Should Early Hyperglycemia Be Considered a Risk Factor for Post-Transplant Diabetes Mellitus? Findings from a Retrospective Cohort Study in Kidney Transplant Recipients Without Diabetes Mellitus Prior to Transplant
Abstract
1. Introduction
2. Materials and Methods
2.1. Immunosuppression
2.2. Outcomes
2.3. Study Definitions
2.4. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Baseline Characteristics
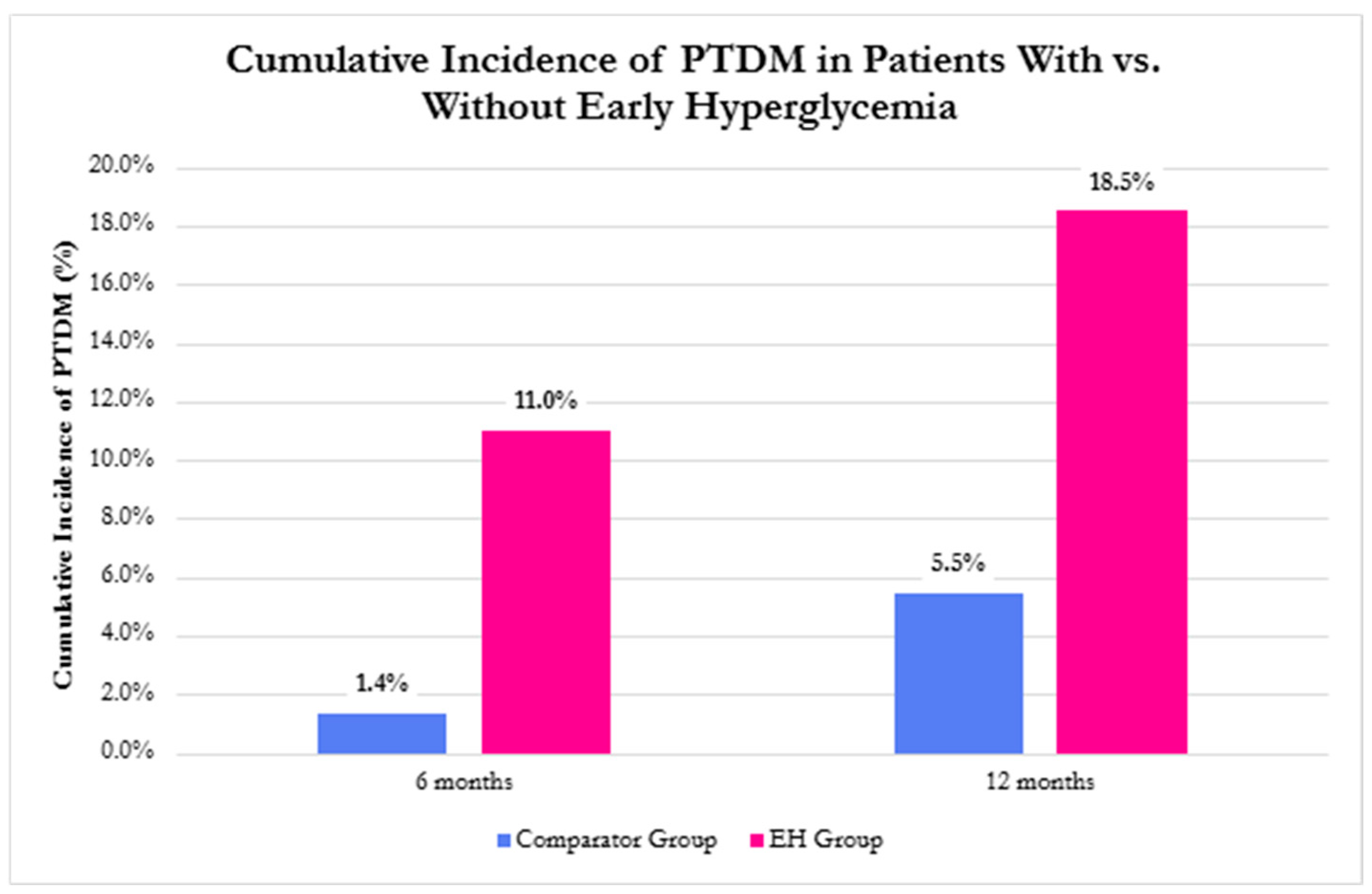
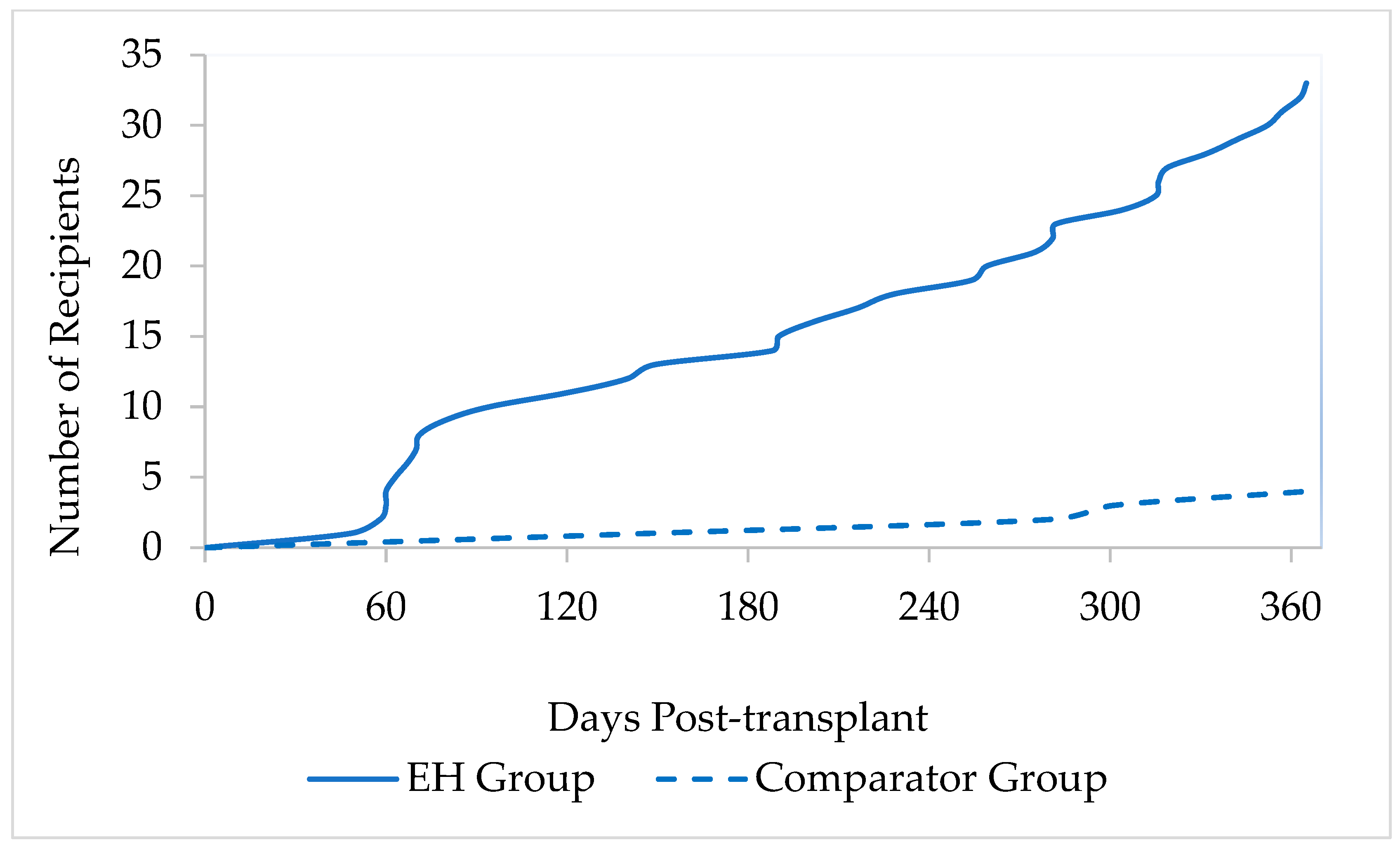
3.3. Incidence of PTDM
3.4. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PTDM | Post-transplant diabetes mellitus |
| KTRs | Kidney transplant recipients |
| EH | Early hyperglycemia |
| DM | Diabetes mellitus |
| RBG | Random blood glucose |
| FBG | Fasting blood glucose |
| HbA1c | Glycated hemoglobin |
| ESRD | End-stage renal disease |
| HCV | Hepatitis C virus |
| CMV | Cytomegalovirus infection |
| HLA | Human leukocyte antigen |
| IV | Intravenous |
| POD | Post-operative day |
| CV | Coefficient of variation |
| UTI | Urinary tract infection |
| CFU | Colony-forming units |
| CKD-EPI | Chronic kidney disease epidemiology collaboration |
| GLP-1 RA | Glucagon-like peptide 1 receptor agonist |
| DPP-4i | Dipeptidyl peptidase 4 inhibitor |
| FSGS | Focal segmental glomerulosclerosis |
| cPRA | Calculated panel reactive antibodies |
| KDPI | Kidney donor profile index |
| DBD | Donation after brain death |
| DCD | Donation after cardiac death |
| SD | Standard deviation |
| Ab | Antibody |
| NAT | Nucleic acid amplification testing |
| IQR | Interquartile range |
| ALZ | Alemtuzumab |
| ATG | Anti-thymocyte globulin |
References
- Shivaswamy, V.; Boerner, B.; Larsen, J. Post-Transplant Diabetes Mellitus: Causes, Treatment, and Impact on Outcomes. Endocr. Rev. 2016, 37, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Li, T.; Yi, X.; Song, S.; Kang, J.; Jiang, Y. Prevalence of new-onset diabetes mellitus after kidney transplantation: A systematic review and meta-analysis. Acta Diabetol. 2024, 61, 809–829. [Google Scholar] [CrossRef] [PubMed]
- Habibnia, F.; Oliaei, F.; Shirafkan, H.; Abbasi Firoozjah, M.; Rezaei Roshan, M.; Akbari, R. Ten-year incidence of post-transplant Diabetes Mellitus in renal transplant patients. Diabetes Vasc. Dis. Res. 2022, 19, 14791641221137352. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.F.; Jia, Y.; Mansour, S.G.; Reese, P.P.; Hall, I.E.; Alasfar, S.; Doshi, M.D.; Akalin, E.; Bromberg, J.S.; Harhay, M.N.; et al. Post-transplant Diabetes Mellitus in Kidney Transplant Recipients: A Multicenter Study. Kidney360 2021, 2, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, A.E.; Porrini, E.; Hornum, M.; Donate-Correa, J.; Morales-Febles, R.; Khemlani Ramchand, S.; Molina Lima, M.X.; Torres, A. Post-Transplant Diabetes Mellitus and Prediabetes in Renal Transplant Recipients: An Update. Nephron 2021, 145, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.; Hecking, M.; de Vries, A.P.; Porrini, E.; Hornum, M.; Rasoul-Rockenschaub, S.; Berlakovich, G.; Krebs, M.; Kautzky-Willer, A.; Schernthaner, G.; et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: Recommendations and future directions. Am. J. Transplant. 2014, 14, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Zhou, K.; Kashyap, S.R.; Lansang, M.C. Early Post-Renal Transplant Hyperglycemia. J. Clin. Endocrinol. Metab. 2022, 107, 549–562. [Google Scholar] [CrossRef]
- Valderhaug, T.G.; Hjelmesæth, J.; Hartmann, A.; Røislien, J.; Bergrem, H.A.; Leivestad, T.; Line, P.D.; Jenssen, T. The association of early post-transplant glucose levels with long-term mortality. Diabetologia 2011, 54, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Wyzgal, J.; Paczek, L.; Ziolkowski, J.; Pawlowska, M.; Rowiński, W.; Durlik, M. Early hyperglycemia after allogenic kidney transplantation. Ann. Transplant. 2007, 12, 40–45. [Google Scholar] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transplant. 2009, 9 (Suppl. S3), S1–S155. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.S.; Nemati, E.; Pourfarziani, V.; Taheri, S.; Nourbala, M.H.; Einollahi, B. Early hyperglycemia after allogenic kidney transplantation: Does it induce infections. Ann. Transplant. 2007, 12, 23–26. [Google Scholar] [PubMed]
- Chakkera, H.A.; Knowler, W.C.; Devarapalli, Y.; Weil, E.J.; Heilman, R.L.; Dueck, A.; Mulligan, D.C.; Reddy, K.S.; Moss, A.A.; Mekeel, K.L.; et al. Relationship between inpatient hyperglycemia and insulin treatment after kidney transplantation and future new onset diabetes mellitus. Clin. J. Am. Soc. Nephrol. 2010, 5, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, F.; Tapia, G.; Ardiles, L. Early hyperglycemia: A risk factor for posttransplant diabetes mellitus among renal transplant recipients. Transplant. Proc. 2009, 41, 2664–2667. [Google Scholar] [CrossRef] [PubMed]
- Eide, I.A.; Halden, T.A.; Hartmann, A.; Åsberg, A.; Dahle, D.O.; Reisaeter, A.V.; Jenssen, T. Mortality risk in post-transplantation diabetes mellitus based on glucose and HbA1c diagnostic criteria. Transpl. Int. 2016, 29, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Eide, I.A.; Halden, T.A.; Hartmann, A.; Åsberg, A.; Dahle, D.O.; Reisæter, A.V.; Jenssen, T. Limitations of hemoglobin A1c for the diagnosis of posttransplant diabetes mellitus. Transplantation 2015, 99, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Galindo, R.J.; Beck, R.W.; Scioscia, M.F.; Umpierrez, G.E.; Tuttle, K.R. Glycemic Monitoring and Management in Advanced Chronic Kidney Disease. Endocr. Rev. 2020, 41, 756–774. [Google Scholar] [CrossRef] [PubMed]
- Petrosan, A.; Santeusanio, A.D.; Khaim, R.; Delaney, V. Comparison of Intensive Insulin Versus Oral Regimens on Early Glycemic Control Following Kidney Transplant. Prog. Transplant. 2022, 32, 327–331. [Google Scholar] [CrossRef] [PubMed]
| EH (N = 204) | Comparator (N = 75) | p-Value | |
|---|---|---|---|
| Sex, male, n (%) | 119 (58.3) | 29 (38.7) | 0.004 |
| Age at time of transplant, years; median (IQR) | 51 (37–62) | 47 (38.5–55) | 0.092 |
| Race, n (%) | |||
| Black | 70 (34.3) | 44 (58.6) | <0.005 |
| White | 94 (46.1) | 20 (26.7) | 0.004 |
| Other | 40 (19.6) | 11 (14.7) | 0.344 |
| Body mass index (kg/m2), mean (SD) | 28.1 (5.5) | 28.7 (5.6) | 0.446 |
| Etiology of kidney disease, n (%) | |||
| Hypertensive nephrosclerosis | 52 (25.5) | 26 (34.7) | 0.13 |
| FSGS | 23 (11.3) | 11 (14.6) | 0.443 |
| Polycystic kidney disease | 24 (11.7) | 5 (6.7) | 0.216 |
| IgA nephropathy | 13 (6.4) | 7 (9.3) | 0.395 |
| Other | 92 (45.1) | 26 (34.7) | 0.118 |
| cPRA, % median (IQR), [min, max] | 0 [0, 100] | 0 [0, 63] | 0.495 |
| cPRA 0–20, n (%) | 196 (96.1) | 73 (97.3) | 0.617 |
| cPRA 21–60, n (%) | 3 (1.5) | 1 (1.3) | 1 |
| cPRA 61–100, n (%) | 5 (2.4) | 1 (1.3) | 1 |
| Delayed graft function, n (%) | 42 (20.6) | 11 (14.7) | 0.264 |
| KDPI, % for deceased donors, median (IQR) | 38 (33–44) | 30 (15.3–40.3) | 0.081 |
| Donor type, n (%) | |||
| DBD | 99 (48.5) | 42 (56) | 0.269 |
| DCD | 53 (26) | 14 (18.7) | 0.205 |
| Living | 52 (25.5) | 19 (25.3) | 0.979 |
| Cold ischemia time for deceased donors (hours), mean (SD) | 15.4 (5.75) | 13.9 (5.7) | 0.095 |
| Induction agent, n (%) | |||
| ALZ | 111 (54.4) | 51 (68) | 0.041 |
| ATG | 79 (38.7) | 20 (26.7) | 0.062 |
| Basiliximab | 14 (6.9) | 4 (5.3) | 0.645 |
| HCV donor status, n (%) | |||
| HCV Ab + NAT+ | 27 (13.2) | 6 (8) | 0.23 |
| HCV Ab + NAT− | 3 (1.5) | 2 (2.7) | 0.613 |
| Positive HCV in recipient | 9 (4.4) | 1 (1.3) | 0.297 |
| Baseline HbA1c, % mean (SD) | 4.97 (0.48) | 4.82 (0.38) | 0.018 |
| Baseline HbA1c in pre-diabetes range (5.7–6.4%), n (%) | 15 (7.4) | 0 (0) | 0.014 |
| Length of stay, days (mean, SD) | 7.3 (6) | 6 (2.1) | 0.074 |
| Combinations Meeting Criteria in EH Group | PTDM * at 6 Months (N = 22) | Additional PTDM * from 6 Months to 12 Months (N = 15) |
|---|---|---|
| HbA1c AND FBG, n (%) | 3 (13.6) | 1 (6.7) |
| HbA1c AND RBG, n (%) | 0 (0) | 0 (0) |
| RBG AND FBG, n (%) | 3 (13.6) | 0 (0) |
| HbA1c AND RBG AND FBG, n (%) | 1 (4.5) | 0 (0) |
| Antihyperglycemic agent, n (%) | 8 (36.4) | 12 (80) |
| HbA1c AND FBG AND antihyperglycemic agent, n (%) | 1 (4.5) | 0 (0) |
| HbA1c AND RBG AND antihyperglycemic agent, n (%) | 1 (4.5) | 1 (6.7) |
| RBG AND FBG AND antihyperglycemic agent, n (%) | 3 (13.6) | 0 (0) |
| HbA1c AND RBG AND FBG AND antihyperglycemic agent, n (%) | 2 (9.1) | 1 (6.7) |
| EH (N = 204) | Comparator (N = 75) | p-Value | |
|---|---|---|---|
| Tacrolimus trough concentration, ng/mL, mean (SD) | |||
| 0–3 months 3–6 months 6–12 months | 8.1 (1.1) 7.7 (1.3) 7.6 (1.7) | 8.1 (1.4) 7.6 (1.6) 6.9 (1.8) | 0.593 0.301 0.002 |
| Tacrolimus coefficient of variation, mean (SD) | |||
| 0–3 months 3–6 months 6–12 months | 38.3 (10.7) 29.3 (15) 28.4 (16) | 37.1 (11) 30.2 (17.9) 29 (14.7) | 0.412 0.654 0.768 |
| Number of recipients taking prednisone, n (%) | |||
| Discharge 3 months 6 months 12 months | 39 (19.1) 67 (32.8) 77 (37.7) 76 (37.2) | 12 (16) 19 (25.3) 29 (38.7) 31 (41.3) | 0.55 0.229 0.888 0.535 |
| Prednisone dose, mg, mean (SD) | |||
| 3 months 6 months 12 months | 8.8 (12) 6.6 (6.6) 5.8 (4) | 6 (3.6) 6.9 (5.5) 6 (3.9) | 0.334 0.841 0.707 |
| Mycophenolate mofetil mg/day, median (IQR) | |||
| 3 months 6 months 12 months | 1500 (1000–1500) 1500 (1000–1500) 1500 (1000–1500) | 1500 (1000–1500) 1000 (500–1500) 1000 (500–1500) | 0.07 0.194 0.567 |
| Renal function *, ml/min/1.73 m2, mean (SD) | |||
| 3 months 6 months 12 months | 57.2 (21.3) 58.3 (21.7) 59.1 (22.6) | 58.8 (23) 61.4 (19.2) 62.3 (20) | 0.603 0.285 0.337 |
| Infections within 6 months, n (%) | |||
| Bacteremia UTI Recipients with ≥2 UTIs Cytomegalovirus DNAemia BK viremia | 11 (5.4) 33 (16.2) 14 (6.9) 13 (6.4) 26 (12.7) | 1 (1.3) 12 (16) 6 (8) 7 (9.3) 10 (13.3) | 0.191 0.972 0.795 0.434 0.897 |
| Readmissions within 6 months, n (%) | 120 (58.8) | 40 (53.3) | 0.411 |
| Number of readmissions within 6 months, n (%) | |||
| 1 2 3 ≥4 | 65 (54.2) 30 (25) 12 (10) 13 (10.8) | 20 (50) 11 (27.5) 4 (10) 5 (12.5) | 0.647 0.754 1 0.776 |
| Cardiovascular events within 12 months, n (%) | 2 (1) | 1 (1.3) | 1 |
| Myocardial infarction Transient ischemic attack | 1 1 | 0 1 | |
| Biopsy-proven acute rejection within 12 months, n (%) | 15 (7.4) | 8 (10.7) | 0.461 |
| Graft survival within 12 months, n (%) | 204 (100) | 75 (100) | 1 |
| Patient survival within 12 months, n (%) | 199 (97.5) | 72 (96) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allen, R.B.; Stevenson, E.; Goley, A.L.; Alexander, B.; Ma, J.; Raiger, T.B.; Chandran, M.M.; Szempruch, K.R. Should Early Hyperglycemia Be Considered a Risk Factor for Post-Transplant Diabetes Mellitus? Findings from a Retrospective Cohort Study in Kidney Transplant Recipients Without Diabetes Mellitus Prior to Transplant. Transplantology 2025, 6, 30. https://doi.org/10.3390/transplantology6040030
Allen RB, Stevenson E, Goley AL, Alexander B, Ma J, Raiger TB, Chandran MM, Szempruch KR. Should Early Hyperglycemia Be Considered a Risk Factor for Post-Transplant Diabetes Mellitus? Findings from a Retrospective Cohort Study in Kidney Transplant Recipients Without Diabetes Mellitus Prior to Transplant. Transplantology. 2025; 6(4):30. https://doi.org/10.3390/transplantology6040030
Chicago/Turabian StyleAllen, Rachel B., Emily Stevenson, April L. Goley, Bonnie Alexander, Joanna Ma, Taylor B. Raiger, Mary M. Chandran, and Kristen R. Szempruch. 2025. "Should Early Hyperglycemia Be Considered a Risk Factor for Post-Transplant Diabetes Mellitus? Findings from a Retrospective Cohort Study in Kidney Transplant Recipients Without Diabetes Mellitus Prior to Transplant" Transplantology 6, no. 4: 30. https://doi.org/10.3390/transplantology6040030
APA StyleAllen, R. B., Stevenson, E., Goley, A. L., Alexander, B., Ma, J., Raiger, T. B., Chandran, M. M., & Szempruch, K. R. (2025). Should Early Hyperglycemia Be Considered a Risk Factor for Post-Transplant Diabetes Mellitus? Findings from a Retrospective Cohort Study in Kidney Transplant Recipients Without Diabetes Mellitus Prior to Transplant. Transplantology, 6(4), 30. https://doi.org/10.3390/transplantology6040030






