Abstract
Moxonidine is a centrally acting, anti-hypertensive medication that exerts additional metabolic properties. It is unknown whether its effects are mediated by neurotransmitters or sympathetic tone regulators, including Neuropeptide Y (NPY). In this study, we evaluated the effects of moxonidine administration on serum NPY in humans. Methods: Ninety individuals with mild or moderate arterial hypertension that required monotherapy were categorized in three age and gender-matched groups according to their Body Mass Index (BMI) as normal weight (n = 30), overweight (n = 30), and obese (n = 30). Moxonidine was administered in therapeutic doses of up to 0.6 mg daily for 12 weeks, and clinical, biochemical and hormonal parameters were recorded. Results: In all three groups, a decrease in systolic and diastolic blood pressure and heart rate was shown. After treatment, BMI, 24 h urine catecholamines and catecholamines’ metabolites, and serum total cholesterol were also reduced. Most importantly, we found a decrease in serum NPY levels in all study groups, with the largest mean decrease in the group of obese and overweight participants compared to normal weight. Conclusions: Moxonidine administration results in improvement in cardio-metabolic parameters, as well as a decrease in serum NPY levels, which therefore represents it being a potent agent against obesity-associated hypertension. Its involvement in energy balance regulation warrants further investigation.
1. Introduction
Obesity and arterial hypertension have become increasingly prevalent in the Western world, reaching epidemic proportions [1]. Excess of body weight has been associated with shorter life expectancy, mainly due to cardiovascular complications, type 2 diabetes and carcinogenesis [2]. It has been reported that almost 60–70% of patients with obesity have high blood pressure, thereby being at increased risk for cardiovascular disease [1]. Therefore, identifying novel therapeutic pathways and/or finding biomarkers that could be used as prognostic agents for the evaluation of the currently available therapeutic strategies against obesity and its related cardiovascular comorbidities, such as hypertension, are popular research topics.
In this regard, several mechanisms have been proposed to link obesity with arterial hypertension. Among them, sympathetic nervous system regulation seems to play an important role [3,4]. Classical and novel sympathetic tone regulators, including catecholamines as well as anorexigenic and orexigenic neuropeptides, such as leptin and neuropeptide Y (NPY), respectively, seem to contribute to pathogenesis of obesity-associated arterial hypertension [5]. Leptin, the prototype anorexigenic adipokine discovered more than 20 years ago, was initially believed to induce weight loss, raising high expectations as an anti-obesity treatment. Leptin reduces body weight by activating the sympathetic nervous system through NPY inhibition, resulting in increased energy expenditure. However, it was later shown that increasing obesity is characterized by a “leptin resistance” phenomenon, similar to insulin resistance in type 2 diabetes [6]. Thus, upregulated levels of NPY due to leptin resistance in obese patients cause increased catecholamine release and subsequent elevation of blood pressure and heart rate [7,8,9]. Our group has previously shown that circulating NPY levels were higher in hypertensive patients with excessive body weight compared to normal weight individuals, as well as in overweight and obese patients with hypertension compared to participants with normal blood pressure [10].
NPY was initially described as a neurotransmitter with wide distribution in the mammalian central and peripheral nervous system [11]. NPY coexists in terminal neurons with norepinephrine and adenosine triphosphate (ATP) in post-ganglionic sympathetic neurons throughout the body, and is secreted according to the intensity of sympathetic nervous system stimulation [12,13]. It is involved in many physiological functions, such as food intake and energy balance regulation, stress response, and vasoconstriction, as well as other pathophysiological mechanisms, including glucose and lipid metabolism and blood pressure regulation, inducing atherosclerosis [14]. More specifically, although NPY decreases the release of norepinephrine centrally in rats, causing reduction of sympathetic activity and a subsequent decrease in blood pressure and heart rate, at the peripheral nervous system it co-exists with norepinephrine, and after its release it acts as a sympathetic tone regulator inducing strong vasoconstriction [15]. Regarding energy balance, NPY has orexigenic properties and thus increases food intake [14], and it has also been shown to reduce brown adipose tissue function [16], thereby contributing to the development of obesity. In clinical studies, metabolic syndrome patients exhibited high circulating levels of NPY [17]. Therefore, therapeutic strategies that target its reduction may prove useful in the prevention of obesity and its associated comorbidities, like hypertension and atherosclerotic disease.
One of the therapeutic agents that have been proposed to target this pathway is moxonidine, a new-generation, centrally-acting antihypertensive medication. Moxonidine is a selective agonist of the imidazoline receptor subtype 1 (I1), which is found in the medulla oblongata [18]. Besides its antihypertensive properties, in animal models, moxonidine has been shown to exert favorable glycemic and metabolic effects, such as improvements in glucose homeostasis and insulin resistance, body weight reduction, and lowering of lipid levels, thereby representing a promising therapeutic agent for obesity-related arterial hypertension and other related chronic diseases [19,20,21]. Moxonidine was also shown to reduce central NPY synthesis and release in obese Zucker rats [22].
However, it remains unknown whether moxonidine affects NPY serum concentrations in humans. In this study, we aimed at evaluating the effects of moxonidine treatment on serum NPY levels in a group of hypertensive individuals. The secondary aim of the present study was the comparative evaluation of the impact of moxonidine administration on cardiometabolic and other sympathetic tone parameters in patients with normal vs. excessive body weight.
2. Materials and Methods
2.1. Study Participants
Ninety (90) treatment-naïve subjects with mild or moderate hypertension according to JNC 7 and ESH/ESC 2008 (i.e., Stage 1: Systolic Blood Pressure (SBP) = 140–159 mmHg, Diastolic Blood Pressure (DBP) = 90–99 mmHg, and Stage 2: SBP = 160–179 mmHg, DBP = 100–109 mmHg) [23] that required monotherapy were included in this study. Demographic, anthropometric and medical history data were individually recorded. Exclusion criteria were: age beyond 25–75 years, current anti-hypertensive, anti-diabetic, or lipid-lowering medication, and other diseases or conditions that may influence blood pressure, heart rate, and catecholamine and NPY levels, including anemia, fever, stage III hypertension, coronary artery disease, recent (less than 6 months) myocardial infraction or stroke, heart failure, secondary hypertension, diabetes mellitus, abnormal thyroid status or other obesity-related endocrinopathies, pregnancy, kidney failure, alcohol abuse, malignancies, depression, or other psychiatric illnesses, such as schizophrenia, where NPY levels are altered. All participants underwent clinical and laboratory tests (such as complete blood count, biochemical assessment, 24 h urine collection for the determination of total protein and adrenaline, noradrenaline and vanillylmandelic acid, serum thyroid function tests, as well as adrenal/kidney and heart ultrasound) to exclude the presence of secondary hypertension or other exclusion criteria. Τhe patients did not receive, for 15 days before the 24-h collection: Coffee-Tea-Cocoa, Bananas-Citrus. Nuts, Vanilla, a-Methyldopa, Levodopa. Corbidopa, Rezepine, Pyridoxine, Aspirin. Additionally, patients were given stable diets and exercise during the study, and the compliance control and assessment of dietary intake was held through questionnaires. Blood pressure was measured by the same experienced research staff as the mean of three measurements in three different office visits within a week. In the case of non-diagnostic or discrepant measurements, 24 h measurements were employed. Body mass index (BMI) was estimated as BMI = Body Weight (kg)/[Height (meters)]2. According to their BMI status, study participants were categorized as: (a) normal weight (ΒΜΙ < 25 kg/m2), (b) overweight (25 kg/m2 ≤ ΒΜΙ < 30 kg/m2), or (c) obese (ΒΜΙ ≥ 30 kg/m2), in respective gender groups.
2.2. Study Design and Measurements
This study was prospective observational. After the initial evaluation, patients orally received 0.6 mg of moxonidine daily for 12 weeks. The parameters of interest were re-evaluated 12 weeks after treatment. Early-morning blood samples were collected after an overnight fast of at least 12 h at both study timepoints for the quantification of routine biochemistry, using standard laboratory techniques, and serum NPY levels by ELISA (Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA, Intra-assay coefficient of variation < 5%, inter-assay coefficient of variation < 14%, linear range 0.27–2.1 ng/mL). All patients provided written informed consent, and the study protocol was approved by the Aristotle University of Thessaloniki Ethics Committee (No. 7337/20.04.2021) in accordance with the Declaration of Helsinki, and is registered at clinicaltrials.gov as NCT05147753.
2.3. Statistical Analysis
Statistical analysis was performed with SPSS version20.0 for Windows (IBM Corp., Armonk, NY, USA). Data for continuous variables are presented as mean ± standard deviation (SD), unless otherwise stated. Normality of distribution of the continuous variables was assessed with the Kolmogorov-Smirnov or Shapiro-Wilk test. The paired t-test or Wilcoxon signed rank test were used to evaluate for differences between values at baseline and the end of the study protocol, as appropriate. The one-way analysis of variance (ANOVA) or Kruskal-Wallis test were used for between study group comparisons (i.e., normal weight vs. overweight vs. obese), with post hoc Bonferroni correction in the case of statistical significance. A mixed-model repeated-measures ANOVA was used to evaluate and compare the effect of moxonidine administration between our three study groups. The level of statistical significance was set at 0.05 for all analyses.
3. Results
Descriptive characteristics of the study participants are depicted in Table 1. There was no significant difference in the mean age among our study groups (Normal weight: 49.80 ± 11.29 years vs. Overweight: 47.90 ± 12.67 years vs. Obese: 46.47 ± 11.15 years, p = 0.55). Significant differences between the three study groups were found for heart rate, SBP, and DBP at both study timepoints. In addition, triglyceride levels, as well as 24 h urine adrenaline, noradrenaline, and vanillylmandelic acid were significantly different between groups at baseline, but not at the end of the study protocol (Table 1). Total blood count parameters were similar between groups and remained unaffected after treatment. As expected, in all three study groups, 12-week moxonidine administration resulted in decreased SBP, DBP, heart rate, and BMI. We additionally found favorable effects on total cholesterol levels and 24 h urine adrenaline, noradrenaline, and Vanillylmandelic acid (VMA) levels. Most interestingly, a robust decrease of serum NPY in all groups was shown, with a higher mean decrease in the overweight and obese groups compared to normal weight (Table 1).

Table 1.
Descriptive characteristics of study participants and effect of the 12-week intervention.
Although there were no significant correlations between systolic and diastolic blood pressure and serum neuropeptide Y levels at baseline, this was altered post-intervention where both systolic and diastolic blood pressure were positively correlated with serum neuropeptide Y levels (Figure 1, Figure 2, Figure 3 and Figure 4).
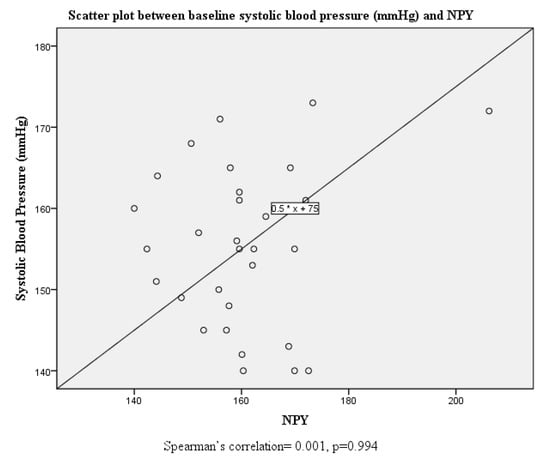
Figure 1.
Scatter plot and correlation between systolic blood pressure and serum neuropeptide Y levels at baseline.
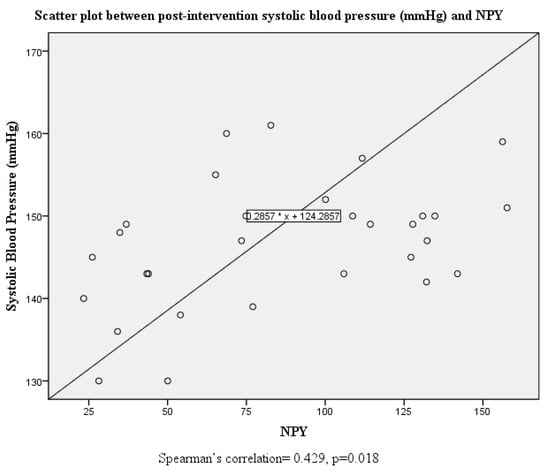
Figure 2.
Scatter plot and correlation between systolic blood pressure and serum neuropeptide Y levels post intervention.
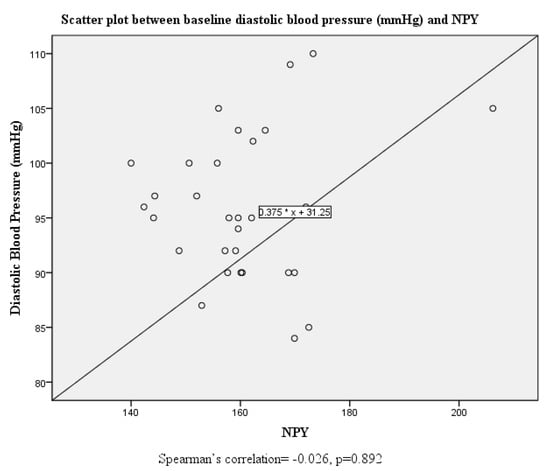
Figure 3.
Scatter plot and correlation between diastolic blood pressure and serum neuropeptide Y levels at baseline.
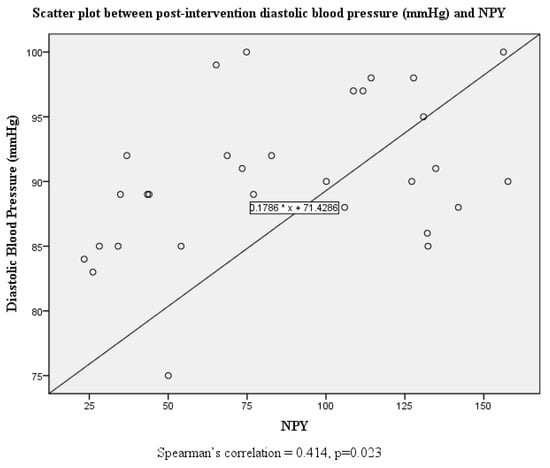
Figure 4.
Scatter plot and correlation between diastolic blood pressure and serum neuropeptide Y levels post intervention.
4. Discussion
This was the first study to explore the effects of moxonidine administration on serum NPY levels in a group of hypertensive humans with a wide range of BMI. We showed that our 12-week intervention resulted in a significant decrease of circulating NPY concentrations, in all study groups, independently of age and gender. Moxonidine treatment also induced significant reduction in SBP and DBP, heart rate, BMI, and lipid profile parameters, as well as 24 h urine catecholamines and catecholamines’ metabolites.
NPY was described as a neurotransmitter involved in many physiological functions, such as food intake and energy balance regulation, stress response, and vasoconstriction, as well as other pathophysiological mechanisms, including glucose and lipid metabolism and blood pressure regulation, inducing atherosclerosis [14]. More specifically, from animal studies, it has been shown that although NPY reduces sympathetic activity and causes decreases in blood pressure and heart rate, at the peripheral nervous system it acts as a sympathetic tone regulator inducing strong vasoconstriction [15].
Moxonidine is a second-generation alpha-2/imidazoline receptor agonist, a centrally acting antihypertensive medication that has been shown to exert favorable metabolic effects, that is, improvements in lipid and glycemic profiles and body weight [21], but no previous human studies have explored whether these actions might be mediated by NPY. Moxonidine acts in I1 imidazoline receptors, resulting in a decrease in sympathetic nervous system activity, thus contributing to a reduction of blood pressure and a subsequent indirect reduction of NPY levels [21]. In an older animal study, moxonidine administration resulted in reduced NPY synthesis and release in the central nervous system, but did not affect peripheral levels [22]. Regarding leptin, which is known to down-regulate NPY in the food intake balance, 24-week moxonidine administration in obese hypertensive patients resulted in decreased serum leptin levels after a glucose load [24]. However, there is paucity of data with regard to the exact effects of moxonidine on food intake in humans, although animal studies have postulated possible antiorexigenic properties [25,26].
Evidence suggests that moxonidine effectively reduces blood pressure compared to diuretics, clonidine, calcium channel blockers, angiotensin converting enzyme (ACE) inhibitors, and a- and b-blockers [27]. Therefore, when moxonidine was compared to aliskiren and hydrochlorothiazides, it did not produce the same effective result as for SBP and DBP [28]. However, in numerous studies, moxonidine, either as monotherapy or as an adjunct to other antihypertensives, has shown to be favorable towards blood pressure and metabolic control [29,30,31,32,33,34,35]. Although, moxonidine results in a similar reduction in blood pressure compared to amlodipine, the first also reduced catecholamine levels, which was not the case with amlodipine [35]. Moreover, monotherapy with moxonidine, in patients with diabetes mellitus type 2 and mild hypertension, improved more glycemic and lipid control than combination with ibersartan [34]. Thus, our study is in accordance with results from several studies (MERSY, CAMUS, OBEZITA, and others) that examined moxonidine as monotherapy or combination therapy in obese hypertensive patients with metabolic syndrome [30,31,32,33] and insulin resistance [29], where moxonidine was proven to improve metabolic control, such as weight and BMI, and also blood lipids, except from reductions in blood pressure and heart rate [29,30,31,32,33].
During our three-month intervention, we confirmed moxonidine’s potency to significantly decrease sympathetic activity by reducing 24 h urine catecholamines and additionally improve cardio-metabolic parameters, such as blood pressure, heart rate, and lipid metabolism, as well as induce weight loss in a group of hypertensive patients of normal and excessive body weight [26]. A possible explanation for the beneficial effects of moxonidine administration on one’s lipid profile could be the reduction of NPY levels [36]. We additionally showed, for the first time, a robust decrease in NPY levels, besides BMI reduction. This pathway may represent a novel mechanism that possibly explains moxonidine’s beneficial cardio-metabolic actions through NPY inhibition. We did not find any difference in hematological parameters after moxonidine treatment in our study, and it could be supported that beyond safety in hematopoiesis [37], NPY might exert additional direct protective effects on endothelial cells in individuals at high risk for atherosclerosis, such as obese hypertensive patients. It is remarkable that these metabolic effects of moxonidine are sustainable in the long-term, since the majority of patients after the end of the study continued their follow-up in our Outpatient Clinic of Hypertension and they presented with well-controlled levels of glucose, lipids, and uric acid while being under moxonidine. Therefore, our results support the role of moxonidine in the therapeutic armamentarium against hypertension in populations bearing the metabolic syndrome phenotype, and in fact, based on the 2013 European Society of Hypertension guidelines, moxonidine, as well as other centrally-acting antihypertensive agents, was suggested as a possible option in combination therapies, especially in cases of resistant hypertension [38].
The strengths of our study include its novel findings as the recruitment of treatment-naïve patients with mild or moderate hypertension, and apparently no other comorbidities. Moreover, although most previous studies have evaluated sympathetic nervous system activity with serum catecholamine levels, we used 24 h urine collection measurements that have been shown to be of higher clinical significance and prognostic accuracy. Thus, we cannot definitely conclude that the effects of NPY are indeed dependent on blood pressure, rather than weight loss. We did not use dietary data to further explore possible moxonidine effects on appetite and food intake. Yet, our findings provide important background information that may help in the design and interpretation of future clinical trials. Furthermore, the small study group is another limitation of our study. Therefore, our observations need to be validated in larger placebo-controlled clinical trials.
In conclusion, moxonidine is an effective medication for obese hypertensive patients, and its possible favorable cardio-metabolic effects may be mediated by NPY reduction that is subsequent of the downregulation of the sympathetic nervous system. Its role in the regulation of obesity-related hypertension and energy balance warrants further investigation with future mechanistic studies and large clinical trials.
Author Contributions
Conceptualization, E.K. (Eleni Karlafti) and C.S.; methodology, E.K. (Eleni Karlafti) and E.B.; software, E.B. validation, E.K. (Eleni Karlafti) and E.B.; formal analysis, E.B.; investigation, E.K. (Eleni Karlafti); resources, T.D. and C.S.; data curation, E.K. (Eleni Karlafti), E.K. (Evangelia Kotzakioulafi) and G.K.; writing—original draft preparation, E.K. (Eleni Karlafti), E.B., E.K. (Evangelia Kotzakioulafi) and T.D.; writing—review and editing, E.K. (Eleni Karlafti), E.K. (Evangelia Kotzakioulafi) and T.D.; visualization, E.B. and E.K. (Eleni Karlafti); supervision, V.K., A.Z., M.D., A.G., T.D. and C.S.; project administration, V.K., A.Z., M.D., A.G., T.D. and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Bioethics Committee of Aristotle University of Thessaloniki (No. 7337/20.04.2021) and is registered in clinicaltrials.gov with registration number NCT05147753.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data will not be shared due to ethical and legal reasons.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jordan, J.; Engeli, S.; Redon, J.; Sharma, A.M.; Luft, F.; Narkiewicz, K.; Grassi, G. European Society of Hypertension Working Group on Obesity: Background, aims and perspectives. J. Hypertens. 2007, 25, 897–900. [Google Scholar] [CrossRef]
- Comerma-Steffensen, S.; Grann, M.; Andersen, C.U.; Rungby, J.; Simonsen, U. Cardiovascular effects of current and future anti-obesity drugs. Curr. Vasc. Pharmacol. 2014, 12, 493–504. [Google Scholar] [CrossRef]
- Parati, G.; Esler, M. The human sympathetic nervous system: Its relevance in hypertension and heart failure. Eur. Heart J. 2012, 33, 1058–1066. [Google Scholar] [CrossRef]
- Landsberg, L. Insulin-mediated sympathetic stimulation: Role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why). J. Hypertens. 2001, 19, 523–528. [Google Scholar] [CrossRef]
- Rahmouni, K.; Haynes, W.G. Leptin and the central neural mechanisms of obesity hypertension. Drugs Today 2002, 38, 807–817. [Google Scholar] [CrossRef]
- Mantzoros, C.S.; Magkos, F.; Brinkoetter, M.; Sienkiewicz, E.; Dardeno, T.A.; Kim, S.-Y.; Hamnvik, O.-P.R.; Koniaris, A. Leptin in human physiology and pathophysiology. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E567–E584. [Google Scholar] [CrossRef] [PubMed]
- Pons, J.; Lee, E.W.; Li, L.; Kitlinska, J. Neuropeptide Y: Multiple receptors and multiple roles in cardiovascular diseases. Curr. Opin. Investig. Drugs 2004, 5, 957–962. [Google Scholar] [PubMed]
- Vonend, O.; Okonek, A.; Stegbauer, J.; Habbel, S.; Quack, I.; Rump, L.C. Renovascular effects of sympathetic cotransmitters ATP and NPY are age-dependent in spontaneously hypertensive rats. Cardiovasc. Res. 2005, 66, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Baltatzi, M.; Hatzitolios, A.; Tziomalos, K.; Iliadis, F.; Zamboulis, C. Neuropeptide Y and alpha-melanocyte-stimulating hormone: Interaction in obesity and possible role in the development of hypertension. Int. J. Clin. Pract. 2008, 62, 1432–1440. [Google Scholar] [CrossRef]
- Baltazi, M.; Katsiki, N.; Savopoulos, C.; Iliadis, F.; Koliakos, G.; Hatzitolios, A.I. Plasma neuropeptide Y (NPY) and alpha-melanocyte stimulating hormone (a-MSH) levels in patients with or without hypertension and/or obesity: A pilot study. Am. J. Cardiovasc. Dis. 2011, 1, 48–59. [Google Scholar] [PubMed]
- Lin, S.; Boey, D.; Herzog, H. NPY and Y receptors: Lessons from transgenic and knockout models. Neuropeptides 2004, 38, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.M.; Franco-Cereceda, A.; Hemsen, A.; Lacroix, J.S.; Pernow, J. Pharmacology of noradrenaline and neuropeptide tyrosine (NPY)-mediated sympathetic cotransmission. Fundam. Clin. Pharmacol. 1990, 4, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Altarejos, J.Y.; Montminy, M. CREB and the CRTC co-activators: Sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 2011, 12, 141–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, P.; Sun, W.; Zhang, C.; Song, Z.; Lin, S. The role of neuropeptide Y in the pathophysiology of atherosclerotic cardiovascular disease. Int. J. Cardiol. 2016, 220, 235–241. [Google Scholar] [CrossRef]
- Morris, M.J.; Tortelli, C.F.; Hart, D.P.; Delbridge, L.M. Vascular and brain neuropeptide Y in banded and spontaneously hypertensive rats. Peptides 2004, 25, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.; Herzog, H.; Shi, Y.C. Regulation of energy homeostasis by the NPY system. Trends Endocrinol. Metab. TEM 2015, 26, 125–135. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Lau, J.; Lin, Z.; Zhang, H.; Zhai, L.; Sperk, G.; Heilbronn, R.; Mietzsch, M.; Weger, S.; Huang, X.-F.; et al. Arcuate NPY controls sympathetic output and BAT function via a relay of tyrosine hydroxylase neurons in the PVN. Cell Metab. 2013, 17, 236–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Qi, Q.; Zheng, Y.; Huang, T.; Lathrop, M.; Zelenika, D.; Bray, G.A.; Sacks, F.M.; Liang, L.; Qi, L. Neuropeptide Y genotype, central obesity, and abdominal fat distribution: The POUNDS LOST trial. Am. J. Clin. Nutr. 2015, 102, 514–519. [Google Scholar] [CrossRef] [Green Version]
- Ernsberger, P.; Damon, T.H.; Graff, L.M.; Schafer, S.G.; Christen, M.O. Moxonidine, a centrally acting antihypertensive agent, is a selective ligand for I1-imidazoline sites. J. Pharmacol. Exp. Ther. 1993, 264, 172–182. [Google Scholar]
- Edwards, L.P.; Brown-Bryan, T.A.; McLean, L.; Ernsberger, P. Pharmacological properties of the central antihypertensive agent, moxonidine. Cardiovasc. Ther. 2012, 30, 199–208. [Google Scholar] [CrossRef]
- Karlafti, E.F.; Hatzitolios, A.I.; Karlaftis, A.F.; Baltatzi, M.S.; Koliakos, G.G.; Savopoulos, C.G. Effects of moxonidine on sympathetic nervous system activity: An update on metabolism, cardio, and other target-organ protection. J. Pharm. Bioallied Sci. 2013, 5, 253–256. [Google Scholar]
- Bing, C.; King, P.; Pickavance, L.; Brown, M.; Ziegler, D.; Kaan, E.; Williams, G. The effect of moxonidine on feeding and body fat in obese Zucker rats: Role of hypothalamic NPY neurones. Br. J. Pharmacol. 1999, 127, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2007, 25, 1105–1187. [Google Scholar] [CrossRef] [PubMed]
- Sanjuliani, A.F.; de Abreu, V.G.; Francischetti, E.A. Selective imidazoline agonist moxonidine in obese hypertensive patients. Int. J. Clin. Pract. 2006, 60, 621–629. [Google Scholar] [CrossRef]
- Ernsberger, P.; Koletsky, R.J.; Collins, L.A.; Bedol, D. Sympathetic nervous system in salt-sensitive and obese hypertension: Amelioration of multiple abnormalities by a central sympatholytic agent. Cardiovasc. Drugs Ther. 1996, 10, 275–282. [Google Scholar] [CrossRef]
- Karlafti, E.; Hatzitolios, A.; Savopoulos, C. The role of moxonidine, a second generation centrally acting antihypertensive agent as antihypertensive therapy in the obese. Hippokratia 2014, 18, 189. [Google Scholar] [PubMed]
- Prichard, B.N.; Graham, B.R. Effective antihypertensive therapy: Blood pressure control with moxonidine. J. Cardiovasc. Pharmacol. 1996, 27, 38–48. [Google Scholar] [CrossRef]
- Dorresteijn, J.A.; Schrover, I.M.; Visseren, F.L.; Scheffer, P.G.; Oey, P.L.; Danser, A.H.; Spiering, W. Differential effects of renin-angiotensin-aldosterone system inhibition, sympathoinhibition and diuretic therapy on endothelial function and blood pressure in obesity-related hypertension: A double-blind, placebo- controlled cross-over trial. J. Hypertens. 2013, 31, 393–403. [Google Scholar] [CrossRef]
- Haenni, A.; Lithell, H. Moxonidine improves insulin sensitivity in insulin- resistant hypertensives. J. Hypertens. Suppl. 1999, 17, S29–S35. [Google Scholar] [PubMed]
- Abellán, J.; Leal, M.; Hernández-Menárguez, F.; García-Galbis, J.A.; Martínez-Pastor, A.; de Vinuesa, S.G.; Luño, J. Efficacy of moxonidine in the treatment of hypertension in obese, noncontrolled hypertensive patients. Kidney Int. Suppl. 2005, S20–S24. [Google Scholar] [CrossRef] [Green Version]
- Chazova, I.; Schlaich, M.P. Improved Hypertension Control with the Imidazoline Agonist Moxonidine in a Multinational Metabolic Syndrome Population: Principal Results of the MERSY Study. Int. J. Hypertens. 2013, 2013, 541689. [Google Scholar] [CrossRef]
- Sharma, A.M.; Wanger, T.; Marsalek, P. Moxonidine in the treatment of overweight and obese patients with the metabolic syndrome: A postmarketing surveillance study. J. Hum. Hypertens. 2004, 18, 669–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupicka, J.; Soucek, M.; Chroust, K. The efficacy and safety of moxonidine in patients with metabolic syndrome (the O.B.E.Z.I.T.A. trial). Vnitr. Lek. 2011, 57, 541–545. [Google Scholar]
- Derosa, G.; Cicero, A.F.; D’Angelo, A.; Fogari, E.; Salvadeo, S.; Gravina, A.; Ferrari, I.; Fassi, R.; Fogari, R. Metabolic and antihypertensive effects of moxonidine and moxonidine plus irbersatan in patients with type 2 diabetes mellitus and mild hypertension: A sequential, randomized double- blind clinical trial. Clin. Ther. 2007, 29, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Sanjuliani, A.F.; Genelhu de Abreu, V.; Ueleres Braga, J.; Francischetti, E. Effects of moxonidine on the Sympathetic Nervous System, Blood Pressure, Plasma Renin Activity, Plasma Aldosterone, Leptin, and Metabolic Profile in Obese Hypertensive Patients. J. Clin. Basic Cardiol. 2004, 7, 19. [Google Scholar]
- Lumb, P.J.; McMahon, Z.; Chik, G.; Wierzbicki, A.S. Effect of moxonidine on lipid subfraction in patients with hypertension. Int. J. Clin. Pract. 2004, 58, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Hoggatt, J.; Kamocka, M.M.; Mohammad, K.S.; Saunders, M.R.; Li, H.; Speth, J.; Carlesso, N.; Guise, T.A.; Pelus, L.M. Neuropeptide Y regulates a vascular gateway for hematopoietic stem and progenitor cells. J. Clin. Investig. 2017, 127, 4527–4540. [Google Scholar] [CrossRef] [Green Version]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Bohm, M.; Christiaens, T.; Cifkova, R.; de Backer, G.; Dominiczak, A. ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).