Primary Hyperaldosteronism: When to Suspect It and How to Confirm Its Diagnosis
Abstract
1. Introduction
2. Cardiovascular Risk Associated with Primary Hyperaldosteronism (PA)
3. Clinical Manifestations
3.1. PA and Atrial Fibrillation
3.2. PA and Hyperparathyroidism
3.3. Hypoklemia
3.4. Hypertension
“Subclinical” PA
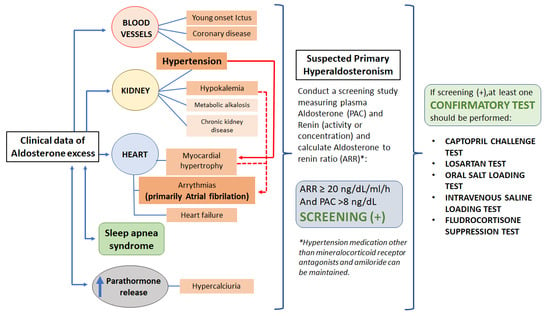
4. Diagnostic Approach
4.1. Screening for PA
Interpretation of Results
4.2. Confirmatory Tests
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conn, J.W. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J. Lab. Clin. Med. 1955, 45, 3–17. [Google Scholar]
- Williams, T.A.; Gomez-Sanchez, C.E.; Rainey, W.E.; Giordano, T.J.; Lam, A.K.; Marker, A.; Mete, O.; Yamazaki, Y.; Zerbini, M.C.N.; Beuschlein, F.; et al. International Histopathology Consensus for Unilateral Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2021, 106, 42–54. [Google Scholar] [CrossRef]
- Mulatero, P.; Monticone, S.; Deinum, J.; Amar, L.; Prejbisz, A.; Zennaro, M.-C.; Beuschlein, F.; Rossi, G.P.; Nishikawa, T.; Morganti, A.; et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: A position statement and consensus of the Working Group on Endocrine Hypertension of The European Society of Hypertension. J. Hypertens. 2020, 38, 1919–1928. [Google Scholar] [CrossRef]
- Vaidya, A.; Carey, R.M. Evolution of the Primary Aldosteronism Syndrome: Updating the Approach. J. Clin. Endocrinol. Metab. 2020, 105, 3771–3783. [Google Scholar] [CrossRef]
- Buffolo, F.; Monticone, S.; Burrello, J.; Tetti, M.; Veglio, F.; Williams, T.; Mulatero, P. Is Primary Aldosteronism Still Largely Unrecognized? Horm. Metab. Res. 2017, 49, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Käyser, S.C.; Dekkers, T.; Groenewoud, H.J.; van der Wilt, G.J.; Carel Bakx, J.; van der Wel, M.C.; Hermus, A.R.; Lenders, J.W.; Deinum, J. Study Heterogeneity and Estimation of Prevalence of Primary Aldosteronism: A Systematic Review and Meta-Regression Analysis. J. Clin. Endocrinol. Metab. 2016, 101, 2826–2835. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Nwankwo, T.; Yoon, S.S.; Burt, V.; Gu, Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief 2013, 1–8. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24171916 (accessed on 1 December 2021).
- Menéndez, E.; Delgado, E.; Fernández-Vega, F.; Prieto, M.A.; Bordiú, E.; Calle, A.; Carmena, R.; Castaño, L.; Catalá, M.; Franch, J.; et al. Prevalence, Diagnosis, Treatment, and Control of Hypertension in Spain. Results of the Di@bet.es Study. Rev. Española Cardiol. 2016, 69, 572–578. [Google Scholar] [CrossRef]
- Käyser, S.C.; Deinum, J.; de Grauw, W.J.; Schalk, B.W.; Bor, H.J.; Lenders, J.W.; Schermer, T.R.; Biermans, M.C. Prevalence of primary aldosteronism in primary care: A cross-sectional study. Br. J. Gen. Pract. 2018, 68, e114–e122. [Google Scholar] [CrossRef]
- Funder, J. Primary Aldosteronism: The Next Five Years. Horm. Metab. Res. 2017, 49, 977–983. [Google Scholar] [CrossRef]
- Handgriff, L.; Reincke, M. Primärer Hyperaldosteronismus—warum diagnostizieren wir immer noch so wenige Patienten? DMW Dtsch. Med. Wochenschr. 2020, 145, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Milliez, P.; Girerd, X.; Plouin, P.-F.; Blacher, J.; Safar, M.E.; Mourad, J.-J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 2005, 45, 1243–1248. [Google Scholar] [CrossRef]
- Stowasser, M.; Sharman, J.; Leano, R.; Gordon, R.D.; Ward, G.; Cowley, D.; Marwick, T.H. Evidence for Abnormal Left Ventricular Structure and Function in Normotensive Individuals with Familial Hyperaldosteronism Type I. J. Clin. Endocrinol. Metab. 2005, 90, 5070–5076. [Google Scholar] [CrossRef] [PubMed]
- Hundemer, G.L.; Curhan, G.C.; Yozamp, N.; Wang, M.; Vaidya, A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: A retrospective cohort study. Lancet Diabetes Endocrinol. 2018, 6, 51–59. [Google Scholar] [CrossRef]
- Meng, Z.; Dai, Z.; Huang, K.; Xu, C.; Zhang, Y.-G.; Zheng, H.; Liu, T.-Z. Long-Term Mortality for Patients of Primary Aldosteronism Compared with Essential Hypertension: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Abad-Cardiel, M.; Álvarez-Álvarez, B.; Luque-Fernandez, L.; Fernández, C.; Fernández-Cruz, A.; Martell-Claros, N. Hipertensión por hiperaldosteronismo: Más lesión cardiaca, mayor riesgo cardiovascular. Rev. Española Cardiol. 2013, 66, 47–52. [Google Scholar] [CrossRef]
- Vogt, B.; Burnier, M. Aldosterone and cardiovascular risk. Curr. Hypertens. Rep. 2009, 11, 450–455. [Google Scholar] [CrossRef]
- Prejbisz, A.; Warchoł-Celińska, E.; Lenders, J.; Januszewicz, A. Cardiovascular Risk in Primary Hyperaldosteronism. Horm. Metab. Res. 2015, 47, 973–980. [Google Scholar] [CrossRef]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef]
- Pratt-Ubunama, M.N.; Nishizaka, M.K.; Boedefeld, R.L.; Cofield, S.S.; Harding, S.M.; Calhoun, D.A. Plasma Aldosterone Is Related to Severity of Obstructive Sleep Apnea in Subjects with Resistant Hypertension. Chest 2007, 131, 453–459. [Google Scholar] [CrossRef]
- Di Murro, A.; Petramala, L.; Cotesta, D.; Zinnamosca, L.; Crescenzi, E.; Marinelli, C.; Saponara, M.; Letizia, C. Renin-angiotensin-aldosterone system in patients with sleep apnoea: Prevalence of primary aldosteronism. J. Renin Angiotensin Aldosterone Syst. 2010, 11, 165–172. [Google Scholar] [CrossRef]
- Pecori, A.; Buffolo, F.; Pieroni, J.; Forestiero, V.; Sconfienza, E.; Veglio, F.; Mulatero, P.; Monticone, S. Primary Aldosteronism and Obstructive Sleep Apnea: Casual Association or Pathophysiological Link? Horm. Metab. Res. 2020, 52, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Kawarazaki, W.; Fujita, T. The Role of Aldosterone in Obesity-Related Hypertension. Am. J. Hypertens. 2016, 29, 415–423. [Google Scholar] [CrossRef]
- Gershuni, V.M.; Herman, D.S.; Kelz, R.R.; Roses, R.E.; Cohen, D.L.; Trerotola, S.O.; Fraker, D.L.; Wachtel, H. Challenges in obesity and primary aldosteronism: Diagnosis and treatment. Surgery 2020, 167, 204–210. [Google Scholar] [CrossRef]
- Ohno, Y.; Sone, M.; Inagaki, N.; Yamasaki, T.; Ogawa, O.; Takeda, Y.; Kurihara, I.; Umakoshi, H.; Ichijo, T.; Katabami, T.; et al. Obesity as a Key Factor Underlying Idiopathic Hyperaldosteronism. J. Clin. Endocrinol. Metab. 2018, 103, 4456–4464. [Google Scholar] [CrossRef]
- Monticone, S.; Sconfienza, E.; D’Ascenzo, F.; Buffolo, F.; Satoh, F.; Sechi, L.A.; Veglio, F.; Mulatero, P. Renal damage in primary aldosteronism. J. Hypertens. 2020, 38, 3–12. [Google Scholar] [CrossRef]
- Fernández-Argüeso, M.; Pascual-Corrales, E.; Bengoa Rojano, N.; García Cano, A.; Jiménez Mendiguchía, L.; Araujo-Castro, M. Higher risk of chronic kidney disease and progressive kidney function impairment in primary aldosteronism than in essential hypertension. Case-control study. Endocrine 2021, 73, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Akehi, Y.; Yanase, T.; Motonaga, R.; Umakoshi, H.; Tsuiki, M.; Takeda, Y.; Yoneda, T.; Kurihara, I.; Itoh, H.; Katabami, T.; et al. High Prevalence of Diabetes in Patients with Primary Aldosteronism (PA) Associated with Subclinical Hypercortisolism and Prediabetes More Prevalent in Bilateral Than Unilateral PA: A Large, Multicenter Cohort Study in Japan. Diabetes Care 2019, 42, 938–945. [Google Scholar] [CrossRef]
- Hanslik, G.; Wallaschofski, H.; Dietz, A.; Riester, A.; Reincke, M.; Allolio, B.; Lang, K.; Quack, I.; Rump, L.C.; Willenberg, H.S.; et al. Increased prevalence of diabetes mellitus and the metabolic syndrome in patients with primary aldosteronism of the German Conn’s Registry. Eur. J. Endocrinol. 2015, 173, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Liu, W.; Su, X. Determining the Prevalence of Primary Aldosteronism in Patients with New-Onset Type 2 Diabetes and Hypertension. J. Clin. Endocrinol. Metab. 2020, 105, 1079–1085. [Google Scholar] [CrossRef]
- Monticone, S.; D’Ascenzo, F.; Moretti, C.; Williams, T.A.; Veglio, F.; Gaita, F.; Mulatero, P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018, 6, 41–50. [Google Scholar] [CrossRef]
- Pan, C.-T.; Tsai, C.-H.; Chen, Z.-W.; Chang, Y.-Y.; Wu, V.-C.; Hung, C.-S.; Lin, Y.-H. Atrial Fibrillation in Primary Aldosteronism. Horm. Metab. Res. 2020, 52, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Maiolino, G.; Flego, A.; Belfiore, A.; Bernini, G.; Fabris, B.; Ferri, C.; Giacchetti, G.; Letizia, C.; Maccario, M.; et al. Adrenalectomy Lowers Incident Atrial Fibrillation in Primary Aldosteronism Patients at Long Term. Hypertension 2018, 71, 585–591. [Google Scholar] [CrossRef]
- Reincke, M.; Fischer, E.; Gerum, S.; Merkle, K.; Schulz, S.; Pallauf, A.; Quinkler, M.; Hanslik, G.; Lang, K.; Hahner, S.; et al. Observational study mortality in treated primary aldosteronism: The German Conn’s registry. Hypertension 2012, 60, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Mulatero, P.; Monticone, S.; Bertello, C.; Viola, A.; Tizzani, D.; Iannaccone, A.; Crudo, V.; Burrello, J.; Milan, A.; Rabbia, F.; et al. Long-Term Cardio- and Cerebrovascular Events in Patients With Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2013, 98, 4826–4833. [Google Scholar] [CrossRef]
- Chang, Y.; Chung, S.-D.; Wu, C.-H.; Chueh, J.S.; Chen, L.; Lin, P.-C.; Lin, Y.-H.; Huang, K.-H.; Wu, V.-C.; Chu, T.-S. Surgery decreases the long-term incident stroke risk in patients with primary aldosteronism. Surgery 2020, 167, 367–377. [Google Scholar] [CrossRef]
- Seccia, T.M.; Caroccia, B.; Maiolino, G.; Cesari, M.; Rossi, G.P. Arterial Hypertension, Aldosterone, and Atrial Fibrillation. Curr. Hypertens. Rep. 2019, 21, 94. [Google Scholar] [CrossRef]
- Nishikawa, T.; Omura, M.; Satoh, F.; Shibata, H.; Takahashi, K.; Tamura, N.; Tanabe, A. Guidelines for the diagnosis and treatment of primary aldosteronism—The Japan Endocrine Society 2009. Endocr. J. 2011, 58, 711–721. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Gruber, S.; Beuschlein, F. Hypokalemia and the Prevalence of Primary Aldosteronism. Horm. Metab. Res. 2020, 52, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Monticone, S.; Burrello, J.; Tizzani, D.; Bertello, C.; Viola, A.; Buffolo, F.; Gabetti, L.; Mengozzi, G.; Williams, T.A.; Rabbia, F.; et al. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J. Am. Coll. Cardiol. 2017, 69, 1811–1820. [Google Scholar] [CrossRef]
- Rossi, G.P.; Cesari, M.; Cuspidi, C.; Maiolino, G.; Cicala, M.V.; Bisogni, V.; Mantero, F.; Pessina, A.C. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension 2013, 62, 62–69. [Google Scholar] [CrossRef]
- Rosenberg, M.A.; Manning, W.J. Diastolic Dysfunction and Risk of Atrial Fibrillation. Circulation 2012, 126, 2353–2362. [Google Scholar] [CrossRef]
- Born-Frontsberg, E.; Reincke, M.; Rump, L.C.; Hahner, S.; Diederich, S.; Lorenz, R.; Allolio, B.; Seufert, J.; Schirpenbach, C.; Beuschlein, F.; et al. Cardiovascular and cerebrovascular comorbidities of hypokalemic and normokalemic primary aldosteronism: Results of the German Conn’s Registry. J. Clin. Endocrinol. Metab. 2009, 94, 1125–1130. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Chen, Y.-L.; Pan, C.-T.; Lin, Y.-T.; Lee, P.-C.; Chiu, Y.-W.; Liao, C.-W.; Chen, Z.-W.; Chang, C.-C.; Chang, Y.-Y.; et al. New-Onset Atrial Fibrillation in Patients with Primary Aldosteronism Receiving Different Treatment Strategies: Systematic Review and Pooled Analysis of Three Studies. Front. Endocrinol. 2021, 12, 578. [Google Scholar] [CrossRef] [PubMed]
- Mourtzinis, G.; Adamsson Eryd, S.; Rosengren, A.; Björck, L.; Adiels, M.; Johannsson, G.; Manhem, K. Primary aldosteronism and thyroid disorders in atrial fibrillation: A Swedish nationwide case-control study. Eur. J. Prev. Cardiol. 2018, 25, 694–701. [Google Scholar] [CrossRef]
- Seccia, T.M.; Letizia, C.; Muiesan, M.L.; Lerco, S.; Cesari, M.; Bisogni, V.; Petramala, L.; Maiolino, G.; Volpin, R.; Rossi, G.P. Atrial fibrillation as presenting sign of primary aldosteronism: Results of the Prospective Appraisal on the Prevalence of Primary Aldosteronism in Hypertensive (PAPPHY) Study. J. Hypertens. 2020, 38, 332–339. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, B. Association of serum parathyriod hormone and calcium levels with primary aldosteronism: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 14625–14633. [Google Scholar] [PubMed]
- Chhokar, V.S.; Sun, Y.; Bhattacharya, S.K.; Ahokas, R.A.; Myers, L.K.; Xing, Z.; Smith, R.A.; Gerling, I.C.; Weber, K.T. Hyperparathyroidism and the Calcium Paradox of Aldosteronism. Circulation 2005, 111, 871–878. [Google Scholar] [CrossRef]
- Asbach, E.; Bekeran, M.; König, A.; Lang, K.; Hanslik, G.; Treitl, M.; Ladurner, R.; Bidlingmaier, M.; Beuschlein, F.; Quinkler, M.; et al. Primary and Secondary Hyperparathyroidism in Patients with Primary Aldosteronism-Findings from the German Conn’s Registry. Exp. Clin. Endocrinol. Diabetes 2020, 128, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Maniero, C.; Fassina, A.; Guzzardo, V.; Lenzini, L.; Amadori, G.; Pelizzo, M.R.; Gomez-Sanchez, C.; Rossi, G.P. Primary hyperparathyroidism with concurrent primary aldosteronism. Hypertension 2011, 58, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, G.; Aragona, F.; Malendowicz, L.K.; Nussdorfer, G.G. PTH and PTH-related peptide enhance steroid secretion from human adrenocortical cells. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E209–E213. [Google Scholar] [CrossRef] [PubMed]
- Lenzini, L.; Prisco, S.; Vanderriele, P.E.; Lerco, S.; Torresan, F.; Maiolino, G.; Seccia, T.M.; Iacobone, M.; Rossi, G.P. PTH Modulation by Aldosterone and Angiotensin II is Blunted in Hyperaldosteronism and Rescued by Adrenalectomy. J. Clin. Endocrinol. Metab. 2019, 104, 3726–3734. [Google Scholar] [CrossRef] [PubMed]
- Brunaud, L.; Germain, A.; Zarnegar, R.; Rancier, M.; Alrasheedi, S.; Caillard, C.; Ayav, A.; Weryha, G.; Mirallie, E.; Bresler, L. Serum aldosterone is correlated positively to parathyroid hormone (PTH) levels in patients with primary hyperparathyroidism. Surgery 2009, 146, 1035–1041. [Google Scholar] [CrossRef]
- Nelson, J.A.; Alsayed, M.; Milas, M. The role of parathyroidectomy in treating hypertension and other cardiac manifestations of primary hyperparathyroidism. Gland Surg. 2020, 9, 136–141. [Google Scholar] [CrossRef]
- Rossi, G.P.; Ragazzo, F.; Seccia, T.M.; Maniero, C.; Barisa, M.; Calò, L.A.; Frigo, A.C.; Fassina, A.; Pessina, A.C. Hyperparathyroidism Can Be Useful in the Identification of Primary Aldosteronism Due to Aldosterone-Producing Adenoma. Hypertension 2012, 60, 431–436. [Google Scholar] [CrossRef]
- Mosso, L.; Carvajal, C.; González, A.; Barraza, A.; Avila, F.; Montero, J.; Huete, A.; Gederlini, A.; Fardella, C.E. Primary Aldosteronism and Hypertensive Disease. Hypertension 2003, 42, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Burrello, J.; Monticone, S.; Losano, I.; Cavaglià, G.; Buffolo, F.; Tetti, M.; Covella, M.; Rabbia, F.; Veglio, F.; Pasini, B.; et al. Prevalence of Hypokalemia and Primary Aldosteronism in 5100 Patients Referred to a Tertiary Hypertension Unit. Hypertension 2020, 75, 1025–1033. [Google Scholar] [CrossRef]
- Ito, Y.; Takeda, R.; Takeda, Y. Subclinical primary aldosteronism. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 485–495. [Google Scholar] [CrossRef]
- Ramos, E.; Barrio, E.; Moraga, I.; Cuesta, M.; Pazos, M.; Pallares, R.; Saez, d.P.V.; Fernandez, L.; Calle, P.A.; Runkle, I. Indications for aldosterone/renin screening presented by patients later diagnosed with hyperaldosteronism in a general endocrinology outpatient clinic. Endocr. Abstr. 2020, 70, 66. [Google Scholar] [CrossRef]
- Vasan, R.S.; Evans, J.C.; Larson, M.G.; Wilson, P.W.F.; Meigs, J.B.; Rifai, N.; Benjamin, E.J.; Levy, D. Serum Aldosterone and the Incidence of Hypertension in Nonhypertensive Persons. N. Engl. J. Med. 2004, 351, 33–41. [Google Scholar] [CrossRef]
- Rossier, B.C.; Baker, M.E.; Studer, R.A. Epithelial Sodium Transport and Its Control by Aldosterone: The Story of Our Internal Environment Revisited. Physiol. Rev. 2015, 95, 297–340. [Google Scholar] [CrossRef]
- Brown, J.M.; Siddiqui, M.; Calhoun, D.A.; Carey, R.M.; Hopkins, P.N.; Williams, G.H.; Vaidya, A. The Unrecognized Prevalence of Primary Aldosteronism. Ann. Intern. Med. 2020, 173, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Markou, A.; Pappa, T.; Kaltsas, G.; Gouli, A.; Mitsakis, K.; Tsounas, P.; Prevoli, A.; Tsiavos, V.; Papanastasiou, L.; Zografos, G.; et al. Evidence of Primary Aldosteronism in a Predominantly Female Cohort of Normotensive Individuals: A Very High Odds Ratio for Progression into Arterial Hypertension. J. Clin. Endocrinol. Metab. 2013, 98, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Baudrand, R.; Guarda, F.J.; Fardella, C.; Hundemer, G.; Brown, J.; Williams, G.; Vaidya, A. Continuum of Renin-Independent Aldosteronism in Normotension. Hypertension 2017, 69, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou-Athanassiou, I.; Athanassiou, L.; Spyropoulos, P.; Xanthakou, E.; Fortis, A.; Kalogirou, T.; Athanassiou, P. Primary hyperaldosteronism. A clinical profile of the disease without arterial hypertension. Endocr. Abstr. 2021, 73, 68. [Google Scholar] [CrossRef]
- Tanabe, A.; Naruse, M.; Takagi, S.; Tsuchiya, K.; Imaki, T.; Takano, K. Variability in the Renin/Aldosterone Profile under Random and Standardized Sampling Conditions in Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2003, 88, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Stowasser, M.; Ahmed, A.H.; Pimenta, E.; Taylor, P.J.; Gordon, R.D. Factors affecting the aldosterone/renin ratio. Horm. Metab. Res. 2012, 44, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.L.; Turner, S.T. Screening for Primary Aldosteronism in Essential Hypertension: Diagnostic Accuracy of the Ratio of Plasma Aldosterone Concentration to Plasma Renin Activity. Clin. Chem. 2005, 51, 386–394. [Google Scholar] [CrossRef]
- Łebek-Szatańska, A.; Papierska, L.; Glinicki, P.; Zgliczyński, W. Withdrawal of all medications is not necessary for accurate primary aldosteronism screening—Preliminary results. Pol. Arch. Intern. Med. 2021, 131, 578–581. [Google Scholar] [CrossRef]
- Rossi, G.P.; Ceolotto, G.; Rossitto, G.; Maiolino, G.; Cesari, M.; Seccia, T.M. Effects of Mineralocorticoid and AT1 Receptor Antagonism on The Aldosterone-Renin Ratio in Primary Aldosteronism—The EMIRA Study. J. Clin. Endocrinol. Metab. 2020, 105, 2060–2067. [Google Scholar] [CrossRef]
- Stowasser, M.; Gordon, R.D. Primary aldosteronism—Careful investigation is essential and rewarding. Mol. Cell. Endocrinol. 2004, 217, 33–39. [Google Scholar] [CrossRef]
- Piazza, M.; Seccia, T.M.; Caroccia, B.; Rossitto, G.; Scarpa, R.; Persichitti, P.; Basso, D.; Rossi, G.P. AT1AA (Angiotensin II Type-1 Receptor Autoantibodies). Hypertension 2019, 74, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Kawamura, M.; Onodera, S.; Hiramori, K. Controlled study of circadian rhythm of blood pressure in patients with aldosterone-producing adenoma compared with those with essential hypertension. J. Hypertens. 2000, 18, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.-C.; Chang, H.-W.; Liu, K.-L.; Lin, Y.-H.; Chueh, S.-C.; Lin, W.-C.; Ho, Y.-L.; Huang, J.-W.; Chiang, C.-K.; Yang, S.-Y.; et al. Primary Aldosteronism: Diagnostic Accuracy of the Losartan and Captopril Tests. Am. J. Hypertens. 2009, 22, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, W.R.; New, M.I.; Coolidge, C.; Lifton, R.P.; Dluhy, R.G. Evaluation of the Dexamethasone Suppression Test for the Diagnosis of Glucocorticoid-Remediable Aldosteronism1. J. Clin. Endocrinol. Metab. 1997, 82, 3570–3573. [Google Scholar] [CrossRef][Green Version]
- Tsiavos, V.; Markou, A.; Papanastasiou, L.; Kounadi, T.; Androulakis, I.I.; Voulgaris, N.; Zachaki, A.; Kassi, E. A new highly sensitive and specific overnight combined screening and diagnostic test for primary aldosteronism. Eur. J. Endocrinol. 2016, 175, 21–28. [Google Scholar] [CrossRef]
- Alexandraki, K.I.; Markou, A.; Papanastasiou, L.; Tyfoxylou, E.; Kapsali, C.; Gravvanis, C.; Katsiveli, P.; Kaltsas, G.A.; Zografos, G.N.; Chrousos, G.P.; et al. Surgical treatment outcome of primary aldosteronism assessed using new modified diagnostic tests. Hormones 2021, 20, 359–368. [Google Scholar] [CrossRef]
- Rossi, G.P.; Belfiore, A.; Bernini, G.; Desideri, G.; Fabris, B.; Ferri, C.; Giacchetti, G.; Letizia, C.; Maccario, M.; Mallamaci, F.; et al. Comparison of the Captopril and the Saline Infusion Test for Excluding Aldosterone-Producing Adenoma. Hypertension 2007, 50, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yang, J.; Hu, J.; Song, Y.; He, W.; Yang, S.; Luo, R.; Li, Q. Confirmatory tests for the diagnosis of primary aldosteronism: A systematic review and meta-analysis. Clin. Endocrinol. 2019, 90, 641–648. [Google Scholar] [CrossRef]
- Xiang, Q.; Wang, W.; Chen, T.; Yu, K.; Li, Q.; Zhang, T.; Tian, H.; Ren, Y. The value of the post-captopril aldosterone/renin ratio for the diagnosis of primary aldosteronism and the influential factors: A meta-analysis. J. Renin. Angiotensin. Aldosterone. Syst. 2020, 21, 1470320320972032. [Google Scholar] [CrossRef] [PubMed]
- Lyons, D.F.; Kem, D.C.; Brown, R.D.; Hanson, C.S.; Carollo, M.L. Single dose captopril as a diagnostic test for primary aldosteronism. J. Clin. Endocrinol. Metab. 1983, 57, 892–896. [Google Scholar] [CrossRef] [PubMed]
| 2009-JES-Guidelines [39] | 2016-ES-Guidelines [20] | 2020-ESH-Consensus [3] |
|---|---|---|
| All patients with hypertension |
|
|
| Effect in Renin Secretion | Effect in Aldosterone Secretion | ARR | |
|---|---|---|---|
| Drugs | |||
| ACEi | ↑↑ | ↓ | ↓ |
| ARB | ↑↑ | ↓ | ↓ |
| MRB | ↑↑↑ | ↑↑ | ↓ |
| Diuretics | ↑↑↑ | ↑↑ | ↓ |
| Amiloride | |||
| Thiazide | |||
| Loop diuretics | |||
| Ca++-channel antagonist * | ↑ | N/↓ * | N/↓ |
| NSAI | ↓ | ↓ | N/↑ |
| β-blockers | ↓ | ↓ | N/↑ |
| Clonidine | ↓ | ↓ | N/↑ |
| Methyldopa | ↓ | ↓ | N/↑ |
| Kalemia | |||
| Hyperkalemia | N | ↑↑↑ | ↑ |
| Hypokalemia | N | ↓↓↓ | ↓ |
| Physiological | |||
| Menstrual cycle | |||
| Follicular phase | N | N | N |
| Ovulation | ↑ | ↑ | N |
| Luteal phase | ↑ | ↑↑ | ↑ |
| Pregnancy | ↑↑↑ | ↑↑ | ↓ |
| Orthostatism | ↑↑ | ↑↑ | ↓ |
| Salt intake | |||
| Low-salt diet | ↑↑↑ | ↑↑ | ↓ |
| High-salt diet | ↓↓↓ | ↓↓ | ↓ |
| Captopril Challenge Test (*) | Oral Salt Loading | Intravenous Saline Loading | |
|---|---|---|---|
| Methodology | Preparation: Correction of hypokalemia Modification of drugs interfering with the RAAS | ||
| Salt intake during the 3 previous days should be at least 7.6 g/d when possible [80]. Keep in sitting position from at least 20–30 min before to the end of the test. Test should be performed before 9 a.m. | Salt intake should be increased for at least 3 days before urine collection to reach at least 11.7 g/d. | Increased salt intake is not necessary. Keeping a sitting position from at least 30 min before and during test. Test should be performed before 9 a.m. | |
| Procedure: Oral administration of Captopril. Blood testing at basal and 60–90 min (with captopril 50 mg), or basal an 2 h (with captopril 25 mg) | Procedure: After 3 days of a high-salt diet, 24 h urine collection is performed. | Procedure: Infusion of 2 L of NaCl 0.9% through 4 h. Blood testing at basal and 4 h | |
| Diagnostic criteria (*) | At the end of the test: PAC ≥ 12 ng/dL, or ARR ≥ 50 ng/dL/mL/h (≥5 in ng/dL * pg/mL), or decrement of PAC above 30% as compared to baseline. | Urinary aldosterone ≥12 µg/24 h with urinary Na ≥ 200 mmol/24 h. | At 4 h post infusion: PAC ≥ 6 ng/dL. |
| Contraindication | Allergy to ACEi | Active heart failure Hypokalemia Uncontrolled hypertension Coronary disease | |
| Precaution | Blood pressure could drop. | Hypokalemia could occur in next 48 h after salt loading is begun. Heart failure might be triggered. Blood pressure could increase during salt loading. | |
| Hospitalization Required | No | No | Yes (ambulatory hospitalization) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Sánchez, J.G.; Pazos Guerra, M.; Meneses, D.; Runkle, I. Primary Hyperaldosteronism: When to Suspect It and How to Confirm Its Diagnosis. Endocrines 2022, 3, 29-42. https://doi.org/10.3390/endocrines3010003
Ruiz-Sánchez JG, Pazos Guerra M, Meneses D, Runkle I. Primary Hyperaldosteronism: When to Suspect It and How to Confirm Its Diagnosis. Endocrines. 2022; 3(1):29-42. https://doi.org/10.3390/endocrines3010003
Chicago/Turabian StyleRuiz-Sánchez, Jorge Gabriel, Mario Pazos Guerra, Diego Meneses, and Isabelle Runkle. 2022. "Primary Hyperaldosteronism: When to Suspect It and How to Confirm Its Diagnosis" Endocrines 3, no. 1: 29-42. https://doi.org/10.3390/endocrines3010003
APA StyleRuiz-Sánchez, J. G., Pazos Guerra, M., Meneses, D., & Runkle, I. (2022). Primary Hyperaldosteronism: When to Suspect It and How to Confirm Its Diagnosis. Endocrines, 3(1), 29-42. https://doi.org/10.3390/endocrines3010003