Abstract
Many patients with a hematologic malignancy have other pre-existing conditions at the time of consideration of an allogeneic stem cell transplant (allo-HSCT). Among these, mild-to-moderate chronic kidney disease (CKD) is a common comorbid condition that can potentially impact the rates of non-relapse mortality among transplant patients. While the risk of severe CKD on allo-HSCT is well recognized, there remains a paucity of data in terms of the impact of mild-to-moderate CKD on patient outcomes in this setting. Using data from the National Inpatient Sample database, we aimed to investigate the impact of mild-to-moderate CKD on hospitalization outcomes for patients undergoing an allo-HSCT. Multivariate analysis revealed that CKD patients had a 31% higher risk of all-cause mortality (OR = 1.31, 95% CI: 1.01–1.70; p = 0.04) and a higher risk of other common hospitalization complications, including acute kidney injury, acute pulmonary edema, cardiac arrhythmias, and septic shock. While this study has limitations, including its retrospective nature and lack of specific medication data, it underscores the importance of considering CKD as a significant risk factor in allo-HSCT outcomes.
1. Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains the standard potentially curative therapy option for advanced hematologic malignant blood disorders [1]. In clinical practice, patients with end stage, renal disease, or hemodialysis dependency are often deemed as transplant ineligible due to the risk associated with transplant-related mortality and morbidity. Additionally, advanced kidney dysfunction is usually accompanied by a constellation of other cardiovascular risk factors that are predictive of poor outcomes [2]. For these reasons, advanced CKD is included as a detrimental comorbidity when risk-stratifying patients using clinical calculators [3]. However, there is paucity of data in regard to the impact of mild-to-moderate chronic kidney disease (CKD) on the hospitalization outcomes of allo-HSCT. As the scarcity of research in this area underscores the necessity for a rigorous investigation, we conducted a nationwide retrospective study employing the National Inpatient Sample (NIS) Database.
2. Methods and Materials
The data used for this nationwide retrospective study were collected from the NIS database, the largest publicly available database in the United States. The NIS database can be accessed by submission of request through the NIS website [4]. It stores data annually that are representative of hospitalizations in all healthcare facilities across all 50 states of insured and uninsured patients via stratified probability sampling. The database is actively managed and overseen by the Agency for Healthcare Research and Quality (AHRQ) through a federal–state–industry partnership called the Healthcare Cost and Utilization Project (HCUP). Overall, the NIS database stores an array of information totaling over 50 data points for each hospitalization including up to 40 clinical diagnoses and 25 procedures billed for during the hospital study [4]. Data regarding patient comorbidities, insurance status, median income, length of hospitalizations, and cost of stay are also stored for each individual hospitalization. Additionally, information regarding hospital characteristics such as the geographic location, urban or rural setting, and teaching or nonteaching institution is also available for each hospitalization. Furthermore, the hospitals are classified as ‘small, medium, or large’ in the database according to the American Hospital Association Annual Survey of Hospitals, where in the urban teaching setting, a small hospital has 1–299 beds, a medium sized hospital has 300–499 beds, and a large hospital has >500 beds. HCUP recommends weighing using the trend and discharge weight to generate a large nationwide population sample of hospitalized patients, which allows researchers to conduct studies gauging causal relationships between various patient characteristics and diseases [4,5]. As the NIS only stores de-identified information, neither Institutional Review Board (IRB) approval nor informed consent was required for this study (Supplementary Index Table S1). We accessed the NIS database to retrieve data for hospitalizations for allo-HSCT in patients >18 years of age using the relevant International Classification of Diseases 9th and 10th Revision (ICD-9 & ICD-10) codes (Supplementary Index Table S2). Thereafter, we removed any cases with missing data for age, gender, or race. All patients with severe CKD were also removed. We then identified patients with mild-to-moderate CKD (Stage I-III) using the relevant ICD codes. As the NIS database does not store laboratory values for serum creatinine or glomerular filtration rate (GFR), the ICD code billing was the sole measure used to identify hospitalizations for patients with CKD. The data were then subclassified into hospitalizations of patients with mild-to-moderate CKD (CKD group) and patients without CKD (control group).
The initial obtained raw data were recorded as frequencies for categorical variables and the median with a standard deviation for age. The Chi-square and Fisher exact test were used to compare the baseline patient and hospital characteristics between the two groups. The independent student t-test was deployed to assess for statistically significant differences in the continuous variables. A multivariate logistic regression model was designed, taking into consideration all the demographics, comorbidities, and hospital characteristics as previously applied in multiple NIS based studies, to assess for the primary and secondary outcomes [6,7]. The primary endpoint was all-cause mortality. Secondary endpoints included common hospitalization complications seen in hospitalizations for allo-HSCT like acute kidney injury (AKI), acute pulmonary edema, cardiogenic shock, cardiac arrhythmias, graft-versus-host disease (GVHD), septic shock, and the need for mechanical ventilation and hemodialysis (HD). The odds ratio with a 95% confidence interval (CI) was calculated for all the endpoints. A two-tailed p value of <0.05 was used to determine the statistical significance. All statistical analyses were performed using Statistical Package for Social Science version 28 (IBM Corp, Armonk, NY, USA).
3. Results
The weighted data for 84,626 hospitalizations allogenic HSCT were collected. Among these, 920 hospitalizations (1.1%) were for patients with early-to-moderate CKD. The patients with CKD were older, more likely to be male, and black. The prevalence of co-morbidities like coronary artery disease (CAD), chronic obstructive lung disease (COPD), congestive heart disease (CHF), diabetes (DM), hypertension (HTN), and obesity was also significantly higher in the CKD group compared to the control group (Table 1). Multivariate logistic regression revealed that after adjusting for the potential confounders, patients with CKD had a higher risk of all-cause mortality: OR = 1.31, 95% CI: 1.01–1.70, p = 0.04. CKD patients also had a statistically higher risk of developing AKI (OR = 4.85, 95% CI: 4.19–5.61, p < 0.01), acute pulmonary edema (OR = 2.16, 95% CI: 1.42–3.26, p < 0.01), cardiac arrhythmias (OR = 1.61, 95% CI: 1.33–1.95, p < 0.01), and septic shock (OR = 2.64, 95% CI: 2.16–3.23, p < 0.01). Though there was a higher trend seen in the CKD group, there was no statistical difference in the risk for GVHD (OR = 1.10, 95% CI: 0.90–1.34, p = 0.37) and the need for HD (OR = 1.67, 95% CI: 0.97–2.87, p = 0.07) or mechanical ventilation (OR = 1.20, 95% CI: 0.93–1.54, p = 0.17) (Table 2 and Figure 1).

Table 1.
Demographics, comorbidities, and hospitalization characteristics of the two groups.

Table 2.
Adjusted and unadjusted odds ratios of the primary and secondary endpoints.
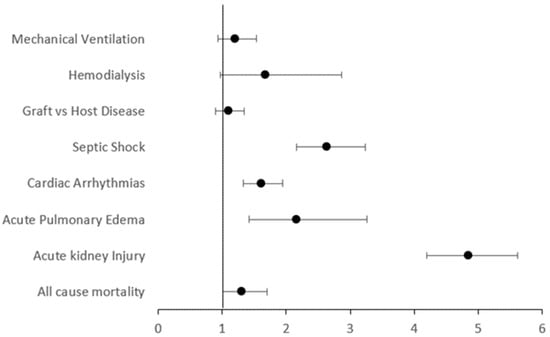
Figure 1.
Forest plot depicting the odds ratio of primary and secondary endpoints.
4. Discussion
Our comprehensive national-level retrospective study offers important insights into the risks associated with allo-HSCT in patients with CKD. Given that patients with CKD have an impaired baseline function, a further decline in their renal functioning is highly probable following allo-HSCT, which places them at a higher risk of all-cause mortality as highlighted by the results of this study. Our group had previously demonstrated that early-stage CKD is associated with poor hospital outcomes in patients admitted for induction therapy for acute myeloid leukemia (AML) [6]. Since allo-HSCT is the only potentially curative option for AML, we wanted to determine the impact of early CKD in the transplant population. Like in admission for induction therapy, hospitalizations for allo-HSCT are prolonged, stretching over weeks until adequate count recovery is achieved. These patients are at an exquisitely high risk of infection and other complications and therefore undergo a stringent pretransplant screening for risk stratification and the optimization of comorbidities [8]. Though comorbidities like hypertension and diabetes can be managed effectively by medications, the correction of the underlying CKD secondary to these conditions can take substantially longer to correct beyond the patient’s ‘baseline’. In many cases with hematologic malignancies, the transplant ‘window’ where the disease is in remission may be short, and waiting for the complete correction of renal function may increase the risk of disease relapse [9]. Consequently, only a modest degree of CKD is usually not considered a major barrier for transplant and awaiting complete renal recovery is not considered a necessity [10]. Hospitalization complications like AKI are commonly seen among transplant recipients, especially those with a lower baseline kidney function due to multifactorial causes, including the renal adverse effects of conditioning chemotherapy, immunosuppressants, and antibiotics [11]. Pre-existing CKD also complicates the drug dosing strategies during complication management and when choosing conditioning regimens due to altered pharmacokinetics and pharmacodynamics in the setting of renal insufficiency [12]. Most transplant programs have dedicated pharmacists to assist in such clinical decision making, and most electronic medical record systems have built-in alerts for drug interactions and dosages in light of compromised renal function to prevent further renal injury. Apart from medications, repeated blood transfusions causing significant volume shifts, thrombotic microangiopathy, and viral culprits such as BK polyomavirus, adenovirus, and cytomegalovirus have all been shown to complicate the hospitalization course for allo-HSCT patients by causing significant alteration in kidney function [13]. Additionally, sepsis-induced AKI may also occur due to systemic vasodilation with hypotension and kidney hypoperfusion, cytokine-related tubulointerstitial injury, and intrarenal endothelial dysfunction with capillary thrombosis [14]. Ongoing research into biomarkers that can predict and detect early signs of sepsis-induced AKI would hopefully improve our ability to predict certain complications such as worsening kidney functions and make proactive changes such as limiting nephrotoxic medications to decrease the risk of clinically overt renal injury [15].
Another notable finding of our study is the increased risk of pulmonary edema and cardiac arrhythmias within the CKD cohort. Although there are limited data to determine the relationship and impact of pre-transplant kidney disease and the development of pulmonary edema, studies have theorized that inflammatory cytokines, tumor necrosis factor α, macrophage inflammatory protein 2, nuclear factor-κB, chemokines, and activated innate immune cells in acute and chronic kidney injury provoke and initiate pathological cascades that lead to acute lung injury and acute respiratory distress syndrome [16]. Most centers have patients undergo a thorough cardiac work up including a baseline echocardiogram to recognize high-risk patients and correct cardiac function. In patients with known arrhythmias like atrial fibrillation, a cardiology consultation is usually sought to optimize rate/rhythm control regimens [17]. During hospitalization for allo-HSCT, CKD likely predisposes to arrhythmias due to the increased risk of electrolyte imbalances especially involving potassium and magnesium [18]. Most immunosuppressants like tacrolimus are notorious for causing hypomagnesemia, which is associated with fatal arrhythmias like torsade de pointes [19]. Finally, certain transplant-related complications such as mucositis might prevent patients from taking their home oral cardiac medications, predisposing them to increased risk of arrhythmias [8].
Overall, the combined increased risk of these complications translated into a higher risk of mortality in our study. Though our results show a slight trend of increased risk for GVHD in patients with CKD, the reasons behind this are unknown. Speculations could include possible differences in GVHD preventative regimens between the two cohorts due to renal function, e.g., switching tacrolimus with sirolimus, etc.
Although our study has an impressive sample size, it has considerable limitations, all inherent to the NIS data. Firstly, the retrospective nature of the data does not allow to establish causative relationships. Secondly, there is a lack of information regarding medication use during the hospital course, which has significant impact pertaining to CKD, especially for the type and dosing of conditioning therapy, immunosuppressants, and antibiotics. Thirdly, laboratory investigations, like on the serum creatinine level, were unavailable, and the presence of AKI was determined by ICD 10 billing, not by serum creatinine levels. Nonetheless, despite these limitations, the results provide a foundation for future research endeavors, encouraging a more nuanced exploration of the factors influencing outcomes in this vulnerable cohort of allo-HSCT recipients with even mild-to-moderate CKD.
5. Conclusions
In conclusion, clinicians should be aware of mild-to-moderate CKD as a potential independent risk factor for adverse hospitalization outcomes among patients admitted to undergo allo-HSCT. Prospective studies evaluating the benefits of a multidisciplinary approach, where continued outpatient nephrology follow-up among these patients can help determine the impact of early-stage CKD on quality of life and long-term non-relapse mortality given the myriad of challenges that come with long-term immunosuppressive medications and fluid imbalances.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/transplantology5030014/s1, Table S1: RECORD statement; Table S2: The International Classification of Diseases, 9th and 10th Revision, Clinical Modification/Procedure Coding System (ICD-9-CM/PCS) used for the analysis. Reference [20] are cited in the Supplementary Materials.
Author Contributions
M.A.U.D.: conception and design of the project, data curation, formal analysis, software, and writing of the original manuscript draft. Q.A.: literature search, data curation, validation, and writing of the manuscript. M.S.U.A.: construction of tables and writing of the manuscript. M.S.: data curation, validation, writing of the manuscript. M.U.M.: formal analysis, validation, supervision and review and editing of the final draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: HCUP Database Online Ordering—Database Catalog (ahrq.gov).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wildes, T.M.; Stirewalt, D.L.; Medeiros, B.; Hurria, A. Hematopoietic stem cell transplantation for hematologic malignancies in older adults: Geriatric principles in the transplant clinic. J. Natl. Compr. Canc. Netw. 2014, 12, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Thakar, M.S.; Broglie, L.; Logan, B.; Artz, A.; Bunin, N.; Burroughs, L.M.; Fretham, C.; Jacobsohn, D.A.; Loren, A.W.; Kurtzberg, J.; et al. The Hematopoietic Cell Transplant Comorbidity Index predicts survival after allogeneic transplant for nonmalignant diseases. Blood 2019, 133, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- HCUP-US NIS Overview. Available online: https://www.hcup-us.ahrq.gov/nisoverview.jsp (accessed on 16 June 2023).
- Trend Weights for HCUP NIS Data. Available online: https://www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp (accessed on 16 June 2023).
- Ammad Ud Din, M.; Saeed, H.; Shahzad, M.; Liaqat, H.; Sweet, K. The impact of mild-to-moderate chronic kidney disease on hospitalization outcomes in patients with acute myeloid leukemia. Leuk. Lymphoma 2023, 64, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Ammad Ud Din, M.; Mahmud, A.; Mostafa, M.; Shahzad, M.; Liaqat, H.; Pinilla-Ibarz, J.; Jaglal, M. Risks and outcomes of hospitalizations in patients with chronic lymphocytic leukemia admitted with immune thrombocytopenia: An analysis of the National Inpatient Sample Database. Ann. Hematol. 2023, 102, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Toprak, S.K.; Atilla, P.A.; Atilla, E.; Demirer, T. An overview of infectious complications after allogeneic hematopoietic stem cell transplantation. J. Infect Chemother. 2016, 22, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Hirose, N.; Tachibana, T.; Izumi, A.; Sato, S.; Tadera, N.; Tamai, Y.; Kanamori, H.; Tanaka, M.; Nakajima, H. Bridging to transplant and post-transplant maintenance therapy with FLT3 inhibitors in patients with relapsed or refractory FLT3 mutated acute myeloid leukemia. Hematology 2023, 28, 2220518. [Google Scholar] [CrossRef] [PubMed]
- Farhadfar, N.; Dias, A.; Wang, T.; Fretham, C.; Chhabra, S.; Murthy, H.S.; Broglie, L.; D’Souza, A.; Gadalla, S.M.; Gale, R.P.; et al. Impact of pretransplantation renal dysfunction on outcomes after allogeneic hematopoietic cell transplantation. Transplant. Cell. Ther. 2021, 27, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Shouval, R.; de Jong, C.N.; Fein, J.; Broers, A.E.; Danylesko, I.; Shimoni, A.; Reurs, M.R.; Baars, A.E.; van der Schaft, N.; Nagler, A.; et al. Baseline renal function and albumin are powerful predictors for allogeneic transplantation-related mortality. Biol. Blood Marrow Transplant. 2018, 24, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Bodge, M.N.; Reddy, S.; Thompson, M.S.; Savani, B.N. Preparative regimen dosing for hematopoietic stem cell transplantation in patients with chronic kidney disease: Analysis of the literature and recommendations. Biol. Blood Marrow Transplant. 2014, 20, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.A.; Jorge, S.; Neves, M. Acute kidney injury in HCT: An update. Bone Marrow Transplant. 2016, 51, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Renaghan, A.D.; Jaimes, E.A.; Malyszko, J.; Perazella, M.A.; Sprangers, B.; Rosner, M.H. Acute Kidney Injury and CKD Associated with Hematopoietic Stem Cell Transplantation. Clin. J. Am. Soc. Nephrol. 2020, 15, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, P.; Coelho, L.; Dal-Pizzol, F.; Ferrer, R.; Huttner, A.; Conway Morris, A.; Nobre, V.; Ramirez, P.; Rouze, A.; Salluh, J.; et al. How to use biomarkers of infection or sepsis at the bedside: Guide to clinicians. Intensive Care Med. 2023, 49, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Farha, N.; Munguti, C. A Dramatic Presentation of Pulmonary Edema Due to Renal Failure. Kans. J. Med. 2020, 13, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Singh, J. Cardio-oncology and transplantation for acute myeloid leukemia. Best Pract. Res. Clin. Haematol. 2023, 36, 101465. [Google Scholar] [CrossRef] [PubMed]
- Turakhia, M.P.; Blankestijn, P.J.; Carrero, J.J.; Clase, C.M.; Deo, R.; Herzog, C.A.; Kasner, S.E.; Passman, R.S.; Pecoits-Filho, R.; Reinecke, H.; et al. Chronic kidney disease and arrhythmias: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur. Heart J. 2018, 39, 2314–2325. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, S.D.; Sankarasubbaiyan, S.; Gross, M.D.; Jeevanantham, V.; Monk, R.D. Tacrolimus-associated hypomagnesemia in renal transplant recipients. Transplant. Proc. 2006, 38, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sørensen, H.T.; von Elm, E.; Langan, S.M.; The RECORD Working Committee. The Reporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med. 2015, 10, e0125620. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).