Abstract
Uterus transplantation (UTx) is a rapidly evolving treatment for uterine factor infertility. New centers offering this treatment must decide whether to utilize living donors, deceased donors, or both. Although limiting UTx to deceased donors eliminates the surgical risks for living donors, an adequate supply of suitable deceased uterus donors in the United States is an emerging concern. Previous studies describing the paucity of deceased uterus donors failed to consider key donor characteristics, potentially overestimating the available organ pool. To estimate the United States’ supply of deceased donor uteri; we extrapolated detailed clinical and demographic information from the regional donor datasets available from three organ procurement organizations to the national Organ Procurement and Transplantation Network donor pool. We estimate there are approximately 3700 possible and 400 optimal uterus donors annually in the United States. Given these projections and the number of women with uterine factor infertility in the U.S. who pursue parenthood through alternative strategies, we conclude that, as uterus transplant transitions from research to established clinical care, demand could quickly exceed the deceased donor supply. The liberalization of deceased donor selection criteria may be insufficient to address this imbalance; therefore, fulfilling the anticipated increased demand for uterus transplantation may require and justify greater use of living donors.
1. Introduction
Uterus transplantation (UTx) is a rapidly evolving treatment option for individuals with uterine factor infertility (UFI). The first successful surgical transplantation of a deceased uterus to a recipient was performed in Turkey in 2011, although the first live birth from a UTx occurred in 2014 from a living donor in Sweden and in 2017 from a deceased donor in Brazil [1,2,3,4]. Following early feasibility studies, there have been over 37 uterus transplants and 25 live births since 2016 in the United States (US) alone [5,6,7]. There have also been 45 additional uterus transplants performed, with 19 associated live births, recorded in the first report of the Registry of the International Society of Uterus Transplantation [3]. UTx is now performed outside of clinical trials in some US centers, given its demonstrated safety, efficacy, and reproducibility [3,8,9]. However, less than 1% of applicants to US clinical trials offering UTx have actually undergone transplantation. Gestational surrogacy is the only alternative reproductive therapy permitting patients with UFI to have a biological child—a treatment not without its own drawbacks, including considerable care coordination, a limited pool of surrogates, prohibitive costs, legal restrictions that vary regionally, and personal or cultural concerns. Despite these limitations, we estimate that over 900 individuals with UFI attempt to achieve parenthood through the use of a gestational carrier per year [10]. Given the number of individuals with UFI currently seeking fertility care to parent a biological child via surrogacy, and the more than 5000 individuals who applied to the three US-based UTx clinical programs, coupled with the ongoing success of UTx, growing demand for this procedure is imminent. It is critical that US transplantation centers have an accurate estimate of the number of donors that may be available for this life-propagating procedure.
Prior estimations of deceased donor availability for UTx in the US likely overestimated the available donors as they were extrapolated from national data with limited medical details about the donor. Estimations were based on donor sex and age alone, without consideration of other critical selection parameters such as a history of gynecologic disease and/or significant medical comorbidities [11]. A subsequent French analysis similarly raised concerns about a small available national deceased donor pool [12]. Though these studies are informative, there are country-specific considerations, including unique distribution channels for organ allocation and the need for explicit consent for uterine donation, and practical considerations, including the vast geography of the US, that would benefit from a country-specific analysis. Accurate estimates of potential deceased UTx donors will be essential for the advancement of the field to inform the establishment of UTx centers, the performance of organ procurement organizations, and United Network for Organ Sharing (UNOS) allocation policies [13]. In this study, we provide a detailed estimate of the potential, appropriate, and optimal deceased uterus donors in the United States based on regional and national organ procurement data.
2. Material and Methods
Although the American Society for Reproductive Medicine (ASRM) provided early guidance for the recommended criteria uterus donors should meet, specific requirements vary by center and evolving experience challenges some initial recommendations [8]. To build our model, we triaged characteristics to define “possible”, “appropriate”, and “optimal” uterus donors based on ASRM recommendations and the growing body of clinical experience. In our model, “possible” uterus donors are at minimum female and donating after brain death (DBD). The ischemic tolerance of human uterine grafts remains undefined, unlike more commonly transplanted organs such as the kidney or liver [14]. Therefore, deceased donors donating after circulatory death (DCD) are currently excluded, given the increased warm ischemic time and other challenges associated with organ transplantation from this group [15]. “Appropriate” donors are “possible donors” who are of reproductive age (18–50 years) without active hepatitis or diabetes [16]. “Optimal” donors are “appropriate donors” who are less than 45 years old (given the risk of age-related cardiovascular disease compromising the vascular integrity of the graft), non-smokers, parous, and without gynecological disease (e.g., stage III/IV endometriosis, cervical dysplasia) or pregnancy contraindications (e.g., previous obstetrical complications, endometrial ablation, or Asherman syndrome). The final criterion defining an “optimal” donor is specialized consent. Uterus transplants are currently classified as vascularized composite allograft (VCA) transplants. VCA transplantation is a relatively nascent field encompassing diverse multi-tissue allografts including the face, upper limb, larynx, abdominal wall, penis, and the uterus. Although VCAs are classified as organs, organ donation consent in most registries does not routinely include consent for VCA-designated organs. The Organ Procurement and Transplantation Network (OPTN) policy defining VCAs as “human organs” implemented in 2014 sought to increase transparency and equity in the procurement and distribution of these grafts [17]. In accordance with this policy, the OPTN requires a separate and specific authorization for any VCA donation by the donor or surrogate decision maker following solid organ donation consent [18,19].
To further examine efforts to increase the available donor pool, we calculated the number of added donors when “increased-risk” donors were included. We identified donors with a Public Health Service increased risk (PHS-IR) designation, e.g., those at increased risk for hepatitis B, C, and human immunodeficiency virus (HIV) [20]. These donors were initially excluded, and a separate analysis was performed, further categorizing donors as “appropriate” and “optimal” in this subgroup.
The OPTN national annual report lacks the clinical and demographic details required to determine the number of donors in the “optimal” category. Therefore, regional procurement organizations, which include more granular information about donors, were utilized to generate a more detailed understanding of the size and composition of the “possible”, “appropriate”, and “optimal” donor pools nationally. To estimate the number of regional donors in each category, we reviewed clinical and demographic data from three procurement organizations (Gift of Life Donation Program [GLDP], Philadelphia, PA, USA; LifeGift, Houston, TX, USA; Southwest Transplant Alliance [STA], Dallas, TX, USA). These specific organizations were selected because of their close geographic proximity to active US uterus transplant centers. To assess national availability, we used annual data provided by the OPTN. Sufficient donor information was provided by the OPTN to determine the number of “possible” and “appropriate” donors nationally; however, in order to estimate the number of “optimal” donors available nationally we extrapolated from the data generated based on the regional datasets. The OPTN data system includes information on all donors, wait-listed candidates, and transplant recipients in the US, submitted by the members of the OPTN.
This study was deemed exempt by University of Pennsylvania and Baylor University Medical Center Institutional Review Boards.
3. Results
3.1. Regional Data
The 2017 regional data from Houston, Dallas, and Philadelphia indicated 565 deceased organ donors in the GLDP registry, 381 in LifeGift, and 385 in STA, for a total of 1331 male and female donors (Figure 1A). Donor demographics were largely comparable between the three OPOs. Overall, one third of donors in these registries were female and donated after brain death, meeting the criteria for “possible” uterus donors (437/1331). The proportion of reproductive-aged donors was 51% in GLDP, 58% in LifeGift, and 60% in STA, and PHS-IR donor prevalence was the highest in GLDP (53%) compared to LifeGift (26%) and STA (23%). While 10% (135/1331) of donors met the “appropriate” criteria, only 3% (37/1331) were “optimal” candidates. Most donors or their surrogate decision makers provided special authorization for VCA transplantation in the regional OPO organizations (29/33, 88% in GLDP; 45/54, 83% in STA; and 40/48, 83% in LifeGift).
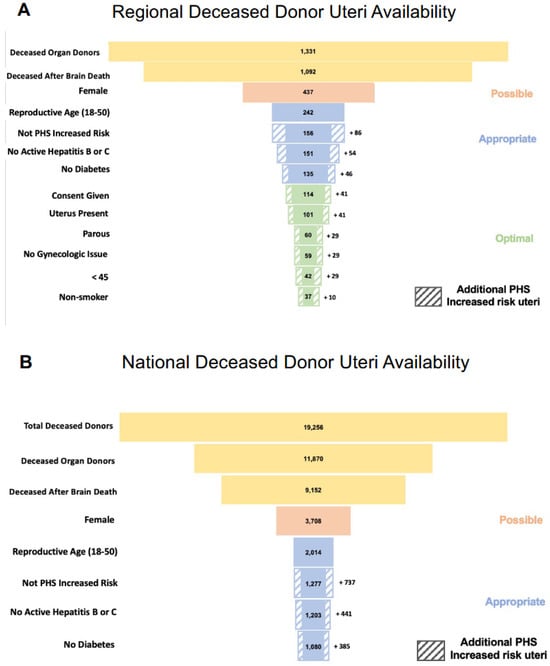
Figure 1.
Panel (A) demonstrates the theoretical regional deceased donor uteri that would be available based on the “possible”, “appropriate”, and “optimal” criteria presented in this manuscript. Panel (B) similarly shows the projected number of available deceased donor uteri. The striped boxes in both panels demonstrate the additional number of available uteri if Public Health Service increased risk (PHS-IR) designated donors were to be eligible.
Of note, 6% (86/1331) of OPO deceased donors were DBD and PHS-IR reproductive-aged females. Inclusion of all PHS-IR donors yielded 46 additional “appropriate” candidates (3% of all deceased donors). Inclusion of only the lowest risk PHS-IR donors (excluding those with active intravenous drug use) yielded 10 additional candidates (1% of all deceased donors).
3.2. National Data
Annual OPTN data from 2019 indicated 19,256 US organ donors, of which 62% were deceased (11,870) (Figure 1B). Almost 80% of deceased donors were DBD (9152/11,870) and approximately 40% of DBD donors (3708/9152) were female. Nationally, we estimate at most only 3708 “possible” (32%) and 1080 “appropriate” (8%) uterus donors annually. Given that the granular data required to determine if donors meet the criteria to be considered “optimal” is not captured in the OPTN annual report, we extrapolated our regional findings to the publicly reported national data. Assuming that 3% of all deceased donors are “optimal” uterus donors, as was observed regionally, only 356 “optimal” uterus donors would be available nationally each year.
Of the 11,870 deceased donors, 737 are DBD, PHS-IR reproductive-aged females. Inclusion of all PHS-IR donors would yield 385 additional candidates, whereas the inclusion of only the lowest-risk PHS-IR donors would yield 119 additional candidates.
4. Discussion
Our estimates of “possible”, “appropriate”, and “optimal” deceased uterus donors complement prior donor supply estimates and portend a future in which UFI treatment, like many interventions reliant on organ transplantation, could be inaccessible due to inadequate organ supply [11]. As demand increases, a data-driven approach to increase organ availability is paramount. Based on this study, we demonstrate that, among deceased donors, female sex and reproductive age most significantly limit the donor pool in regional and national OPOs. Efforts to expand the donor pool include broadening age restrictions to include older donors. However, this may sacrifice graft survival and function for only modest gains. Although births have occurred after uterus donation from living, parous donors in their sixties [3,21], there has also been a reported case of a uterus from a 61-year-old donor that demonstrated a profound resistance of the uterine arteries following removal and poor intraoperative perfusion testing, ultimately leading to abortion of the transplantation [9]. Age-related vascular pathology may cause immediate graft failure or potentially impair vascular-dependent uterine functions, including implantation, placentation, and fetal growth, due to the high risk of vessel narrowing or thrombosis [16]. In our experience, the average age of deceased uterus transplant donors in the US was 29 years and the oldest donor was only 45 years old. Living donors are often older than deceased donors, as they must have completed their own childbearing. However, there is more opportunity to thoroughly assess candidates for cardiovascular disease or risk factors and obtain advanced vascular imaging to identify age-related vasculopathy [7,22]. Deceased donors do not have the opportunity to undergo invasive vascular testing. Given this, we instead chose to adopt a conservative age cutoff of 45 years for optimal donors, given that the known lifetime risk of developing cardiovascular disease approaches 50% among women aged 50 and above [23].
Parity requirements reduce the number of available donors most significantly within the “optimal” stratum. The importance of parity is greatly debated, as live births have occurred using nulliparous donors [24]. However, given the significant resources required to undergo UTx, some assurance that the organ previously achieved an uncomplicated live birth is reasonable.
There is limited information about donors who consent to and complete VCA organ transplantation and those who decline VCA organ transplantation [25,26]. There is also little understanding of the implications additional authorizations incur on the consenting process or overall donor pool size. At the regional level, we observed more than 80% of eligible donors and surrogate decision makers ultimately provided a specific authorization for VCA donation. UTx has largely benefited from its formal designation as a VCA, and an organ regulated by the OPTN. However, specific authorization may produce another unnecessary barrier, without the benefits of risk mitigation that are needed for other VCA transplants. Unlike face or limb transplants, uterus donation does not alter the appearance of the donor, nor does it carry the future risk of violating donor anonymity by posthumous facial or fingerprint recognition, begging the question of the need for specialized authorization. Currently, the Department of Motor Vehicles is responsible for most donor registrations and does not provide an opportunity to specifically authorize VCA donations. As the number of uterus transplants continues to rapidly eclipse the number of other VCA transplants performed in the United States, there may be a need to reevaluate the relevance and necessity of specialized consent [25].
The use of PHS-IR donors has been approved by UTx programs as another potential opportunity to reduce organ non-utilization, although, at this time, a UTx from a PHS-IR donor has not been reported [27,28,29]. The ongoing opioid epidemic coupled with rising rates of intravenous drug overdose has led to a higher proportion of increased-risk donors [30]. Although PHS-IR donors carry increased risks of recently acquired viral infections and may inadvertently cause transmission despite negative serologic testing, the advent of nucleic acid testing (NAT) significantly reduces this risk [31]. An OPTN study suggested greater non-utilization of otherwise high-quality organs as a result of this designation. Non-utilization of these organs likely has a marginal risk reduction benefit; comparable rates of unexpected HIV, HBV, and HCV transmission and graft survival were noted independently from the deceased donor’s a priori designated risk [28]. Regardless of risk, our analysis suggests that liberalization to include PHS-IR donors will still not sufficiently increase the organ supply.
Donor body mass index (BMI) limits and cytomegalovirus (CMV) status are stipulated by some UTx programs, but these considerations were not included in the present analysis to estimate the available donor pool. The relevance of donor BMI remains controversial. Given that 40% of all US women are obese (BMI 30 kg/m2 or greater), incorporating BMI cutoffs would likely further restrict organ availability without a proven benefit [32]. Of the 12 deceased donor uterus transplants performed in the United States (as of late 2021), the mean BMI was 28.5 kg/m2 and 58% were obese. Similarly, the implications of CMV discordance for recipients remain understudied, particularly after a uterus transplant [33,34]. CMV reactivation or seropositivity have unique risks for pregnancy and offspring [35] and may contribute to suboptimal delays in post-operative embryo transfer. The seroconversion of a CMV-negative recipient following the transplantation of a uterus from a CMV-positive donor has been reported [36]. The recipient was successfully treated with ganciclovir and after negative PCR testing went on to have a healthy, unaffected livebirth—evidence that discordance and seroconversion can be successfully managed after transplantation [36]. Based on OPTN data, approximately 70% of deceased organ donors are CMV-positive. Though small, findings from our cohort of US deceased donors mirror this estimation; 9 of 12 donors (75%) were CMV-positive (Table 1).

Table 1.
Preoperative recipient and donor demographics with associated intraoperative surgical and postoperative outcomes.
Historically, most deceased donor uterus transplants have involved regional matches to avoid increased ischemia time due to long-distance transport. The optimal cold ischemia time limits for UTx have not been established. Specialized centers can better match supply and demand if procurement teams can travel farther to retrieve organs. The Cleveland Clinic and University of Pennsylvania expanded their donor radius beyond local OPO and have both recovered uteri from OPOs in nearby states, resulting in successful transplantations and live births.
Evidence of limited optimal donor availability is clearly seen in the time recipients spent awaiting a uterus transplant in the US (Table 1). The median days on the waitlist was 135, although this ranged from 1 to 610. Moreover, one third of the recipients waited over a year to receive a deceased donor UTx.
Though the focus of this study was to rigorously estimate the deceased donor pool and evaluate efforts to optimize its size, the most effective strategy to address the mismatch between supply and demand is likely the inclusion of living donors. In fact, the initial successful human uterus transplant trial in Gothenburg included exclusively living donors; worldwide, more than 80% of UTx procedures have been performed using living donors [35]. The inclusion of living donors affords medical staff time to comprehensively evaluate the donor, including a complete obstetric/gynecologic history, preoperative imaging of the intrauterine cavity and uterine vasculature, and to schedule the surgery at the convenience of the donor, recipient, and surgical team. The major disadvantage of using living donors is the risk of complications to the donor. Reviews estimate the risk of Clavien–Dindo Grade IIIb complications to the donor (those requiring intervention or repair under anesthesia) are between 11.1% and 17.2% [35,37]. However, it is reasonable to assume that uterus transplantation will mirror the evolution of other organ transplants and that donor complications will decline with increasing center experience [5,38]. Regardless, the use of living donors must be pursued with caution, proper planning, and informed consent before they can bridge the gap between supply and demand.
Other studies have reported comparable findings about the relatively limited pool of uteri available from deceased donors, supporting our conclusion about living donors [11,12]. One study estimating available grafts in France suggested expanding donor criteria by raising the BMI cutoff to 35 and the age limitations to 18–60. Notably, this analysis did not include parity in their identification of ideal candidates. The current study shows that parity was one of the greatest contributors to donor attrition in the optimal category. Even their very ideal donor, which was restricted to a normal BMI, failed to incorporate parity.
There are important limitations to acknowledge in this analysis. We extrapolated proportions from regional to national data, which does not account for the regional variability present in donor populations. We did observe a similar proportion of “possible”, “appropriate”, and “optimal” donors in each of the OPOs studied, despite them covering distinct regions; however, the difference in the proportion of PHS-IR donors in the two regions demonstrates that regional differences exist and caution must be used in applying these regional trends nationally. A more precise estimate of the national availability of organs for uterus transplant would require OPOs to collect and record additional donor characteristics that are currently not captured by the OPTN. Additionally, the number of deceased donors available has consistently increased over time and it is possible that this will alter the supply and demand ratio for uterus transplant in the future. Finally, although this analysis includes important donor features that could impact their suitability for uterus transplant, it is not all-encompassing; important aspects of donor management including, but not limited to, donor downtime, length of hospital stay, the presence of concomitant infections, and the use of medications to maintain hemodynamic stability were not considered in this analysis.
In summary, the broader adoption of uterus transplantation may face the recurring challenge experienced by other types of organ transplantation: demand threatens to exceed organ supply. Our results showcase the importance of and opportunity for increasing organ availability by minimizing deceased donor organ discard and promoting the broad sharing of organs across geographic borders. Another potential way to minimize organ discard is to include DCD donors in the pool of uterus donors. Despite these strategies, the incorporation of living donors will likely be necessary to alleviate the supply constraints caused by an exclusive deceased donor approach and to provide UTx to individuals living in regions of the country without a regional UTx center (e.g., the west coast). This dilemma in uterus transplantation is not unique to the US [11,35,38], and as a field we must heed the call to develop organ allocation systems [13,39] to ensure equitable access to this life-generating organ transplant.
Author Contributions
Conceptualization, K.O., J.W., S.W., R.H., G.T., S.K., T.F., R.F., N.L., A.T. and L.J.; investigation, K.O., E.G.R., J.W., S.W., R.H. and L.J.; writing—original draft preparation, K.O., E.G.R. and L.J.; writing—review and editing, J.W., S.W., R.H., G.T., S.K., T.F., R.F. and N.L.; supervision, K.O., A.T. and L.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This work, utilizing non-identifiable registry data, was deemed exempt from IRB review.
Informed Consent Statement
Given the use of non-identifiable registry data, patient consent was not applicable to our study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Acknowledgments
This work would not have been possible without the collaboration of our partners at the Gift of Life Donor Program, LifeGift, and Southwest Transplant Alliance. We would also like to acknowledge Paige Porrett and David Mulligan for their contributions to the conception of this paper, and Jordana Cohen for her preliminary data analysis.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| BMI | Body mass index |
| CMV | Cytomegalovirus |
| GLDP | Gift of Life Donation Program |
| HCV | Hepatitis C Virus |
| NAT | Nucleic acid testing |
| OPTN | Organ Procurement and Transplantation Network |
| PHS-IR | Public Health Service increased risk |
| STA | Southwest Transplant Alliance |
| UTx | Uterus transplantation |
| UFI | Uterine factor infertility |
| US | United States |
| UNOS | United Network for Organ Sharing |
| VCA | Vascularized composite allograft transplant |
References
- Ozkan, O.; Akar, M.E.; Ozkan, O.; Erdogan, O.; Hadimioglu, N.; Yilmaz, M.; Gunseren, F.; Cincik, M.; Pestereli, E.; Kocak, H.; et al. Preliminary results of the first human uterus transplantation from a multiorgan donor. Fertil. Steril. 2013, 99, 470–476. [Google Scholar] [CrossRef]
- Ozkan, O.; Akar, M.E.; Erdogan, O.; Ozkan, O.; Hadimioglu, N. Uterus transplantation from a deceased donor. Fertil. Steril. 2013, 100, e41. [Google Scholar] [CrossRef]
- Brännström, M.; Tullius, S.G.; Brucker, S.; Dahm-Kähler, P.; Flyckt, R.; Kisu, I.; Andraus, W.; Wei, L.; Carmona, F.; Ayoubi, J.M.; et al. Registry of the International Society of Uterus Transplantation: First Report. Transplantation 2023, 107, 10–17. [Google Scholar] [CrossRef]
- Ejzenberg, D.; Andraus, W.; Baratelli Carelli Mendes, L.R.; Ducatti, L.; Song, A.; Tanigawa, R.; Rocha-Santos, V.; Macedo Arantes, R.; Soares, J.M., Jr.; Serafini, P.C.; et al. Livebirth after uterus transplantation from a deceased donor in a recipient with uterine infertility. Lancet 2019, 392, 2697–2704. [Google Scholar] [CrossRef]
- Testa, G.; McKenna, G.J.; Bayer, J.; Wall, A.; Fernandez, H.; Martinez, E.; Gupta, A.; Ruiz, R.; Onaca, N.; Gunby, R.T.; et al. The Evolution of Transplantation From Saving Lives to Fertility Treatment: DUETS (Dallas UtErus Transplant Study). Ann Surg. 2020, 272, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Flyckt, R.; Falcone, T.; Quintini, C.; Perni, U.; Eghtesad, B.; Richards, E.G.; Farrell, R.M.; Hashimoto, K.; Miller, C.; Ricci, S.; et al. First birth from a deceased donor uterus in the United States: From severe graft rejection to successful cesarean delivery. Am. J. Obstet. Gynecol. 2020, 223, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, L.; Richards, E.; Reddy, V.; Walter, J.; Olthoff, K.; Quintini, C.; Tzakis, A.; Latif, N.; Porrett, P.; O’Neill, K.; et al. The First 5 Years of Uterus Transplant in the US: A Report From the United States Uterus Transplant Consortium. JAMA Surg. 2022, 157, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Electronic address aao, Practice Committee of the American Society for Reproductive, M. American Society for Reproductive Medicine position statement on uterus transplantation: A committee opinion. Fertil. Steril. 2018, 110, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Brännström, M.; Dahm-Kähler, P.; Kvarnström, N.; Enskog, A.; Olofsson, J.I.; Olausson, M.; Mölne, J.; Akouri, R.; Järvholm, S.; Nilsson, L.; et al. Reproductive, obstetric, and long-term health outcome after uterus transplantation: Results of the first clinical trial. Fertil. Steril. 2022, 118, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Murugappan, G.; Farland, L.V.; Missmer, S.A.; Correia, K.F.; Anchan, R.M.; Ginsburg, E.S. Gestational carrier in assisted reproductive technology. Fertil. Steril. 2018, 109, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Kristek, J.; Johannesson, L.; Testa, G.; Chmel, R.; Olausson, M.; Kvarnström, N.; Karydis, N.; Fronek, J. Limited Availability of Deceased Uterus Donors: A Transatlantic Perspective. Transplantation 2019, 103, 2449–2452. [Google Scholar] [CrossRef] [PubMed]
- Dion, L.; Santin, G.; Nyangoh Timoh, K.; Boudjema, K.; Jacquot Thierry, L.; Gauthier, T.; Carbonnel, M.; Ayoubi, J.M.; Kerbaul, F.; Lavoue, V. Procurement of Uterus in a Deceased Donor Multi-Organ Donation National Program in France: A Scarce Resource for Uterus Transplantation? J. Clin. Med. 2022, 11, 730. [Google Scholar] [CrossRef]
- Johannesson, L.; Testa, G.; Flyckt, R.; Farrell, R.; Quintini, C.; Wall, A.; O’neill, K.; Tzakis, A.; Richards, E.G.; Gordon, S.M.; et al. Guidelines for standardized nomenclature and reporting in uterus transplantation: An opinion from the United States Uterus Transplant Consortium. Am. J. Transplant. 2020, 20, 3319–3325. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, A.; Dion, L.; Lavoué, V.; Chazelas, P.; Marquet, P.; Piver, P.; Sallée, C.; Aubard, Y.; Guellec, C.B.-L.; Favreau, F.; et al. The Key Role of Warm and Cold Ischemia in Uterus Transplantation: A Review. J. Clin. Med. 2019, 8, 760. [Google Scholar] [CrossRef]
- Smith, M.; Dominguez-Gil, B.; Greer, D.M.; Manara, A.R.; Souter, M.J. Organ donation after circulatory death: Current status and future potential. Intensive Care Med. 2019, 45, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Koon, E.C.; Johannesson, L.; McKenna, G.J.; Anthony, T.; Klintmalm, G.B.; Gunby, R.T.; Warren, A.M.; Putman, J.M.; Deprisco, G.; et al. Living Donor Uterus Transplantation: A Single Center’s Observations and Lessons Learned From Early Setbacks to Technical Success. Am. J. Transplant. 2017, 17, 2901–2910. [Google Scholar] [CrossRef]
- The Organ Procurement and Transplantation Network. Guidance to Organ Procurement Programs (OPOs) for VCA Deceased Donor Authorization. 2014.
- The Organ Procurement and Transplantation Network. Policy 12: Allocation of Vascularized Composite Allografts (VCA). 2020.
- McDiarmid, S.V. Donor and procurement related issues in vascularized composite allograft transplantation. Curr. Opin. Organ. Transplant. 2013, 18, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M.; Kracalik, I.; Levi, M.E.; Bowman, J.S.; Berger, J.J.; Bixler, D.; Buchacz, K.; Moorman, A.; Brooks, J.T.; Basavaraju, S.V. Assessing Solid Organ Donors and Monitoring Transplant Recipients for Human Immunodeficiency Virus, Hepatitis B Virus, and Hepatitis C Virus Infection—U.S. Public Health Service Guideline, 2020. MMWR Recomm Rep. 2020, 69, 1–16. [Google Scholar] [CrossRef]
- Brännström, M.; Dahm-Kähler, P.; Kvarnström, N.; Akouri, R.; Rova, K.; Olausson, M.; Groth, K.; Ekberg, J.; Enskog, A.; Sheikhi, M.; et al. Live birth after robotic-assisted live donor uterus transplantation. Acta Obstet. Gynecol. Scand. 2020, 99, 1222–1229. [Google Scholar] [CrossRef]
- Veroux, M.; Scollo, P.; Giambra, M.M.; Roscitano, G.; Giaquinta, A.; Setacci, F.; Veroux, P. Living-Donor Uterus Transplantation: A Clinical Review. J. Clin. Med. 2024, 13, 775. [Google Scholar] [CrossRef]
- Garcia, M.; Mulvagh, S.L.; Merz, C.N.; Buring, J.E.; Manson, J.E. Cardiovascular Disease in Women: Clinical Perspectives. Circ. Res. 2016, 118, 1273–1293. [Google Scholar] [CrossRef] [PubMed]
- Chmel, R.; Pastor, Z.; Novackova, M.; Matecha, J.; Cekal, M.; Fronek, J. Clinical pregnancy after deceased donor uterus transplantation: Lessons learned and future perspectives. J. Obstet. Gynaecol. Res. 2019, 45, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, L.; Wall, A.; Tzakis, A.; Quintini, C.; Richards, E.G.; O’neill, K.; Porrett, P.M.; Testa, G. Life underneath the VCA umbrella: Perspectives from the US Uterus Transplant Consortium. Am. J. Transplant. 2021, 21, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Wainright, J.L.; Wholley, C.L.; Rosendale, J.; Cherikh, W.S.; Di Battista, D.; Klassen, D.K. VCA Deceased Donors in the United States. Transplantation 2019, 103, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Volk, M.L.; Wilk, A.R.; Wolfe, C.; Kaul, D.R. The “PHS Increased Risk” Label Is Associated with Nonutilization of Hundreds of Organs per Year. Transplantation. 2017, 101, 1666–1669. [Google Scholar] [CrossRef] [PubMed]
- Pruett, T.L.; Clark, M.A.; Taranto, S.E. Deceased Organ Donors and PHS Risk Identification: Impact on Organ Usage and Outcomes. Transplantation 2017, 101, 1670–1678. [Google Scholar] [CrossRef]
- Ariyamuthu, V.K.; Sandikci, B.; AbdulRahim, N.; Hwang, C.; MacConmara, M.P.; Parasuraman, R.; Atis, A.; Tanriover, B. Trends in utilization of deceased donor kidneys based on hepatitis C virus status and impact of public health service labeling on discard. Transpl. Infect. Dis. 2020, 22, e13204. [Google Scholar] [CrossRef] [PubMed]
- Zibbell, J.E.; Asher, A.K.; Patel, R.C.; Kupronis, B.; Iqbal, K.; Ward, J.W.; Holtzman, D. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am. J. Public Health 2018, 108, 175–181. [Google Scholar] [CrossRef]
- Seem, D.L.; Lee, I.; Umscheid, C.A.; Kuehnert, M.J.; United States Public Health, S. PHS guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission through organ transplantation. Public Health Rep. 2013, 128, 247–343. [Google Scholar] [CrossRef]
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016, 315, 2284–2291. [Google Scholar] [CrossRef]
- Camargo, J.F.; Komanduri, K.V. Emerging concepts in cytomegalovirus infection following hematopoietic stem cell transplantation. Hematol. Oncol. Stem. Cell Ther. 2017, 10, 233–238. [Google Scholar] [CrossRef]
- Johnson, R.J.; Clatworthy, M.R.; Birch, R.; Hammad, A.; Bradley, J.A. CMV mismatch does not affect patient and graft survival in UK renal transplant recipients. Transplantation 2009, 88, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.P.; Saso, S.; Bracewell-Milnes, T.; Thum, M.-Y.; Nicopoullos, J.; Diaz-Garcia, C.; Friend, P.; Ghaem-Maghami, S.; Testa, G.; Johannesson, L.; et al. Human uterine transplantation: A review of outcomes from the first 45 cases. BJOG 2019, 126, 1310–1319. [Google Scholar] [CrossRef]
- Rosenzweig, M.; Wall, A.; Spak, C.W.; Testa, G.; Johannesson, L. Pregnancy after CMV infection following uterus transplantation: A case report from the Dallas Uterus Transplant Study. Transpl. Infect. Dis. 2021, 23, e13653. [Google Scholar] [CrossRef] [PubMed]
- Ayoubi, J.M.; Carbonnel, M.; Racowsky, C.; de Ziegler, D.; Gargiulo, A.; Kvarnström, N.; Dahm-Kähler, P.; Brännström, M. Evolving clinical challenges in uterus transplantation. Reprod. Biomed. Online 2022, 45, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.M.; Kauffman, H.M.; McBride, M.A.; Davies, D.B.; Rosendale, J.D.; Smith, C.M.; Edwards, E.B.; Daily, O.P.; Kirklin, J.; Shield, C.F.; et al. Center-specific graft and patient survival rates: 1997 United Network for Organ Sharing (UNOS) report. JAMA 1998, 280, 1153–1160. [Google Scholar] [CrossRef][Green Version]
- Bayefsky, M.J.; Berkman, B.E. The Ethics of Allocating Uterine Transplants. Camb. Q. Healthc. Ethics 2016, 25, 350–365. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).