Abstract
Although the majority of patients with Hodgkin lymphoma (HL) are cured with initial therapy, in 85–90% of early stage and 70–80% of advanced-stage disease cases, relapse remains a major problem. Autologous stem-cell transplantation (auto-HCT) after salvage chemotherapy is currently considered to be the standard of care for patients who relapse after first-line chemotherapy or for whom first-line treatment fails. The curative capacity of auto-HCT has been improving with the introduction of new drug-based salvage strategies and consolidation strategies after auto-HCT. Allogeneic stem-cell transplantation (allo-HCT) represents a reasonable treatment option for young patients who relapse or progress after auto-HCT and have chemosensitive disease at the time of transplantation. Allo-HCT is a valid treatment strategy for patients with relapse/refractory HL (r/r HL) because the results have improved over time, mainly with the safe combination of allo-HCT and new drugs. Bearing in mind that outcomes after haploidentical stem-cell transplantation (haplo-HCT) are comparable with those for matched sibling donors and matched unrelated donors, haplo-HCT is now the preferred alternative donor source for patients with r/r HL without a donor or when there is urgency to find a donor if a matched related donor is not present. The development of new drugs such as anti-CD 30 monoclonal antibodies and checkpoint inhibitors (CPI) for relapsed or refractory HL has demonstrated high response rates and durable remissions, and challenged the role and timing of HCT. The treatment of patients with HL who develop disease recurrence or progression after allo-HCT remains a real challenge and an unmet need.
1. Introduction
Hodgkin lymphoma (HL) accounts for approximately 10% of cases of newly diagnosed lymphoma. HL is an uncommon malignancy with approximately 8830 estimated new cases in the United States and 960 estimated deaths in 2021, according to the American Cancer Society, Cancer Statistic Center; the crude incidence in the European Union is 2.3, with a mortality rate of 0.7/100.000 cases per year [1,2]. Affecting both young adults and elderly patients older than 60 years, this disease has bimodal distribution [3]. The factors associated with this disease include immunosuppression, familial factors, and exposure to viruses such as the Epstein–Barr virus, although there is no clear evidence yet [4]. The condition sine qua non for a confirmed diagnosis of HL is the pathohistological result from a sufficiently large surgical specimen or excisional lymph-node biopsy providing enough material for freshly frozen and formalin-fixed samples.
HL has evolved from a disease, which historically has had a poor outcome to a potentially curable lymphoma with distinct histology, biological behavior, and clinical characteristics [5]. Today, we use risk-adapted initial therapy for HL patients based on the stage of the disease (limited or advanced), the presence of poor prognostic features, of constitutional symptoms, and bulky disease (single site of disease >10 cm) [6,7]. The goals of this treatment strategy are to potentially increase the likelihood for a cure and to minimize toxicity. Patients with HL are typically managed on the basis of fluorodeoxyglucose - positron emission tomography (FDG-PET) results [8]. The result of an interim FDG-PET scan after two cycles of treatment may help in the decision for further plans of therapy. A positive PET scan at the end of treatment may result in the addition of consolidation radiotherapy (RT) on the positive sites. It is generally accepted that the treatment strategy for patients with early-stage disease is an abbreviated course of combination chemotherapy (CT), followed by consolidation RT in a subset of patients and a more prolonged course of combination CT alone for patients with advanced disease. The optimal initial management of patients with HL results in an excellent outcome in a large cohort of patients, but there is still a subset of patients who subsequently progress and need further salvage CT, often followed by autologous stem-cell transplantation (auto-HCT). Allogeneic stem-cell transplantation (allo-HCT) has been a treatment option for patients who relapse after an auto-HCT, and for a significant proportion of patients that require an auto-HCT but are not eligible for the procedure because of an inadequate response to a previously administered salvage strategy. The cohort of patients who relapse after auto-HCT, who are not candidates for high-dose therapy, or who relapse or progress after allo-HCT are candidates to receive target agents including anti-CD 30 and checkpoint inhibitors [9,10,11].
2. Autologous Stem-Cell Transplantation
More than 80% of patients with advanced-stage classical HL (cHL) can be cured after first-line CT or combined treatment [12]. Unfortunately, up to 10% of those treated with ABVD are primary refractory, and up to 30% to 40% of patients that achieve complete remission (CR) with first-line treatment eventually relapse. There are only two randomized trials in the relapsed/refractory HL (r/r HL) setup exploring salvage CT alone versus salvage, followed by high-dose chemotherapy and auto-HCT for responding patients. These two prospective randomized clinical trials indicate that auto-HCT is able to significantly improve freedom from treatment failure in these subsets of patients over conventional salvage CT [13,14]; because of this, auto-HCT is the standard of care in this situation [15]. Both these trials showed superior progression-free survival (PFS), but no advantage in terms of overall survival (OS) (Table 1).

Table 1.
Randomized studies of auto-HCT in cHL.
There are two main reasons why these randomized trials did not show increased OS. The first one was the small sample size in the British randomized trial because patients refused single-arm salvage CT. The second reason was that, in both trials, patients underwent transplantation at the time of second relapse in the salvage CT arm.
Nowadays, the use of high-dose CT (Table 2 and Table 3) followed by auto-HCT is considered to be the standard of care for patients with relapsed or refractory HL that respond to salvage strategies [15,16].

Table 2.
Salvage regimens—conventional chemotherapy.

Table 3.
Salvage regiments before auto-SCT—new drugs with chemotherapy-combination strategies.
Auto-HCT is generally not recommended for patients with cHL in first CR. Almost 20 years ago, upfront auto-HCT as a consolidation strategy was considered for high-risk patients. In a prospective clinical trial that included 163 patients with unfavorable HL (presence of at least 2/5 poor prognostic factors: high lactate dehydrogenase (LDH) level, large mediastinal mass, >1 extranodal site, low hematocrit level, and inguinal involvement), those patients who had achieved CR or partial remission (PR) with four initial courses of chemotherapy (ABVD or other doxorubicin-containing regimens) were randomized to undergo high-dose therapy and auto-HCT (83 patients, arm A) or to receive four additional courses of CT (80 patients, arm B). This study demonstrated that there was no statistically significant difference in the CR rate, 5-year failure-free survival, and OS between the two arms [28].
The curative capacity of auto-HCT is improving with the introduction of new drug-based salvage strategies and consolidation strategies after auto-HCT [17,18,19,20,21,22,23,24,25,26,27] (Table 2 and Table 3). The incorporation of new drugs in the salvage regimens is a promising strategy to induce a high rate of metabolic CR and improve disease status before auto-HCT with favorable tolerability. Brenduximab vedotin (BV) was also combined with conventional CT to increase the percentage of patients achieving a metabolic CR. LaCasce [24] published results from a Phase I study including 55 patients who had received up to six cycles of BV-Benda as first salvage therapy. After a median follow-up of 21 months, 74% of the patient population achieved a CR, and the estimated 2-year PFS was 63% for patients who received auto-HCT.
Herrera AF in a Phase 1/2 study used a chemotherapy-free salvage regimen with BV and nivolumab as the initial salvage therapy in 62 patients with r/r HL [27]. CR was achieved in 61% of patients, a result firmly proving chemotherapy-free regiment as an active, well-tolerated salvage regimen that can be used as effective bridge to auto-HCT.
The combination of pembrolizumab and gemcitabine, vinorelbine, and liposomal doxorubicin (GVD) as salvage therapy for r/rHL, is associated with a CR rate of 95%, especially in those patients receiving BV in the frontline setting [29].
Nevertheless, a significant proportion of patients that require an auto-HCT are not eligible for the procedure because of an inadequate response to the salvage strategy that is administered, and those patients have historically had a poor outcome. Recently published data from 22 centers in the USA included 78 HL patients who had undergone auto-HCT after receiving an anti-PD-1 mAb (alone or in combination) as third-line or later therapy, including 42 patients (54%) who had been refractory to more than two consecutive systemic therapies immediately before anti-PD-1 treatment. After a median post-auto-HCT follow-up of 19.6 months, patients who had responded to anti PD-1 therapy had particularly favorable outcomes, with an 18-month PFS of 88%. These results strongly suggest that the use of PD-1 blockades as a therapeutic option in salvage therapy beyond the second-line setting can serve as a bridge to auto-SCT. Patients with previously chemorefractory relapsed HL who had responded to anti-PD-1 therapy could then be considered for auto-HCT [30].
Maintenance strategies after auto-HCT in high-risk patients also have important impact in improving the results of auto-HCT in patients with r/r HL. AETHERA is a randomized, double-blind, placebo-controlled Phase III trial that established BV as a consolidative treatment option for adult patients with cHL at a high risk of relapse or progression after auto-HCT. Results showed that BV significantly improved PFS vs. placebo. With a 5-year follow-up, BV continued to provide patients with a sustained PFS benefit 5-year PFS of 59% with BV vs. 41% with the placebo, and reduced the need for subsequent therapy. Patients with more than two risk factors experienced significantly higher PFS at five years, and the clinical benefit was more pronounced [31].
Tandem auto-HCT was also tested in patients with high risk r/r HL. The H96 prospective clinical trial was a Phase II trial that offered a tandem auto-HCT to patients that presented risk factors such as relapse in less than 12 months, Stages III–IV at the time of relapse, or relapse in a previously irradiated area after combined modality therapy. After a median follow-up of 10.3 years, the 10-year PFS and OS rates were 64% and 70% for the intermediate groups, and 41% and 47% for the poor-risk groups [32]. Although, the H96 was not a randomized trial, the authors claimed that the long-term outcome of patients with poor risk factors before transplant was improved with the tandem procedure with respect to the results achieved with a HCT alone.
Tandem auto-HCT is a safe procedure and is associated with an NRM of 0–5%, a 5-year OS of 58–84%, and a 5-year PFS of 49–55% [33]. Nevertheless, and bearing in mind the integration of positron emission tomography findings, and new drugs such as anti-CD30 and checkpoint inhibitors, the role of tandem auto-HCT, even in high-risk patients, is less defined.
In spite of all the advances made before auto-HCT, there is still a significant proportion of patients that fail the procedure. Patients failing auto-HCT are candidates to receive new drugs. In a multinational, open-label, Phase II study, 102 patients with histologically documented CD30-positive r/r HL after auto-HCT or at least two prior regimens received BV. The study reported an ORR of 75% and a metabolic CR rate of 34%, with a median duration of response of 20.5 months for patients achieving CR [34].
The Checkmate 205 study enrolled 243 patients with r/r HL, including patients “naïve to BV”, patients who received BV at different times relative to auto-HCT, and patients refractory to prior lines of therapy. All patients had received nivolumab 3 mg/kg every 2 weeks until disease progression or unacceptable toxicity. The ORR rate is 69%, with a CR rate of 16% and PFS of 22.2 months for patients achieving CR [35]. These results suggested that patients with r/r HL with or without prior BV exposure, achieved durable responses after treatment with nivolumab.
Programmed death −1 inhibitor pembrolizumab also provides durable and deep responses with acceptable tolerability for patients with r/r cHL and difficult to treat chemorefractory cHL. The 2-year follow-up of the KEYNOTE-087 study that included 210 patients with r/r cHL receiving pembrolizumab after auto-HCT and BV, salvage chemotherapy with BV and progression after auto-HCT without BV, reported an ORR of 71.9%, with a CR of 27.6% and a median response duration of 16.5 months [36].
Although auto-HCT is the evidence-based standard of care for r/r HL, emerging new drugs, post-autologous stem-cell consolidation, and managing postautologous stem-cell failure are rapidly changing the landscape. There is an unmet need for properly designed randomized clinical trials that can answer these emerging questions regarding the role of new drugs in the treatment algorithm of patients with HL and indication for auto-HCT.
3. Allogeneic Stem-Cell Transplantation (allo-HCT)
In spite of all the above-mentioned treatment strategies, a significant proportion of patients still relapse after auto-HCT. A retrospective study from the Lymphoma Working party (LWP) of the European Group for Blood and Marrow Transplantation (EBMT) and the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) in a cohort of 511 adult patients that relapsed after auto-HCT indicated that early relapse, Stage IV, bulky disease, poor PS, and age >50 years at auto-HCT failure were adverse prognostic factors for long-term outcome [37].
Allo-HCT is still considered to be a curative treatment approach for patients with HL who relapse or progress after auto-HCT [16]. The first published reports on allo-HCT in patients with HL appeared in the mid-1980s. These studies included a low number of patients and did not offer a realistic evaluation of the therapeutic potential of allo-HCT [38,39].
Two large registry-based retrospective analyses on allo-HCT on HL were published in 1996. These studies gave disappointing results regarding the role of myeloablative allo-HCT in patients and were hampered by an exceedingly high non relapse-mortality (NRM) so the potential benefit of an allo-HCT was not seen at that point [40,41].
To reduce transplant-related mortality, limit systemic toxicity, and facilitate donor engraftment associated with allo-HCT, reduced intensity conditioning (RIC) regimens were introduced in patients with HL. The enthusiasm of the scientific community for the use of allo-HCT in relapsed HL was renewed by using RIC protocols that have opened allo-HCT to elderly patients and those with significant comorbidities [42].
The Lymphoma Working Party (LWP) of the EBMT published the first retrospective comparison between RIC and myeloablative conditioning protocols (MAC) in patients with r/r HL. Non-relapse mortality (NRM) significantly decreased in the RIC/allo-SCT group (23% vs. 46% at 1 year, p < 0.001). Although relapse rate was higher in the RIC group, this decrease in NRM was translated into a better PFS and OS (hazard ratio of 1.28, 995% CI 0.92–1.78, p = 0.1 and hazard ratio of 1.62 (95% CI 1.15–2.28), p = 0.005, respectively) [44].
Additional information on the role of RIC/allo-HCT in r/r HL mostly comes from another large study from the LWP of the EBMT, which included 285 patients with heavily pretreated disease treated with RIC/allo-HCT. Disease status at the time of allotransplant was CR for 47 patients (17%), chemosensitive disease in 123 patients (43%), and chemoresistant disease or untested relapse in 115 (40%). The 100-day NRM was 12%, but increased to 20% at 12 months and to 22% at 3 years; it was significantly higher for patients with chemoresistant disease with a 2-year PFS of 29% (p < 0.001). [45].
Results of allo-HCT have changed overtime. A second retrospective analysis of the LWP of the EBMT comparing RIC vs MAC protocols included patients with HL allografted in a more recent period of time, from 2006 to 2010. A cohort of 312 patients was included, out of which 63 had received a MAC and the remaining 249 received an RIC approach. After median follow-up for the patients alive at 56 months, there was no significant difference in NRM between MAC and RIC. Relapse rate was lower in the MAC group (41% vs. 52% at 24 months, p = 0.16), and this was translated into marginal improvement in EFS for the MAC group (48% vs. 36% at 24 months, p = 0.09) with no significant difference in overall survival (73% for MAC and 62% for RIC at 24 months, p = 0.13). Our study showed that NRM associated with MAC decreased over time, and that the intensity of conditioning regimens should be individually designed (Table 4) [46].

Table 4.
Allogeneic SCT transplant for relapsed/refractory Hodgkin lymphoma.
Lastly, and in order to analyze potential changes in patient characteristics and outcomes over time, the LWP of the EBMT conducted the largest retrospective study, published in 2020 and included 13,639 adult patients with relapse/refractory HL receiving an auto-SCT or allo-SCT who were reported in the EBMT registry [44]. The aim of the study was to assess changes in the patient population and characteristics of hematopoietic stem-cell transplantation for relapsed/refractory Hodgkin lymphoma over a 25-year period. Results suggest that the number of transplanted patients with relapsed/refractory HL has increased over time. Transplant centers are autografting older patients earlier in the course of disease and with a higher percentage of patients with chemosensitive disease. The major cause of failure is the relapse/progression of disease (Figure 1 and Figure 2, reproduced with permission) [43].
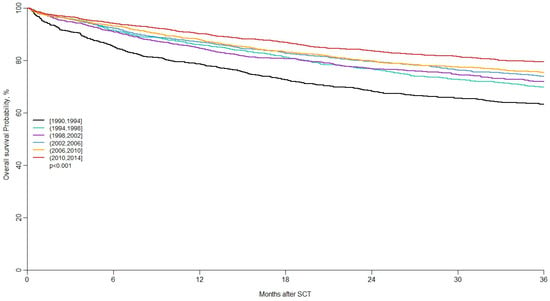
Figure 1.
Long-term outcomes of auto-SCT in patients with r/r HL. Reproduced with permission [43], 2020.
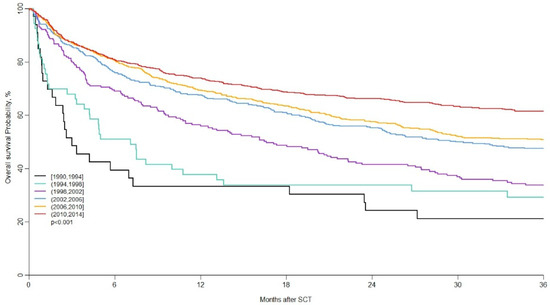
Figure 2.
Long-term outcome (OS) of allo-SCT in patients with r/r cHL. Reproduced with permission [43], 2020.
In the next decade, according to the lymphoma database of the EBMT, there was an increasing number of patients with HL treated with an allo-SCT. The outcomes of allotransplantation in HL have improved over time. These benefits are likely due to improved supportive care and more effective salvage therapies.
Historically, in the early days, allo-SCT was used as the first transplant after disease refractory to multiple lines of therapy, and myeloablative conditioning was used. In contrast, patients are now younger and fitter, have a shorter interval from diagnosis to transplant, and reduced intensity conditioning is more frequently used. As in auto-SCT settings, the major cause of failure is disease relapse. Reduced intensity conditioning is associated with decreased NRM, but it does not offer sufficient disease control. Increasing experience in auto-HCT or allo-HCT resulted in significant improvement in long-term outcomes, NRM, PFS, and OS over time [43].
Allo-HCT generally remains a reasonable treatment option for patients who relapse or progress after an auto-HCT; in high-risk HL patients, allo-HCT was demonstrated to be potentially valid as a first transplant in young patients and those with a short time interval from diagnosis to transplant [50].
The combination of the effectiveness of high-dose CT and the graft-versus-lymphoma effect should be considered to be the backbone of the clinical benefit of an allo-HCT. Results from some retrospective studies and prospective clinical trials, and evidence from donor lymphocyte infusion (DLI) studies indicated the existence of a beneficial graft-versus-HL effect, but this was not proven in prospective clinical trials [51].
DLI is a therapeutic option with low NRM, and with the potential for a durable response in patients with r/r cHL who relapse after allo-HCT [52]. A total of 49 patients with multiply relapsed HL had reduced intensity allogeneic stem-cell transplantation; 31 of them had HLA-related matched donors, and 18 had unrelated donors. This cohort of patients was heavily pretreated, with a median number (5) of previous treatment courses (range 3–8), and time from diagnosis was 4.8 years (range 0.6–4.8). A total of 16 (33%) patients had progression or residual disease and received donor lymphocyte infusion 3 months after allogeneic stem-cell transplantation. Non-relapse-related mortality was 16.3% at 730 days with a projected 4-year OS of 55.7% and a PFS of 39%.
The use of new drugs as a bridging strategy before allo-HCT is feasible and possibly has allowed to improve the results of allo-HCT. A large EBMT study looked at the use of BV as bridge to transplant for patients with r/r HL and in multivariate analysis demonstrated that pretransplant BV had no significant effect on NRM, RI, or OS. Refractory patients who respond to BV and achieve remission prior to transplant obtain advantageous disease status prior to allo-HCT, which is one of the most important prognostic factors [53].
A French retrospective trial performed between 2011 and 2013 enrolled 240 patients with r/r HL. Among 145 responders to BV, 54 patients received consolidation allo-HCT with median PFS of 18.8 versus 8.7 months for the 91 patients without transplant. This study suggested high-dose therapy with stem-cell transplantation for responders as soon as possible, having in mind the short-term responses in most patients. BV is effective and safe with manageable toxicity, and, according to this real-life study, can be considered to be a bridge to transplant [54].
Anti-PD-1 mAb can also be used as bridge to allotransplant. Merryman et al. published a retrospective study of 209 HL patients who had been treated with anti-PD-1 and undergone allo-HCT. With a median follow-up of 24 months, the 2-year cumulative incidence of NRM and relapse were 14% and 18% respectively, and the 2-year GRFS, PFS, and OS were 47%, 69%, and 82% respectively. These data suggested that anti-PD-1 mAb is associated with impact in post-allo-HCtT period, both in efficacy and toxicity. Patients undergoing alloHCT after PD-1 blockade are candidates for PTCy-based GVHD prophylaxis as a strategy with significantly improved outcomes [55].
4. Haploidentical Stem-Cell Transplantation
Multiple studies were published supporting haploidentical stem cell transplantation (haplo-HCT) with Pt-Cy as a promising therapeutic option to treat patients with r/r HL when an HLA-identical donor, either matched related or unrelated, is not available [56].
Most of them suggested that the outcomes of patients with HL after haplo-HCT can be influenced by pretransplant disease status (CR vs. not CR or CR/PR vs. active disease), patient performance status (Karnofsky), and hematopoietic stem-cell transplantation comorbidity index (HCT-CI) (either 0 vs. >0 or 3 vs. ≥3) [57].
According to the Genova experience based on a study of 41 patients, disease status assessed with positron emission tomography/computed tomography (PET-CT) (Deauville score ≥ 4) and hematopoietic stem-cell transplantation comorbidity index ≥ 3 were identified as the strongest predictors for worse outcomes of (relapse incidence) RI, progression-free survival (PFS), and relapse-free survival (RFS). Patients with HCT-CI ≥ 3 have increased NRM and lower OS. Achieving a greater level of disease response before transplant is greatly important for better post-transplant outcomes [58].
Two retrospective studies from the Center for International Blood and Marrow Transplant Research (CIBMTR) compared outcomes after haplo-HCT with matched unrelated donor (MUD) and matched sibling donor (MSD) transplantation for patients with lymphoma including HL (22% of the global population of patients). In the first study, Kanate compared haplo with MUD with and without ATG. Of the 185 patients with lymphoma who had received haplo, 46 patients had r/r HL. PFS at 3 years was 45%, compared with 45% for MUD without ATG and significantly better than for MUD with ATG at 34%. Haploidentical transplant had a significantly lower incidence of severe GVHD compared with MUD without ATG. There was difference in relapse risk and NRM between haplo and MUD transplant [59]. The second study included 180 patients with lymphoma who had received haplo-HCT, but only 44 with HL. A study compared haplo-HCT with MSD and reported similar survival outcomes, with a lower risk of cGVHD for patients who had received haploidentical stem-cell transplantation [60] (Table 5).

Table 5.
Haplo-SCT in Hodgkin disease using PTCy.
The most important European study was performed by EBMT, and it is the largest retrospective registry-based analysis that evaluated 709 adult patients with r/r HL who underwent allogeneic transplantation. The study compares the outcome of patients with HL receiving MSD (338), MUD (273), and haplo-HCT with PT-Cy (98). This study reported no significant difference in OS and PFS between haplo-HCT and MSD or MUD transplantation. Results of 2-year OS and PFS were 67% and 43% for haplo-HCT, 71% and 38% for MSD, and 62% and 45% for MUD, respectively. Haplo-SCT results showed a lower risk of cGVHD vs. MUD transplantation. Multivariate analysis showed that, relative to MSD, NRM was similar after haplo-SCT and higher after MUD, and that the cumulative incidence (CI) of relapse was lower in both haplo-SCT and MUD. The most important factors predicting prognosis are disease status at transplant and chemorefractory disease [63].
The recommendation for haplo-SCT in patients with r/r HL from the LWP of the EBMT is largely based upon retrospective studies. Due to the lack of prospective clinical trials, recent recommendations suggested that haplo-HCT with PT-Cy is an effective treatment option for r/r HL patients relapsing after autologous stem-cell transplantation [64].
Results from retrospective analyses showed no significant difference in OS between haplo-HCT and HLA-matched transplants. An additional advantage is a significantly lower risk of cGVHD, which results in a better relapse-free survival. Bearing in mind that outcomes after haplo-HCT are comparable with those for MSD and MUD, haplo-HCT is now a preferred alternative donor source for those patients with r/r HL, without MSD, or 10/10 MUD, or when there is an urgency to find a donor if MRD is not present. [57].
There is still debate concerning the superiority of one source of stem cells (bone-marrow or peripheral stem cells) over another. Recent studies suggested the advantage of peripheral blood stem cells versus bone marrow in terms of enhanced OS, PFS, and relapse-free survival, most probably due to a lower chance for relapse mediated by the higher lymphocyte content of peripheral blood stem cells versus that in the bone marrow [65,66].
As the best strategy for choosing a haploidentical donor is selecting a younger haploidentical donor, a sibling is preferable to a parent donor, and a father donor is better than a mother in terms of improving outcomes [67].
Nevertheless, the issue of which the best donor type is for patients who are candidates for allo-HCT is not yet resolved. There are data coming from retrospective analyses that indicate that haplodonors might be associated to a lower relapse rate, and thus to a better PFS. Castagna and colleagues reported results from a multicenter study that included 198 patients with chemosensitive HL (CR or PR before allo-HCT), performed from 2010 to 2014. Haplo-HCT was performed in 65 patients, and 133 patients underwent HLA-identical transplantation. Two-year PFS was significantly better for haplo than HLA-identical transplantation (63% vs 37%; p = 0.03), with lower relapse incidence in the haplo group (24% vs 44%; p = 0.008), without difference in OS. PFS, OS, and RI were significantly better in CR compared to in PR. This study suggests that haplo-HCT is more effective than HLA, identical in patients with chemosensitive HL, raising the question of donor choice [68].
Patients with HL who developed disease relapse or progression after allo-HCT often have a disease that is refractory to chemotherapy and are heavily pretreated. These patients have poor prognosis, and treatment is challenging.
Bazarbachi et al. assessed the outcomes in 184 adult patients with HL who had developed disease recurrence or progression after allo-HCT (MUD: matched unrelated donor or UD-unrelated donor) between 2010 and 2014. A total of 80 patients in the BV group who had received a median of six doses of post-transplant BV were compared with 104 who did not. Although there was a risk of selection bias [the BV group was younger (median age: 30 years vs. 34) and more likely to receive pretransplant BV (65%vs. 46%) or post-transplant DLI (66% vs. 33%) and there was a longer median follow-up for surviving patients in the BV group], this approach resulted in a CR rate of 29%, a PR rate of 45%, and a stable disease rate of 26%. The use of BV before DLI was associated with the highest probability of being alive and in CR (40%). These results indicate the benefit of BV as a safe and effective therapy for patients with HL who had developed disease recurrence or progression after undergoing allo-SCT, even after prior exposure to BV. Post-transplantation BV may synergize with immune intervention such as DLI to achieve sustained control of HL recurring after allo-HCT. Prospective randomized trials are needed to address this question [69].
Brentuximab vedotin is also a therapeutic option for r/r cHL recurring after allogeneic stem cell transplantation. A cohort of 25 patients with recurrent disease and without active GVHD received BV at 100 days after allo-SCT. Results reported an ORR rate of 50% and a complete response rate of 38%, supporting BV as a potential therapeutic option for selected patients relapsing after allo-SCT [70].
Another effective treatment with an acceptable safety profile for those patients relapsing after allo-SCT is nivolumab. Nivolumab as a single agent was administrated in 20 patients with cHL relapsing after allo-HCT with an ORR of 95%. Nivolumab initiation resulted in GVHD in all patients who had a previous history of aGVHD [71]
Patients with r/r HL relapsing after allo-HCT are candidates for PD-1 blockade as a therapeutic strategy that can provide durable disease control and prolonged survival. Those patients with prior history of GvHD have an increased risk for GvHD after checkpoint inhibition (30–55%).
The use of PD-1 blockades for patients progressing after more standard approaches resulted in very high response rates, and many of these responses were durable. These recent data challenged the timing of allo-HCT after relapse or progression after auto-HCT [72,73].
5. Conclusions
According to the 2019 annual report of EBMT, the current activity of hematopoietic stem-cell transplantation in Europe during 2019, in patients with r/r HL included 434 allotransplants and 2185 autotransplants. These data confirm that HCT is widely used in the treatment of patients with r/r Hodgkin disease [74].
HCT plays an important and clear role in the treatment of r/r HL. Auto-HCT remains the standard of care for most patients in the first chemosensitive relapse. Allo-HCT is most frequently used in young patients with failure after auto-HCT and with chemosensitive disease. Haplo-HCT is considered the preferred alternative donor source for those patients with r/r HL without a donor, or when there is urgency to find a donor if a matched related donor is not present.
Without any doubt, the transplant outcomes, NRM, PFS, and OS are significantly improved in both auto-HCT and allo-HCT due to the experience of transplant centers, a better selection of patients, and improvements in supportive measures.
The curative capacity of auto-HCT is improving with the introduction of new drug-based salvage strategies and consolidation strategies after auto-HCT.
Allo-HCT is a valid treatment strategy for patients with r/r cHL because results have improved over time, mainly with a safe combination of allo-HCT and new drugs.
New strategies are appearing in this field. CD30 CAR-T cell therapy is a promising treatment option for patients with r/r HL who progress after prior immunotherapies including BV and checkpoint inhibitors. In a two-center study, 41 heavily pretreated patients with relapsed or refractory Hodgkin lymphoma received fludarabine-based lymphodepletion followed by CD30.CAR-T cells. The patients in this study had poor prognosis, with a median of seven prior lines of therapy, including BV, checkpoint inhibitors, auto-HCT and allo-HCT. CD30.CAR-Ts had a high rate of durable responses, with an ORR of 72%, a CR rate of 59%, and a PFS of 41% at 1-year follow-up. The treatment was well-tolerated with an excellent safety profile [75].
AFM13 is CD30/CD16A-bispecific, first-in-class tetravalent antibody that provides a bridge between CD30 on HL cells and the CD 16A receptor on natural killer cells and macrophages, and induces tumor cell killing. This antibody demonstrated efficacy as a monotherapy for r/r Hl, but also in combination with pembrolizumab. The combination of AFM13 and pembrolizumab as novel immunotherapy is a promising therapeutic option for heavily pretreated patients with r/r HL. This combination provides an ORR of 83% [76].
Seven patients with r/r HL after allo-HCT using oral ibrutinib as an alternative for patients with prior history of GvHD were reported by Badar et al. The drug was well-tolerated, and four patients achieved a response [77].
Relapse is still the major cause of failure. Disease status at the time of transplant is one of the most important predictive factors. Achieving a greater level of disease response before transplant is greatly important for better post-transplant outcomes.
The introduction and availability of novel drugs such as the anti-CD 30 antibody, checkpoint inhibitors, and CAR-T cell therapy significantly influence the treatment algorithm in patients with r/r HL, and their use may pave the way for improving transplant outcomes (Table 6). As novel target drugs are incorporated earlier in the disease course, they could further optimize the outcome of HL by preventing relapse and by improving disease status prior to transplant.

Table 6.
Treatment of r/r HL recurring after stem-cell transplantation with brentuximab vedotin, nivolumab, pembrolizumab, and CAR-T cells.
Author Contributions
Conceptualization, writing—original draft preparation, review, and editing, S.G.S. and A.S. Both authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Eichenauer, D.A.; Aleman, B.M.P.; Andre, M.; Federico, M.; Hutchings, M.; Illidge, T.; Engert, A.; Ladetto, M. Hodgkin lymphoma: ESMO Clinical Practice. Guidelines for diagnosis, treatment and follow up. Ann. Oncol. 2018, 29 (Suppl. 4), iv19–iv29. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistic. Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.; Ambinder, R. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J. Clin. 2018, 68, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Alexander, F.E.; Jarrett, R.F.; Lawrence, D.; Armstrong, A.A.; Freeland, J.; Gokhale, D.A.; Kane, E.; Taylor, G.M.; Wright, D.H.; Cartwright, R.A. Risk factors for Hodgkin’s disease by Epstein-Barr virus (EBV) ststus: Prior infection by EBV and other agents. Br. J. Cancer 2000, 82, 1117–1121. [Google Scholar] [CrossRef] [Green Version]
- Ekstrand, B.C.; Horning, S.J. Hodgkin’s disease. Blood Rev. 2002, 16, 111–117. [Google Scholar] [CrossRef]
- Diehl, V.; Stein, H.; Hummel, M.; Zollinger, R.; Connors, J.M. Hodgkin’s lymphoma: Biology and treatment strategies for primary, refractory and relapsed disease. Hematology 2003, 2003, 225–247. [Google Scholar] [CrossRef] [Green Version]
- Hasenclever, D.; Diehl, V.; Armitage, J.O.; Assouline, D.; Björkholm, M.; Brusamolino, E.; Canellos, G.P.; Carde, P.; Crowther, D.; Cunningham, D. A prognostic scvore for advanced Hodgkin’s diosease. International Prognostic Factors Project on Advanced Hodgkin’s disease. N. Engl. J. Med. 1998, 339, 1506–1514. [Google Scholar] [CrossRef]
- Gallamini, A.; Hutchings, M.; Riggaci, L.; Specht, L.; Merli, F.; Hansen, M.; Patti, C.; Loft, A.; Di Raimondo, F.; D’Amore, F.; et al. Early intertrim 2-[18F]Fluoro-2-deoxy-d-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma:a report from a joint Italian-Danish study. J. Clin. Oncol. 2007, 25, 3746–3752. [Google Scholar] [CrossRef] [Green Version]
- Ansell, S.M. Brenduximab vedotin. Blood 2014, 124, 3197–3200. [Google Scholar] [CrossRef]
- Shah, G.; Moskowitz, C.H. Transplant strategies in relapsed/reafractory Hodgkin lymphoma. Blood 2018, 131, 1689–1697. [Google Scholar] [CrossRef]
- Sureda, A.; Barbosa Pereira, M.I.; Dreger, P. Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. The role of hematopoietic stem cell transplantation in the treatment of relapsed/refractory Hodgkin’s lymphoma. Curr. Opin. Oncol. 2012, 24, 727–732. [Google Scholar] [CrossRef]
- Diehl, V.; Franklin, J.; Pfreundschuh, M.; Lathan, B.; Paulus, U.; Hasenclever, D.; Tesch, H.; Herrmann, R.; Dörken, B.; Müller-Hermelink, H.K.; et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N. Engl. J. Med. 2003, 348, 2386–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linch, D.C.; Winfield, D.; Goldstone, A.H.; Moir, D.; Hancock, B.; McMillan, A.; Chopra, R.; Milligan, D.; Hudson, G.V. Dose intensification with autologous bone-marrow transplantation in relapsed and resist-ant Hodgkin’s disease: Results of a BNLI randomised trial. Lancet 1993, 341, 1050–1054. [Google Scholar] [CrossRef]
- Schmitz, N.; Pfistner, B.; Sextro, M.; Sieber, M.; Carella, A.M.; Haenel, M.; Boissevain, F.; Zschaber, R.; Müller, P.; Kirchner, H.; et al. Aggressive conventional chemotherapy compared with high dose chemotherapy with autologous haematopoietic stem cell transplantation for relapsed chemosensitive Hodgkin’s disease: A randomised trial. Lancet 2002, 359, 2065–2071. [Google Scholar] [CrossRef]
- Duarte, R.F.; Labopin, M.; Bader, P.; Basak, G.W.; Bonini, C.; Chabannon, C.; Corbacioglu, S.; Dreger, P.; Dufour, C.; Gennery, A.R.; et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: Current practice in Europe, 2019. Bone Marrow Transpl. 2019, 54, 1525–1552. [Google Scholar] [CrossRef]
- Carreras, E.; Dufour, E.; Mohty, M.; Kroger, N. The EBMT Handbook: Hematopietic Stem Cell Transplantation and Cellular Therapie; Springer: Cham, Switzerland, 2019; pp. 653–662. [Google Scholar]
- Santoro, A.; Mazza, R.; Pulsoni, A.; Re, A.; Bonfichi, M.; Zilioli, V.R.; Salvi, F.; Merli, F.; Anastasia, A.; Luminari, S.; et al. bendamustine in combination woth gemcitabine and vinorelbine is an effective regimen as induction chemotherapy before autologous stem-cell transplantation for relapsed or refractory Hodgkin lymphoma: Final results of a multicenter ophase II study. J. Clin. Oncol. 2016, 34, 3293–3299. [Google Scholar] [CrossRef] [Green Version]
- Moskowitz, A.J.; Yahalom, J.; Kewalramani, T.; Maragulia, J.C.; Vanak, J.M.; Zelenetz, A.D.; Moskowitz, C.H. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. 2010, 116, 4934–4937. [Google Scholar] [CrossRef] [Green Version]
- Josting, A.; Rudolph, C.; Reiser, M.; Mapara, M.; Sieber, M.; Kirchner, H.H.; Dörken, B.; Hossfeld, D.K.; Diehl, V.; Engert, A.; et al. Time-intensified dexamethasone/cisplatin/cytarabine: An effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin’s disease. Ann. Oncol. 2002, 13, 1628–1635. [Google Scholar] [CrossRef]
- Bartlett, N.L.; Niedzwiecki, D.; Jonson, J.L.; Friedberg, J.W.; Johnson, K.B.; Van Besien, K.; Zelenetz, A.D.; Cheson, B.D.; Canellos, G.P.; Cancer Leukemia Group B. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin’s lymphoma: CALGB 59804. Ann. Oncol. 2007, 18, 1071–1079. [Google Scholar] [CrossRef]
- Moskovitz, A.J.; Schoder, H.; Yahalom, J.; McCall, S.J.; Fox, S.Y.; Gerecitano, J.; Grewal, R.; Hamlin, P.A.; Horwitz, S.; Kobos, R.; et al. PET -adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosfamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin’s lymphoma: A non-randomised, oper-label, single center, phase 2 study. Lancet Oncol. 2015, 16, 284–292. [Google Scholar] [CrossRef]
- Chen, R.; Palmer, J.M.; Martin, P.; Tsai, N.; Kim, Y.; Chen, B.T.; Popplewell, L.; Siddiqi, T.; Thomas, S.H.; Mott, M.; et al. Results of a multiventer phase II trial of brentuximab vedotin as second -line therapy before autologous transplantation in relapsed/refractpry Hodgkin lymphoma. Biol. Blood Marrow Transpl. 2015, 21, 2136–2140. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Sanz, R.; Sureda, A.; Gonzales, A.P.; La Cruz, F.; Sanches-Gonzales, B.; Rodriguez, A.; Domingo-Domenech, E.; Miriam, M.; Lopez, J.; Jose, L.P.; et al. Brenduximab vedotin plus ESHAP (BRESHAP) is a highly effective combination for inducing remission in refractory and relapsed Hodgkin lymphoma patients prior to autologous stem cell transplant: A trial of the Spanish Group of Lymphoma and Bone Marrow Transplantation (GELTAMO) (abstract). Blood 2016, 128, 1109. [Google Scholar]
- LaCasce, A.S.; Bociek, R.G.; Sawas, A.; Caimi, P.; Agura, E.; Matous, J.; Ansell, S.M.; Crosswell, H.E.; Islas-Ohlmayer, M.; Behler, C.; et al. Brentuximab vedotin plus bendamustine: A highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood 2018, 132, 40–48, PMCID:PMC60735. [Google Scholar] [CrossRef] [PubMed]
- Cassaday, R.D.; Fromm, J.; Cowan, A.J.; Libby, E.N.; Phillip, M.; Behnja, S.; Nartea, M.; Press, O.; Gopal, A. Safety and activity of brenduximab vedotin (BV) plus ifosfamide, carboplatin, and etoposide (ICE) for relapsed/refractory (Rel/Ref) classical Hodgkin lymphoma (cHL) initial result of a phase I/II trial (abstract). Blood 2016, 128, 1834. [Google Scholar] [CrossRef]
- Hagenbeek, A.; Zijlstra, J.; Lugtenburg, P.; van Imhoff, G.; Nijland, M.; Tonino, S.; Hutchings, M.; Spiering, M.; Liu, R.; Burggraaf, C.N.; et al. Transplant BRaVE: Combining brenduximab-vedotin with DHAP as salvage treatment in relapsed/refractory Hodgkin lymphoma: A phase 1 dose-escalation study. Hematologica 2016, 101, 44. [Google Scholar]
- Herrera, A.F.; Moskowitz, A.J.; Bartlett, N.L.; Vose, J.M.; Ramchandren, R.; Feldman, T.A.; LaCasce, A.S.; Ansell, S.M.; Moskowitz, C.H.; Fenton, K.; et al. Interim results from a phase ½ study of brenduximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 2018, 131, 1183–1194. [Google Scholar] [CrossRef]
- Frederico, M.; Bellei, M.; Brice, P.; Brugiatelli, M.; Nagler, A.; Gisselbrecht, C.; Moretti, L.; Colombat, P.; Luminari, S.; Fabbiano, F.; et al. High-dose therapy and autologous stem-cell transplantation versus conventional therapy for patients with advanced Hodgkin’s lymphoma responding to front-line therapy. J. Clin. Oncol. 2003, 21, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, A.J.; Shah, G.; Schöder, H.; Ganesan, N.; Drill, E.; Hancock, H.; Davey, T.; Perez, L.; Ryu, S.; Sohail, S.; et al. Phase II Trial of Pembrolizumab Plus Gemcitabine, Vinorelbine, and Liposomal Doxorubicin as Second-Line Therapy for Relapsed or Refractory Classical Hodgkin Lymphoma. J. Clin. Oncol. 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Merryman, R.; Redd, R.; Nishihori, T.; Chavez, J.; Nieto, Y.; Darrah, J.M.; Rao, U.; Byrne, M.T.; Bond, D.A.; Maddocks, K.J.; et al. Autologous stem cell transplantation after anti-PD-1 therapy for multiply relapsed or refractory Hodgkin lymphoma. Blood Adv. 2021, 6, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, C.H.; Walewski, J.; Nademanee, A.; Masszi, T.; Agura, E.; Holowiecki, J.; Abidi, M.H.; Chen, A.I.; Stiff, P.; Viviani, S.; et al. Five-year PFS from the AETHERA trial of brenduximab vedotin for Hodgkin lymphoma at risk of progression or relapse. Blood 2018, 123, 2639–2642. [Google Scholar] [CrossRef] [Green Version]
- Shibon, D.; Morschhauser, F.; Eche-Rigon, M.; Ghez, D.; Dupuis, J.; Marçais, A.; Deau-Fischer, B.; Bouabdallah, R.; Sebban, C.; Salles, G.; et al. Single or tandem autologous stem-cell transplantation for first -relapsed or refractory Hodgkin lymphoma: 10-years follow-up of the prospective H96 trial by the LYSA/SFGM-TC study group. Hematologica 2016, 101, 474–481. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.P.; Li, H.; Friedberg, J.W.; Constine, L.S.; Rimsza, L.M.; Cook, J.R.; Laport, G.G.; Popplewell, L.L.; Holmberg, L.A.; Smith, S.M.; et al. Tandem Autologous Hematopoietic Cell Transplantation for Patients with Primary Progressive or Recurrent Hodgkin Lymphoma: A SWOG and Blood and Marrow Transplant Clinical Trials Network Phase II Trial (SWOG S0410/BMT CTN 0703). Biol Blood Marrow Transplant. 2018, 24, 700–707. [Google Scholar] [CrossRef] [Green Version]
- Younes, A.; Gopal, A.K.; Smith, S.E.; Ansell, S.M.; Rosenblatt, J.D.; Savage, K.J.; Ramchandren, R.; Bartlett, N.L.; Cheson, B.D.; de Vos, S.; et al. Results of a pivotal phase II study brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J. Clin. Oncol. 2012, 30, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Armand, P.; Engert, A.; Younes, A.; Fanale, M.; Santoro, A.; Zinzani, P.L.; Timmerman, J.M.; Collins, G.P.; Ramchandren, R.; Cohen, J.B.; et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: Extended follow-up of the multicohort single-arm phase CheckMate 205 trial. J. Clin. Oncol. 2018, 36, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zinzani, P.L.; Lee, H.J.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow up of KEYNOTE-087. Blood 2019, 134, 1144–1153. [Google Scholar] [CrossRef] [Green Version]
- Martinez, C.; Canals, C.; Sarina, B.; Alessandrino, E.P.; Karakasis, D.; Pulsoni, A.; Sica, S.; Trneny, M.; Snowden, J.A.; Kanfer, E.; et al. Identification of prognostic factors predicting outcome in Hodgkin’s lymphoma patients relapsing after autologous stem cell transplantation. Ann. Oncol. 2013, 24, 2430–2434. [Google Scholar] [CrossRef]
- Appelbaum, F.R.; Sullivan, K.M.; Deeg, H.J.; Neiman, P.E.; Sanders, J.E.; Stewart, P.; Storb, R. Allogeneic bone marrow transplanatation in the treatment of MOPP-resistant Hodgkin’s disease. J. Clin. Oncol. 1985, 3, 1490–1494. [Google Scholar] [CrossRef]
- Phillips, G.L.; Reece, D.E.; Barnett, M.J.; Connors, J.M.; Fay, J.W.; Herzig, G.P.; Herzig, R.H.; Klingemann, H.G.; Shepherd, J.D.; Wolff, S.N. Allogeneic marrow transplanatation for refractory Hodgkin disease. J. Clin. Oncol. 1989, 7, 1039–1045. [Google Scholar] [CrossRef]
- Gajewski, J.L.; Philips, G.L.; Sobocinski, K.A.; Armitage, J.O.; Gale, R.P.; Champlin, R.E.; Herzig, R.H.; Hurd, D.D.; Jagannath, S.; Klein, J.P.; et al. Bone marrow transplants from HLA-identical siblings in advanced Hodgkin’s disease. J. Clin. Oncol. 1996, 14, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Milpied, N.; Fielding, A.K.; Perace, R.M.; Ernst, P.; Goldstone, A.H. Allogeneic bone marrow transplant is not better than autologous transplant for patients with relapsed Hodgkin’s disease. J. Clin. Oncol. 1996, 14, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Schmitz, N. Role of allogeneic stem cell transplantation in relapse or refractory Hodgkin’s disease. Ann. Oncol. 2002, 13 (Suppl. 1), 128–132. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Genadieva Stavrik, S.; Boumendil, A.; Finel, H.; Khvedelidze, I.; Dietricht, S.; Dreger, P.; Hermine, O.; Kyriakou, C.; Robinson, S.; et al. Changes in patients population and characteristics of hematopoietic stem cell transplantation for relapsed/refractory Hodgkin lymphoma: An analysis of the Lymphoma Working Party of the EBMT. Bone Marrow Transpl. 2020, 55, 2170–2179. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Robinson, S.; Canals, C.; Carella, A.M.; Boogaerts, M.A.; Caballero, D.; Hunter, A.E.; Kanz, L.; Slavin, S.; Cornelissen, J.J.; et al. Reduced-intensity conditioning compared with conventional allogeneic stem cell transplantation in relapsed or refractory Hodgkin’s lymphoma: An analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J. Clin. Oncol. 2008, 26, 455–462. [Google Scholar] [CrossRef]
- Robinson, S.P.; Sureda, A.; Canals, C.; Russell, N.; Caballero, D.; Bacigalupo, A.; Iriondo, A.; Cook, G.; Pettitt, A.; Socie, G.; et al. Reduced intensity conditioning allogeneic stem cell transplantation for Hodgkin’s lymphoma: Identification of prognostic factors predicting outcome. Hematologica 2009, 94, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genadieva-Stavrik, S.; Boumendil, A.; Dreger, P.; Peggs, K.; Briones, J.; Corradini, P.; Bacigalupo, A.; Socié, G.; Bonifazi, F.; Finel, H.; et al. Myeloablative versus reduced intensity allogeneic stem cell transplantation for relapsed/refractory Hodgkin’s lymphoma in recent years: A retrospective analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Ann. Oncol. 2016, 27, 2251–2257. [Google Scholar] [CrossRef]
- Anderlini, P.; Saliba, R.; Acholonu, S.; Okoroji, G.J.; Donato, M.; Giralt, S.; Andersson, B.; Ueno, N.T.; Khouri, I.; De Lima, M.; et al. Reduced-intensity allogeneic stem cell transplantation in relapse and refractory Hodgkin’s disease: Low transplant-related mortality and impact of intensity of conditioning regimen. Bone Marrow Transplant. 2005, 35, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Alvares, I.; Sureda, A.; Caballero, M.D.; Urbano-Ispizua, A.; Ribera, J.M.; Canales, M.; García-Conde, J.; Sanz, G.; Arranz, R.; Bernal, M.T.; et al. Nonmyeloablative stem cell transplantation is an effective therapy for refractory or relapsed Hodgkin lymphoma:result of a Spanish prospective cooperative protocol. Biol. Blood Marrow Transplant. 2006, 12, 172–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peggs, K.S.; Sureda, A.; Qian, W.; Caballero, D.; Hunter, A.; Urbano-Ispizua, A.; Cavet, J.; Ribera, J.M.; Parker, A.; Canales, M.; et al. Reduced intensity conditioning for allogeneic haematopoetic stem cell transplantation in relapsed and refractory Hodgkin lymphoma; impact of alemtuzumab and donor lymphocyte infusion on long term outcomes. Br. J. Hematol. 2007, 139, 70–80. [Google Scholar] [CrossRef]
- Das-Gupta, E.; Tomson, K.J.; Bloor, A.J.C.; Clark, A.D.; Mackinnon, S.; Kayani, I.; Clifton-Hadley, L.; Patrick, P.; El-Mehidi, N.; Lawrie, A.; et al. Allo-HSCT in transplant-naïve patients with Hodgkin lymphoma: A single-arm, multicenter study. Blood Adv. 2019, 3, 4264–4270. [Google Scholar] [CrossRef] [Green Version]
- Peggs, K.S.; Kayani, I.; Edwards, N.; Kottaridis, P.; Goldstone, A.H.; Linch, D.C.; Hough, R.; Morris, E.C.; Fielding, A.; Chakraverty, R.; et al. Donor lymphocyte infusion modulate relapse risk in mixed chimeras and induce durable salvage in relapsed patients after T-cell-depleted allogeneic transplantation for Hodgkin’s lymphoma. J. Clin. Oncol. 2011, 29, 971–978. [Google Scholar] [CrossRef]
- Peggs, K.S.; Hunter, A.; Chopra, R.; Parker, A.; Mahendra, P.; Milligan, D.; Craddock, C.; Pettengell, R.; Dogan, A.; Thomson, K.J.; et al. Clinical evidence of a graft-versus Hodgkin’s lymphoma effect after reduced-intensity allogeneic transplantation. Lancet 2005, 365, 1934–1941. [Google Scholar] [CrossRef]
- Bazarbachi, A.; Boumendil, A.; Finel, H.; Mohty, M.; Castagna, L.; Peggs, K.S.; Blaise, D.; Afanasyev, B.; Diez-Martin, J.L.; Sierra, J.; et al. Brentuximab vedotin prior to allogeneic stem cell transplantation in Hodgkin lymphoma: A report from the EBMT Lymphoma Working Party. Br. J. Haematol. 2018, 181, 86–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrot, A.; Monjanel, H.; Bouabdallah, R.; Quittet, P.; Sarkozy, C.; Bernard, M.; Stamatoullas, A.; Borel, C.; Bouabdallah, K.; Nicolas-Virelizier, E.; et al. Impact of post-brentuximab vedotin consolidation on relapsed/refractory CD30+ Hodgkin lymphomas: A large retrospective study on 240 patients enrolled in the French Named-Patient Program. Haematologica 2016, 101, 466–473, PMCID:PMC5004383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merryman, R.W.; Castagna, L.; Giordano, L.; Ho, V.T.; Corradini, P.; Guidetti, A.; Casadei, B.; Bond, D.A.; Jaglowski, S.; Spinner, M.A.; et al. Allogeneic transplantation after PD-1 blockade for classic Hodgkin lymphoma. Leukemia 2021, 35, 2672–2683. [Google Scholar] [CrossRef] [PubMed]
- Castagna, L.; Bramanti, S.; Devillier, R.; Sarina, B.; Crocchiolo, R.; Furst, S.; El-Cheikh, J.; Granata, A.; Faucher, C.; Harbi, S.; et al. Haploidentical transplantation with post-infusion cyclophosphamide in advanced Hodgkin lymphoma. Bone Marrow Transpl. 2017, 52, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, J.; Bramanti, S.; Santoro, A.; Castagna, L. Haploidentical stem cell Transplantation in lymphomas-expectation and ptifals. J. Clin. Med. 2020, 9, 3589–3605. [Google Scholar] [CrossRef]
- Marani, C.; Raiola, A.M.; Morbelli, S.; Dominietto, A.; Ferrarazzo, G.; Avenoso, D.; Giannoni, L.; Varaldo, R.; Gualandi, F.; Grazia, D.; et al. Haploidentical transplant with post-transplant cyclophosphamide for relapsed or refractory Hodgkin lymphoma: The role of comorbidity index and pretransplant positron emission tomography. Biol. Blood Marrow Transpl. 2018, 24, 2501–2508. [Google Scholar] [CrossRef] [Green Version]
- Kanate, A.S.; Mussetti, A.; Kharfan-Dabaja, M.A.; Ahn, K.W.; DiGilio, A.; Beitinjaneh, A.; Chhabra, S.; Fenske, T.S.; Freytes, C.; Gale, R.P.; et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs. HLA-mached unrelated donors. Blood 2016, 127, 938–947. [Google Scholar] [CrossRef]
- Ghosh, N.; Karmali, R.; Rocha, V.; Ahn, K.W.; DiGilio, A.; Hari, P.N.; Bachanova, V.; Bacher, U.; Dahi, P.; de Lima, M.; et al. Reduced-intensity transplantation for lymphomas using haploidentical realated donors versus hla-matched sibling donors: A center for International Blood and Marrow transplant Research Analysis. J. Clin. Oncol. 2016, 34, 3141–3149. [Google Scholar] [CrossRef]
- Burroughs, L.M.; O’Donnell, P.V.; Sandmaier, B.M.; Storer, B.E.; Luznik, L.; Symons, H.J.; Jones, R.J.; Ambinder, R.F.; Maris, M.B.; Blume, K.G.; et al. Comparison of outcome of HLA-matched related, unrelated or HLA-haploidentical related hematopoetic ceklkl transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol. Blood Marrow Transpl. 2008, 14, 1279–1287. [Google Scholar] [CrossRef] [Green Version]
- Gayoso, J.; Balsalobre, P.; Pascual, M.J.; Castilla-Llorente, C.; López-Corral, L.; Kwon, M.; Serrano, D.; Piñana, J.L.; Herrera, P.; Ferrá, C.; et al. Busulfan-based reduced intensity conditioning regimens for haploidentical transplantation in relapsed/refractory Hodgkin lymphoma:Spanish multicenter experience. Bone Marrow Transpl. 2016, 51, 1307–1312. [Google Scholar] [CrossRef] [Green Version]
- Martinez, C.; Gayoso, J.; Canals, C.; Finel, H.; Peggs, K.; Dominietto, A.; Castagna, L.; Afanasyev, B.; Robinson, S.; Blaise, D.; et al. Post-transplantation cyclophosphamide-based haploidentical transplantation as alternative to matched sibling or unrelated donor transplantation for Hodgkin lymphoma: A registry study of the lymphoma working party of the European Society for Blood and marrow transplantation. J. Clin. Oncol. 2017, 35, 3425–3432. [Google Scholar] [PubMed] [Green Version]
- Dietrich, S.; Dreger, P.; Hermine, O.; Kyriakou, C.; Montoto, S.; Robinson, S.; Schmitz, N.; Schouten, H.C.; Sureda, A.; Tanase, A. Haploidentical stem cell transplantation for patients with lymphoma: A position statement from the Lymphoma Working Party-European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2020, 55, 317–324. [Google Scholar] [CrossRef]
- Bazarbachi, A.; Boumendil, A.; Finel, H.; Castagna, L.; Dominietto, A.; Blaise, D.; Diez-Martin, J.L.; Tischer, J.; Gülbas, Z.; Wallet, H.L.; et al. Influence of donor type, stem cell source and conditioning on outcomes after haploidentical transplant for lymphoma—A LWP-EBMT study. Br. J. Haematol. 2020, 188, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, J.; Devillier, R.; Bramanti, S.; Giordano, L.; Sarina, B.; Furst, S.; Granata, A.; Maisano, V.; Pagliardini, T.; De Philippis, C.; et al. peripheral blood stem cell versus Bone Marrow for the T-cell deplete haploidentical transplantation with post-transplant cyclophosphamide in Hodgkin lymphoma. Biol. Blood Marrow Transpl. 2019, 25, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, J.; Raiola, A.M.; Evangelista, A.; Carella, A.M.; Martino, M.; Patriarca, F.; Risitano, A.; Bramanti, S.; Busca, A.; Giaccone, L.; et al. Impact of donor age and kinship on clinical outcome after T-cell -replete haploidentical transplantation with PT-Cy. Blood Adv. 2020, 4, 3900–3912. [Google Scholar] [CrossRef] [PubMed]
- Castagna, L.; Busca, A.; Bramanti, S.; Raiola Anna, M.; Malagola, M.; Ciceri, F.; Arcese, W.; Vallisa, D.; Patriarca, F.; Specchia, G.; et al. Haploidentical related donor compared to HLA-identical donor transplantation for chemosensitive Hodgkin lymphoma patients. BMC Cancer 2020, 20, 1140, PMCID:PMC7685618. [Google Scholar] [CrossRef] [PubMed]
- Bazarbachi, A.; Boumendil, A.; Finel, H.; Mohty, M.; Castagna, L.; Blaise, D.; Peggs, K.S.; Afanasyev, B.; Diez-Martin, J.L.; Corradini, P.; et al. Brentuximab Vedotin for Recurrent Hodgkin Lymphoma After Allogeneic Hematopoetic Stem cell Transplantation: A Report from the EBMT Lymphoma Working Party. Cancer 2019, 125, 90–98. [Google Scholar] [CrossRef]
- Gopal, A.K.; Ramchandren, R.; O’Conor, O.A.; Berryman, R.B.; Advani, R.H.; Chen, R.; Smith, S.E.; Cooper, M.; Rothe, A.; Matous, J.V.; et al. Safety and efficacay of brenduximab vedotin for Hodgkin lymphoma recurring after allogeneic stem cell transplanatation. Blood 2012, 120, 560–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbaux, C.; Gauthier, J.; Brice, P.; Drumez, E.; Ysebaert, L.; Doyen, H.; Fornecker, L.; Bouabdallah, K.; Manson, G.; Ghesquières, H.; et al. Efficacy and tolerability of nivolumab after allogeneic transplanatation for relapsed Hodgkin lymphoma. Blood 2017, 129, 2471–2478. [Google Scholar] [CrossRef] [Green Version]
- Ansell, S.M.; Lesokhin, A.M.; Borrelo, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 blocade with nivolumab in relapsed or refractory Hodgkin lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armand, P.; Shipp, M.A.; Ribarg, V.; Michot, J.M.; Zinzani, P.L.; Kuruvilla, J.; Snyder, E.S.; Ricart, A.D.; Balakumaran, A.; Rose, S.; et al. Programmed death-1 blocade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J. Clin. Oncol. 2016, 34, 3733–3739. [Google Scholar] [CrossRef]
- Passweg, J.R.; Baldomero, H.; Chabannon, C.; Basak, G.W.; de la Cámara, R.; Corbacioglu, S.; Dolstra, H.; Duarte, R.; Glass, B.; Greco, R.; et al. European Society for Blood and Marrow Transplantation (EBMT). Hematopoietic cell transplantation and cellular therapy survey of the EBMT: Monitoring of activities and trends over 30 years. Bone Marrow Transpl. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.A.; Grover, N.S.; Beaven, A.W.; Lulla, P.D.; Wu, M.F.; Ivanova, A.; Wang, T.; Shea, T.C.; Rooney, C.M.; Dittus, C.; et al. Anti-CD30 CAR-T cell therapy in relapsed and refractory Hodgkin Lymphoma. J. Clin. Oncol. 2020, 38, 3794–3804. [Google Scholar] [CrossRef]
- Bartlett, N.L.; Herrera, A.F.; Domingo-Domenech, E.; Mehta, A.; Forero-Torres, A.; Garcia-Sanz, R.; Armand, P.; Devata, S.; Izquierdo, A.R.; Lossos, I.S.; et al. A phase 1b study of AFM13 in combination with pembrolizumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 2020, 136, 2401–2409, PMCID:PMC7685206. [Google Scholar] [CrossRef] [PubMed]
- Badar, T.; Astle, J.; Kakar, I.K.; Zellner, K.; Hari, P.N.; Hamadani, M. Clinical activity of ibrutinib in classical Hodgkin lymphoma relapsing after allogeneic stem cell transplantation is independent of tumor BTK expression. Br. J. Haematol. 2020, 190, e98–e101. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).