Non-Invasive Diagnosis of Pediatric Intestinal Graft-Versus-Host Disease: A Case Series
Abstract
1. Introduction
2. Patients
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Ferrara, J.L.M.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Acute graft-versus-host disease—biologic process, prevention, and therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; Blazar, B.R. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N. Engl. J. Med. 2017, 377, 2565–2579. [Google Scholar] [CrossRef] [PubMed]
- Filipovich, A.H.; Weisdorf, D.; Pavletic, S.; Socie, G.; Wingard, J.R.; Lee, S.J.; Martin, P.; Chien, J.; Przepiorka, D.; Couriel, D.; et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging working group report. Biol. Blood Marrow Transplant. 2005, 11, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Mohty, M. New insight for the diagnosis of gastrointestinal acute graft-versus-host disease. Mediat. Inflamm. 2014, 2014, 701013. [Google Scholar] [CrossRef]
- Naymagon, S.; Naymagon, L.; Wong, S.Y.; Ko, H.M.; Renteria, A.; Levine, J.; Colombel, J.F.; Ferrara, J. Acute graft-versus-host disease of the gut: Considerations for the gastroenterologist. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, M.J.; Özbek, U.; Holler, E.; Renteria, A.S.; Major-Monfried, H.; Reddy, P.; Aziz, M.; Hogan, W.J.; Ayuk, F.; Efebera, Y.A.; et al. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight 2018, 2, e89798. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.E.; Braun, T.M.; Harris, A.C.; Holler, E.; Taylor, A.; Miller, H.; Magenau, J.; Weisdorf, D.J.; Ho, V.T.; Bolaños-Meade, J.; et al. A prognostic score for acute graft-versus-host disease based on biomarkers: A multicentre study. Lancet Haematol. 2015, 2, e21–e29. [Google Scholar] [CrossRef]
- MacMillan, M.L.; Robin, M.; Harris, A.C.; DeFor, T.E.; Martin, P.J.; Alousi, A.; Ho, V.T.; Bolaños-Meade, J.; Ferrara, J.L.M.; Jones, R.; et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol. Blood Marrow Transplant. 2015, 21, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Vander Lugt, M.T.; Braun, T.M.; Hanash, S.; Ritz, J.; Ho, V.T.; Antin, J.H.; Zhang, Q.; Wong, C.-H.; Wang, H.; Chin, A.; et al. ST2 as a Marker for Risk of Therapy-Resistant Graft-versus-Host Disease and Death. N. Engl. J. Med. 2013, 369, 529–539. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, S.R.; Radojcic, V.; Tsai, H.L.; Vulic, A.; Thompson, E.; Ivcevic, S.; Kanakry, C.G.; Powell, J.D.; Lohman, B.; Adom, D.; et al. Signatures of GVHD and relapse after posttransplant cyclophosphamide revealed by immune profiling and machine learning. Blood 2022, 139, 608–623. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, E.; Bruno, B.; McDonald, G.B.; Paolicchi, A.; Caracciolo, F.; Papineschi, F.; Pelosini, M.; Campani, D.; Galimberti, S.; Petrini, M. Prospective qualitative and quantitative non-invasive evaluation of intestinal acute GVHD by contrast-enhanced ultrasound sonography. Bone Marrow Transplant. 2013, 48, 1421–1428. [Google Scholar] [CrossRef]
- Weber, D.; Weber, M.; Hippe, K.; Ghimire, S.; Wolff, D.; Hahn, J.; Evert, M.; Herr, W.; Holler, E.; Jung, E.M. Non-invasive diagnosis of acute intestinal graft-versus-host disease by a new scoring system using ultrasound morphology, compound elastography, and contrast-enhanced ultrasound. Bone Marrow Transplant. 2019, 54, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Schreyer, A.G.; Landfried, K.; Zorger, N.; Hoffstetter, P.; Ammer, J.; Fellner, C.; Friedrich, C.; Andreesen, R.; Holler, E.; Jung, E.M. Transmural penetration of intravenously applied microbubbles during contrast-enhanced ultrasound as a new diagnostic feature in patients with GVHD of the bowel. Bone Marrow Transplant. 2011, 46, 1006–1011. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Glucksberg, H.; Storb, R.; Fefer, A.; Buckner, C.D.; Neiman, P.E.; Clift, R.A.; Lerner, K.G.; Thomas, E.D. Clinical manifestations of graft-versus-host disease in human recipients of marrow from hl-a-matched sibling donors1. Transplantation 1974, 18, 295–304. [Google Scholar] [CrossRef]
- Thomas, E.; Hows, J.; Klingemann, H.; Przepiorka, D.; Weisdorf, D.; Martin, T. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995, 15, 825–828. [Google Scholar]
- Nishida, M.; Shigematsu, A.; Sato, M.; Kudo, Y.; Omotehara, S.; Horie, T.; Iwai, T.; Endo, T.; Iguchi, A.; Shibuya, H.; et al. Ultrasonographic evaluation of gastrointestinal graft-versus-host disease after hematopoietic stem cell transplantation. Clin. Transplant. 2015, 29, 697–704. [Google Scholar] [CrossRef]
- Cartoni, C.; Dragoni, F.; Micozzi, A.; Pescarmona, E.; Mecarocci, S.; Chirletti, P.; Petti, M.C.; Meloni, G.; Mandelli, F. Neutropenic enterocolitis in patients with acute leukemia: Prognostic significance of bowel wall thickening detected by ultrasonography. J. Clin. Oncol. 2001, 19, 756–761. [Google Scholar] [CrossRef]
- Penack, O.; Socié, G.; Van Den Brink, M.R.M. The importance of neovascularization and its inhibition for allogeneic hematopoietic stem cell transplantation. Blood 2011, 117, 4181–4189. [Google Scholar] [CrossRef]
- Pausch, A.M.; Kammerer, S.; Weber, F.; Herr, W.; Stroszczynski, C.; Holler, E.; Edinger, M.; Wolff, D.; Weber, D.; Jung, E.M.; et al. Parametric Imaging of Contrast-Enhanced Ultrasound (CEUS) for the Evaluation of Acute Gastrointestinal Graft-Versus-Host Disease. Cells 2021, 10, 1092. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.; Ramprasad, J.; Jensen, M.K.; Margolis, D.; Werlin, S. Endoscopic diagnosis of pediatric acute gastrointestinal graft-versus-host disease. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 417–420. [Google Scholar] [CrossRef]
- Hiejima, E.; Nakase, H.; Matsuura, M.; Honzawa, Y.; Higuchi, H.; Saida, S.; Umeda, K.; Hiramatsu, H.; Adachi, S.; Izawa, K.; et al. Diagnostic accuracy of endoscopic features of pediatric acute gastrointestinal graft-versus-host disease. Dig. Endosc. 2016, 28, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Schwarzenberg, S.J.; Sharp, H.; Jessurun, J.; Gulbahce, H.E.; DeFor, T.; Nagarajan, R. Diagnostic endoscopy in children after hematopoietic stem cell transplantation. Gastrointest. Endosc. 2006, 64, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Mudambi, K.; Sandberg, J.; Bass, D.; Rubesova, E. Contrast enhanced ultrasound: Comparing a novel modality to MRI to assess for bowel disease in pediatric Crohn’s patients. Transl. Gastroenterol. Hepatol. 2020, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Kljucevsek, D.; Vidmar, D.; Urlep, D.; Dezman, R. Dynamic contrast-enhanced ultrasound of the bowel wall with quantitative assessment of Crohn’s disease activity in childhood. Radiol. Oncol. 2015, 50, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamad, S.; Hackam, D.J.; Goldstein, S.D.; Huisman, T.A.G.M.; Darge, K.; Hwang, M. Contrast-Enhanced Ultrasound and Near-Infrared Spectroscopy of the Neonatal Bowel: Novel, Bedside, Noninvasive, and Radiation-Free Imaging for Early Detection of Necrotizing Enterocolitis. Am. J. Perinatol. 2018, 35, 1358–1365. [Google Scholar] [CrossRef] [PubMed]


| Patient | Disease | Age at HSCT, Sex | Donor | HLA Matching | Stem Cell Source | Conditioning Regimen | GVHD Prophylaxis |
|---|---|---|---|---|---|---|---|
| 1 | FA | 8 y.o., M | MFD | 10/10 | BM | CTX-Flu | CSA-MTX |
| 2 | X-ALD | 8 y.o., M | MUD | 10/10 | BM | Bu-Flu-TT | ATG-Grafalon-CSA-MTX |
| 3 | DBA | 9 y.o., F | MUD | 10/10 | PBSC | Treo-Flu-TT | ATG-Grafalon-CSA-MTX |
| Patient | Day at Presentation, Clinical Picture at Presentation | GVHD Staging and Overall Grading | Segment Involved, US and PD Findings at Diagnosis | Therapy and Outcome | US and PD Findings At Flare | Therapy and Outcome | US and PD Findings at 2nd Flare | Salvage Therapy and Outcome | US and PD Findings at Last Follow-Up | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | +120 days post HSCT, abdominal cramping with intermittent diarrhea (600 ml daily max) | Skin 0, liver 0, GI + Overall II | T. Ileum A. and T. Colon; BWT 4-5 mm ileum; 7-8 mm A. and T. colon PD: Moderate increase | MPDN 2 mg/kg/day, complete response | NA | NA | NA | NA | Complete normalization | Complete remission, patient alive and well |
| 2 | +40 days post HSCT, abdominal cramping, watery and green diarrhea (3000 ml daily max) | Skin 0, liver 0, GI ++++ Overall IV | Colon; BWT 3.8-5.2mm PD: Moderate increase | MPDN 2 mg/kg/day, steroid refractory | T.ileum+ Colon; BWT 3.5-5.7 mm ileum; 2 mm colon due to abnormal distension PD: Strong increase | Ruxolitinib+ oral budesonide, refractory | Unchanged | Infliximab (4 doses), complete response | Complete normalization | Complete remission, patient alive and well |
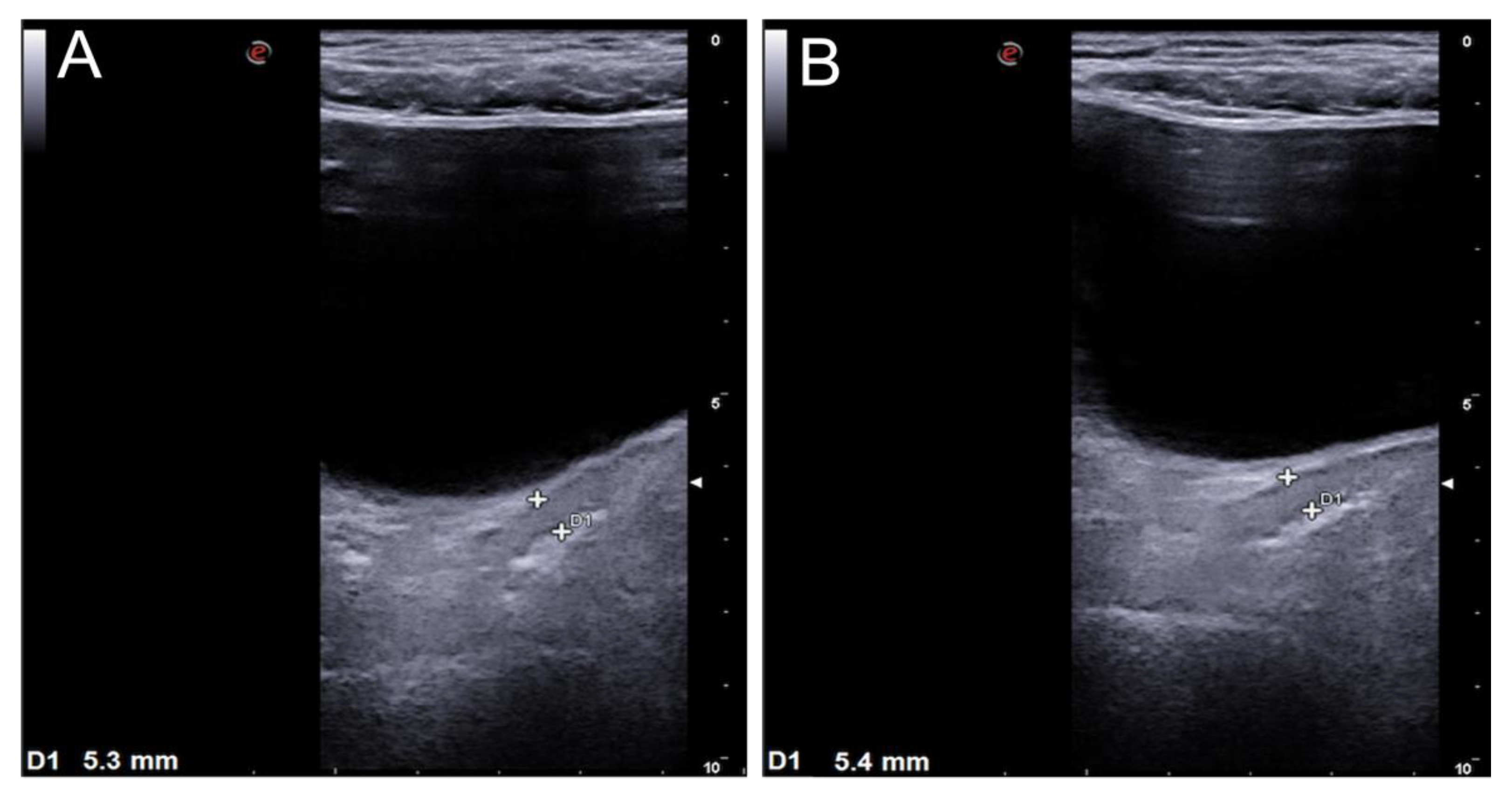
| 3 | +35 days post HSCT, watery and green diarrhea inconstantly containing fresh blood (700 ml daily max) | Skin 2, liver 0, GI + Overall II | Sigma colon, rectum; BWT 4 mm PD not evaluable | MPDN 2 mg/kg/day, steroid refractory | Sigma colon, rectum; BWT 5.3-5.4 mm; PD not evaluable | Ruxolitinib, complete response | NA | NA | Complete normalization | Complete remission, patient alive and well |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spadea, M.; Saglio, F.; Opramolla, A.; Rigazio, C.; Cisarò, F.; Berger, M.; Quarello, P.; Calvo, P.L.; Fagioli, F. Non-Invasive Diagnosis of Pediatric Intestinal Graft-Versus-Host Disease: A Case Series. Transplantology 2022, 3, 115-123. https://doi.org/10.3390/transplantology3020012
Spadea M, Saglio F, Opramolla A, Rigazio C, Cisarò F, Berger M, Quarello P, Calvo PL, Fagioli F. Non-Invasive Diagnosis of Pediatric Intestinal Graft-Versus-Host Disease: A Case Series. Transplantology. 2022; 3(2):115-123. https://doi.org/10.3390/transplantology3020012
Chicago/Turabian StyleSpadea, Manuela, Francesco Saglio, Anna Opramolla, Caterina Rigazio, Fabio Cisarò, Massimo Berger, Paola Quarello, Pier Luigi Calvo, and Franca Fagioli. 2022. "Non-Invasive Diagnosis of Pediatric Intestinal Graft-Versus-Host Disease: A Case Series" Transplantology 3, no. 2: 115-123. https://doi.org/10.3390/transplantology3020012
APA StyleSpadea, M., Saglio, F., Opramolla, A., Rigazio, C., Cisarò, F., Berger, M., Quarello, P., Calvo, P. L., & Fagioli, F. (2022). Non-Invasive Diagnosis of Pediatric Intestinal Graft-Versus-Host Disease: A Case Series. Transplantology, 3(2), 115-123. https://doi.org/10.3390/transplantology3020012








