Abstract
Background/Objectives: Calcineurin inhibitors (CNI) contribute to renal dysfunction post-transplant. Belatacept is a renal sparing immunosuppressive agent. We sought to determine if the use of belatacept, as an alternative to a CNI-based maintenance immunosuppressive regimen ameliorates the effects of CNI-related nephrotoxicity in lung transplant recipients, while preserving graft function. Methods: Retrospective case series of adult lung transplant recipients (LTR) converted to belatacept with CNI elimination between 2020 and 2023. Primary outcomes were estimated glomerular filtration rate (eGFR) and pulmonary function testing. Secondary outcomes included incidence of rejection, mortality, donor specific antibody (DSA), chronic lung allograft dysfunction, infection, malignancies, and drug discontinuation. Results: Five LTR converted to belatacept with a median follow up of 3.49 years (IQR 16.4). eGFR improved with a median change of +18 mL/min/1.73 m2 (IQR 6–34) at 12 months, this was sustained at last-follow-up (+19 mL/min/1.73 m2 (IQR 6–34)). Force expiratory volume in 1 s (FEV1) declined from baseline to last follow-up (median change −0.53 L). At a median of 199 days post-conversion (IQR 108–453), belatacept was discontinued in 4/5 (80%) LTR, primarily due to graft dysfunction (3/4), and CNI therapy resumed. No LTR developed CLAD, DSA, malignancy, or died on belatacept. Infection (primarily pulmonary bacterial or fungal) occurred in all LTR on belatacept. Conclusions: Belatacept with complete CNI elimination in LTR resulted in a sustained improvement in renal function in this series but was accompanied by a high discontinuation rate due to worsening graft function. The risks to the graft associated with belatacept and calcineurin inhibitor elimination outweigh any potential renal benefits.
1. Introduction
Calcineurin inhibitors (CNIs) remain the standard of care for maintenance immunosuppression after lung transplantation [1]. Despite their efficacy in the prevention of rejection, CNIs are associated with significant adverse effects that may require discontinuation, including thrombotic microangiopathy and posterior reversible encephalopathy (PRES) [2,3,4]. Additionally, CNIs are known to cause both acute and chronic nephrotoxicity. Acute nephrotoxicity is a dose-dependent effect secondary to afferent arteriole vasoconstriction, whereas chronic nephrotoxicity is characterized by irreversible interstitial fibrosis and tubular atrophy [5,6].
Kidney disease is an important predictor of mortality in lung transplantation. Chronic kidney disease in non-renal solid organ transplant recipients is associated with a four- to fivefold increased risk of death after transplantation [7]. Risk of developing stage 4–5 CKD has been reported as 15.8% in lung transplant recipients in the first 5 years post-transplant [7]. Additionally, the high immunogenicity of the lungs requires maintenance of CNI at higher goal troughs than what is required for other solid organ transplants, which increases the risk of CNI toxicities [8]. Thus, it has become of interest to pursue a maintenance immunosuppressive strategy that prevents the development and progression of renal insufficiency in the lung transplant population while maintaining graft function.
Mammalian target of rapamycin inhibitors (mTORis) may be utilized as renal-sparing agents in conjunction with reduced CNI exposure in select cases, but are also associated with proteinuria and nephrotoxicity, which can further potentiate renal insufficiency [9]. Use of mTORis in lung transplantation has also been largely limited by their adverse effect profile, with reported discontinuation rates due to adverse effects of 50–71% [10]. Additionally, the significant risk of impaired wound healing and bronchial anastomotic dehiscence with mTORis precludes de novo use of mTORis early after lung transplantation. Typically, mTORis are not initiated within the first 3 months post-lung transplant due to this [11,12]. Given the adverse effect profile of mTORis that limit their use, belatacept may be an appropriate alternative renal-sparing agent in lung transplantation.
Belatacept is a CTLA-4 inhibitor that binds to CD80 and CD86 receptors on antigen-presenting cells, preventing CD28-mediated co-stimulation of T-lymphocytes. Belatacept was approved by the US Food and Drug Administration (FDA) in 2011 for prevention of rejection in adult kidney transplant recipients seropositive for Epstein–Barr virus (EBV) [13] Evidence for belatacept in lung transplant recipients (LTR) in the setting of CNI intolerance is limited to case reports [14,15,16]. We sought to determine if the use of belatacept, as an alternative to a CNI-based maintenance immunosuppressive regimen, ameliorates the effects of CNI-related nephrotoxicity in LTR while preserving graft function.
2. Materials and Methods
2.1. Study Design
This was a retrospective case series of all adult LTR at University of Michigan initiated on belatacept for any indication between 2020 and 2023. Reason for belatacept initiation, time to initiation of belatacept, dosing and duration of belatacept therapy, immunosuppression at the time of belatacept initiation, management of concomitant maintenance immunosuppression with belatacept initiation, and reason for discontinuation of belatacept were collected. Efficacy outcomes included mortality, pulmonary function tests (PFTs), estimated glomerular filtration rate (eGFR) using the CKD-EPI 2021 equation, donor-specific antibodies (DSA), biopsy-proven acute cellular rejection (ACR), antibody-mediated rejection (AMR), and chronic lung allograft function (CLAD) stage [17]. Pulmonary function tests (PFTs) and chest computed tomography results were also evaluated to characterize the development and progression of chronic lung allograft function (CLAD) stage. Safety outcomes included viral, bacterial, and fungal infections and malignancies. Outcomes were assessed while on belatacept therapy and at last follow-up. Descriptive statistics were used to summarize demographics and outcomes. Outcomes were collected until last follow-up or date of death.
2.2. Immunosuppression
All LTR received IV methylprednisolone without antibody induction at the time of transplant, per standard institutional protocol. Standard post-operative maintenance immunosuppression included tacrolimus, azathioprine, and corticosteroids, per institutional protocol at the time. Mycophenolate may be used in lieu of azathioprine per treating pulmonologist’s discretion. Target tacrolimus troughs were 10–14 ng/mL until 6 months post-transplant, 8–12 ng/mL from 6 to 24 months post-transplant, and 6–8 ng/mL after 24 months post-transplant. Tacrolimus troughs were reduced per pulmonologists’ discretion for renal dysfunction.
LTR were considered for belatacept maintenance immunosuppression for indications of renal insufficiency or severe CNI adverse effects. Only Epstein–Barr virus (EBV) IgG-positive patients were considered for belatacept immunosuppression. For indications where the calcineurin inhibitor was tapered off, belatacept was dosed at 5 mg/kg day 1, 15, 29, 45, 57 ± 71 (5–6 induction doses) followed by 5 mg/kg monthly thereafter. If tapered off, tacrolimus was kept at 100% of the starting dose on day 1, reduced to 50% on day 15, reduced to 25% on day 29, and discontinued on day 45. For indications where immediate discontinuation of the calcineurin inhibitor was required, belatacept was dosed at 10 mg/kg on day 1, 7, 14, 28, 56, and 84 (6 induction doses) followed by 5 mg/kg monthly thereafter. The dosing weight for belatacept was weight at the time of transplant; if weight changed by >10%, then the belatacept dose was adjusted accordingly [18].
3. Results
3.1. Patient Population
Five LTR were switched from a CNI-based regimen to a belatacept-based regimen (Table 1). Of these patients, four were switched for an indication of renal insufficiency and one was switched given microangiopathic hemolytic anemia secondary to tacrolimus. Median age at the time of initiation was 64 years (IQR 16.4) and belatacept was initiated at a median of 575 (IQR 3–98) days post-transplant, with a median follow-up period of 3.49 years (IQR 2.9–7.4). Immunosuppression management at the time of belatacept initiation is shown in Table 2. Of the 4 patients who were switched to belatacept for renal insufficiency, 2/4 (patients 2 and 3) had tacrolimus goal troughs reduced prior to belatacept initiation. This did not result in improvement in eGFR, prompting the decision to pursue belatacept.

Table 1.
Baseline Characteristics.

Table 2.
Immunosuppression at Time of Belatacept Initiation and Belatacept Conversion Dosing.
Median duration of belatacept therapy was 199 days (IQR 108–453). Median baseline eGFR was 22 mL/min/1.73m2 (IQR 14–32) One LTR had pre-existing CLAD stage 3 with bronchiolitis obliterans syndrome (BOS) phenotype at the time of belatacept initiation. Prior to belatacept initiation, one LTR had positive DSAs, two were treated for presumptive ACR, and one had A1BX ACR that was not treated, given stable PFTs.
3.2. Outcomes
3.2.1. Efficacy
Renal function, defined by eGFR at 12 months post-belatacept initiation, was stabilized or increased from pre-belatacept values in all patients, with a median change of +18 mL/min/1.73 m2 (IQR 6–34) (Figure 1). At a median follow-up period of 3.49 years (IQR 2.9–7.4), this eGFR increase was sustained with a median change of +19 mL/min/1.73 m2 (IQR 0–41). The largest increase in eGFR from baseline to 12 months (+39 mL/min/1.73 m2) was observed in patient 5, who converted from CNI to belatacept therapy for an indication of a thrombotic microangiopathy secondary to tacrolimus.
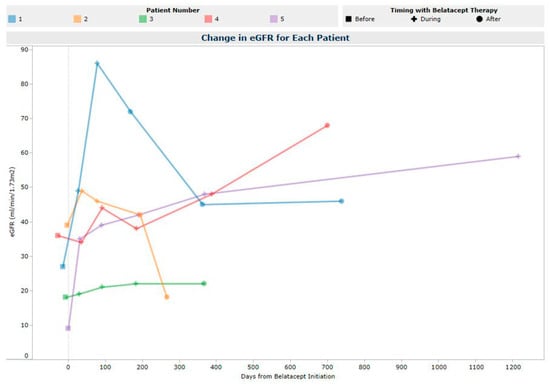
Figure 1.
Estimated Glomerular Filtration Rate Post-Belatacept Initiation.
Forced expiratory volume in one second (FEV1 L) values are depicted in Figure 2. FEV1 declined from baseline to last follow-up (median change −0.53 L). Treated acute cellular rejection occurred in 3/5 (60%) LTR on belatacept; of these, 2 (patients 1, 2) were presumed acute cellular and treated with intravenous methylprednisolone, and 1 (patient 5) was symptomatic A1B0 rejection which was treated with an oral prednisone burst. No donor-specific antibodies (DSA) developed on belatacept. One LTR (patient 1) had DSA prior to initiation (DQA1*05 in association with DQ2 with MFI 1072), which cleared during belatacept therapy and remained negative at last follow-up 377 days after belatacept discontinuation. One LTR (patient 4) developed antibody-mediated rejection 142 days post-belatacept discontinuation that was treated with methylprednisolone, plasma exchange, carfilzomib, and IVIG. No LTR experienced development of CLAD (patient 1, 2, 4, 5), and no progression of pre-existing CLAD occurred during belatacept therapy (patient 3). Post-belatacept discontinuation, two LTR developed CLAD. Patient 1 developed CLAD stage 2 (165 days post-discontinuation) and patient 2 developed CLAD stage 2 (90 days post-discontinuation). Both patients 1 and 2 progressed to CLAD stage 3 and patient 3 remained in CLAD stage 3 throughout the last follow-up.
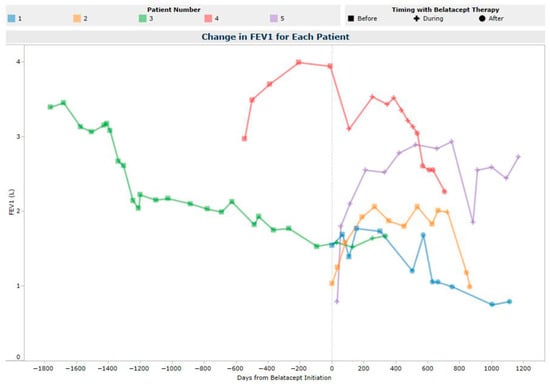
Figure 2.
FEV1 (L) Post-Belatacept Initiation.
Belatacept was discontinued in four of the five LTR at a median of 199 days (IQR 108–453), at which point CNI therapy was resumed. Of the LTR who discontinued belatacept, patients 1 and 2 discontinued due to concern for presumed acute cellular rejection (ACR); patient 4 had belatacept held after 470 days of therapy due to concern for post-transplant lymphoproliferative disorder (PTLD) and resumed treatment once PTLD was ruled out, but later discontinued belatacept due to declining PFTs; and patient 3 transitioned to home hospice, at which point maintenance immunosuppression was discontinued. Patient 5 remains on belatacept. When examining LTR 1–4 who discontinued belatacept and resumed a CNI, the median change in eGFR from pre-belatacept to belatacept discontinuation was +31.5 mL/min/1.73 m2 (IQR 19–34). This improvement in eGFR was partially reversed with CNI resumption, with a median change in eGFR from belatacept discontinuation to last follow-up of −22 mL/min/1.73 m2 (IQR 43–62). Overall, renal function improved from their pre-belatacept baseline with a median net change of +9.5 mL/min/1.73 m2 (IQR 19–63) at last follow-up.
3.2.2. Safety
Infection occurred in all 5 LTR during belatacept treatment. Most were bacterial or fungal pulmonary infections and did not require a modification to the belatacept regimen. No LTR developed EBV or CMV viremia during belatacept therapy. Time to infection and infection type are listed in Table 3. No malignancy developed on belatacept therapy, and none died on therapy. Four LTR were alive at last follow-up.

Table 3.
Infections.
4. Discussion
In this case series, belatacept use as an alternative to calcineurin inhibitor therapy in LTR was associated with an improvement or stabilization in kidney function. Despite high belatacept discontinuation rates, improvements in kidney function were sustained post-belatacept discontinuation after CNI therapy was reinitiated. The primary reason for belatacept use was graft dysfunction/treated acute rejection. Despite all cases of acute rejection being treated, two of the three LTR with treated acute rejection later developed CLAD post-belatacept discontinuation. All LTR developed either a bacterial or fungal infection while on belatacept therapy, and none developed a viral infection. The absence of viral infections in this cohort is surprising, as belatacept-based immunosuppression has been associated with an increased risk of viral (CMV, EBV) infections, which is thought to be due to the effect of belatacept on naïve CD4 T cells, impairing immune responses to primary viral infections [19,20,21]. While infection was seen in all patients in this series, our data cannot conclude that the bacterial and fungal infections seen were resultant of belatacept therapy, as these are common types of infection seen in the lung transplant population overall [21]. Overall, the maintenance dosing regimen of belatacept and CNI withdrawal taper used in this study may have contributed to tolerability. It would be prudent to explore alternative belatacept dosing and concurrent maintenance immunosuppressive regimens, such as low dose CNI regimens or a prolonged CNI taper, that would minimize the risk of infection, preserve renal function, and maintain adequate graft function.
Acceptable rejection outcomes with renal function improvement have been previously reported with both calcineurin minimization and elimination strategies when utilizing belatacept in the lung transplant population. Timofte et al. evaluated belatacept use in eight lung transplant recipients with renal insufficiency where the CNI was decreased or temporarily discontinued. All had stabilization or improvement in renal function, with two patients weaning off dialysis. FEV1 remained stable in this cohort, with only one steroid-responsive ACR [16]. Iasella et al. had similar findings when evaluating belatacept use with CNI withdrawal in nine lung transplant recipients. Belatacept was initiated in place of the CNI for thrombotic thrombocytopenic purpura, PRES, and renal insufficiency. Renal function was significantly higher post-belatacept and two patients were weaned off dialysis. There was no difference in FEV1 or ACR pre- and post-conversion to belatacept [15]. Our findings support a potential renal-sparing benefit of belatacept, as all patients had an increase or stabilization in eGFR during and post-belatacept therapy. However, our findings do not support the conclusion of the aforementioned studies with respect to graft function. This may be due to inherent differences in the immunological baseline risk of our population compared to other reports, time post-transplant to conversion, differences in concurrent maintenance immunosuppression, and belatacept dosing regimens utilized.
ACR remains a significant concern in the lung transplant population given that it is associated with development of CLAD. Brugiere et al. examined a CNI-free belatacept regimen and a belatacept/low-dose CNI regimen in a total of ten lung transplant recipients. Belatacept was initiated for severe renal failure for nine patients and for immunosuppression-related toxicities in one patient. Concordant with our findings, they found that eGFR significantly improved after belatacept initiation; however, they found a high incidence of recurrent ACR exclusively in patients with CNI-free belatacept-based immunosuppression [14].
There have been a few proposed mechanisms for ACR risk associated with belatacept. Belatacept works by inhibiting the co-stimulatory signal; however, pre-sensitized memory T-cells are resistant to co-stimulatory blockage by belatacept [22]. Additionally, terminally differentiated memory CD4+ and CD8+ T-cells have downregulation of CD28 expression and become insensitive to the lack of CD28/B7 co-stimulation [23]. Increased numbers of CD4+ and CD8+ CD28− memory T-cells have been associated with poor outcomes in lung transplant recipients [24]. Belatacept’s mechanism of preventing signaling through CTLA-4 can also have negative effects on the functions of regulatory T-cells. Calcineurin inhibitors suppress cytokine secretion from memory T-cells, which may counter the risk of rejection that comes with the activation of memory T-cells [25]. Based upon the findings from Timofte and Iasella, renal function improvements were found with both CNI minimization and elimination strategies [15,16]. Thus, utilizing a CNI minimization strategy with a lower goal trough in indications where continuation of CNI is feasible (e.g., renal insufficiency) may decrease immunologic risk.
Additionally, it is unclear at what time after transplant or degree of renal insufficiency belatacept should be initiated to maximize renal improvements. De novo use of belatacept in lung transplant is associated with increased risk of mortality, which precludes its widespread use early post-transplant [26]. However, earlier initiation of belatacept may be prudent to avoid the onset of chronic CNI nephrotoxicity. In the Timofte case series, patients initiated on belatacept between POD 140 and 400 exhibited improvements in renal function, and patients initiated on belatacept between POD 347 and 2176 had stabilization of renal function, suggesting that earlier initiation may be of greater benefit. However, with the case series from Iasella, timing of belatacept initiation was not associated with degree of renal improvement, as patients initiated up to 9 years post-transplant still had renal improvements [15,16]. In our study, belatacept was initiated at a range of POD 61–3778, and there was no correlation between timing of initiation and renal function changes.
This case series is limited by its retrospective study design and small sample size. LTR were converted to belatacept at varying times post-transplant and adjustments to other maintenance immunosuppression regimens throughout the study period were not examined. Furthermore, maintenance immunosuppression amongst LTR varied, which poses a confounding variable that we could not control for. Our cohort also had high discontinuation rates of belatacept, so we were unable to evaluate whether ACR influences long-term graft outcomes while on belatacept-based immunosuppression. Additionally, given lack of matching to a control cohort, it is difficult to exclude that renal function changes may have occurred in the absence of belatacept immunosuppression therapy.
5. Conclusions
Belatacept with complete CNI elimination in LTR resulted in a sustained improvement in renal function in this case series, but was accompanied by a high discontinuation rate due to worsening graft function. The risks to the graft associated with belatacept and calcineurin inhibitor elimination outweigh any potential renal benefits. Further studies investigating alternative maintenance immunosuppressive regimens that preserve renal function while maintaining adequate graft function are warranted.
Author Contributions
A.C.: concept/design, data collection, data analysis/interpretation, statistics, drafting article, critical revisions of article, approval of article. J.H.: concept/design, data analysis/interpretation, critical revisions of article, approval of article. E.B.: critical revisions of article, approval of article. M.C.: critical revisions of article, approval of article. D.L.: critical revisions of article, approval of article. K.W.: concept/design, data collection, data analysis/interpretation, critical revisions of article, approval of article and agreed to the published version of the manuscript. R.S.: concept/design, data analysis/interpretation, critical revisions of article, approval of article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was originally approved by the Institutional Review Board of the University of Michigan (HUM00014506) on 15 August 2007. The study was amended on 2 August 2024 to include current study personnel. This current study falls within the scope of this IRB ((HUM00014506) approval as confirmed by the institutional IRB.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
Data is unavailable due to privacy restrictions.
Acknowledgments
University of Michigan Internal Medicine Clinical Experience & Quality Program for assistance with creation of the figures.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CNI | Calcineurin inhibitor |
| LTR | Lung transplant recipients |
| DSA | Donor specific antibody |
| mTORi | Mammalian target of rapamycin inhibitors |
| FEV1 | Forced expiratory volume in 1 s |
| eGFR | Estimated glomerular filtration rate |
| CLAD | Chronic lung allograft dysfunction |
| ACR | Acute cellular rejection |
References
- Valapour, M.; Lehr, C.J.; Schladt, D.P.; Smith, J.M.; Swanner, K.; Weibel, C.J.; Weiss, S.; Snyder, J.J. OPTN/SRTR 2022 Annual Data Report: Lung. Am. J. Transplant. 2024, 24 (Suppl. S1), S394–S456. [Google Scholar] [CrossRef]
- Abraham, K.A.; Little, M.A.; Dorman, A.M.; Walshe, J.J. Hemolytic-uremic syndrome in association with both cyclosporine and tacrolimus. Transpl. Int. 2000, 13, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Cicora, F.; Paz, M.; Mos, F.; Roberti, J. Use of belatacept as alternative immunosuppression in three renal transplant patients with de novo drug-induced thrombotic microangiopathy. Case Rep. Med. 2013, 2013, 260254. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, G.; Heck, S.; Sienel, W.; Irlbeck, M.; Kneidinger, N.; Michel, S.; Forbrig, R.; Walter, J.; Zimmermann, J.; Kovács, J.; et al. Posterior reversible encephalopathy syndrome after lung transplantation: Risk factors and management. Clin. Transplant. 2023, 37, e14850. [Google Scholar] [CrossRef]
- Issa, N.; Kukla, A.; Ibrahim, H.N. Calcineurin inhibitor nephrotoxicity: A review and perspective of the evidence. Am. J. Nephrol. 2013, 37, 602–612. [Google Scholar] [CrossRef]
- Zaltzman, J.S.; Pei, Y.; Maurer, J.; Patterson, A.; Cattran, D.C. Cyclosporine Nephrotoxicity in Lung Transplant Recipients. Transplantation 1992, 54, 875–878. [Google Scholar] [CrossRef]
- Ojo, A.O.; Held, P.J.; Port, F.K.; Wolfe, R.A.; Leichtman, A.B.; Young, E.W.; Arndorfer, J.; Christensen, L.; Merion, R.M. Chronic renal failure after transplantation of a nonrenal organ. N. Engl. J. Med. 2003, 349, 931–940. [Google Scholar] [CrossRef]
- Monchaud, C.; Marquet, P. Pharmacokinetic optimization of immunosuppressive therapy in thoracic transplantation: Part I. Clin. Pharmacokinet. 2009, 48, 419–462. [Google Scholar] [CrossRef]
- Paluri, R.K.; Sonpavde, G.; Morgan, C.; Rojymon, J.; Mar, A.H.; Gangaraju, R. Renal toxicity with mammalian target of rapamycin inhibitors: A meta-analysis of randomized clinical trials. Oncol. Rev. 2019, 13, 455. [Google Scholar] [CrossRef]
- Schmucki, K.; Hofmann, P.; Fehr, T.; Inci, I.; Kohler, M.; Schuurmans, M.M. Mammalian Target of Rapamycin Inhibitors and Kidney Function After Thoracic Transplantation: A Systematic Review and Recommendations for Management of Lung Transplant Recipients. Transplantation 2023, 107, 53–73. [Google Scholar] [CrossRef]
- King-Biggs, M.B.; Dunitz, J.M.; Park, S.J.; Kay Savik, S.; Hertz, M.I. Airway anastomotic dehiscence associated with use of sirolimus immediately after lung transplantation. Transplantation 2003, 75, 1437–1443. [Google Scholar] [CrossRef]
- Groetzner, J.; Kur, F.; Spelsberg, F.; Behr, J.; Frey, L.; Bittmann, I.; Vogeser, M.; Ueberfuhr, P.; Meiser, B.; Hatz, R.; et al. Airway anastomosis complications in de novo lung transplantation with sirolimus-based immunosuppression. J. Heart Lung Transplant. 2004, 23, 632–638. [Google Scholar] [CrossRef]
- Vincenti, F.; Larsen, C.; Durrbach, A.; Wekerle, T.; Nashan, B.; Blancho, G.; Lang, P.; Grinyo, J.; Halloran, P.F.; Solez, K.; et al. Costimulation blockade with belatacept in renal transplantation. N. Engl. J. Med. 2005, 353, 770–781. [Google Scholar] [CrossRef]
- Brugière, O.; Vallée, A.; Raimbourg, Q.; Peraldi, M.-N.; de Verdière, S.C.; Beaumont, L.; Hamid, A.; Zrounba, M.; Roux, A.; Picard, C.; et al. Conversion to belatacept after lung transplantation: Report of 10 cases. PLoS ONE 2023, 18, e0281492. [Google Scholar] [CrossRef] [PubMed]
- Iasella, C.J.; Winstead, R.J.; Moore, C.A.; Johnson, B.A.; Feinberg, A.T.; Morrell, M.R.; Hayanga, J.W.A.; Lendermon, E.A.; Zeevi, A.; McDyer, J.F.; et al. Maintenance Belatacept-Based Immunosuppression in Lung Transplantation Recipients Who Failed Calcineurin Inhibitors. Transplantation 2018, 102, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Timofte, I.; Terrin, M.; Barr, E.; Sanchez, P.; Kim, J.; Reed, R.; Britt, E.; Ravichandran, B.; Rajagopal, K.; Griffith, B.; et al. Belatacept for renal rescue in lung transplant patients. Transpl. Int. 2016, 29, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Belatacept (Nulojix) [Package Insert]. U.S. Food and Drug Administration Website. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125288s075lbl.pdf (accessed on 5 May 2024).
- Chavarot, N.; Divard, G.; Scemla, A.; Amrouche, L.; Aubert, O.; Leruez-Ville, M.; Timsit, M.O.; Tinel, C.; Zuber, J.; Legendre, C.; et al. Increased incidence and unusual presentations of CMV disease in kidney transplant recipients after conversion to belatacept. Am. J. Transplant. 2021, 21, 2448–2458. [Google Scholar] [CrossRef]
- Karadkhele, G.; Hogan, J.; Magua, W.; Zhang, W.; Badell, I.R.; Mehta, A.; Lyon, M.; Pastan, S.; Pearson, T.C.; Larsen, C.P. CMV high-risk status and posttransplant outcomes in kidney transplant recipients treated with belatacept. Am. J. Transplant. 2021, 21, 208–221. [Google Scholar] [CrossRef]
- Abdinoor, A.; Wyatt, T.; Hareesh, S.; Bregman, A.; Obeid, K. The Infectious Complications After Belatacept-Use in Kidney and/or Pancreas Transplant Recipients. Open Forum Infect. Dis. 2025, 12 (Suppl. S1), ofae631.2404. [Google Scholar] [CrossRef]
- Mathews, D.V.; Wakwe, W.C.; Kim, S.C.; Lowe, M.C.; Breeden, C.; Roberts, M.E.; Farris, A.B.; Strobert, E.A.; Jenkins, J.B.; Larsen, C.P.; et al. Belatacept-Resistant Rejection Is Associated with CD28(+) Memory CD8 T Cells. Am. J. Transplant. 2017, 17, 2285–2299. [Google Scholar] [CrossRef]
- Benichou, G.; Gonzalez, B.; Marino, J.; Ayasoufi, K.; Valujskikh, A. Role of Memory T Cells in Allograft Rejection and Tolerance. Front. Immunol. 2017, 8, 170. [Google Scholar] [CrossRef]
- Studer, S.M.; George, M.P.; Zhu, X.; Song, Y.; Valentine, V.G.; Stoner, M.W.; Sethi, J.; Steele, C.; Duncan, S.R. CD28 down-regulation on CD4 T cells is a marker for graft dysfunction in lung transplant recipients. Am. J. Respir. Crit. Care Med. 2008, 178, 765–773. [Google Scholar] [CrossRef][Green Version]
- Tsuda, K.; Yamanaka, K.; Kitagawa, H.; Akeda, T.; Naka, M.; Niwa, K.; Nakanishi, T.; Kakeda, M.; Gabazza, E.C.; Mizutani, H. Calcineurin inhibitors suppress cytokine production from memory T cells and differentiation of naïve T cells into cytokine-producing mature T cells. PLoS ONE 2012, 7, e31465. [Google Scholar] [CrossRef]
- Huang, H.J.; Schechtman, K.; Askar, M.; Bernadt, C.; Mittler, B.; Dore, P.; Witt, C.; Byers, D.; Vazquez-Guillamet, R.; Halverson, L.; et al. A pilot randomized controlled trial of de novo belatacept-based immunosuppression following anti-thymocyte globulin induction in lung transplantation. Am. J. Transplant. 2022, 22, 1884–1892. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).