef]
- Hintz, R.L. Growth Hormone Neurosecretory Dysfunction as a Clinical Entity. Clin. Pediatr. Endocrinol. 1995, 4, 9–19. [Google Scholar] [CrossRef]
- Hilczer, M.; Smyczynska, J.; Lewinski, A. Limitations of clinical utility of growth hormone stimulating tests in diagnosing children with short stature. Endocr. Regul. 2006, 40, 69–75. [Google Scholar]
- Loche, S.; Bizzarri, C.; Maghnie, M.; Faedda, A.; Tzialla, C.; Autelli, M.; Casini, M.R.; Cappa, M. Results of early reevaluation of growth hormone secretion in short children with apparent growth hormone deficiency. J. Pediatr. 2002, 140, 445–449. [Google Scholar] [CrossRef]
- Hilczer, M.; Smyczyńska, J.; Stawerska, R.; Lewiński, A. Stability of IGF-I concentration despite divergent results of repeated GH stimulating tests indicates poor reproducibility of test results. Endocr. Regul. 2006, 40, 37–45. [Google Scholar]
- Wit, J.M.; Joustra, S.D.; Losekoot, M.; Van Duyvenvoorde, H.A.; De Bruin, C. Differential diagnosis of the short IGF-I-deficient child with apparently normal growth hormone secretion. Horm. Res. Paediatr. 2021, 94, 81–104. [Google Scholar] [CrossRef]
- Obara-Moszynska, M.; Kedzia, A.; Korman, E.; Niedziela, M. Usefulness of growth hormone (GH) stimulation tests and IGF-I concentration measurement in GH deficiency diagnosis. J. Pediatr. Endocrinol. Metab. 2008, 21, 569–579. [Google Scholar] [CrossRef]
- Smyczyńska, J.; Stawerska, R.; Lewiński, A.; Hilczer, M. Limited usefulness of the test of spontaneous growth hormone (GH) nocturnal secretion as a screening procedure in diagnosing GH deficiency in children with short stature. Ann. Agric. Environ. Med. 2014, 21, 893–897. [Google Scholar] [CrossRef][Green Version]
- Rose, S.R.; Ross, J.L.; Uriarte, M.; Barnes, K.M.; Cassorla, F.G.; Cutler, G.B.J. The advantage of measuring stimulated as compared with spontaneous growth hormone levels in the diagnosis of growth hormone deficiency. N. Engl. J. Med. 1988, 319, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Spiliotis, B.E.; August, G.P.; Hung, W.; Sonis, W.; Mendelson, W.; Bercu, B.B. Growth hormone neurosecretory dysfunction. JAMA 1984, 251, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Radetti, G.; Buzi, F.; Cassar, W.; Paganini, C.; Stacul, E.; Maghnie, M. Growth hormone secretory pattern and response to treatment in children with short stature followed to adult height. Clin. Endocrinol. 2003, 59, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Rogol, A.D.; Blethen, S.L.; Sy, J.P.; Veldhuis, J.D. Do growth hormone (GH) serial sampling, insulin-like growth factor-I (IGF-I) or auxological measurements have an advantage over GH stimulation testing in predicting the linear growth response to GH therapy? Clin. Endocrinol. 2003, 58, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Wit, J.M.; Deeb, A.; Bin-Abbas, B.; Al Mutair, A.; Koledova, E.; Savage, M.O. Achieving optimal short- and long-term responses to paediatric growth hormone therapy. J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 329–340. [Google Scholar] [CrossRef]
- Binder, G.; Hähnel, J.; Weber, K.; Schweizer, R. Adult height after treatment of neurosecretory dysfunction and comparison to idiopathic GHD. Clin. Endocrinol. 2022, 96, 184–189. [Google Scholar] [CrossRef]
- Inoue-Lima, T.H.; Vasques, G.A.; Scalco, R.C.; Nakaguma, M.; Mendonca, B.B.; Arnhold, I.J.P.; Jorge, A.A.L. IGF-1 assessed by pubertal status has the best positive predictive power for GH deficiency diagnosis in peripubertal children. J. Pediatr. Endocrinol. Metab. 2019, 32, 173–179. [Google Scholar] [CrossRef]
- Blum, W.F.; Albertsson-Wikland, K.; Rosberg, S.; Ranke, M.B. Serum levels of insulin-like growth factor I (IGF-I) and IGF binding protein 3 reflect spontaneous growth hormone secretion. J. Clin. Endocrinol. Metab. 1993, 76, 1610–1616. [Google Scholar] [CrossRef]
- Ho, K.K.Y. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: A statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur. J. Endocrinol. 2007, 157, 695–700. [Google Scholar] [CrossRef]
- Ibba, A.; Corrias, F.; Guzzetti, C.; Casula, L.; Salerno, M.; Di Iorgi, N.; Tornese, G.; Patti, G.; Radetti, G.; Maghnie, M.; et al. Igf1 for the diagnosis of growth hormone deficiency in children and adolescents: A reappraisal. Endocr. Connect. 2020, 9, 1095–1102. [Google Scholar] [CrossRef]
- Iwayama, H.; Kitagawa, S.; Sada, J.; Miyamoto, R.; Hayakawa, T.; Kuroyanagi, Y.; Muto, T.; Kurahashi, H.; Ohashi, W.; Takagi, J.; et al. Insulin-like growth factor-1 level is a poor diagnostic indicator of growth hormone deficiency. Sci. Rep. 2021, 11, 16159. [Google Scholar] [CrossRef] [PubMed]
- Smyczyńska, J.; Lewiński, A.; Stawerska, R.; Hilczer, M.; Karasek, M. Assessment of insulin-like growth factor-I serum concentration as a screening procedure in diagnosing children with short stature. Neuroendocrinol. Lett. 2007, 28, 274–278. [Google Scholar] [PubMed]
- Meazza, C.; Elsedfy, H.H.; Khalaf, R.I.; Lupi, F.; Pagani, S.; El Kholy, M.; Tinelli, C.; Radetti, G.; Bozzola, M. Serum α-klotho levels are not informative for the evaluation of growth hormone secretion in short children. J. Pediatr. Endocrinol. Metab. 2017, 30, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Savage, M.O.; Blum, W.F.; Ranke, M.B.; Postel-Vinay, M.C.; Cotterill, A.M.; Hall, K.; Chatelain, P.G.; Preece, M.A.; Rosenfeld, R.G. Clinical features and endocrine status in patients with growth hormone insensitivity (Laron syndrome). J. Clin. Endocrinol. Metab. 1993, 77, 1465–1471. [Google Scholar] [CrossRef]
- Rudman, D.; Kutner, M.H.; Blackston, R.D.; Cushman, R.A.; Bain, R.P.; Patterson, J.H. Children with normal-variant short stature: Treatment with human growth hormone for six months. N. Engl. J. Med. 1981, 305, 123–131. [Google Scholar] [CrossRef]
- Jorge, A.A.; Souza, S.C.; Arnhold, I.J.; Mendonca, B.B. Poor reproducibility of IGF-I and IGF binding protein-3 generation test in children with short stature and normal coding region of the GH receptor gene. J. Clin. Endocrinol. Metab. 2002, 87, 469–472. [Google Scholar] [CrossRef][Green Version]
- Smyczyńska, J.; Smyczyńska, U.; Hilczer, M.; Stawerska, R.; Lewiński, A. Significance of direct confirmation of growth hormone insensitivity for the diagnosis of primary IGF-I deficiency. J. Clin. Med. 2020, 9, 240. [Google Scholar] [CrossRef]
- Smyczyńska, J.; Hilczer, M.; Stawerska, R.; Lewiński, A. Significant increase of IGF-I concentration and of IGF-I/IGFBP-3 molar ratio in generation test predicts the good response to growth hormone (GH) therapy in children with short stature and normal results of GH stimulating tests. Neuroendocrinol. Lett. 2013, 34, 222–228. [Google Scholar]
- Hilczer, M.; Smyczyńska, J.; Lewiński, A. Monitoring and optimising of growth hormone (GH) therapy in GH-deficient children—The role of assessment of insulin-like growth factor-I (IGF-I) secretion and IGF-I/IGF binding protein-3 molar ratio. Med. Sci. Technol. 2006, 47, 219–223. [Google Scholar]
- Gaddas, M.; Périn, L.; Le Bouc, Y. Evaluation of IGF1/IGFBP3 molar ratio as an effective tool for assessing the safety of growth hormone therapy in small-for-gestational-age, growth hormone-deficient and prader-willi children. JCRPE J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 253–261. [Google Scholar] [CrossRef]
- Renes, J.S.; van Doorn, J.; Hokken-Koelega, A.C. Ternary complex formation and IGFBP-3 proteolytic activity during childhood: Age-dependent changes. J. Clin. Endocrinol. Metab. 2014, 99, E1988–E1996. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Renes, J.S.; van Doorn, J.; Hokken-Koelega, A.C.S. Current Insights into the Role of the Growth Hormone-Insulin-Like Growth Factor System in Short Children Born Small for Gestational Age. Horm. Res. Paediatr. 2019, 92, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Bøtkjær, J.A.; Noer, P.R.; Oxvig, C.; Andersen, C.Y. A common variant of the pregnancy-associated plasma protein-A (PAPPA) gene encodes a protein with reduced proteolytic activity towards IGF-binding proteins. Sci. Rep. 2019, 9, 13231. [Google Scholar] [CrossRef] [PubMed]
- Bang, P.; Thorell, A.; Carlsson-Skwirut, C.; Ljungqvist, O.; Brismar, K.; Nygren, J. Free dissociable IGF-I: Association with changes in IGFBP-3 proteolysis and insulin sensitivity after surgery. Clin. Nutr. 2016, 35, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Elmlinger, M.W.; Kühnel, W.; Weber, M.M.; Ranke, M.B. Reference ranges for two automated chemiluminescent assays for serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 (IGFBP-3). Clin. Chem. Lab. Med. 2004, 42, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Blum, W.F.; Schweizer, R. Insulin-like growth factors and their binding proteins. In Diagnostics of Endocrine Function in Children and Adolescents; Ranke, M.B., Ed.; Karger: Basel, Switzerland, 2003; pp. 166–169. [Google Scholar]
- Siemens Healthcare Diagnostics Inc. Introducing the Restandardized Insulin-Like Growth Factor-I (IGF-I) Assay. Cust. Bull. 2016, 5, 1–12. [Google Scholar]
- Bang, P.; Bjerknes, R.; Dahlgren, J.; Dunkel, L.; Gustafsson, J.; Juul, A.; Kriström, B.; Tapanainen, P.; Åberg, V. A comparison of different definitions of growth response in short prepubertal children treated with growth hormone. Horm. Res. Paediatr. 2011, 75, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Bang, P.; Ahmed, S.F.; Argente, J.; Backeljauw, P.; Bettendorf, M.; Bona, G.; Coutant, R.; Rosenfeld, R.G.; Walenkamp, M.-J.; Savage, M.O. Identification and management of poor response to growth-promoting therapy in children with short stature. Clin. Endocrinol. 2012, 77, 169–181. [Google Scholar] [CrossRef]
- Straetemans, S.; De Schepper, J.; Thomas, M.; Tenoutasse, S.; Beauloye, V.; Rooman, R. Criteria for First-Year Growth Response to Growth Hormone Treatment in Prepubertal Children with Growth Hormone Deficiency: Do They Predict Poor Adult Height Outcome? Front. Endocrinol. 2019, 10, 792. [Google Scholar] [CrossRef]
- Straetemans, S.; Thomas, M.; Craen, M.; Rooman, R.; De Schepper, J. Poor growth response during the first year of growth hormone treatment in short prepubertal children with growth hormone deficiency and born small for gestational age: A comparison of different criteria. Int. J. Pediatr. Endocrinol. 2018, 2018, 9. [Google Scholar] [CrossRef]
- Straetemans, S.; Rooman, R.; De Schepper, J. Is a Two-Year Growth Response to Growth Hormone Treatment a Better Predictor of Poor Adult Height Outcome Than a First-Year Growth Response in Prepubertal Children with Growth Hormone Deficiency? Front. Endocrinol. 2021, 12, 678094. [Google Scholar] [CrossRef] [PubMed]
- Wit, J.M.; Ranke, M.B.; Albertsson-Wikland, K.; Carrascosa, A.; Rosenfeld, R.G.; Van Buuren, S.; Kristrom, B.; Schoenau, E.; Audi, L.; Hokken-Koelega, A.C.S.; et al. Personalized Approach to Growth Hormone Treatment: Clinical Use of Growth Prediction Models. Horm. Res. Paediatr. 2013, 79, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Hindmarsh, P.C.; Cole, T.J. Evidence-based growth hormone therapy prediction models. J. Pediatr. Endocrinol. Metab. 2000, 13 (Suppl. S6), 1359–1364. [Google Scholar] [CrossRef]
- Smyczyńska, U.; Smyczyńska, J.; Tadeusiewicz, R. Neural modelling of growth hormone therapy for the prediction of therapy results. Bio-Algorithms Med-Syst. 2015, 11, 33–45. [Google Scholar] [CrossRef]
- Carel, J.C.; Ecosse, E.; Nicolino, M.; Tauber, M.; Leger, J.; Cabrol, S.; Bastie-Sigeac, I.; Chaussain, J.L.; Coste, J. bservational follow up study of the FAdult height after long term treatment with recombinant growth hormone for idiopathic isolated growth hormone deficiency: Orench population based registry. BMJ 2002, 325, 70. [Google Scholar] [CrossRef]
- De Ridder, M.A.J.; Stijnen, T.; Hokken-Koelega, A.C.S. Prediction of adult height in growth-hormone-treated children with growth hormone deficiency. J. Clin. Endocrinol. Metab. 2007, 92, 925–931. [Google Scholar] [CrossRef]
- Ranke, M.B.; Lindberg, A.; Chatelain, P.; Wilton, P.; Cutfield, W.; Albertsson-Wikland, K.; Price, D.A. Derivation and Validation of a Mathematical Model for Predicting the Response to Exogenous Recombinant Human Growth Hormone (GH) in Prepubertal Children with Idiopathic GH Deficiency. J. Clin. Endocrinol. Metab. 1999, 84, 1174–1183. [Google Scholar] [CrossRef]
- Ranke, M.B.; Lindberg, A.; Martin, D.D.; Bakker, B.; Wilton, P.; Albertsson-Wikland, K.; Cowell, C.T.; Price, D.A.; Reiter, E.O. The mathematical model for total pubertal growth in idiopathic growth hormone (GH) deficiency suggests a moderate role of GH dose. J. Clin. Endocrinol. Metab. 2003, 88, 4748–4753. [Google Scholar] [CrossRef]
- Smyczyńska, U.; Smyczyńska, J.; Hilczer, M.; Stawerska, R.; Tadeusiewicz, R.; Lewiński, A. Pre-treatment growth and IGF-I deficiency as main predictors of response to growth hormone therapy in neural models. Endocr. Connect. 2018, 7, 239–249. [Google Scholar] [CrossRef]
- Attanasio, A.; Attie, K.; Baxter, R.; Bengtsson, B.-A.; Black, A.; Blethen, S.; Carlsson, L.; Casaneuva, F.; Chipman, J.; Christiansen, J.S.; et al. Consensus guidelines for the diagnosis and treatment of adults with growth hormone deficiency: Summary statement of the growth hormone research society workshop on adult growth hormone deficiency. J. Clin. Endocrinol. Metab. 1998, 83, 379–381. [Google Scholar] [CrossRef]
- Zucchini, S.; Pirazzoli, P.; Baronio, F.; Gennari, M.; Bal, M.O.; Balsamo, A.; Gualandi, S.; Cicognani, A. Effect on adult height of pubertal growth hormone retesting and withdrawal of therapy in patients with previously diagnosed growth hormone deficiency. J. Clin. Endocrinol. Metab. 2006, 91, 4271–4276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Penta, L.; Cofini, M.; Lucchetti, L.; Zenzeri, L.; Leonardi, A.; Lanciotti, L.; Galeazzi, D.; Verrotti, A.; Esposito, S. Growth hormone (GH) therapy during the transition period: Should we think about early retesting in patients with idiopathic and isolated GH deficiency? Int. J. Environ. Res. Public Health 2019, 16, 307. [Google Scholar] [CrossRef] [PubMed]
- Krukowska-Andrzejczyk, B.J.; Kalina, M.; Kalina-Faska, B.; Małecka-Tendera, E. Growth hormone therapy in children with partial growth hormone deficiency. Are we treating the right patients? Pediatr. Endocrinol. Diabetes Metab. 2020, 26, 65–72. [Google Scholar] [CrossRef]
- Ranke, M.B.; Schweizer, R.; Binder, G. Basal characteristics and first year responses to human growth hormone (GH) vary according to diagnostic criteria in children with non-acquired GH deficiency (naGHD): Observations from a single center over a period of five decades. J. Pediatr. Endocrinol. Metab. 2018, 31, 1257–1266. [Google Scholar] [CrossRef]
- Felício, J.S.; Janaú, L.C.; Moraes, M.A.; Zahalan, N.A.; de Souza Resende, F.; de Lemos, M.N.; de Souza Neto, N.J.K.; Farias de Franco, I.I.; Leitão, L.T.C.; Silva, L.D.S.D.A.; et al. Diagnosis of Idiopathic GHD in Children Based on Response to rhGH Treatment: The Importance of GH Provocative Tests and IGF-1. Front. Endocrinol. 2019, 10, 638. [Google Scholar] [CrossRef]
- Cohen, P.; Rogol, A.D.; Deal, C.L.; Saenger, P.; Reiter, E.O.; Ross, J.L.; Chernausek, S.D.; Savage, M.O.; Wit, J.M. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: A summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J. Clin. Endocrinol. Metab. 2008, 93, 4210–4217. [Google Scholar] [CrossRef]
- Henry, R.K. Childhood growth hormone deficiency, a diagnosis in evolution: The intersection of growth hormone history and ethics. Growth Horm. IGF Res. 2020, 55, 101358. [Google Scholar] [CrossRef]
- Rodari, G.; Profka, E.; Giacchetti, F.; Cavenaghi, I.; Arosio, M.; Giavoli, C. Influence of biochemical diagnosis of growth hormone deficiency on replacement therapy response and retesting results at adult height. Sci. Rep. 2021, 11, 14553. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).