Dynamic Expression of the Homeobox Factor PBX1 during Mouse Testis Development †
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture
2.3. RNA Preparation and PCR
2.4. Immunohistochemistry
2.5. Immunofluorescence
2.6. Protein Purification and Western Blots
3. Results
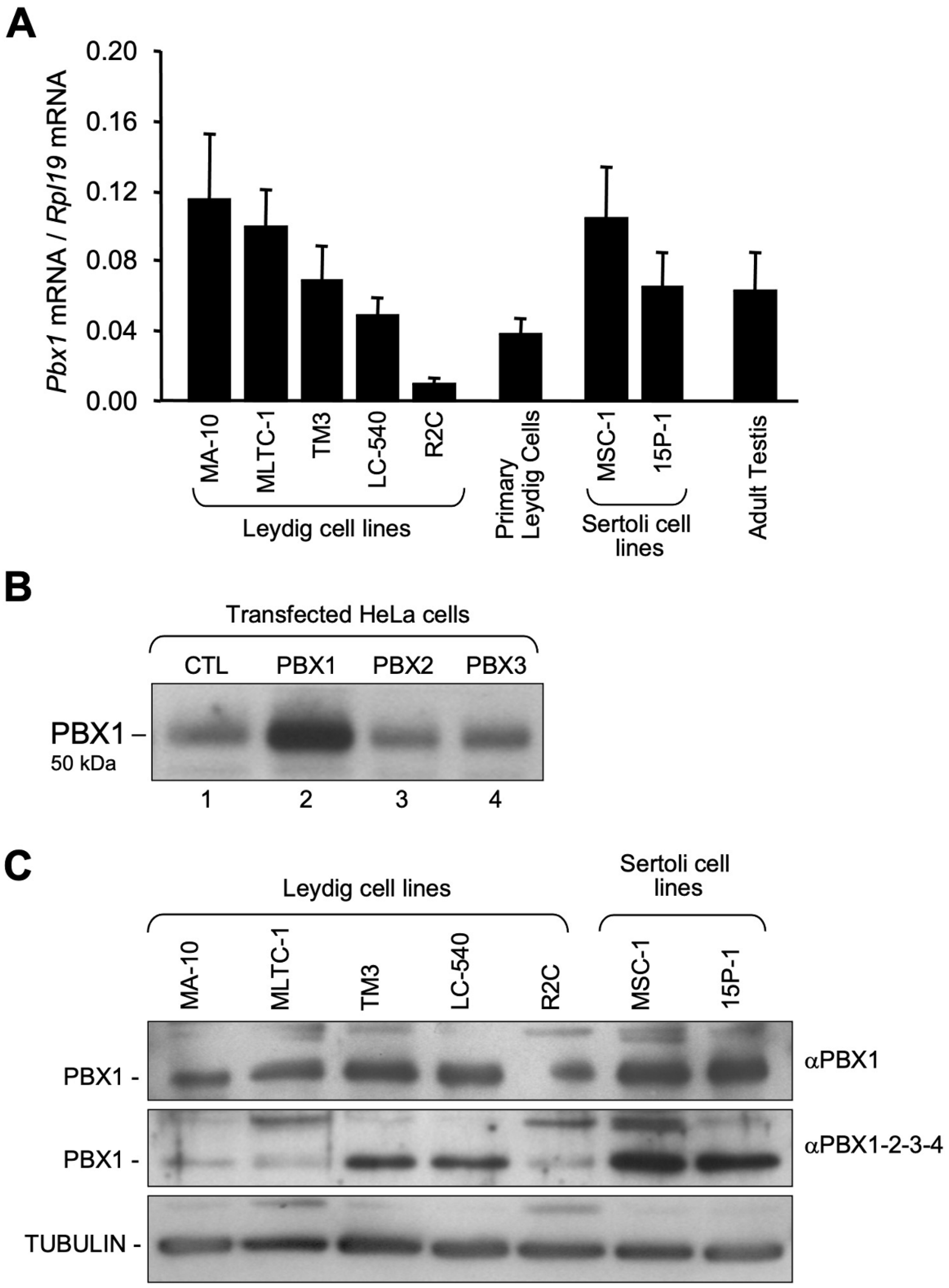
3.1. Pbx1 mRNA and Protein in Leydig and Sertoli Cells
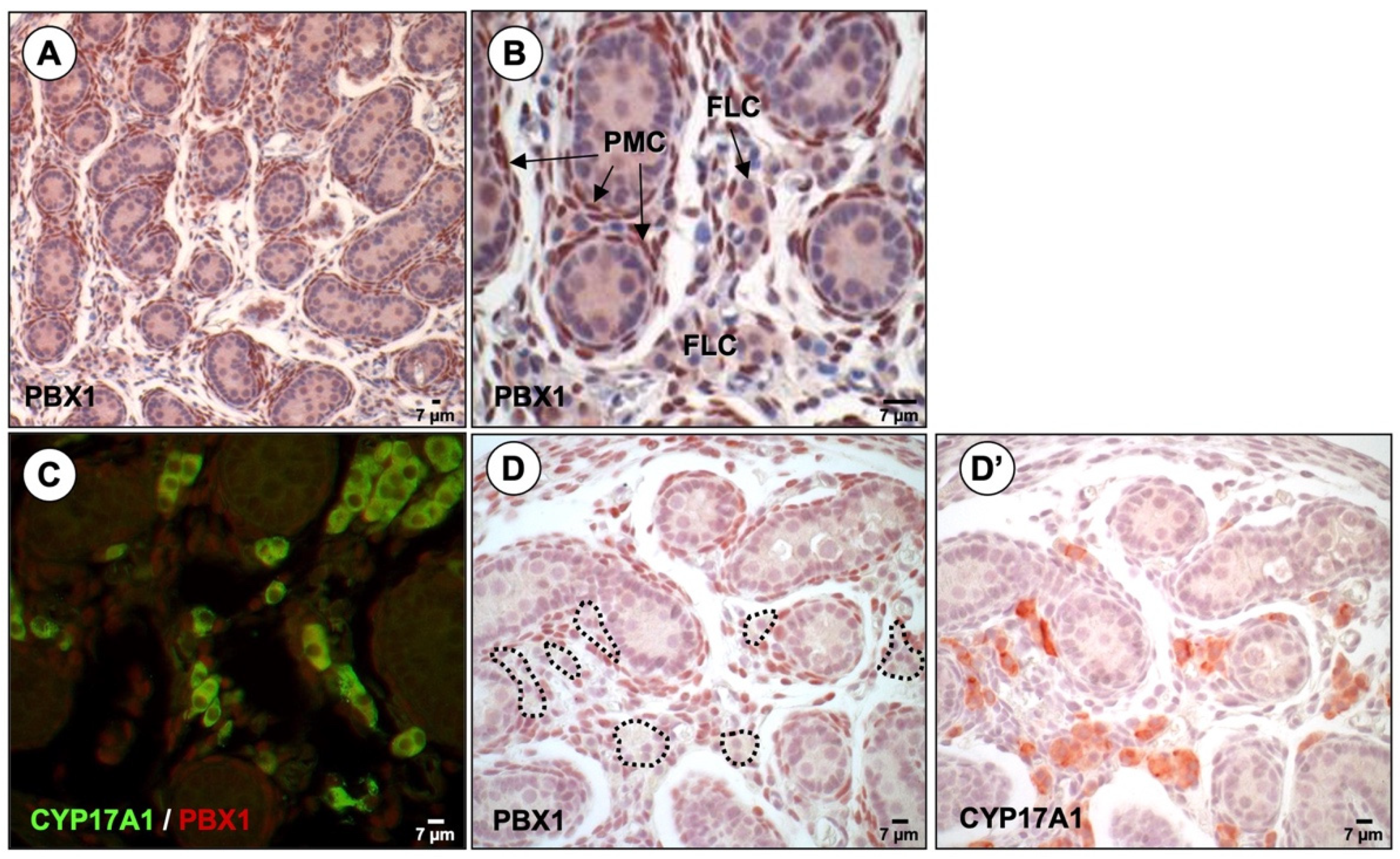
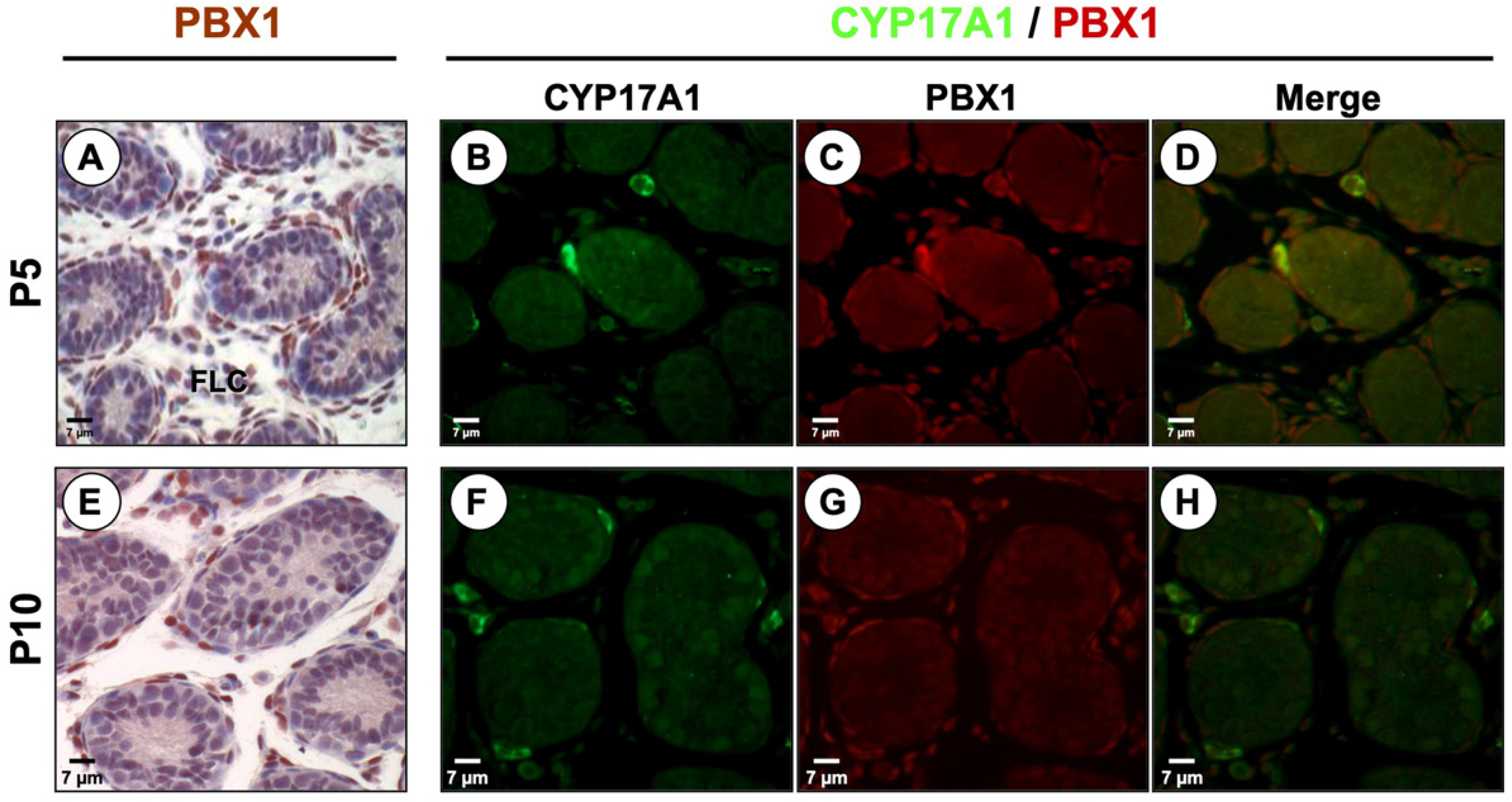
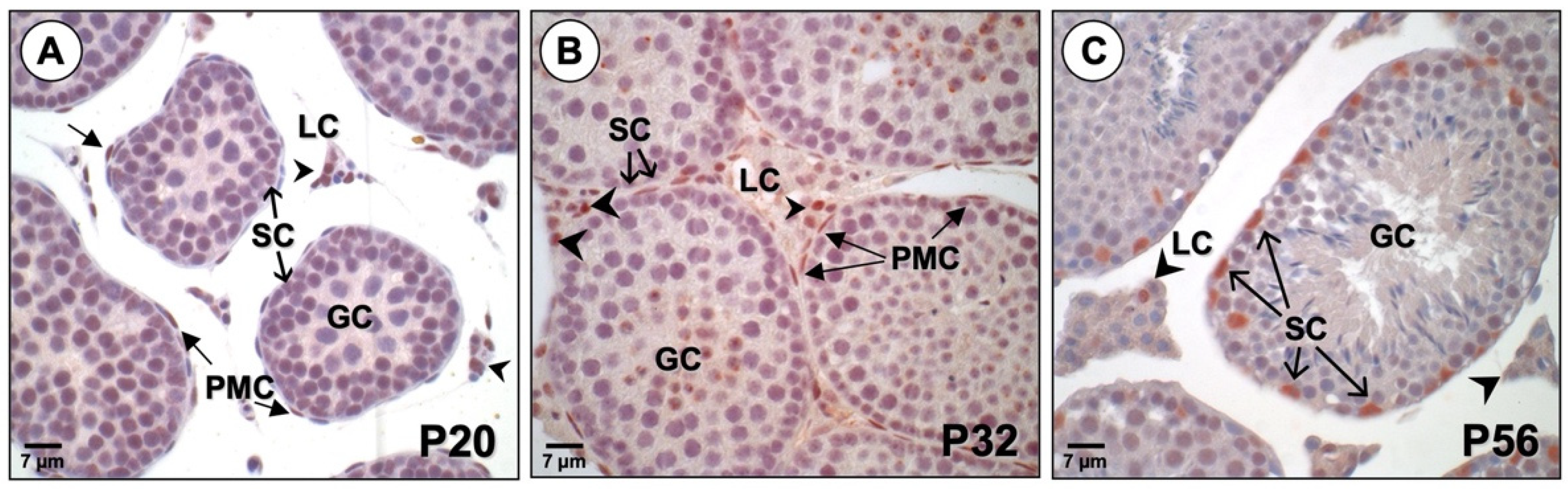
3.2. PBX1 Is Dynamically Expressed in the Mouse Testis throughout Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hacker, A.; Capel, B.; Goodfellow, P.; Lovell-Badge, R. Expression of Sry, the mouse sex determining gene. Development 1995, 121, 1603–1614. [Google Scholar] [CrossRef]
- Goodfellow, P.N.; Lovell-Badge, R. SRY and sex determination in mammals. Annu. Rev. Genet. 1993, 27, 71–92. [Google Scholar] [CrossRef]
- Josso, N.; Picard, J.Y.; Rey, R.; di Clemente, N. Testicular anti-Mullerian hormone: History, genetics, regulation and clinical applications. Pediatr. Endocrinol. Rev. 2006, 3, 347–358. [Google Scholar]
- Teixeira, J.; Maheswaran, S.; Donahoe, P.K. Mullerian inhibiting substance: An instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr. Rev. 2001, 22, 657–674. [Google Scholar] [CrossRef]
- Josso, N.; Picard, J.Y.; Imbeaud, S.; di Clemente, N.; Rey, R. Clinical aspects and molecular genetics of the persistent mullerian duct syndrome. Clin. Endocrinol. 1997, 47, 137–144. [Google Scholar] [CrossRef]
- Nef, S.; Parada, L.F. Cryptorchidism in mice mutant for Insl3. Nat. Genet. 1999, 22, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Steding, G.; Emmen, J.M.; Brinkmann, A.O.; Nayernia, K.; Holstein, A.F.; Engel, W.; Adham, I.M. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol. Endocrinol. 1999, 13, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Pepe, A.; Gianesello, L.; Garolla, A.; Feng, S.; Giannini, S.; Zaccolo, M.; Facciolli, A.; Morello, R.; Agoulnik, A.I.; et al. Mutations in the insulin-like factor 3 receptor are associated with osteoporosis. J. Bone Miner. Res. 2008, 23, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Ingraham, H.A.; Lala, D.S.; Ikeda, Y.; Luo, X.; Shen, W.H.; Nachtigal, M.W.; Abbud, R.; Nilson, J.H.; Parker, K.L. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994, 8, 2302–2312. [Google Scholar] [CrossRef]
- Manuylov, N.L.; Zhou, B.; Ma, Q.; Fox, S.C.; Pu, W.T.; Tevosian, S.G. Conditional ablation of Gata4 and Fog2 genes in mice reveals their distinct roles in mammalian sexual differentiation. Dev. Biol. 2011, 353, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.C.; Okumura, L.M.; Page, D.C. Gata4 is required for formation of the genital ridge in mice. PLoS Genet. 2013, 9, e1003629. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.W.; Dominguez-Steglich, M.A.; Guioli, S.; Kowk, G.; Weller, P.A.; Stevanovic, M.; Weissenbach, J.; Mansour, S.; Young, I.D.; Goodfellow, P.N.; et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 1994, 372, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Meeks, J.J.; Weiss, J.; Jameson, J.L. Dax1 is required for testis determination. Nat. Genet. 2003, 34, 32–33. [Google Scholar] [CrossRef]
- Kamps, M.P.; Murre, C.; Sun, X.H.; Baltimore, D. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell 1990, 60, 547–555. [Google Scholar] [CrossRef]
- Monica, K.; Galili, N.; Nourse, J.; Saltman, D.; Cleary, M.L. PBX2 and PBX3, new homeobox genes with extensive homology to the human proto-oncogene PBX1. Mol. Cell Biol. 1991, 11, 6149–6157. [Google Scholar]
- Wagner, K.; Mincheva, A.; Korn, B.; Lichter, P.; Popperl, H. Pbx4, a new Pbx family member on mouse chromosome 8, is expressed during spermatogenesis. Mech. Dev. 2001, 103, 127–131. [Google Scholar] [CrossRef]
- Selleri, L.; Zappavigna, V.; Ferretti, E. ‘Building a perfect body’: Control of vertebrate organogenesis by PBX-dependent regulatory networks. Genes Dev. 2019, 33, 258–275. [Google Scholar] [CrossRef]
- Shimamoto, T.; Ohyashiki, K.; Toyama, K.; Takeshita, K. Homeobox genes in hematopoiesis and leukemogenesis. Int. J. Hematol. 1998, 67, 339–350. [Google Scholar] [CrossRef]
- Schnabel, C.A.; Selleri, L.; Cleary, M.L. Pbx1 is essential for adrenal development and urogenital differentiation. Genesis 2003, 37, 123–130. [Google Scholar] [CrossRef]
- Selleri, L.; Depew, M.J.; Jacobs, Y.; Chanda, S.K.; Tsang, K.Y.; Cheah, K.S.; Rubenstein, J.L.; O’Gorman, S.; Cleary, M.L. Requirement for Pbx1 in skeletal patterning and programming chondrocyte proliferation and differentiation. Development 2001, 128, 3543–3557. [Google Scholar] [CrossRef]
- Nourse, J.; Mellentin, J.D.; Galili, N.; Wilkinson, J.; Stanbridge, E.; Smith, S.D.; Cleary, M.L. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell 1990, 60, 535–545. [Google Scholar] [CrossRef]
- DiMartino, J.F.; Selleri, L.; Traver, D.; Firpo, M.T.; Rhee, J.; Warnke, R.; O’Gorman, S.; Weissman, I.L.; Cleary, M.L. The Hox cofactor and proto-oncogene Pbx1 is required for maintenance of definitive hematopoiesis in the fetal liver. Blood 2001, 98, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Selleri, L.; DiMartino, J.; van Deursen, J.; Brendolan, A.; Sanyal, M.; Boon, E.; Capellini, T.; Smith, K.S.; Rhee, J.; Popperl, H.; et al. The TALE homeodomain protein Pbx2 is not essential for development and long-term survival. Mol. Cell Biol. 2004, 24, 5324–5331. [Google Scholar] [CrossRef]
- Di Giacomo, G.; Koss, M.; Capellini, T.D.; Brendolan, A.; Popperl, H.; Selleri, L. Spatio-temporal expression of Pbx3 during mouse organogenesis. Gene. Expr. Patterns 2006, 6, 747–757. [Google Scholar] [CrossRef]
- Rhee, J.W.; Arata, A.; Selleri, L.; Jacobs, Y.; Arata, S.; Onimaru, H.; Cleary, M.L. Pbx3 deficiency results in central hypoventilation. Am. J. Pathol. 2004, 165, 1343–1350. [Google Scholar] [CrossRef]
- Longobardi, E.; Penkov, D.; Mateos, D.; De Florian, G.; Torres, M.; Blasi, F. Biochemistry of the tale transcription factors PREP, MEIS, and PBX in vertebrates. Dev. Dyn. 2014, 243, 59–75. [Google Scholar] [CrossRef]
- Mann, R.S.; Chan, S.K. Extra specificity from extradenticle: The partnership between HOX and PBX/EXD homeodomain proteins. Trends Genet. 1996, 12, 258–262. [Google Scholar] [CrossRef]
- Mann, R.S.; Affolter, M. Hox proteins meet more partners. Curr. Opin. Genet. Dev. 1998, 8, 423–429. [Google Scholar] [CrossRef]
- Moens, C.B.; Selleri, L. Hox cofactors in vertebrate development. Dev. Biol. 2006, 291, 193–206. [Google Scholar] [CrossRef]
- Berkes, C.A.; Bergstrom, D.A.; Penn, B.H.; Seaver, K.J.; Knoepfler, P.S.; Tapscott, S.J. Pbx marks genes for activation by MyoD indicating a role for a homeodomain protein in establishing myogenic potential. Mol. Cell 2004, 14, 465–477. [Google Scholar] [CrossRef]
- Knoepfler, P.S.; Bergstrom, D.A.; Uetsuki, T.; Dac-Korytko, I.; Sun, Y.H.; Wright, W.E.; Tapscott, S.J.; Kamps, M.P. A conserved motif N-terminal to the DNA-binding domains of myogenic bHLH transcription factors mediates cooperative DNA binding with pbx-Meis1/Prep1. Nucleic Acids Res. 1999, 27, 3752–3761. [Google Scholar] [CrossRef] [PubMed]
- Kia, F.; Sarafoglou, K.; Mooganayakanakote Siddappa, A.; Roberts, K.D. Partial gonadal dysgenesis associated with a pathogenic variant of PBX1 transcription factor. BMJ Case Rep. 2019, 12, e227986. [Google Scholar] [CrossRef]
- Eozenou, C.; Bashamboo, A.; Bignon-Topalovic, J.; Merel, T.; Zwermann, O.; Lourenco, D.; Lottmann, H.; Lichtenauer, U.; Rojo, S.; Beuschlein, F.; et al. The TALE homeodomain of PBX1 is involved in human primary testis-determination. Hum. Mutat. 2019, 40, 1071–1076. [Google Scholar] [CrossRef]
- Moisan, V.; Bomgardner, D.; Tremblay, J.J. Expression of the Ladybird-like homeobox 2 transcription factor in the developing mouse testis and epididymis. BMC Dev. Biol. 2008, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Moisan, V.; Robert, N.M.; Tremblay, J.J. Expression of ladybird-like homeobox 2 (LBX2) during ovarian development and folliculogenesis in the mouse. J. Mol. Histol. 2010, 41, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Garon, G.; Bergeron, F.; Brousseau, C.; Robert, N.M.; Tremblay, J.J. FOXA3 is expressed in multiple cell lineages in the mouse testis and regulates Pdgfra expression in Leydig cells. Endocrinology 2017, 158, 1886–1897. [Google Scholar] [CrossRef]
- Martin, L.J.; Boucher, N.; Brousseau, C.; Tremblay, J.J. The orphan nuclear receptor NUR77 regulates hormone-induced StAR transcription in Leydig cells through a cooperation with CaMKI. Mol. Endocrinol. 2008, 22, 2021–2037. [Google Scholar] [CrossRef]
- Mendoza-Villarroel, R.E.; Di-Luoffo, M.; Camire, E.; Giner, X.C.; Brousseau, C.; Tremblay, J.J. The INSL3 gene is a direct target for the orphan nuclear receptor, COUP-TFII, in Leydig cells. J. Mol. Endocrinol. 2014, 53, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Villarroel, R.E.; Robert, N.M.; Martin, L.J.; Brousseau, C.; Tremblay, J.J. The nuclear receptor NR2F2 activates Star expression and steroidogenesis in mouse MA-10 and MLTC-1 Leydig cells. Biol. Reprod. 2014, 91, 26. [Google Scholar] [CrossRef]
- Laguë, E.; Tremblay, J.J. Antagonistic effects of testosterone and the endocrine disruptor mono-(2-ethylhexyl) phthalate on INSL3 transcription in Leydig cells. Endocrinology 2008, 149, 4688–4694. [Google Scholar] [CrossRef]
- Ascoli, M. Characterization of several clonal lines of cultured Leydig tumor cells: Gonadotropin receptors and steroidogenic responses. Endocrinology 1981, 108, 88–95. [Google Scholar] [CrossRef]
- McGuinness, M.P.; Linder, C.C.; Morales, C.R.; Heckert, L.L.; Pikus, J.; Griswold, M.D. Relationship of a mouse Sertoli cell line (MSC-1) to normal Sertoli cells. Biol. Reprod. 1994, 51, 116–124. [Google Scholar] [CrossRef]
- Chari, R.; Lonergan, K.M.; Pikor, L.A.; Coe, B.P.; Zhu, C.Q.; Chan, T.H.; MacAulay, C.E.; Tsao, M.S.; Lam, S.; Ng, R.T.; et al. A sequence-based approach to identify reference genes for gene expression analysis. BMC Med. Genom. 2010, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lim, Q.E.; Wan, G.; Too, H.P. Normalization with genes encoding ribosomal proteins but not GAPDH provides an accurate quantification of gene expressions in neuronal differentiation of PC12 cells. BMC Genom. 2010, 11, 75. [Google Scholar] [CrossRef]
- Chiao, Y.C.; Cho, W.L.; Wang, P.S. Inhibition of testosterone production by propylthiouracil in rat Leydig cells. Biol. Reprod. 2002, 67, 416–422. [Google Scholar] [CrossRef]
- Ronen-Fuhrmann, T.; Timberg, R.; King, S.R.; Hales, K.H.; Hales, D.B.; Stocco, D.M.; Orly, J. Spatio-temporal expression patterns of steroidogenic acute regulatory protein (StAR) during follicular development in the rat ovary. Endocrinology 1998, 139, 303–315. [Google Scholar] [CrossRef]
- Tremblay, M.A.; Mendoza-Villarroel, R.E.; Robert, N.M.; Bergeron, F.; Tremblay, J.J. KLF6 cooperates with NUR77 and SF1 to activate the human INSL3 promoter in mouse MA-10 leydig cells. J. Mol. Endocrinol. 2016, 56, 163–173. [Google Scholar] [CrossRef][Green Version]
- Chen, C.; Okayama, H. High efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell Biol. 1987, 7, 2745–2752. [Google Scholar] [PubMed]
- Schreiber, E.; Matthias, P.; Muller, M.M.; Schaffner, W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989, 17, 6419. [Google Scholar] [CrossRef]
- Dubé, C.; Bergeron, F.; Vaillant, M.J.; Robert, N.M.; Brousseau, C.; Tremblay, J.J. The nuclear receptors SF-1 and LRH-1 are expressed in endometrial cancer cells and regulate steroidogenic gene transcription by cooperating with AP-1 factors. Cancer Lett. 2009, 275, 127–138. [Google Scholar] [CrossRef]
- Ascoli, M. Immortalized Leydig cell lines as models for studying Leydig cell physiology. In The Leydig Cell in Health and Disease, 2nd ed.; Payne, A.H., Hardy, M.P., Eds.; Humana Press: Totowa, NJ, USA, 2007; pp. 373–381. [Google Scholar]
- Rahman, N.A.; Huhtaniemi, I.T. Testicular cell lines. Mol. Cell Endocrinol. 2004, 228, 53–65. [Google Scholar] [CrossRef]
- Rebois, R.V. Establishment of gonadotropin-responsive murine leydig tumor cell line. J. Cell Biol. 1982, 94, 70–76. [Google Scholar] [CrossRef]
- Shin, S.I.; Yasumura, Y.; Sato, G.H. Studies on interstitial cells in tissue culture. II. Steroid biosynthesis by a clonal line of rat testicular interstitial cells. Endocrinology 1968, 82, 614–616. [Google Scholar] [CrossRef]
- Jacobs, B.B.; Huseby, R.A. Transplantable Leydig cell tumors in Fischer rats: Horomone responsivity and hormone production. J. Natl. Cancer Inst. 1968, 41, 1141–1153. [Google Scholar]
- Mather, J.P. Establishment and characterization of two distinct mouse testicular epithelial cell lines. Biol. Reprod. 1980, 23, 243–252. [Google Scholar]
- Peschon, J.J.; Behringer, R.R.; Cate, R.L.; Harwood, K.A.; Idzerda, R.L.; Brinster, R.L.; Palmiter, R.D. Directed expression of an oncogene to Sertoli cells in transgenic mice using Müllerian inhibiting substance regulatory sequences. Mol. Endocrinol. 1992, 6, 1403–1411. [Google Scholar]
- Paquis-Flucklinger, V.; Michiels, J.F.; Vidal, F.; Alquier, C.; Pointis, G.; Bourdon, V.; Cuzin, F.; Rassoulzadegan, M. Expression in transgenic mice of the large T antigen of polyomavirus induces Sertoli cell tumours and allows the establishment of differentiated cell lines. Oncogene 1993, 8, 2087–2094. [Google Scholar]
- Berthelsen, J.; Zappavigna, V.; Mavilio, F.; Blasi, F. Prep1, a novel functional partner of Pbx proteins. EMBO J. 1998, 17, 1423–1433. [Google Scholar] [CrossRef]
- Greco, T.L.; Payne, A.H. Ontogeny of expression of the genes for steroidogenic enzymes P450 side-chain cleavage, 3 beta-hydroxysteroid dehydrogenase, P450 17 alpha-hydroxylase/C17-20 lyase, and P450 aromatase in fetal mouse gonads. Endocrinology 1994, 135, 262–268. [Google Scholar] [CrossRef]
- Zhang, P.; Compagnone, N.A.; Fiore, C.; Vigne, J.L.; Culp, P.; Musci, T.J.; Mellon, S.H. Developmental gonadal expression of the transcription factor SET and its target gene, P450c17 (17alpha-hydroxylase/c17,20 lyase). DNA Cell Biol. 2001, 20, 613–624. [Google Scholar] [CrossRef]
- Roberts, V.J.; van Dijk, M.A.; Murre, C. Localization of Pbx1 transcripts in developing rat embryos. Mech. Dev. 1995, 51, 193–198. [Google Scholar] [CrossRef]
- Schnabel, C.A.; Selleri, L.; Jacobs, Y.; Warnke, R.; Cleary, M.L. Expression of Pbx1b during mammalian organogenesis. Mech. Dev. 2001, 100, 131–135. [Google Scholar] [CrossRef]
- Buehr, M.; Gu, S.; McLaren, A. Mesonephric contribution to testis differentiation in the fetal mouse. Development 1993, 117, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Davidoff, M.S.; Middendorff, R.; Enikolopov, G.; Riethmacher, D.; Holstein, A.F.; Muller, D. Progenitor cells of the testosterone-producing Leydig cells revealed. J. Cell Biol. 2004, 167, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Merchant-Larios, H.; Moreno-Mendoza, N. Mesonephric stromal cells differentiate into Leydig cells in the mouse fetal testis. Exp. Cell Res. 1998, 244, 230–238. [Google Scholar] [CrossRef]
- Nishino, K.; Yamanouchi, K.; Naito, K.; Tojo, H. Characterization of mesonephric cells that migrate into the XY gonad during testis differentiation. Exp. Cell Res. 2001, 267, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, I.B.; Kaur, J.; Ge, R.S.; Cooke, P.S.; Yao, H.H. Dynamic changes in fetal Leydig cell populations influence adult Leydig cell populations in mice. FASEB J. 2013, 27, 2657–2666. [Google Scholar] [CrossRef]
- Chen, H.; Ge, R.S.; Zirkin, B.R. Leydig cells: From stem cells to aging. Mol. Cell Endocrinol. 2009, 306, 9–16. [Google Scholar] [CrossRef]
- Haider, S.G. Cell biology of Leydig cells in the testis. Int. Rev. Cytol. 2004, 233, 181–241. [Google Scholar]
- Landreh, L.; Spinnler, K.; Schubert, K.; Hakkinen, M.R.; Auriola, S.; Poutanen, M.; Soder, O.; Svechnikov, K.; Mayerhofer, A. Human testicular peritubular cells host putative stem leydig cells with steroidogenic capacity. J. Clin. Endocrinol. Metab. 2014, 99, E1227–E1235. [Google Scholar] [CrossRef]
- Kilcoyne, K.R.; Smith, L.B.; Atanassova, N.; Macpherson, S.; McKinnell, C.; van den Driesche, S.; Jobling, M.S.; Chambers, T.J.; De Gendt, K.; Verhoeven, G.; et al. Fetal programming of adult Leydig cell function by androgenic effects on stem/progenitor cells. Proc. Natl. Acad. Sci. USA 2014, 111, E1924–E1932. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.S.; Dong, Q.; Sottas, C.M.; Papadopoulos, V.; Zirkin, B.R.; Hardy, M.P. In search of rat stem Leydig cells: Identification, isolation, and lineage-specific development. Proc. Natl. Acad. Sci. USA 2006, 103, 2719–2724. [Google Scholar] [CrossRef]
- Ge, R.; Hardy, M.P. Regulation of Leydig cells during pubertal development. In The Leydig Cell in Health and Disease, 2nd ed.; Payne, A.H., Hardy, M.P., Eds.; Humana Press: Totowa, NJ, USA, 2007; pp. 55–70. [Google Scholar]
- Chen, P.; Zirkin, B.R.; Chen, H. Stem Leydig Cells in the Adult Testis: Characterization, Regulation and Potential Applications. Endocr. Rev. 2020, 41, 22–32. [Google Scholar] [CrossRef]
- Mendis-Handagama, S.M.; Ariyaratne, H.B. Differentiation of the adult Leydig cell population in the postnatal testis. Biol. Reprod. 2001, 65, 660–671. [Google Scholar] [CrossRef]
- Gondos, B.; Berndtson, W.E. Postnatal and pubertal development. In The Sertoli Cell; Russell, L.D., Griswold, M.D., Eds.; Cache River Press: Clearwater, FL, USA, 1993; pp. 115–154. [Google Scholar]
- Shah, W.; Khan, R.; Shah, B.; Khan, A.; Dil, S.; Liu, W.; Wen, J.; Jiang, X. The Molecular Mechanism of Sex Hormones on Sertoli Cell Development and Proliferation. Front. Endocrinol. 2021, 12, 648141. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moisan, V.; Brousseau, C.; Tremblay, J.J. Dynamic Expression of the Homeobox Factor PBX1 during Mouse Testis Development. Endocrines 2022, 3, 16-28. https://doi.org/10.3390/endocrines3010002
Moisan V, Brousseau C, Tremblay JJ. Dynamic Expression of the Homeobox Factor PBX1 during Mouse Testis Development. Endocrines. 2022; 3(1):16-28. https://doi.org/10.3390/endocrines3010002
Chicago/Turabian StyleMoisan, Vanessa, Catherine Brousseau, and Jacques J. Tremblay. 2022. "Dynamic Expression of the Homeobox Factor PBX1 during Mouse Testis Development" Endocrines 3, no. 1: 16-28. https://doi.org/10.3390/endocrines3010002
APA StyleMoisan, V., Brousseau, C., & Tremblay, J. J. (2022). Dynamic Expression of the Homeobox Factor PBX1 during Mouse Testis Development. Endocrines, 3(1), 16-28. https://doi.org/10.3390/endocrines3010002






