Abstract
There is uncertainty about the best approach to replacement treatment for kidney transplant recipients with chronic terminal graft dysfunction, since a retransplant could be performed before the resumption of dialysis, thus avoiding this treatment and the dilemma of whether or not to suspend immunosuppressive therapy. However, there is limited experience in pre-emptive repeat transplantations, and none from deceased donors. This study aims to assess the results of a pre-emptive retransplantation program with brain-dead deceased donors. We designed a retrospective matched cohort study, including 36 recipients in the pre-dialysis group and 36 controls who were already on dialysis, matched for donor age and transplant date, which could not differ by more than 7 days between pairs. The variables used to standardize the cohorts were donor and recipient age and sex, blood group, duration of the first graft, time on the waitlist to receive the second graft, cold ischemia time, induction and maintenance of immunosuppression, and HLA antibodies (-) prior to retransplantation. The efficacy variables were early graft loss, acute rejection, delay in graft function, renal function at the end of follow-up, survival time, and recipient and graft survival at 24 and 48 months’ follow-up. The pre-dialysis group presented a significantly shorter waitlist time, lower immunization status, and a significantly longer duration of the first graft than the control group. The percentage of recipients who presented early graft loss, delayed renal function, or acute rejection was similar between groups. No significant differences were observed in kidney function or in the survival of the recipient or graft. Retransplantation yields good outcomes in patients with terminal chronic dysfunction, helping to avoid recurrence to dialysis, shortening the time spent on the waitlist, reducing the risk of producing antibodies, and resolving the dilemma of whether or not to stop immunosuppression.
1. Introduction
Kidney transplant is considered the best treatment option for patients with end-stage chronic kidney failure. Transplant patients survive longer [1], have better quality of life [2], and consume fewer health resources than patients who remain on dialysis [3].
In December 2020, there were a total of 33,784 functioning kidney transplant recipients in Spain [4]. Kidney transplant is not a definitive treatment for patients with chronic kidney failure, as about 4% of them have to restart dialysis annually, according to data published by the United States Renal Data System (USRDS) [5]. Transplant failure is the fifth cause of entry into dialysis in this setting [5]. Extrapolating these data to Spain, more than 871 patients per year would restart dialysis after loss of graft [4].
There is uncertainty about the best approach to replacement treatment for kidney transplant recipients with chronic terminal graft dysfunction, since a retransplant could be performed before resuming dialysis [6,7,8,9], and a dilemma exists regarding the discontinuance of immunosuppressive therapy [2,3]. Different studies have shown longer survival in patients and grafts receiving a first pre-emptive transplant than those transplanted after dialysis [10,11,12]. While this evidence was not confirmed in the only Spanish series published on the subject, other advantages have been observed for pre-emptive transplantation [8,13], together with a harmful effect of pre-transplant dialysis time [7,12,14,15]. However, different conditions exist in the case of retransplantation, such as prior immunosuppression, the dilemma of its discontinuance [16], nutritional status [17], and the presumed chronic inflammation in the potential recipient [17,18].
This group of patients represents a high-risk group on returning to dialysis, since they have greater morbidity and mortality in the first months due to infectious and cardiovascular causes [3]. Patients who continue with immunosuppressive treatment have a 3.4 times higher risk of mortality than those in whom immunosuppression is withdrawn [19], but the latter group are more likely to develop sensitization and subsequently less likely to have a successful retransplantation; moreover, they often develop graft intolerance syndrome [9,20]. Pre-emptive transplant in transplant graft failure patients would solve all these problems since the patient would not return to dialysis and would thus maintain their immunosuppression. However, if pre-emptive transplantation is performed too soon, the recipient could lose months to years of renal function.
At the same time, there is an ethical dilemma about transplanting a patient who is not yet on dialysis when there are other potential candidates already undergoing replacement treatment. This debate would be forestalled by the existence of a living donor [6,7,8], which would remove the competitive aspect, but a deceased donor could be used as long as there were no candidates already on dialysis for that graft. Then, the denial of a renal transplant to a qualified patient on dialysis would never happen.
This study aims to assess the outcomes of a pre-emptive retransplantation program based exclusively on deceased donors.
2. Materials and Methods
We designed a retrospective matched cohort study. Inclusion criteria were all adult transplant patients in pre-dialysis who received a second kidney transplant between 2008 and 2021 at our hospital (pre-dialysis group) or adult patients who received a second transplant when they were on renal replacement therapy (control group). Pre-emptive transplant in transplant graft failure patients was defined when the recipients’ first functioning graft was in a situation of chronic dysfunction and they did not resume dialysis for more than 7 days. In the pre-dialysis group, patients had to have a pre-transplant glomerular filtration rate of less than 15 mL/min (measured by CKD-EPI), an estimated time to entry into dialysis of less than 6 months, and post-transplant follow-up of more than 12 months. Each case in the pre-dialysis group was matched to a control according to donor age and transplant date, which could not differ by more than 7 days.
The general immunosuppressive treatment regimen at the time of kidney transplantation consisted of anti-thymocyte globulin (thymoglobulin); prolonged-release tacrolimus (0.2 mg/kg/day, with adjustment of subsequent doses to maintain a tacrolimus trough concentration of 8 ng/mL to 10 ng/mL during the first month and subsequently 6 ng/mL to 8 ng/mL); mycophenolate mofetil (500 mg/12 h orally) or sirolimus (2 mg/24 h); and tapering doses of corticosteroids. The choice of sirolimus instead of mycophenolate mofetil was due to a change in the immunosuppression protocol in the department, which took place in 2017. Sirolimus was avoided if the possible transplant recipient had chronic hypertriglyceridemia or obesity.
The initial dose of thymoglobulin was 1 mg/kg/24 h and its effect was monitored daily by the count of peripheral T lymphocytes. Flow cytometric analysis of T cells was performed using antiCD3 fluorescence monoclonal antibodies (Caltag, BD Biosciences, San Jose, CA, USA) and a cytometer (FACS Canto II flow cytometer, Becton Dickinson, San Jose, CA, USA). Then, the dose of thymoglobulin was omitted if the T-cell count had decreased to 10 cells/L.
The measure of tacrolimus and sirolimus levels was carried out by enzyme immunoassay (Dimension®, Siemens, Erlangen, Germany).
Cohort standardization variables were donor and recipient age and sex, blood group, duration of the first graft, time on the waitlist to receive the second graft, cold ischemia time, induction and maintenance immunosuppression, and HLA antibodies prior to retransplantation. These data were extracted from an electronic medical record system belonging to the Alicante General University Hospital, Alicante (Spain). Immunological risk in the recipients was assessed as donor–recipient histocompatibility; the rate of class I and II antibodies pre-transplant measured by single antigen Luminex technology (One Lambda, West Hills, CA, USA); and the percentage of patients who received rituximab at induction following immunological risk assessment by the Immunology Department.
The duration of the first transplant was considered as the period of time from the day when the first transplant was performed to the day of the first dialysis or the date when the second transplant was performed in transplant graft failure patients.
The time spent on the waitlist was defined as the period of time from the day when the patient was enrolled on the waitlist to the day when the patient was transplanted.
Cold ischemia time was considered to be the period of time in hours between the interruption of blood supply to the graft inside the donor and the reestablishment of blood to the graft inside the recipient.
The efficacy variables were the incidence of early graft loss (within 48 h), acute rejection (on a histological basis), delay in graft function (evaluated as the need for dialysis in the first week post transplant), kidney function at the end of follow-up (serum creatinine level, glomerular filtration rate calculated by CKD-EPI, and albuminuria–creatinine ratio), survival time, and actual graft and recipient survival at 2 and 4 years’ follow-up. These data were extracted from an electronic medical record system belonging to Alicante General University Hospital, Alicante (Spain).
For the statistical analysis, continuous variables were expressed as means and 95% confidence intervals (CIs), or as medians and interquartile ranges (IQRs), depending on the normality of the distribution, and they were compared using Student’s t-test or the Mann–Whitney U test, respectively. Categorical variables were described as absolute and relative frequencies and compared using Fisher’s exact test. A Kaplan–Meier survival analysis was also performed to analyze the percentage of patients and grafts lost during follow-up. Both groups were compared using the log-rank statistical test. The significance level was set at 0.05. The statistical analysis was undertaken with SPSS software (version 24).
Informed consent was obtained from all subjects involved in this study through an interview between the transplant nephrologist and the recipient in which the patient was informed of the aim of the study.
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Alicante Hospital General (recorded on 28 April 2021, number 2021-4).
3. Results
The total sample was 72 kidney recipients: 36 in the pre-dialysis group and 36 matched controls already on dialysis. The median glomerular filtration rate of the pre-dialysis group before transplantation was 10.5 mL/min (IQR 9.5–12.5). Median follow-up in the pre-dialysis group was 29.5 months (IQR 15.0–47.7) versus 32.0 months (IQR 14.7–45.7) in the control group (p = 0.7).
The time spent on the waitlist was significantly lower in the pre-dialysis group compared to the controls (mean 2 months, 95% CI 0.8–4.0 versus 5 months, 95% CI 2.8–11.0; p = 0.002), but the duration of the first graft was significantly longer (mean 138.50 months, 95% CI 80.3–173.50 versus 88.6.0 (1.0–145.5) 95% CI p = 0.047). No significant differences were observed between the groups in terms of donor age or sex, but recipients in the control group were younger than those in the pre-dialysis group 52.3 (48.4–56.1) versus 58.2 (54.3–62.1) p = 0.037.
Both groups were similar with regard to cold ischemia time, the rate of induction with anti-thymocyte globulin, blood groups, HLA antibodies, and the use of different immunosuppressants, although the use of rituximab was twice as high in the control group (33.3% vs. 16.3%). Standardization variables are shown in Table 1.

Table 1.
Characteristics of the different groups of recipients receiving their second grafts.
The incidence of delayed graft function was much lower in the pre-dialysis group than in controls (3.3% vs. 16.7%), but the difference did not reach statistical significance (p = 0.20). Likewise, no significant differences were observed with regard to acute rejection (pre-dialysis 10% vs. controls 6.7%; p = 1.000) or early graft loss (pre-dialysis 0% vs. controls 6.7%; p = 0.49). Kidney function was similar between groups at the end of follow-up (Table 2).

Table 2.
Variables of efficacy.
Pre-dialysis recipients survived much longer than controls (mean 131 months, 95% CI 94–168 vs. 97 months, 95% CI 69–124), although without statistical significance (p = 0.70). Graft survival showed a similar pattern (mean 131 months, 95% CI 94–168 vs. 74 months, 95% CI 47–102; Table 2), although this difference was not significant (p = 0.052). Actual patient survival rate at 2, 4, and 5 years was 97.2% in both groups (Figure 1), but graft survival was 97.2% in the pre-dialysis group compared to 81.8% in the control group at 2, 4, and 5 years (Figure 2).
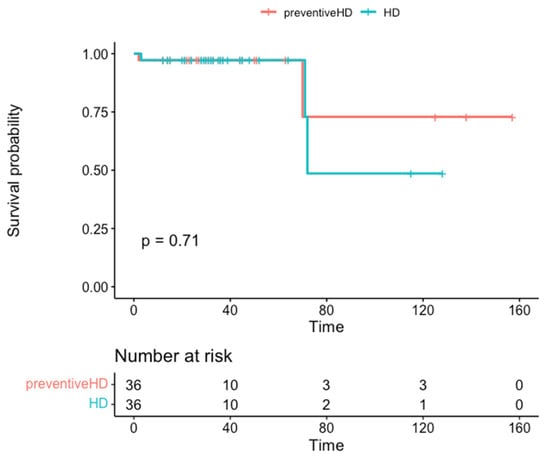
Figure 1.
Patient survival in months stratified by group.
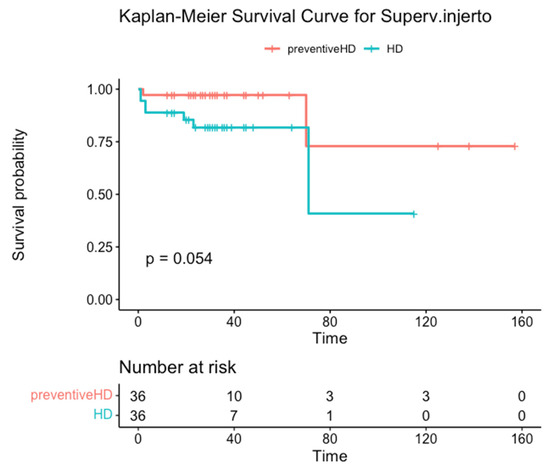
Figure 2.
Graft survival in months stratified by group.
4. Discussion
Using data from the US Renal Data System from 1990 to 2000, Goldfarb et al. came to the surprising conclusion that survival in patients receiving a pre-emptive retransplanted graft was lower than in patients retransplanted after a period of dialysis. The authors justified these striking results by citing the possible benefit of temporary dialysis in recovering the uremic state of the recipient and the possible state of malnutrition in candidates for retransplantation who did not undergo dialysis [21]. In contrast, Johnston [6], using that same database years later (1995 to 2007), concluded that pre-emptive transplant recipients had better survival thanks to the reduced number of deceased patients with a functioning graft. They attributed the discrepancy in results to the heterogeneity of the Goldfarb series [21], since those authors had included adult and pediatric recipients, third and fourth transplants, and reno-pancreatic transplants, which could explain the poor outcomes in the pre-emptive (preventive) transplant series. On the other hand, Johnston et al. used similar selection criteria to ours, including only simple second transplants in adults [6].
In a multicenter study in France, Girerd et al. concluded unequivocally that pre-emptive transplant from deceased donors in transplant graft failure patients results in better graft survival than retransplantation after dialysis, similar to that from a living donor, whether pre-emptive or not [7]. This evidence prompted us to perform pre-emptive transplants from deceased donors in transplant graft failure patients when the recipients did not have a living donor and there was no other candidate already on dialysis for that graft. In our series, as in Girerd et al.’s [7], we also observed a notable, borderline significant increase in graft survival.
The lower incidence of delayed graft function in our pre-dialysis group (3.3% vs. 16.7% in controls) is consistent with other published series and can be attributed to the existence of residual renal function [6,7,9,15]. In addition, the duration of the first transplant was significantly longer in our pre-dialysis group, in keeping with previous studies [6,7,9], possibly indicating a greater immunological tolerance in these patients [6,7,9], as reflected in a lower percentage of patients with pre-formed antibodies prior to transplant [6,7,8,9], and in our case, a lower need for rituximab on induction. A longer duration of the first graft has also been associated with better survival of the second [7,22].
It is notable that comparisons of pre-emptive versus non-pre-emptive retransplant cohorts find patient sensitization is significantly higher in the latter group [6,7,8,9], which could be due to the decrease in or suspension of pharmacological immunosuppression in patients reinstated on dialysis [20]. This increase in sensitization makes subsequent retransplantation difficult for this group, as demonstrated by the significantly longer time spent on the waitlist for retransplantation in published series [7,9,15], as in ours. This risk confers an advantage to pre-emptive transplants, in which immunosuppression is maintained until retransplantation, avoiding both sensitization and possible graft intolerance syndrome in the recipient. The percentage of our transplant graft failure patients treated with rituximab was far lower than the percentage in the control group, which could show a higher immunological risk in this group.
Several authors have concluded that the longer the time on the waitlist, the worse the patient and graft survival outcomes in retransplantation [12,21,22]. Wong et al. observed a linear relationship between time on waitlist before the second transplant and all-cause mortality, where each year on the waitlist increased the risk of death by 13% [15]. These data, together with the 3.4-fold higher risk of mortality in patients who continue rather than stop immunosuppressive treatment on dialysis [19], plus the difficulty in managing immunosuppressants in dialysis [23] and the side effects of immunosuppressive treatment such as cataracts and fractures [24], warrant efforts to minimize this waiting time as much as possible, an objective that would be fully met with pre-emptive transplant in transplant graft failure patients. Moreover, pre-emptive transplant in transplant graft failure patients would also avoid the negative outcomes associated with dialysis, such as the placement of catheters, the frequent onset of depression at the time of restarting dialysis [25,26], and the high proportion of patients who refuse a new transplant [3].
Published pre-emptive series describe a significantly higher percentage of transplant recipients with a living versus deceased donor [6,7,8], with the largest difference reported by Florit et al. (89.9% vs. 19.3%, respectively) [8], as this type of transplant is encouraged so as not to lengthen the waiting time for patients already on dialysis, given the limited supply of deceased donors. In Girerd et al.’s series, the difference is smaller, although still significant (29% vs. 7%) [7]—the same as observed in the US registry [6]. In our case, all donors were deceased, which affords both great homogeneity to the series, avoiding possible bias, and also a certain degree of originality compared to other studies.
The potential for the adoption of this strategy holds promise, with published series showing a steady increase in the proportion of pre-emptive transplants in transplant graft failure patients over time [6,7]. Girerd et al. reported that 2.8% of retransplantations were pre-emptive from 2000 to 2004, rising to 8% in 2005–2009 and to 9% in 2010–2014 (p = 0.003) [7]. In the USA, this type of transplant increased from 15.1% in 1995–1998 to 29% in 2003–2007 [6].
Although the benefits of this approach seem certain, we consider, as do the authors of a French multicenter study [7], that ethical issues arise from retransplanting recipients who are not yet in dialysis (undoubtedly some with special characteristics) instead of patients already on replacement treatment. For this reason, we insist on performing this type of transplant only in the event that a candidate already on dialysis is not available for the graft. Then, the denial of a renal transplant to a qualified patient on dialysis can never happen.
Our study’s strengths reside in its well-matched cohorts, with very homogeneous characteristics and management, along with complete follow-up data, with none of the missingness that occurs in registry studies on the topic [6,7]. Furthermore, it is novel in that it is the first study to collect data only from recipients with deceased donors. On the other hand, the main limitation is the small size of the cohorts, resulting in a lack of statistical power to draw definitive conclusions; thus, our results are only suggestive of an association that needs to be confirmed in larger series. Although the data of the pre-emptive group are far better than those of the control group, including less delayed renal function and far longer recipient and graft survival time, the small size of the cohorts prevents these results from having statistical power.
5. Conclusions
Pre-emptive transplant in transplant graft failure patients shows good outcomes, avoids the recipient’s return to dialysis, shortens their time on the waiting list, reduces the risk of producing antibodies, and eliminates the dilemma of whether or not to suspend immunosuppression.
Our results are only suggestive of an association that needs to be confirmed in larger series to have statistical power and to arrive at definitive conclusions.
Author Contributions
Conceptualization, A.F.E.; Methodology, A.F.E., P.M.-S., F.M.M., E.G.C. and F.J.P.C.; Formal analysis, A.F.E. and P.M.-S.; Investigation, F.J.P.C.; Data curation, P.M.-S. and E.G.C.; Writing—original draft, A.F.E., P.M.-S. and F.J.P.C.; Writing—review and editing, A.F.E., P.M.-S., F.M.M., E.G.C. and F.J.P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Alicante General Hospital (recorded on 28 April 2021, approval number 2021-4).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data supporting the reported results can be found in the database of the Department of Nephrology, Alicante General Hospital Dr Balmis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McDonald, S.P.; Russ, G.R. Survival of cadaveric kidney transplants compared with those reciving dialysis treatment in Australia and New Zealand 1991–2001. Nephrol. Dial. Transplant 2002, 17, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.; Held, P.J.; Port, F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Cattran, D.C.; Fenton, S.S.A. Contemporary management of renal failure: Outcome of the failed allograft recipient. Kidney Int. 1993, 43 (Suppl. S41), S36–S39. [Google Scholar]
- Franco, A.; Mas, P.; Gonzalez, Y.; Andres, A.; Zarraga, S.; Esforzado, N.; Alonso, A.; Gonzalez, F.; Hernandez, D.; Beneyto, I. There are 33,784 functioning kidney grafts in Spain. Who monitors them and how? Transplant Proc. 2021, 53, 2672–2674. [Google Scholar] [CrossRef] [PubMed]
- USRDS. USRDS 2008 anual data report: Atlas of end stage renal disease in the United States national Institute of diabetes and digestive and kidney diseases: Bethedsa. Natl. Inst. Odf Health 2008, 147. [Google Scholar]
- Johnston, O.; Rose, C.L.; Gill, J.S.; Gill, J.S. Risks and Benefits of Preemptive Second Kidney Transplantation. Transplantation 2013, 95, 705–710. [Google Scholar] [CrossRef]
- Girerd, S.; Girerd, N.; Duarte, K.; Giral, M.; Legendre, C.; Mourad, G.; Garrigue, V.; Morelon, E.; Buron, F.; Kamar, N.; et al. Preemptive second kidney transplantation is associated with better graft survival compared with non-preemptive second transplantation: A multicenter French 2000-2014 cohort study. Transpl. Int. 2017, 31, 408–423. [Google Scholar] [CrossRef]
- Florit, E.A.; Bennis, S.; Rodríguez, E.; Revuelta, I.; De Souza, E.; Esforzado, N.; Cofan, F.; Ricart, M.J.; Torregrosa, J.B.; Campistol, J.M.; et al. Pre-emtivere transplantation in patients with chronic kidney graft failure. Transplant. Proc. 2015, 47, 2351–2353. [Google Scholar] [CrossRef]
- Girerd, S.; Girerd, N.; Aarnink, A.; Solimando, E.; Ladrière, M.; Kennel, A.; Rossignol, P.; Kessler, M.; Frimat, L. Temporal trend and time-varying effect of preemptive second kidney transplantation on graft survival: A 30-year single-center cohort study. Transplant. Proc. 2016, 48, 2663–2668. [Google Scholar] [CrossRef]
- Papalois, V.E.; Moss, A.; Gillingham, K.J.; Sutherland, D.E.R.; Matas, A.J.; Humar, A. PRE-emptive transplants for patients with renal failure. Transplantation 2000, 70, 625–631. [Google Scholar] [CrossRef]
- Prezelin-Reydit, M.; Combe, C.; Harambat, J.; Jacquelinet, C.; Merville, P.; Couzi, L.; Leffondré, K. Prolonged dialysis duration is associated with graft failure and mortality after kidney transplantation: Results from the French transplant data base. Nephrol. Dial. Transplant. 2019, 34, 538–545. [Google Scholar] [CrossRef]
- A Milton, C.; Russ, G.R.; Mcdonald, S.P. Pre-emptive renal transplantation from living donors in Australia: Effect on allograft and patient survival. Nephrology 2008, 13, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Más-Serrano, P.; González, Y.; Balibrea, N.; Rodríguez, D.; López, M.I.; Contreras, F.J.P. Una aproximación al trasplante renal anticipado de donante cadáver. Estudio de cohortes emparejadas. Nefrologia 2020, 40, 32–37. [Google Scholar] [CrossRef]
- Meier-Kriesche, H.-U.; Kaplan, B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes. Transplantation 2002, 74, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Chua, S.; Chadban, S.J.; Clayton, P.; Pilmore, H.; Hughes, P.D.; Ferrari, P.; Lim, W.H. Waiting time between failure of first graft and second kidney transplant and graft and patient survival. Transplantation 2016, 100, 1767–1775. [Google Scholar] [CrossRef]
- Gill, J.S. Mananging patients with a failer kidney transplant: How can we do better? Curr. Opin. Nephrol. Hipertens. 2011, 20, 616. [Google Scholar] [CrossRef]
- López Gómez, J.M.; Pérez Flores, Y.; Jofre, R.; Carretero, D.; Rodríguez-Benitez, P.; Villaverde, M.; Pérez-García, R.; Nassar, G.M.; Niembro, E.; Ayus, J.C. Presence of a failed kidney transplant in patients who are on hemodialysis is associated with chronic inflammatory state and erythropoietin resistance. J. Am. Soc. Nephrol. 2004, 15, 2494–2501. [Google Scholar] [CrossRef]
- Mourad, G.; Minguet, J.; Pernin, V.; Garrigue, V.; Peraldi, M.-N.; Kessler, M.; Jacquelinet, C.; Couchoud, C.; Duny, Y.; Daurès, J.-P. Similar patient survival following kidney allograft failure compared with non-transplanted patients. Kidney Int. 2014, 86, 191–198. [Google Scholar] [CrossRef]
- Gregoor, P.S.; Zietse, R.; Van Saase, J.; De Hoek, C.O.; Ijzermans, J.; Lavrijssen, A.; De Jong, G.; Kramer, P.; Weimar, W. Immunosuppression should be stopped in patients with renal allograft failure. Clin. Transplant. 2001, 15, 397–401. [Google Scholar] [CrossRef]
- Augustine, J.J.; Woodside, K.J.; Padiyar, A.; Sanchez, E.Q.; Hricik, D.E.; Schulak, J.A. Independent of nephrectomy, weaning immunosuppression leads to late sensitization after kidney transplant failure. Transplantation 2012, 94, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb-Rumyantzev, A.S.; Hurdle, J.F.; Baird, B.C.; Stoddard, G.; Wang, Z.; Scandling, J.D.; Barenbaum, L.L.; Cheung, A.K. The role of pre-emptive re-transplant in graft and recipient outcome. Nephrol. Dial. Transplant. 2006, 21, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Arnol, M.; Prather, J.C.; Mittalhenkle, A.; Barry, J.M.; Norman, D.J. Long-term kidney regraft survival from deceased donors: Risk factors and outcomes in a single center. Transplantation 2008, 86, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Altieri, P.; Sau, G.; Cao, R.; Barracca, A.; Menneas, A.; Micchittu, B.; Cabiddu, G.; Esposito, P.; Pani, A. Immunosuppressive treatment in dialysis patients. Nephrol. Dial. Transplant. 2002, 17 (Suppl. S8), S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Kiberd, B.A.; Belistsky, P. The fate of failed renal transplant. Transplantation 1995, 59, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Perl, J.; Zhang, J.; Gillespie, B.; Wikström, B.; Fort, J.; Hasegawa, T.; Fuller, D.S.; Pisoni, R.L.; Robinson, B.M.; Tentori, F. Reduced survival and quality of life following return to dialysis after transplant failure: The Dialysis Outcomes and Practice Patterns Study. Nephrol. Dial. Transplant. 2012, 27, 4464–4472. [Google Scholar] [CrossRef]
- Akman, B.; Özdemir, F.; Sezer, S.; Miçozkadioǧlu, H.; Haberal, M. Depression levels before and after renal transplantation. Transplant. Proc. 2004, 36, 111–113. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).